Abstract
Background
Depressive symptoms are common in Parkinson’s disease (PD); however, it is unclear whether there are specific depressive symptom patterns in patients with PD and co-morbid depression (dPD).
Objective
The goal of this study is to examine the frequency and correlates of specific depressive symptoms in PD.
Method
A sample of 158 individuals with PD completed the self-rated Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS). By multiple-regression analysis, the authors examined the association between HANDS total and subscale scores and various demographic variables.
Results
The frequency of depression was 37% (N=58). Patients with a history of depression before PD had significantly more serious depression than those who had no such history. Of those who were more depressed, the most common symptoms of depression endorsed were low energy, difficulty with concentration/making decisions, feeling blue, feeling hopeless, and having poor sleep.
Conclusion
There is a relatively high prevalence of dPD. Items on the HANDS that discriminated best between depressed and nondepressed subjects with PD included feeling blue, feeling hopeless, feeling worthless, lack of interest, and self-blame. It remains to be defined whether dPD should be understood primarily as a psychological reaction to a physical disability or perceived impending one, or as a direct expression of the neuropathology of PD.
Parkinson’s disease (PD), first described almost two centuries ago (1817) by James Parkinson as the “shaking palsy,” is the second most common neurodegenerative disorder, affecting approximately 500,000 individuals in the United States.1 Depression is a significant health problem, and is associated with worsened health status across a variety of diseases.2 Since the 1920s, depression has been reported as a common feature of PD,3 and, indeed, is the most studied psychiatric disorder in PD patients. Reports of prevalence rates vary, ranging from 7% to 90%, with a general consensus that depression in some form (i.e., either major or non-major depression) appears to occur in approximately 40% of PD patients.4,5 It can be difficult to diagnose depression accurately in PD patients (dPD), in part because the motor impairments of PD may overlap with common depressive symptoms, such as reduced energy, psychomotor retardation, mental slowing, difficulties concentrating, and insomnia.6 As part of the NINDS/NIMH Work Group on Depression and Parkinson’s Disease, Marsh and colleagues7 reviewed the difficulties of accurately diagnosing depression within the context of PD. Symptoms of PD may mask symptoms of depression, leading to symptoms that cannot be identified. It is also common for clinicians, as well as patients, to discount depressive symptoms when they occur in the presence of a major medical illness such as PD. They endorse an “inclusive” strategy for managing symptom assessment; counting symptoms toward both conditions, not one or the other.7 In terms of rating scales, there are several from which to choose, depending on the clinical or research goal.8 Currently, there is no specific dPD scale.
Disabilities due to motor impairments, such as tremor, rigidity, and slowness, dominate the clinical manifestations of PD, but mood changes and even psychoses are being recognized as major sources of disability. Depression may be associated with specific impairments in PD. Weintraub and others9 found that depression significantly contributes to a PD patient’s disability. Norman and colleagues5 found that depression in PD was associated with more rapid disease progression, cognitive decline, memory difficulties, and functional disability. Yamamoto3 proposed that depression accounts for about 58.2% of impairment in quality of life (QOL) in PD patients, affecting QOL more than severity of motor disability. Given the co-occurrence of dPD and the impact of dPD, we conducted a cross-sectional study to identify the prevalence and specific symptoms of depression in a cohort of PD patients attending an outpatient clinic. This is an exploratory analysis; we did not have a priori hypotheses, and we were interested in seeing whether we could detect depressive symptom patterns.
METHOD
We invited 200 consecutive individuals with a research diagnosis of PD, who attended the Massachusetts General Hospital Movement Disorders Unit between March 3, 2004, and June 2, 2005, to complete the Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS).10 This study was approved by the Institutional Review Board at MGH. Of the invited patients, 158 completed the questionnaire, and their data were available for analysis. Of the 42 who did not participate, 15 left certain items on the HANDS unanswered, and 19 refused to complete the HANDS. We found no evidence of differences between those who filled out the questionnaires completely and those who did not (see Results section). Because of scheduling conflicts, we were not able to offer the HANDS to eight patients. The HANDS is a 10-item self-report scale, each item of which assesses one of the following symptom areas over the previous 2 weeks: decreased energy, self-blame, appetite, sleep, hopelessness, sadness, interest, feelings of worthlessness, suicidal thoughts, and loss of concentration. Each item is graded on a 0 (normal)-to-3 (severe) scale, with a maximum Depression score of 30; that is, the HANDS Total score or sum of the items. Typically, a score of >9 indicates depression. In order to increase sensitivity while maintaining an acceptable level of specificity, a score of > 6 on the HANDS was used to identify depression.
