Abstract
Background and Purpose
Angiotensin II produces oxidative stress and endothelial dysfunction in cerebral arteries, and angiotensin II type I receptors may play a role in longevity and vascular aging. Angiotensin converting enzyme type 2 (ACE2) converts angiotensin II to angiotensin (1–7) and thus may protect against effects of angiotensin II. We hypothesized that ACE2 deficiency increases oxidative stress and endothelial dysfunction in cerebral arteries, and examined the role of ACE2 in age-related cerebrovascular dysfunction.
Methods
Endothelial function, expression of angiotensin system components, NADPH oxidase subunits, and proinflammatory cytokines were examined in cerebral arteries from adult [12 mo old] and old [24 mo old] ACE2 knockout (KO) and wild type (WT) mice. The superoxide scavenger tempol was used to examine the role of oxidative stress on endothelial function.
Results
Vasodilatation to acetylcholine was impaired in adult ACE2 KO [24±6% (mean +/− SE)] compared to WT mice [52±7%, p<0.05]. In old mice, vasodilatation to acetylcholine was impaired in WT mice [29±6%] and severely impaired in ACE2 KO mice [7±5%]. Tempol improved endothelial function in adult and old ACE2 KO and WT mice. Aging increased mRNA for TNFα in WT mice, and significantly increased mRNA levels of Nox2, p47phox, and Rcan1 in both ACE2 KO and WT mice. mRNA levels of angiotensin system components did not change during aging.
Conclusions
ACE2 deficiency impaired endothelial function in cerebral arteries from adult mice and augmented endothelial dysfunction during aging. Oxidative stress plays a critical role in cerebrovascular dysfunction induced by ACE2 deficiency and aging.
Keywords: Endothelium, angiotensin converting enzyme 2, aging, cerebral arteries, oxidative stress
INTRODUCTION
Stroke is the second most frequent cause of death from cardiovascular events in the US1. Hypertension and endothelial dysfunction increase with age and are risk factors for stroke2–5. Aging is associated with endothelial dysfunction and oxidative stress in cerebral arteries6–8. In people without coronary artery disease, endothelial dysfunction is associated with a four-fold increase in the risk of cerebrovascular events4. Cerebral endothelial dysfunction may also have a role in the pathophysiology of vascular cognitive impairment and Alzheimer’s disease9–11, 2.
Angiotensin II increases reactive oxygen species (ROS) and superoxide levels via increases in expression and activation of NADPH oxidases, a major source of superoxide anion in the vasculature12. Superoxide reacts with the vasodilator nitric oxide (NO.) to produce peroxynitrite, resulting in decreased NO bioavailability and endothelial dysfunction13, 14, 4. Angiotensin II impairs endothelial function in cerebral arteries and the microcirculation14–16. Interestingly, genetic deletion of angiotensin II type 1 (AT1) receptors markedly attenuates cerebrovascular dysfunction during aging8, 6. Pharmacological modulation of angiotensin signaling in patients with stroke is associated with decreased inflammation, better functional outcome, and decreased risk for future cardiovascular events17.
Angiotensin converting enzyme type 2 (ACE2), a homolog of ACE with different substrate specificity, metabolizes angiotensin II into angiotensin 1–718, 19. Binding of angiotensin (1–7) to the Mas receptor20 attenuates signaling cascades activated by angiotensin II, decreases activity of NAPDH oxidase 21, and produces vasodilatation20, 22. Several studies suggest that ACE2 levels may be reduced with aging23, 24, which should result in magnification of effects of angiotensin II. However, little is known about the function of ACE2 in cerebral arteries or in endothelial dysfunction during aging. In this study, we tested the hypothesis that ACE2 deficiency increases oxidative stress and vasomotor dysfunction in cerebral arteries, and examined effects of ACE2 on endothelial function in adult animals and during aging.
