Abstract
FR901464 is a natural product isolated from a bacterium source that activates a reporter gene, inhibits pre-mRNA splicing, and shows antitumor activity. We previously reported the development of a more potent analogue, meayamycin, through the total synthesis of FR901464. Herein, we report detailed structure-activity relationships of FR901464 that revealed the significance of the epoxide, carbon atoms in the left tetrahydropyran ring, the Z-geometry of the side chain, the 1,3-diene moiety, the C-4 hydroxy group, and the C2"-carbonyl group. Importantly, the methyl group of the acetyl substituent was found to be inessential, leading to a new potent analogue. Additionally, partially based on in vivo data, we synthesized and evaluated potentially more metabolically stable analogues for their antiproliferative activity. These structural insights into FR901464 may contribute to the simplification of the natural product for further drug development.
Keywords: carbamate, FR901464, meayamycin, cancer, structure-activity relationship
Introduction
Development of natural product-based anticancer agents
Development of small molecule ligands for specific protein targets is a contemporary approach in drug discovery processes and chemical biology (reverse chemical genetics). The success of imatinib (Gleevec®) exemplifies this approach,[1] but the generality of this rational approach remains to be seen,[2] as small molecules often bind to off-targets. Historically, especially in cancer medicine, major breakthroughs occurred through the target identifications of previously known anticancer natural products (forward chemical genetics). These targets were usually not obvious prior to these studies. For example, paclitaxel was first identified as an anticancer compound;[3] however, until Horwitz reported that this natural product bound to microtubule, this protein was presumably not viewed as a viable target.[4] Similarly, although lactacystin was discovered earlier,[5] the proteasome was not widely accepted as a viable target to fight cancer until lactacystin's target was found to be a proteasome unit.[5] Consequently, Bortezomib® has been approved by the FDA as the first proteasome inhibitor for cancer treatment.[6]
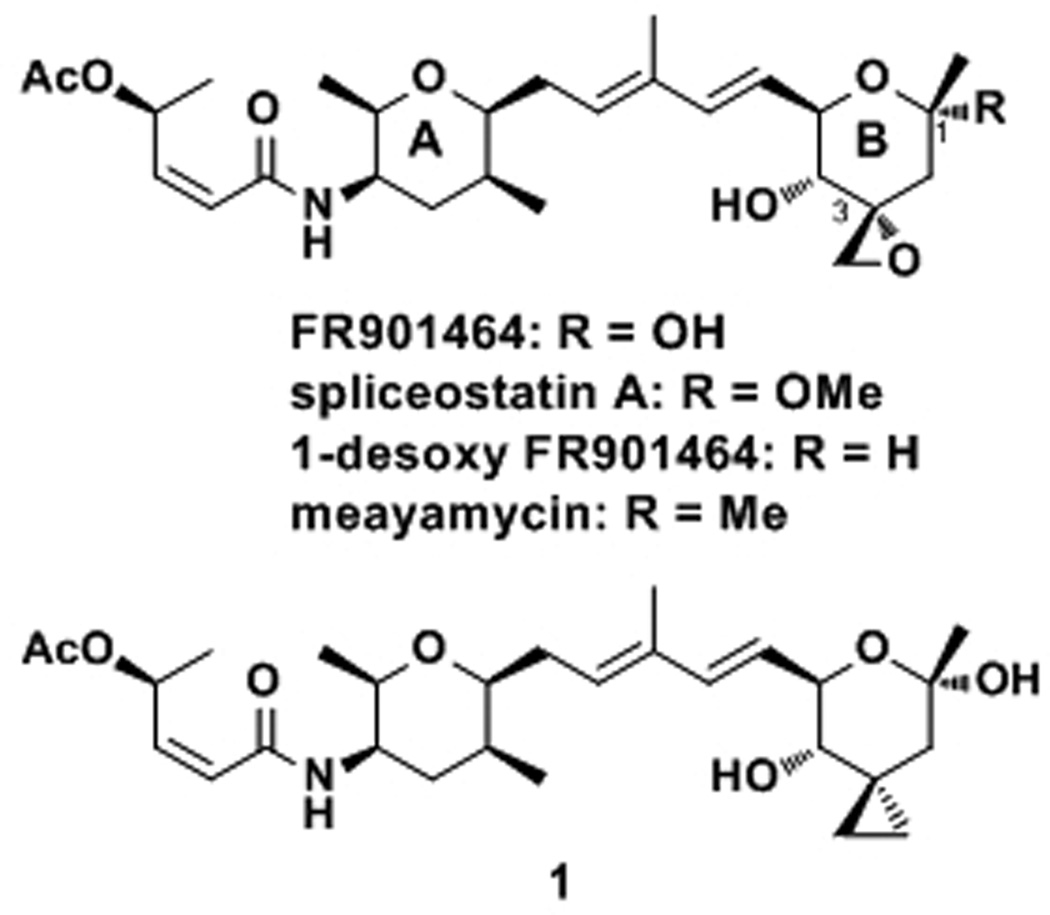
FR901464 (Figure 1) may contribute to cancer therapy in a similar fashion. The isolation and antitumor activity of FR901464 was reported in 1996 by Nakajima and his co-workers.[7] Although its biological profile was unique and indicative of a new mode of action, the molecular mechanisms of this antitumor agent were not known for a long time. In 2007, the Yoshida-Kitahara group reported that FR901464 inhibited pre-mRNA splicing and that its target was splicing factor 3b (SF3b).[8] SF3b, consisting of seven proteins,[9] is required for pre-mRNA splicing and is part of the spliceosome, a complex of approximately 200 proteins and small nuclear RNAs (snRNAs) that catalyzes RNA splicing.[10] Although the specific molecular target of FR901464 has not yet been determined, it is likely that it is either spliceosomal associated protein (SAP)135 or SAP155.[8] The same research group also showed that the splicing inhibition of p27 by FR901464 was linked to cell cycle arrest.[8] These studies indicate that the anticancer activity of FR901464 is directly linked to pre-mRNA splicing inhibition. This is potentially a breakthrough because splicing processes have never been exploited as therapeutic targets or biomarkers in cancer medicine. Moreover, post-transcriptional RNA modifications are an increasingly important theme in biology,[11] for which FR901464 or its analogue may be used as a chemical tool. Very recently, the Webb group reported the promising in vivo antitumor activity of an FR901464 analogue, which further supports the idea that FR901464 analogues could be antitumor drugs.[12]