The HANDS scale was selected to detect the presence of depression and identify specific symptoms of depression because it is a brief questionnaire with a high degree of correspondence with DSM–III-R criteria for major depression.10 Furthermore, the HANDS has been shown to have adequate reliability, as measured by internal consistency; correlation of each item with the total score (minus the item) was moderate-to-high, and its coefficient α was 0.87.10 Completion time for the HANDS is approximately 1–3 minutes. For each of the 158 patients, per chart review, we also collected demographic, historical, and clinical data, including age, gender, history of depression or other psychiatric illness before developing PD, presence of dementia, age at PD onset, and PD severity, as judged by the Hoehn and Yahr scale.11 The Hoehn and Yahr Scale was designed to include the entire range of Parkinson’s states. It is a clinician-rated instrument, allocating a 0-to-5 scale to indicate the relative level of disability the patient is experiencing (0: no visible symptoms of PD; to 5: PD symptoms on both sides of the body and unable to walk).11
Data Analyses
With respect to data analysis methods, the distribution of the key dependent variable, the HANDS Total score, was positively skewed in violation of the normality assumptions of some of the significance tests we used. Therefore, where needed, a more normally distributed log-transformation of this score was analyzed first to establish statistical significance of effects. Significant findings were then followed with the same analysis, but with the raw HANDS Total scores, to obtain more interpretable effect estimates (regression coefficients, correlation coefficients, mean differences, etc.). Multiple-regression analyses examined the relation of the HANDS Total to various demographic variables adjusted for each other, as well as the separate relationship of each individual HANDS item to the same variables. We used the method of backward-elimination of nonsignificant effects. We also used non-parametric tests (i.e., the Mann-Whitney, to test for group differences for continuous variables, and Fisher’s exact test for categorical variables).
The depression frequency-estimate and analysis that examined the relationship of the depressed/nondepressed patient dichotomy to other variables were dependent on our chosen cutoff on the HANDS for depression (≥6). However, the regression analyses relating the HANDS Total or the individual HANDS items to demographic variables (age, duration of PD illness, Hoehn and Yahr stage, history of depression, dementia, and gender) did not involve any predetermined HANDS cutoff.
RESULTS
A total of 158 individuals were screened for dPD (mean age: 66.82 years; standard deviation [SD]: 9.6; there were 108 men [68%], 50 women [32%]). The mean age at PD onset was 57.7 (10.8) years, and the mean Hoehn and Yahr score was 2.4 (1.0), which suggests mild bilateral disease. The mean duration of PD was 9.10 (6.2) years. On the basis of the definition of depression as HANDS (≥6), of the 158 screened individuals with PD, the rate of depression was 37% (N=58; 95% confidence interval [CI]: 29.1%–44.3%); 38 of these 58 subjects (66%) were being treated with an antidepressant. Thirty-two patients (32%) with a HANDS score <6 were also on an antidepressant.
We compared those who filled out the HANDS questionnaire (N=158) with those who refused or only partially filled it out (N=34), and there were no significant differences in age, although there was a significant (p=0.001) overrepresentation of women among the partial completers, for unknown reasons. The items that were most often left unanswered were: “had feelings of worthlessness” (5 subjects); “been feeling no interest in things” (4 subjects); and “been feeling blue” (4 subjects).