METHODS
Experimental animals
Studies were performed in adult [12±0.2 mo old (mean±SE)] and old [24±0.4 mo old] male ACE2 deficient (knockout or KO) and wild type (WT) mice (n=54). The ACE2 gene is located in the X chromosome, and ACE2 KO mice and WT littermates were derived from breeding heterozygous females with WT or KO males25. The mice were bred onto a C57 background for eight generations. All experimental protocols and procedures conform to the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Studies of endothelial function
Mice were euthanized with an overdose of sodium pentobarbital (150 mg/kg ip). The brain was rapidly removed and placed in ice-cold Krebs solution. The basilar artery was carefully isolated, removed, cannulated and pressurized to 60 mmHg in an organ bath. After an equilibration period of 30 minutes, baseline diameter was measured and contraction was examined in response to KCl (50 mmol/L). The arteries were submaximally constricted with the thromboxane A2 analog U46619 before assessment of dilator responses. Endothelium-dependent vasodilatation was tested with acetylcholine (ACh). Endothelium-independent vasodilatation was tested with sodium nitroprusside (SNP) and papaverine. To test the role of ROS, arteries were preincubated (30 min) with Tempol (1 mmol/L), a superoxide scavenger, before treatment with acetylcholine.
Cerebral arteries were also used for analysis of gene expression and immunohistochemical studies. Plasma was collected for measurement of angiotensin levels. Detailed methods are available in the Online-only Data Supplement (http://stroke.ahajournals.org).
Statistics
Results are expressed as mean±SEM. Statistical significance in assays of endothelial function was determined by repeated measures two-way ANOVA on the complete data set. Then, the significance of comparisons within the adult or old data set was evaluated using Tukey post hoc test, and the highest (10−4 M) concentration of acetylcholine. Comparison between adult and old mice was performed with ANOVA followed by Newman-Keuls multiple comparison test. Statistical significance for gene expression and peptide measurement assays was determined by two way ANOVA and Bonferroni post hoc test. Significant differences were identified when P<0.05. The analysis was performed using Prism 5 (Graphpad, La Jolla, CA) and was validated in SAS (SAS Institute Inc, Cary, NC).
RESULTS
Expression of components of the ACE2/Angiotensin 1–7/Mas axis and the renin angiotensin system
ACE2 deficiency was confirmed in ACE2 KO mice using real time-qPCR in samples from kidneys and brain arteries (Table 1 and Table S1.). ACE2 mRNA levels in cerebral arteries and kidneys were not significantly different between adult and old WT mice. ACE2 mRNA levels in brain cortex from adult and old WT mice did not change during aging (data not shown).
Table 1.
Gene expression in cerebral arteries from adult and old ACE2 KO and WT mice
| Adult | Old | |||
|---|---|---|---|---|
| WT | ACE2 KO | WT | ACE2 KO | |