Figure 1.
Structures of FR901464 and Previously Prepared Analogues.
Not surprisingly, several pharmaceutical companies recently used reporter assays related to those that the Nakajima group employed and discovered a series of new natural products with biological profiles similar to that of FR901464.[13, 14] The most notable natural products are the pladienolides,[14] a derivative of which is currently in Phase I trials as the first drug candidate with splicing inhibitory activity.[15]
In addition to the significance of using splicing inhibitors as antitumor agents, there is a great need to develop chemical probes for RNA splicing because the process is not very tractable with currently available biological methods. As the first natural product that inhibits pre-mRNA splicing, FR901464 is now considered a prototype compound for splicing inhibitors. Given the unique mode of action in conjunction with its antitumor activity, we envision that FR901464 or its analogues will be widely used in oncology and RNA biology. Thus, it is important to understand the structure-activity relationships of FR901464, which would enable the rational design of more potent analogues that are compatible with in vivo experiments.
Synthetic studies of FR901464
The Jacobsen group accomplished the first total synthesis of FR901464[16] and systematically studied the structure-activity relationship (SAR) of this natural product.[17] The results of their SAR studies are described throughout this article where they are directly related to our studies. The second total synthesis was accomplished by the Kitahara group,[18] who later improved their synthetic scheme.[19] Our group reported the third total synthesis of FR901464 in 2006,[20, 21] and later disclosed how the combination of the epoxide at the C3 position and the hydroxy group at the C1 position caused the decomposition of FR901464.[22]
C1-Hydroxy group of FR901464
Spliceostatin A (Figure 1), the 1-methoxy analogue prepared by the Kitahara group, is more active than FR901464 in enhancing gene expression of a reporter gene.[23] Unfortunately, their semi-quantitative description of the activity does not allow for complete quantitative assessment. Moreover, the methoxy group at the anomeric center without neighboring electron-withdrawing groups is acid-sensitive,[24] which raises the question of whether it is simply an FR901464-prodrug with enhanced cell permeability. Alternatively, the improved activity could be a result of the improved stability of spliceostatin A as compared to FR901464.[23] 1-Desoxy FR901464, prepared by the Jacobsen group, is slightly more active against Jurkat cells than FR901464.[17] This analogue shows an important feature about FR901464: its active form contains a cyclic B-ring. It is not clear whether the 1-hydroxy group participates in molecular recognition since the improved stability of 1-desoxy FR901464 and loss of the hydroxy group may compromise each other, resulting in slightly better anticancer activity. We recently substituted the 1-hydroxy group with a methyl group and found that this analog, meayamycin, was 100 times more potent against human breast cancer MCF-7 cells than FR901464.[22] Moreover, it is more potent than 1-desoxy FR901464 and should be more stable than spliceostatin A. Therefore, in this work, all of the analogues contain the geminal dimethyl group at the C1 position.