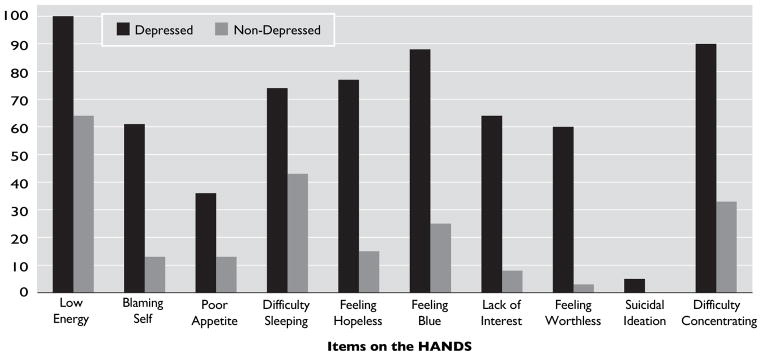
Table 1 shows the endorsement of items on the HANDS for the entire sample. Of those who were currently depressed (HANDS ≥6), the most common symptoms of depression endorsed at least “some of the time” were as follows: low energy (100%), difficulty with concentration/decision-making (90%); blue (88%), hopeless (78%); and poor sleep (74%). Suicidal thoughts were relatively uncommon in this population (5% of depressed respondents), and none of the nondepressed PD patients reported suicidal ideation. Fisher’s exact tests showed highly significant (p <0.0001) differences between the depressed and nondepressed PD patients in their distributions for each individual HANDS item, except in the case of the Suicide item, which was still, however, significant (p=0.0478; Figure 1). Items on the HANDS that were most discriminative between depressed and nondepressed subjects with PD included feeling blue, feeling hopeless, feeling worthless, lack of interest, and self-blame (Figure 1). Although all subjects with dPD reported low energy and many reported poor sleep, these symptoms were also common among PD subjects without depression.
TABLE 1.
Endorsement of Items on the HANDS Among All Parkinson’s Disease Patients
| Over the past 2 weeks, how often have you: | None or Little of the Time | Some of the Time | Most of the Time | All of the Time |
|---|---|---|---|---|
| Been feeling low in energy, slowed down? | 36 (23%) | 92 (58%) | 24 (15%) | 6 (4%) |
| Been blaming yourself for things? | 110 (70%) | 39 (25%) | 7 (4%) | 2 (1%) |
| Had poor appetite? | 124 (79%) | 26 (16%) | 7 (4%) | 1 (1%) |
| Had difficulty falling asleep, staying asleep? | 72 (46%) | 48 (30%) | 28 (18%) | 10 (6%) |
| Been feeling hopeless about the future? | 98 (62%) | 50 (32%) | 8 (5%) | 2 (1%) |
| Been feeling blue? | 82 (52%) | 68 (43%) | 7 (4%) | 1 (1%) |
| Been feeling no interest in things? | 113 (72%) | 34 (22%) | 8 (5%) | 3 (2%) |
| Had feeling of worthlessness? | 120 (76%) | 33 (21%) | 5 (3%) | 0 (0%) |
| Thought about or wanted to commit suicide? | 155 (98%) | 3 (2%) | 0 (0%) | 0 (0%) |
| Had difficulty concentrating or making decisions? | 73 (46%) | 72 (46%) | 12 (7%) | 1 (1%) |
HANDS: Harvard Department of Psychiatry/National Depression Screening Day Scale.
FIGURE 1. Percentage of Subjects Endorsing Each Item on the HANDS.
HANDS: Harvard Department of Psychiatry/National Depression Screening Day Scale. All items were significant in discriminating depressed from nondepressed Parkinson’s disease patients.
As a secondary analysis, we wanted to compare using a HANDS cutoff of ≥6 with ≥9, which is the commonly used cutoff for depression (see Methods section). Using a cutoff of ≥9, the rate of depression would have been 11% (N = 18), versus 37%. Sixty-seven percent (N=12) were men, and 14 of these 18 subjects (78%) were taking an antidepressant. Of those who were currently depressed (with HANDS ≥9), the most common symptoms of depression endorsed at least “some of the time” were the following: low energy (100%), no interest (100%), difficulty with concentration/decision-making (100%); feeling blue (100%), worthless (89%), hopeless (89%); and poor sleep (78%). Suicidal thoughts were slightly more common with this cutoff than with HANDS ≥6 (17% of the depressed patients, versus 5%).