| ACE2 | 1.00 (±0.25) | 0.05 (±0.03)* | 1.39 (±0.17) | 0.06 (±0.06)* |
| Mas | 1.00 (±0.14) | 0.86 (±0.08) | 1.05 (±0.32) | 0.73 (±0.05) |
| AT1 | 1.00 (±0.14) | 0.88 (±0.27) | 1.09 (±0.14) | 1.07 (±0.21) |
| EcSOD # | 1.00 (±0.09) | 1.08 (±0.11) | 1.40 (±0.18)* | 1.42 (±0.10) |
| Catalase # | 1.00 (±0.09) | 1.13 (±0.11) | 1.44 (±0.14)* | 1.78 (±0.18) † |
| Nrf2 | 1.00 (±0.05) | 0.93 (±0.07) | 1.11 (±0.09) | 1.16 (±0.10) |
| IL-6 | 1.00 (±0.21) | 0.57 (±0.21) | 1.27 (±0.41) | 1.76 (±0.70) |
| TNFα # | 1.00 (±0.23) | 0.99 (±0.31) | 2.03 (±0.39) * | 1.64 (±0.28) |
| Rcan1 # | 1.00 (±0.07) | 0.98 (±0.09) | 1.42 (±0.10)* | 1.41 (±0.09) † |
| iNOS | 1.00 (±0.17) | 0.65 (±0.11) | 1.16 (±0.24) | 1.12 (±0.12) |
Data expressed as mean ±SEM
n= 6–7 mice / value
P<0.05 vs adult WT
P=<0.05 vs adult KO
P<0.05 Overall effect of aging (two-way ANOVA)
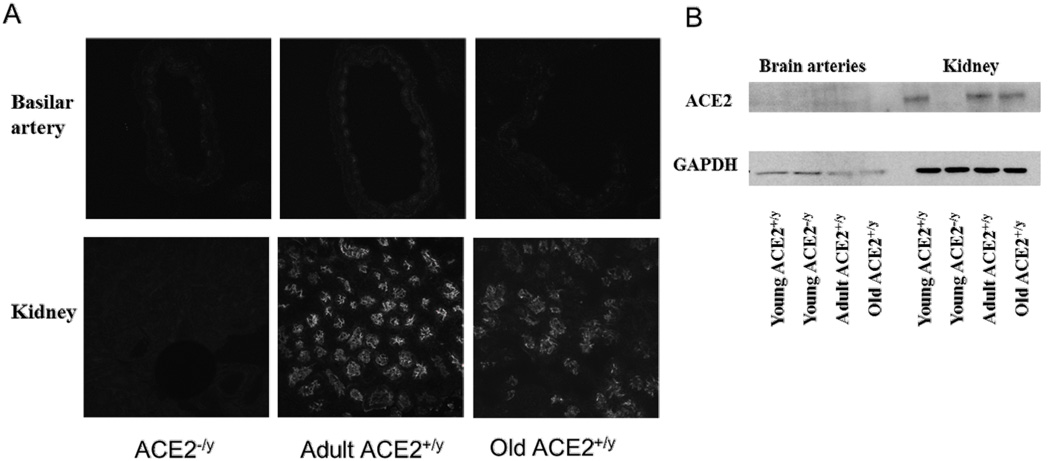
The presence of ACE2 protein was assessed using western blotting and immunostaining. ACE2 expression was confirmed in kidney homogenates from WT mice, but was absent in kidney homogenates from an ACE2 KO mouse (Fig 1B). ACE2 protein was not detected by Western blotting in homogenates from cerebral arteries (Fig 1B). ACE2 expression by immunofluorescence was abundantly localized in epithelium of renal tubules (Fig 1A). Weak positive staining for ACE2 was detected in sections from cerebral arteries, but it was difficult to differentiate from weak background staining in ACE2 KO mice (Fig 1A).
Figure 1.
ACE2 expression in basilar arteries and kidneys. Panel A: ACE2 staining in basilar artery, especially in the smooth muscle layer of adult WT mice. ACE staining was also seen in the epithelium of renal tubules in adult and old WT mice. Staining was absent in sections from ACE2 KO mice. Similar findings were observed in 3 mice from each group. Panel B: Western blots showing ACE2 expression in kidney homogenates from WT mice, and absence of protein in the ACE2 KO mouse. ACE2 staining was not detected by western blotting in brain arteries.
In cerebral arteries and kidneys, mRNA levels of the angiotensin 1–7 receptor Mas and the angiotensin II type 1 receptor (AT1R) were similar in all groups (Table 1 and Table S1).
Effect of ACE2 deficiency on blood pressure
Systolic blood pressure was comparable (P>0.05) between adult ACE2 KO (104±3) and WT mice (106±4 mmHg). Similar results were found in old ACE2 KO (113±12) vs WT mice (109±6 mmHg).
Vascular function in adult ACE2 KO and WT mice
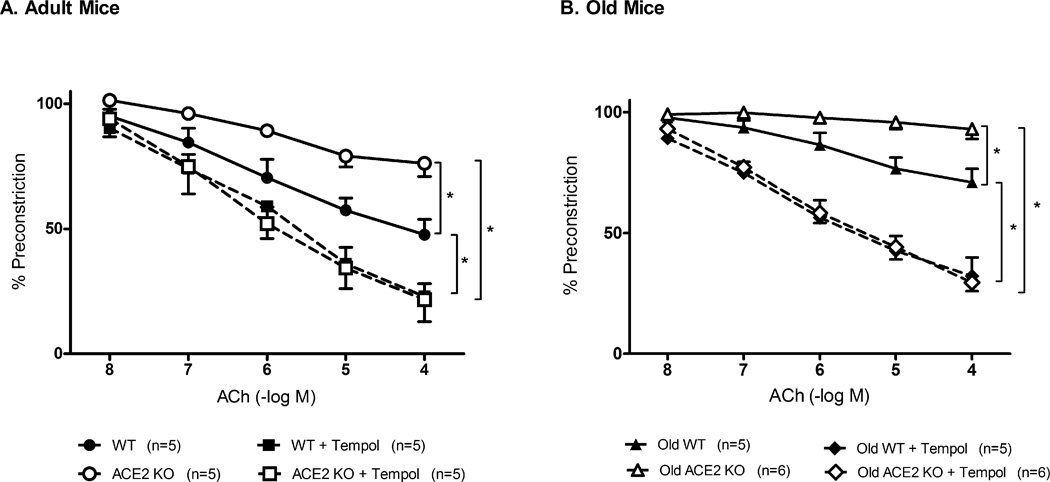
Baseline diameter of the basilar artery under resting conditions (prior to precontraction) was similar in ACE2 KO (163±8 µm) and WT mice (172±6µm). Dilatation to acetylcholine was reduced by ~50% in the basilar artery from adult ACE2 KO mice [24±6%] compared to adult WT mice [52±7%, p<0.05]. Tempol improved responses to acetylcholine in both ACE2 KO (to 78±7%, p<0.05) and WT mice (87±13%, p<0.05) (Fig 2A). Vasodilatation to the endothelium independent agonist, sodium nitroprusside, and papaverine was similar in both groups.