Results and Discussion[25]
The epoxide moiety
The C3-cyclopropyl analogue 1 (Figure 1) was prepared by the Jacobsen group and shown to be inactive even at 4 µm (i.e., >3 orders of magnitude less active).[17] This result implies that the epoxide may be crucial for FR901464’s biological activity, but it was not clear whether the oxygen atom or the electrophilicity of the epoxide was important. If the electrophilicity is important, such a notion would be contradictory to the Yoshida-Kitahara team’s statement that FR901464 does not form a covalent bond with its target protein.[8] In light of this potential discrepancy, we set out to prepare non-epoxide analogues that still contain the oxygen atom at the C3 position (Scheme 1).
Scheme 1.
Preparation of C3 analogues 5, 10, and 13. a Conditions: (a) LiAlH4 (5.0 equiv), Et2O, reflux, 80%. (b) PMPCH(OMe)2 (2.9 equiv), CSA (0.10 equiv), CH2Cl2, 23 °C, 84%. (c) 3 (1.3 equiv), 4 (1.0 equiv), Ru-1 (0.10 equiv), benzoquinone (0.22 equiv), DCE, 42 °C, 41%. (d) AcOH/THF/H2O (3:1:1), 23 °C, 66%. (e) PMBCl (2.9 equiv), Ag2O (1.5 equiv), DMF, 60 °C, 74%. (f) KOH (5.0 equiv), DMSO/H2O (1.5:1), 55 °C, 77%. (g) NaIO4 (1.2 equiv), H2O/THF (2:1), 23 °C; LisBu3BH (3.1 equiv), THF, −78 °C, 69% (2 steps). (h) DDQ (3.0 equiv), CH2Cl2/sat. aq. NaHCO3 (9:1), 23 °C; CSA (0.05 equiv), MeOH, 23 °C; 5% KOH/H2O, 23 °C, 79% (3 steps). (i) PMPCH(OMe)2 (2.9 equiv), CSA (0.10 equiv), CH2Cl2, 23 °C, quant. (j) 4 (1.0 equiv), 9 (1.3 equiv), Ru-1 (0.10 equiv), benzoquinone (0.22 equiv), DCE, 40 °C, 48%. (k) AcOH/THF/H2O (3:1:1), 23 °C, 68%. (l) MeI (2.0 equiv), NaH (2.0 equiv), THF, 23 °C, 94%. (m) DDQ (2.0 equiv), CH2Cl2/sat. aq. NaHCO3 (9:1), 23 °C, 89%. (n) 3,4-dihydro-2H-pyran (1.5 equiv), CSA (0.05 equiv), CH2Cl2, 23 °C, quant. (o) 4 (1.2 equiv), Ru-1 (0.10 equiv), benzoquinone (0.20 equiv), DCE, 45 °C, 40%. (p) AcOH/THF/H2O (3:1:1), 23 °C, 63%. CSA: camphorsulfonic acid. PMB: para-methoxybenzyl. DDQ: 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. PMP: para-methoxyphenyl. DCE: 1,2-dichloroethane.
Compound 2[22] was reduced with LiAlH4 to a diol, which was protected as an acetal to give 3 in 67% yield for the two steps (Scheme 1A). This acetal was subjected to olefin cross metathesis conditions with the previously prepared diene 4 and Ru-1[26] to give the cross-coupled compound in 41% yield. The acetal of this compound was hydrolyzed under acidic conditions to give analogue 5 in 66% yield.