Univariate relationships of relevant demographic variables to the HANDS Total were examined first. Gender, presence of dementia, Hoehn and Yahr stage, and duration of PD illness were not related to the HANDS Total. Age had a significant (r= − 0.2; p <0.02;) correlation. Patients with a history of depression before PD were significantly more depressed than patients who did not have a history of depression before PD (HANDS score of 7.86 [5.43] versus 4.26 [3.66], respectively (p <0.001). The multiple regression of the HANDS total scores for patients simultaneously on age, Hoehn and Yahr PD level, and history of depression (a dichotomous indicator: Yes/No: 1/0) showed significant effects (p <0.05) for all three predictors, with the HANDS increasing a mean of 0.11 points for each 1-year decrease in age, and increasing 0.8 points for each unit increment in Hoehn and Yahr stage. Duration of PD illness and dementia were also initially included in the regression model, but were sequentially removed when found to be nonsignificant.
A multiple regression of each HANDS item separately on the three demographic variables simultaneously, which were found to be significant in the model for the HANDS Total, showed the following especially strong and significant effects: history of depression was associated with symptoms of low energy (p <0.0001), suicidal ideas (p <0.0001), and poor concentration (p<0.0001); age was negatively related to sleep disturbance (p <0.0007); Hoehn and Yahr scores were positively related to hopelessness (p <0.0062; see Table 2).
TABLE 2.
Partial Regression Coefficients from Multiple Regression of HANDS Items/Total on Age, Hoehn–Yahr Stage,11 and History of Depression (0: No/1: Yes), (N=149)
| HANDS Dependent Variable | Age | Hoehn–Yahr Stage | History of Depression | p |
|---|---|---|---|---|
| 1. Low Energy | −0.00882 | 0.09030 | 0.72042 | *** |
| 2. Self Blame | −0.01235* | 0.10136 | 0.47290 | ** |
| 3. Poor Appetite | −0.00921 | 0.05047 | 0.03096 | NS |
| 4. Poor Sleep | −0.02838*** | 0.15094 | −0.05732 | NS |
| 5. Hopeless | −0.01389* | 0.16236** | 0.31449 | * |
| 6. Blue | −0.01035 | 0.09065 | 0.53848 | *** |
| 7. No Interest | −0.01409* | 0.07720 | 0.50169 | ** |
| 8. Worthless | −0.01125* | 0.09896* | 0.29734 | * |
| 9. Suicidal Ideas | −0.00249* | 0.02362* | 0.14480 | *** |
| 10. Difficulty Concentrating/Decision-Making | −0.00006 | −0.02110 | 0.70447 | *** |
| HANDS Total | −0.11089** | 0.82477* | 3.66822 | *** |
HANDS: Harvard Department of Psychiatry/National Depression Screening Day Scale.
p=0.05;
p≤0.01;
p≤0.001.
CONCLUSIONS
Because depression is one of the most disabling symptoms of PD, the value of regular depression screening and treatment has been repeatedly emphasized.12–14 However, very little is known about the factors that influence depression severity or the nature of specific depression symptoms in PD. In general, studies have found certain characteristics of PD to be associated with depression severity. For example, Rojo and colleagues15 found that female gender and high Hoehn and Yahr scores were significantly related to depressive symptoms. Stefanova and colleagues16 found that cognitive impairment in early PD was predicted by depression severity. Cubo and colleagues,17 for example, found that cognitive impairment, high axial bradykinesia, and gait and balance impairment are significant predictors of dPD. Weintraub and others9 found that depression severity is related to global functioning, as measured by the Schwab and England Scale. The purpose of this study was to evaluate symptom patterns of dPD, using a validated self-rating instrument, and to examine which symptoms most clearly differentiate PD patients with and without depression.