Figure 2.
Effects of ACE2 deficiency and oxidative stress on vascular function. Panel A: Vasodilatation to acetylcholine (ACh) in adult ACE2 WT(●, n=5) and KO (○, n=5) mice. Role of oxidative stress was examined after incubation with tempol of basilar arteries from adult ACE2 WT (■) and KO (□) mice. Panel B: Vasodilatation to acetylcholine (ACh), was examined in old ACE2 WT(▲, n=5) and KO (△, n=6) mice. Tempol was also added to arteries from old ACE2 WT (◆) and KO (◇) mice. Values are mean ±SE, *p<0.05.
Vasoconstriction to KCl (50 mmol/L) was comparable between ACE2 KO (43±5%) and WT mice (48±3%). Vasoconstriction to U46619 was also similar in ACE2 KO (26±1%) and WT mice (25±3%).
Effect of ACE2 deficiency and aging in cerebral vascular function
Diameter of the basilar artery was similar in old ACE2 KO (166 ±5 µm) vs WT (182 ±7 µm) mice (p=0.07). Maximal vasodilatation to acetylcholine was significantly less in old WT mice than in adult WT mice (Fig S1). Similarly, maximal responses to acetylcholine were less in old ACE2 KO mice than in adult ACE2 KO mice (p<0.05). In old mice, vasodilatation to acetylcholine was profoundly impaired in ACE2 KO mice, and was significantly less than in old WT mice (Fig 2B). Tempol improved responses to acetylcholine in both old ACE2 KO (p<0.01) and old WT mice (p<0.01). Vasodilatation to sodium nitroprusside and papaverine was similar in both groups.
Vasoconstriction to KCl was also preserved in ACE2 WT (43±4%) and KO mice (51±2%). Vasoconstriction in response to U46619 was similar in old ACE2 WT (28±4%)and KO mice (32±2%).
Oxidative stress and inflammation
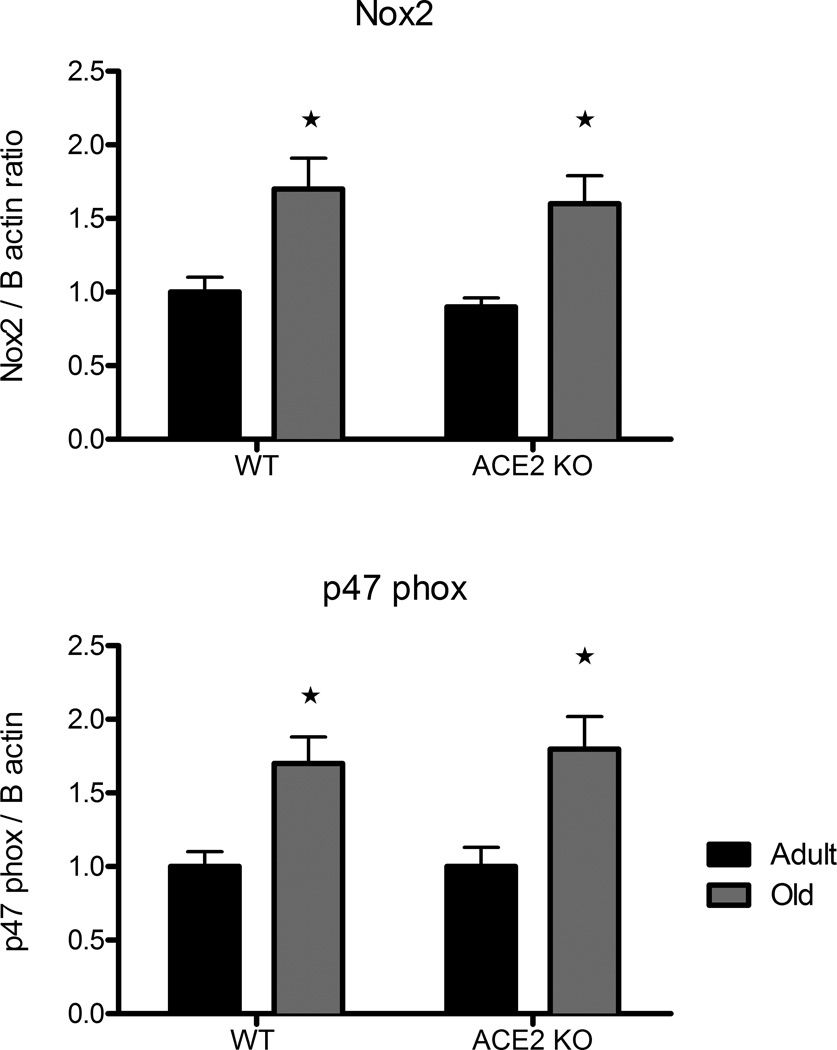