Depression appears to be relatively common in our sample of PD patients. We found that about 37% of our sample was depressed, based upon the cutoff used by our group with the HANDS, which is consistent with a relatively recent review that looked at 45 studies and found that the rate of dPD is about 31%.18 In PD patients, depressive symptoms affect all domains, including physical, affective, and cognitive domains. In a previous study that examined the symptom-profile of dPD patients, it was found that depressed mood, tension, loss of interest, and loss of concentration were the most common depressive symptoms, whereas feelings of guilt, self-blame, appetite disturbance, and suicidal ideation were not as common.19 Another previous study found that low mood, anhedonia, and lack of interest constituted the most prominent symptoms in dPD patients, and that reduced appetite and early morning awakening are two somatic items that discriminate between PD patients with and without depression.20 The symptoms that showed a marked difference (more than 3 times higher) in rates between depressed and non-depressed PD patients were lack of interest (64% versus 8%), feelings of worthlessness (60% versus 3%), and feelings of hopelessness (77% versus 15%), feeling blue (88% versus 25%), and blaming self (61% versus 13%).
In this study, poor sleep appears to be related to younger age, and PD severity to hopelessness. Also, a history of depression before PD was related to symptoms of low energy, suicidal ideas, and poor concentration.
Depression may be part of the PD process, but not necessarily. The clinical implications of our findings are that depressive symptoms appear common, despite attempts to treat depression; 66% of our subjects, who had a HANDS score of ≥6, were on an antidepressant and, and 32%, with a HANDS score of <6, were also on an anti-depressant.
The limitations of this study include reliance on a self-rating scale to detect depression without a clinical interview or semistructured interview to validate the diagnosis. Nevertheless, the HANDS has been validated with Structured Clinical Interview for DSM–III-R diagnosis.10 Also, comorbid psychiatric symptoms, particularly anxiety, were not evaluated and may also have been common in this population. Another limitation of this study is the absence of a depression control group without PD. Thus, we cannot determine the degree to which the pattern of depressive symptoms discerned in this population is related to comorbid PD. Although one previous study did not find differences between depressed individuals with PD compared to those without PD,21 other studies have found differences between depressed patients with PD and those without PD. For example, studies have indicated that anxiety symptoms are more prevalent, and that symptoms such as self-blame, guilt, and suicidality are less prevalent in depressed patients with PD than those without PD.6,22,23 Ehrt and colleagues24 found that depressed PD patients had a different depression profile (significantly less sadness, anhedonia, feelings of guilt, loss of energy, and more concentration problems) than the depressed patients without PD. Despite not having a control group, if we compare the rates of depression in this sample with the National Comorbidity Survey (NCS),25 which found that 12-month prevalence estimates for mood disorders was 9.5% (for non-PD subjects), the rates we report are markedly higher. We cannot rule out that the rates are not unique to PD, or similar to those with other medical illnesses. PD does not necessarily cause depression, but depressive symptoms seem to be higher in the PD group than in the general population. Another potential limitation is that we did not look at the stability of depressive symptoms, given that this was a cross-sectional examination.
Further studies are needed to confirm these findings, and to further increase our understanding of specific signs and symptoms of depression in the context of PD, which are likely to influence treatment decisions. Characterizing depression on a continuum, in addition to symptom profiles, may also increase our understanding of depression in the context of PD.9 Whether dPD should be understood primarily as a psychological reaction to a physical disability or to a perceived impending one, or as a direct expression of the neuropathology of PD remains to be defined. Advances in clinical neuroscience are likely to shed further light on the relationship between specific symptom presentations and neural degenerative changes in PD patients.24 Longitudinal techniques and/or structural-equation models that assess causal directions among biological indices, motor symptoms, and depressive symptoms26 may help unravel the complex interrelationships that contribute to the development of depression in patients with PD and may help identify optimal treatment interventions.
Acknowledgments
Daniel Weintraub, M.D., has research support from Forest Pharmaceutical Laboratories, Inc., the Office of Veterans Affairs, Boehringer Ingelheim, the National Institutes of Health, and the University of Pennsylvania. He is an educational consultant for AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Company, Squib, Eisai Inc., GlaxoSmithKline, Teva Pharmaceutical Industries, Novartis Pharmaceuticals Corporation, Schwarz Pharma, Avanir Pharmaceuticals, and Boehringer Ingelheim.
William M. McDonald, M.D., has research support from the Fuqua Foundation, the National Institute of Mental Health, the National Institute of Neurological Disease and Stroke, NeuroNetics, and Janssen Pharmaceutica. He is a consultant for NeuroNetics, Janssen Pharmaceutica, Forest Pharmaceuticals, Inc., and Bristol-Myers Squibb, and on the speakers’ bureau of Janssen Pharmaceutica, Forest Pharmaceuticals, Inc., Bristol-Myers Squibb, and Solvay Pharmaceuticals, Inc. Dr. McDonald has equity holdings in Amgen, Teva, Pfizer Inc., U.S. Pharmaceuticals Group, and Abbott Laboratories.
Jonathan E. Alpert, M.D., Ph.D., has research support from Abbott Laboratories, Alkermes, Lichtwer Pharma GmbH, Lorex Pharmaceuticals; Aspect Medical Systems, AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc., Eli Lilly and Company, Forest Pharmaceuticals, Inc., GlaxoSmithKline, J & J Pharmaceuticals, Novartis Pharmaceuticals Corporation, Organon, Inc., PamLab, LLC, Pfizer Inc., U.S. Pharmaceuticals Group.; Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., and Wyeth-Ayerst Laboratories. He is on the speakers’ bureau of Eli Lilly and Company and Organon, Inc. and is an adviser/consultant to Pamlab LLC and Pharmavite LLC.
Maurizio Fava, M.D., has research support from Abbott Laboratories, Alkermes, Aspect Medical Systems, AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc., Eli Lilly and Company, Forest Pharmaceuticals, Inc., GlaxoSmithKline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis Pharmaceuticals Corporation, Organon, Inc., PamLab, LLC; Pfizer Inc., U.S. Pharmaceuticals Group; Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., and Wyeth-Ayerst Laboratories. He is an adviser/consultant to Aspect Medical Systems, AstraZeneca, Bayer AG, Biovail Pharmaceuticals, Inc., BrainCells, Inc., Bristol-Myers Squibb Company, Cephalon, Inc., Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eli Lilly and Company, EPIX Pharmaceuticals, Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc., GlaxoSmithKline, Grunenthal GmBH, Janssen Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Lundbeck, MedAvante, Inc., Neuronetics, Novartis Pharmaceuticals Corporation, Nutrition 21, Organon, Inc., PamLab, LLC, Pfizer Inc., U.S. Pharmaceuticals Group, PharmaStar, Pharmavite, Roche, Sanofi/Synthelabo, Sepracor, Solvay Pharmaceuticals, Inc., Somaxon, Somerset Pharmaceuticals, and Wyeth-Ayerst Laboratories. He is on the speakers’ bureau of AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb Company, Cephalon, Inc., Eli Lilly and Company, Forest Pharmaceuticals, Inc., GlaxoSmithKline, Novartis Pharmaceuticals Corporation, Organon, Inc., Pfizer Inc., U.S. Pharmaceuticals Group, PharmaStar, and Wyeth-Ayerst Laboratories. Dr. Fava has equity holdings in Compellis and MedAvante.
Footnotes
Statistical analysis was conducted by Joseph J. Locascio, Ph.D., Massachusetts General Hospital.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.Murr C, Widner B, Sperner-Unterweger B, et al. Immune reactions link disease progression in cancer patients with depression. Med Hypotheses. 2000;55:137–140. doi: 10.1054/mehy.1999.1043. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M. Depression in Parkinson’s disease: its prevalence, diagnosis, and neurochemical background. J Neurol. 2001;238(suppl 3):5–11. doi: 10.1007/pl00022917. [DOI] [PubMed] [Google Scholar]
- 4.Cummings JL. Depression and Parkinson’s disease: a review. Am J Psychiatry. 1992;149:443–454. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- 5.Norman S, Troster AI, Fields JA, et al. Effects of depression and Parkinson’s disease on cognitive functioning. J Neuropsychiatry Clin Neurosci. 2002;14:31–36. doi: 10.1176/jnp.14.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Leentjens AF. Depression in Parkinson’s disease: conceptual issues and clinical challenges. J Geriatr Psychiatry Neurol. 2004;17:120–126. doi: 10.1177/0891988704267456. [DOI] [PubMed] [Google Scholar]