mRNA transcript levels of NADPH oxidase subunits p47phox and Nox2 were higher in cerebral arteries from old vs adult mice (Fig 3). Expression of the subunits was not affected by genotype (Fig 3). Nrf2 levels were similar in the four groups. Expression of EcSOD was increased in old WT mice (Table 1). Catalase was increased significantly in old ACE2 KO and WT mice (Table 1). Nitrotyrosine immunostaining was relatively low in sections of basilar artery from adult WT mice. Quantification of these data was difficult, but the immunostaining appeared to be increased in adult ACE2 KO mice and old ACE2 KO and WT mice (Fig S2).
Figure 3.
Effect of aging on expression of NADPH oxidase subunits. Relative expression levels of Nox2 and p47phox mRNA in cerebral arteries from adult (black bars) and old (gray bars) wild type or ACE2 KO mice. Values are mean ±SE (n=7/group), *p<0.05 vs adult mice.
Aging was associated with increased mRNA levels of TNFα in WT mice. Rcan1 mRNA levels were also significantly increased in cerebral arteries from both ACE2 KO and WT mice. IL-6 and iNOS mRNA levels were not significantly different between groups (Table 1).
Levels of angiotensins in plasma
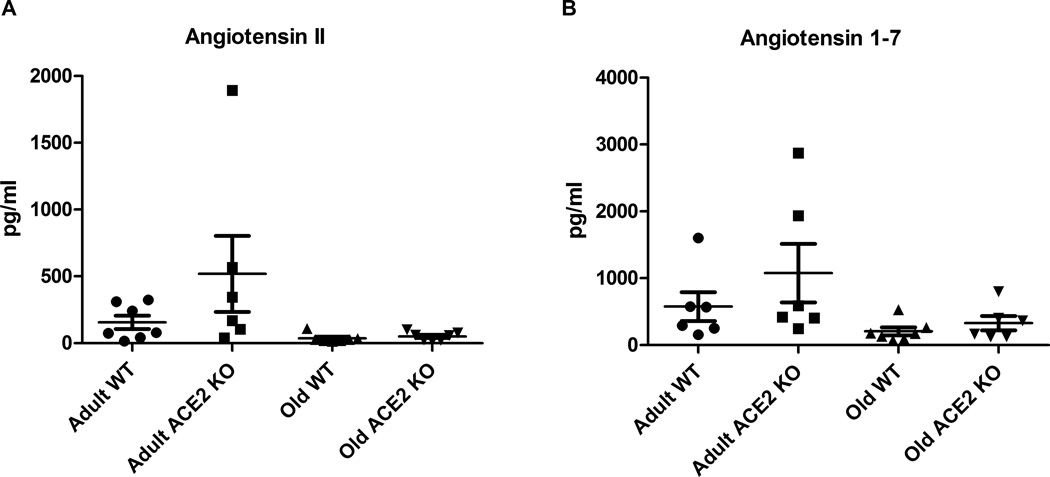
Angiotensin II levels were not significantly different between adult ACE2 KO and WT mice (Fig 4A). Angiotensin II levels were not significantly affected by aging although they tended to be lower in old ACE2 KO and WT mice. Likewise, angiotensin 1–7 levels were not affected by aging or genotype (Fig 4B).
Figure 4.
Plasma levels of angiotensin peptides. Panel A: Angiotensin II levels in plasma from adult WT (n=7), adult ACE2 KO (n=6), old WT (n=7) and old ACE2 KO (n=7) mice. Panel B: Angiotensin 1–7 levels in plasma from adult WT (n=6), adult ACE2 KO (n=6), old WT (n=6) and old ACE2 KO (n=7) mice. There were no significant differences in plasma peptide levels between groups. Values are mean ±SE.
DISCUSSION
There are several major new findings in this study. First, genetic deficiency of ACE2 impaired endothelial function in the cerebral circulation. Second, cerebrovascular dysfunction during aging was greater in ACE2 KO mice than in WT mice. Third, oxidative stress plays a key role in cerebrovascular dysfunction with ACE2 deficiency and during aging. These findings provide evidence for an important role of ACE2 in the maintenance of endothelial function in cerebral arteries under normal conditions and support the overall concept that the renin angiotensin system has a major impact on the cerebrovasculature with aging. We chose to study cerebral vessels, because cerebral arteries are important resistance vessels in the brain14 and play an important role in the pathophysiology of stroke33. Previous data have shown that young ACE2 KO mice have endothelial dysfunction in conduit vessels such as aorta26, but effects on the cerebral circulation and mechanisms responsible for impaired vascular function in ACE2 KO mice have not been explored.
There is a poor understanding of the role of ACE2 in cardiovascular disease or stroke. Studies of ACE2 polymorphisms in patients suggest a weak association of the ACE2 G9570A polymorphism with stroke 27. Decreased expression of ACE2 has been found in kidneys of patients with diabetes and renal disease28. Hypertension is an important risk factor for stroke and one might expect that ACE2 deficiency would be associated with hypertension. ACE2 deficiency however has little or no effect on blood pressure29, and hypertension does not appear to contribute to endothelial dysfunction in ACE2 deficient mice. We did not find any significant differences in blood pressure between WT and ACE2 KO mice in either age group. Our values for blood pressures are comparable to those reported previously for ACE2 KO mice25. These findings in mice are consistent with studies in humans, in which association of ACE2 polymorphisms with hypertension is variable30–32.
We found that endothelial function was impaired in adult ACE2 KO mice. Moreover, we found that cerebrovascular dysfunction during aging was augmented in old ACE2 KO mice. Because a superoxide scavenger restored endothelial responses to acetylcholine, our data suggest that oxidative stress plays a primary role in dysfunction caused by ACE2 deficiency. Furthermore, nitrotyrosine staining appeared to be higher in basilar arteries from ACE2 KO mice which suggest that these vessels are exposed to relatively greater oxidative stress than wild type mice. Consistent with these findings, superoxide has been proposed to be a key mediator of cerebrovascular dysfunction in other models of aging and disease6, 7, 12, 13, 33–35.
ACE2 may play an important role in regulation of oxidative stress in blood vessels. ACE2 overexpression prevents angiotensin II-induced increase in ROS and NADPH oxidase expression in endothelium26. On the other hand, ACE2 inhibition enhanced angiotensin-II-stimulated ROS formation36. We measured expression of antioxidant proteins, NADPH oxidase subunits, and proinflammatory genes to explore possible mechanisms that may contribute to increased dysfunction in ACE2 KO mice. Concordant with previous studies6, 7, we found that aging increased expression of NADPH oxidase subunits in cerebral arteries. In addition, gene expression data indicated that aging had a significant effect on the expression of the proinflammatory molecules TNFα and Rcan 1. Rcan1 modulates vasomotor function37 and its expression is increased by angiotensin II38. We also found an increase in SOD and catalase mRNA levels in cerebral arteries from old mice, which presumably is a compensatory mechanism to limit increases in ROS. We did not find changes in Nrf2, which is thought to be a master regulator of antioxidant expression during aging. Our findings for antioxidant enzymes and Nrf2 in cerebral vessels differ from previous studies in which decreased activity and expression of antioxidant proteins was found in rat aorta 39 and carotid arteries from macaques40.
We initially speculated that expression of ACE2 decreases with aging which, by loss of inhibitory effects on the renin angiotensin system, might lead to higher concentrations of angiotensin II and more angiotensin II-related cardiovascular pathology with aging. Multiple lines of evidence suggest an association between increased angiotensin II signaling and aging. First AT1 receptor deficient mice live longer than WT controls41. Second, cerebrovascular dysfunction with aging is attenuated in AT1 receptor deficient mice8. Third, long term administration of AT1 receptor blockers is associated with improved metabolic profiles during aging, which mimic some of the effects of caloric restriction42. We did not find, however, an effect of aging on expression of ACE2, Mas or AT1 receptors in cerebral arteries, kidney, or brain cortex. These results differ from findings in rat lung, in which expression of ACE2 protein decreases with aging24.