- 7.Marsh L, McDonald WM, Cummings J, et al. Provisional diagnostic criteria for depression in Parkinson’s disease: report of an NINDS/NIMH work group. Mov Disord. 2006;21:148–158. doi: 10.1002/mds.20723. [DOI] [PubMed] [Google Scholar]
- 8.Schrag A, Barone P, Brown RG, et al. Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2007;22:1077–1092. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weintraub D, Moberg PJ, Duda JE, et al. Effect of psychiatric and other non-motor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc. 2004;52:784–788. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- 10.Baer L, Jacobs D, Meszler-Reizes J, et al. Development of a brief screening instrument: The HANDS. Psychother Psychosom. 2000;69:35–41. doi: 10.1159/000012364. [DOI] [PubMed] [Google Scholar]
- 11.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 12.Cubo E, Bernard B, Leurgans S, et al. Cognitive and motor function in patients with Parkinson’s disease with and without depression. Clin Neuropharmacol. 2000;23:331–334. doi: 10.1097/00002826-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Mentis MJ, Delatot D. Depression in Parkinson’s disease. Adv Neurol. 2005;96:26–41. [PubMed] [Google Scholar]
- 14.Rihmer Z, Seregi K, Rihmer A. Parkinson’s disease and depression. Neuropsychopharmacol Hung. 2004;6:82–85. [PubMed] [Google Scholar]
- 15.Rojo A, Aguilar M, Garolera MT, et al. Depression in Parkinson’s disease: clinical correlates and outcome. Parkinsonism Relat Disord. 2003;10:23–28. doi: 10.1016/s1353-8020(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 16.Stefanova E, Potrebic A, Ziropadja L, et al. Depression predicts the pattern of cognitive impairment in early Parkinson’s disease. doi: 10.1016/j.jns.2006.05.031. E-pub 2006, June 13. [DOI] [PubMed] [Google Scholar]
- 17.Cubo E, Bernard B, Leurgans S, et al. Cognitive and motor function in patients with Parkinson’s disease with and without depression. Clin Neuropharmacol. 2000;23:331–334. doi: 10.1097/00002826-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Slaughter JR, Slaughter KA, Nichols D, et al. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2001;13:187–196. doi: 10.1176/jnp.13.2.187. [DOI] [PubMed] [Google Scholar]
- 19.Brown RG, MacCarthy B. Psychiatric morbidity in patients with Parkinson’s disease. Psychol Med. 1990;20:77–87. doi: 10.1017/s0033291700013246. [DOI] [PubMed] [Google Scholar]
- 20.Leentjens AF, Marinus J, Van Hilten JJ, et al. The contribution of somatic symptoms to the diagnosis of depressive disorder in Parkinson’s disease: a discriminant analytic approach. J Neuropsychiatry Clin Neurosci. 2003;15:74–77. doi: 10.1176/jnp.15.1.74. [DOI] [PubMed] [Google Scholar]
- 21.Merschdorf U, Berg D, Csoti I, et al. Psychopathological symptoms of depression in Parkinson’s disease compared to depression. Psychopathology. 2003;36:221–225. doi: 10.1159/000073446. [DOI] [PubMed] [Google Scholar]
- 22.Menza MA, Robertson-Hoffman DE, Banapace AS. Parkinson’s disease and anxiety: comorbidity with depression. Biol Psychiatry. 1993;34:465–470. doi: 10.1016/0006-3223(93)90237-8. [DOI] [PubMed] [Google Scholar]
- 23.Huber SJ, Freidenberg DL, Paulson GW, et al. The pattern of depressive symptoms varies with progression of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1990;53:257–258. doi: 10.1136/jnnp.53.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrt U, Bronnick K, Leentjens AF, et al. Depressive symptom profile in Parkinson’s disease: a comparison with depression in elderly patients without Parkinson’s disease. Int J Geriatr Psychiatry. 2006;21:252–258. doi: 10.1002/gps.1456. [DOI] [PubMed] [Google Scholar]
- 25.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM–IV disorders in The National Comorbidity Survey replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locascio JJ, Lee J, Meltzer HY. The importance of adjusting for correlated concomitant variables in psychiatric research. Psychiatry Res. 1988;23:311–327. doi: 10.1016/0165-1781(88)90022-4. [DOI] [PubMed] [Google Scholar]