We used mice in which ACE2 is knocked out in all tissues. Contrary to what could be expected, we did not find differences in plasma levels of angiotensin II or angiotensin 1–7 between ACE2 KO and WT mice. These results agree with previous studies that demonstrated that plasma angiotensin II or angiotensin 1–7 were not significantly different in plasma from ACE2 deficient and WT mice43, 44. It is possible that other enzymes including prolyl45 or neutral endopeptidases46 compensate for ACE2 deficiency and maintain normal angiotensin 1–7 levels. Moreover, ACE2 metabolizes several peptides including angiotensin II, apelin, des-Arg9 bradykinin, ghrelin and neurotensins18. Some of these peptides modulate vasomotor function, so it is possible that the altered vascular phenotype in ACE2 KO mice can be explained by alterations in signaling pathways other than (or in addition to) the angiotensin II pathway.
In summary, this is the first study to show that ACE2 deficiency impaired function in cerebral arteries, and exaggerates cerebrovascular dysfunction with aging. It is known that angiotensin II impairs neurovascular coupling12, induces oxidative stress and produces vasomotor dysfunction in cerebral arteries15, 16, and plays an important role in cerebrovascular dysfunction with aging8, 9, 22. Therefore it is possible that by modulating effects of angiotensin II, ACE2 plays an important role in the maintenance of vascular function and prevention of cerebrovascular disease. Therapeutic approaches to increase ACE2 levels and activity might be beneficial in the management and prevention of cerebrovascular disease.
ACKNOWLEDGEMENTS
We thank Dr Chantal Allamargot in the Central Microscopy Research Facility and Dr Ana Sierra in Internal Medicine for technical assistance with immunostaining, Dr Rhonda de Cook in the Department of Statistics (University of Iowa) for statistical advising. We also thank Dr Bridget Brosnihan at the Hypertension Core Laboratory at Wake Forest University for measurement of angiotensin peptides.
SOURCES OF FUNDING
This work was supported by NIH grants NS-24621, HL-62984, HL-38901 and HL-113863; a Carver Program of Excellence. R.P was supported by a Fulbright Scholarship and American Heart Association Predoctoral Fellowships (0815525G, 10PRE3780044).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics-2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yavuz BB, Yavuz B, Sener DD, Cankurtaran M, Halil M, Ulger Z, et al. Advanced age is associated with endothelial dysfunction in healthy elderly subjects. Gerontology. 2008;54:153–156. doi: 10.1159/000129064. [DOI] [PubMed] [Google Scholar]
- 3.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. Sirt-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 5.Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 6.Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: Role of oxidative stress. Microcirculation. 2008;15:225–236. doi: 10.1080/10739680701641421. [DOI] [PubMed] [Google Scholar]
- 7.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 8.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2009;296:H1914–H1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuliani G, Cavalieri M, Galvani M, Passaro A, Munari MR, Bosi C, et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer's disease or vascular dementia. J. Neurol. Sci. 2008;272:164–170. doi: 10.1016/j.jns.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 11.Benarroch EE. Neurovascular unit dysfunction: A vascular component of Alzheimer disease? Neurology. 2007;68:1730–1732. doi: 10.1212/01.wnl.0000264502.92649.ab. [DOI] [PubMed] [Google Scholar]
- 12.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ. Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 13.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler. Thromb. Vac. Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 14.Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol. 2011;300:H1566–H1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrissobolis S, Faraci FM. Sex differences in protection against angiotensin II-induced endothelial dysfunction by manganese superoxide dismutase in the cerebral circulation. Hypertension. 2010;55:905–910. doi: 10.1161/HYPERTENSIONAHA.109.147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H397–H407. doi: 10.1152/ajpheart.00679.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Napoli M, Papa F. Angiotensin-converting enzyme inhibitor use is associated with reduced plasma concentration of c-reactive protein in patients with first-ever ischemic stroke. Stroke. 2003;34:2922–2929. doi: 10.1161/01.STR.0000099124.84425.BB. [DOI] [PubMed] [Google Scholar]
- 18.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 19.Turner AJ, Tipnis SR, Guy JL, Rice GI, Hooper NM. Aceh / ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE. Biochemistry (Mosc) 2002;353:346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- 20.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor mas. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 22.Feterik K, Smith L, Katusic ZS. Angiotensin-(1–7) causes endothelium-dependent relaxation in canine middle cerebral artery. Brain Res. 2000;873:75–82. doi: 10.1016/s0006-8993(00)02482-3. [DOI] [PubMed] [Google Scholar]
- 23.Liang W, Zhu Z, Guo J, Liu Z, Zhou W, Chin DP, et al. Severe acute respiratory syndrome, Beijing 2003. Emerg Infect Dis. 2004;10:25–31. doi: 10.3201/eid1001.030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 26.Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 27.Mo YJ, Huang WH, Chen DL, Chen FR. Relationship of angiotensin-converting enzyme 2 gene polymorphism with the prognosis of hypertensive stroke patients. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:84–87. [PubMed] [Google Scholar]
- 28.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- 29.Gurley SB, Coffman TM. Angiotensin-converting enzyme 2 gene targeting studies in mice: Mixed messages. Experimental Physiology. 2008;93:538–542. doi: 10.1113/expphysiol.2007.040014. [DOI] [PubMed] [Google Scholar]
- 30.Fan X, Wang Y, Sun K, Zhang W, Yang X, Wang S, et al. Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of captopril in women. Clin Pharmacol Ther. 2007;82:187–196. doi: 10.1038/sj.clpt.6100214. [DOI] [PubMed] [Google Scholar]
- 31.Benjafield AV, Wang WY, Morris BJ. No association of angiotensin-converting enzyme 2 gene (ACE2) polymorphisms with essential hypertension. Am J Hypertens. 2004;17:624–628. doi: 10.1016/j.amjhyper.2004.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu N, Yang Y, Wang Y, Liu Y, Fu G, Chen D, et al. ACE2 gene polymorphism and essential hypertension: An updated meta-analysis involving 11,051 subjects. Mol Biol Rep. 2012;39:6581–6589. doi: 10.1007/s11033-012-1487-1. [DOI] [PubMed] [Google Scholar]
- 33.Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- 34.Kitayama J, Yi C, Faraci FM, Heistad DD. Modulation of dilator responses of cerebral arterioles by extracellular superoxide dismutase. Stroke. 2006;37:2802–2806. doi: 10.1161/01.STR.0000245134.12145.ae. [DOI] [PubMed] [Google Scholar]
- 35.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 36.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1–7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55:166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riper DV, Jayakumar L, Latchana N, Bhoiwala D, Mitchell AN, Valenti JW, et al. Regulation of vascular function by RCAN1 (ADAPT78) Arch Biochem Biophys. 2008;472:43–50. doi: 10.1016/j.abb.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Esteban V, Mendez-Barbero N, Jimenez-Borreguero LJ, Roque M, Novensa L, Garcia-Redondo AB, et al. Regulator of calcineurin 1 mediates pathological vascular wall remodeling. J Exp Med. 2011;208:2125–2139. doi: 10.1084/jem.20110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demaree SR, Lawler JM, Linehan J, Delp MD. Ageing alters aortic antioxidant enzyme activities in Fischer-344 rats. Acta Physiol. Scand. 1999;166:203–208. doi: 10.1046/j.1365-201x.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- 40.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J. Gerontol. A. Biol. Sci. Med. Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. 2009:119. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Cavanagh EMV, Inserra F, Ferder L. Angiotensin II blockade: A strategy to slow ageing by protecting mitochondria? Cardiovasc. Res. 2010:31–40. doi: 10.1093/cvr/cvq285. [DOI] [PubMed] [Google Scholar]
- 43.Bharadwaj MS, Strawn WB, Groban L, Yamaleyeva LM, Chappell MC, Horta C, et al. Angiotensin-converting enzyme 2 deficiency is associated with impaired gestational weight gain and fetal growth restriction. Hypertension. 2011;58:852–858. doi: 10.1161/HYPERTENSIONAHA.111.179358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel VB, Bodiga S, Basu R, Das SK, Wang W, Wang Z, et al. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: A critical role of the angiotensin II/AT1 receptor axis. Circ. Res. 2012;110:1322–1335. doi: 10.1161/CIRCRESAHA.112.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welches WR, Santos RA, Chappell MC, Brosnihan KB, Greene LJ, Ferrario CM. Evidence that prolyl endopeptidase participates in the processing of brain angiotensin. J. Hypertens. 1991;9:631–638. doi: 10.1097/00004872-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (ec 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692–696. doi: 10.1161/01.hyp.19.6.692. [DOI] [PubMed] [Google Scholar]