To arrive at two similar analogues, alcohol 6[22] was used as the starting material. The alcohol was protected as its PMB ether in 74% yield, and its epoxide was subsequently opened with KOH to give diol 7 in 77% yield (Scheme 1B). Diol 7 was cleaved by the action of NaIO4, and the resulting ketone was immediately reduced with l-Selectride® to give alcohol 8 as a single diastereomer. Alcohol 8 was treated with DDQ to give a mixture of acetals and esters (see Supporting Information), which was treated with acidic methanol and subsequently with KOH to give a diol. This diol was converted to acetal 9 in quantitative yield. Acetal 9 was then subjected to diene 4 and Ru-1 to give a metathesis adduct in 48% yield, which was deprotected under acidic conditions to give 10 in 68% yield.
Alcohol 8 was also methylated by the action of MeI and NaH to give 11 in 94% yield. The PMB ether 11 was exposed to DDQ to give an alcohol in 89% yield, which was then protected as its THP ether 12 in quantitative yield. Compound 12 was treated with diene 4 and Ru-1 to give the desired cross-coupled product in 40% yield. Finally, the THP ether of this product was hydrolyzed to give analogue 13 in 63% yield.
None of these non-epoxy analogues (5, 10, 13) exhibited antiproliferative activity against MCF-7 cells even at 10 µm, meaning that they are at least six orders of magnitude less active than meayamycin (Table 1). These data, together with those by the Jacobsen group,[17] suggest that the epoxide of FR901464 plays a crucial role as an electrophile and presumably forms a covalent bond. Since non-specific epoxide opening by thiols in vivo may not be a major concern,[27] the potential covalent bond formation may be specific for the putative target(s).
Table 1.
Antiproliferative Activities against MCF-7 Cellsa
| FR901464 analogue | GI50 (nm) |
|---|---|
| meayamycin | 0.02 |
| 5 | >10,000 |
| 10 | >10,000 |
| 13 | >10,000 |
| 22 | 0.30 |
| 28 | 2.5 |
| 29 | 85 |
| 30 | 19 |
| meayamycin B | 0.008 |
| 4'-desacetyl meayamycin | 0.48 |
| 4'-epi-desacetyl meayamycin | 4.7 |
| 41 | 5 |
| 48 | 19 |
| 50 | 0.40 |
| 59 | 15 |
| 60 | 10 |
Each experiment consists of at least twelve data points (twelve different concentrations).
The 12 position
Vasella and co-workers studied the conformations of compound 14 in water and found that a significant population (1/3) of this compound existed as conformer 14N-ax despite the bulk of the acetamide group (Scheme 2A).[28] The somewhat surprising stability of the axial conformer prompted us to speculate that the more stable chair conformer of the A-ring of FR901464 might be FR901464N-ax (Scheme 2B), provided that the A-ring is indeed in a chair form (Scheme 2B). This preference can be justified by arguing that in this conformer, if the amide NH is hydrogen bonded to the oxygen atom of the A-ring, only the nitrogen lone pair would point toward the methyl group at the C12 position, minimizing the 1,3-diaxial interaction. On the other hand, in conformer FR901464N-eq, there is a substantial 1,3-diaxial interaction between the methyl group at the C15 position and the C10-allylic methylene group. Therefore, we hypothesized that FR901464 might bind to its target(s) when it assumed the chair form FR901464N-ax in the A-ring.
Scheme 2.
Chair Conformers.
With this hypothesis, it appeared to us that the 12-methyl group in the axial position destabilized this putative binding conformer. Therefore, removal of this methyl group might indeed increase the population of the bound conformer, thereby enhancing the potency of meayamycin. Moreover, inspired by the work of Wender on a series of bryostatin analogues,[29] which was followed by the Smith group on phorboxazole analogues,[30] the substitution of the 12-carbon with an oxygen atom looked very appealing from a synthetic standpoint.
Our synthetic efforts toward analogue 22 began with the one pot DIBALH reduction-Wittig condensation reaction sequence of the l-threonine derivative 15 (Scheme 3A).[31] The resulting enoate was hydrogenated to form the corresponding saturated compound 16 in 87% yield for the two steps. Treatment of this compound with acetic acid promoted the acetonide cleavage and intramolecular transesterification, giving lactone 17 in 73% yield. The allylation of this lactone followed by the BF3•OEt2–Et3SiH mediated reduction of the resulting hemiketal provided tetrahydropyran 18 in 43% yield for the two steps. The same three-step sequence as our previous synthesis (1. Boc-deprotection–amidation with acid 19; 2. olefin cross metathesis with methacrolein; 3. Wittig reaction with Ph3P=CH2)[21] afforded the conjugated diene 21 in 43% yield over the three steps. This left fragment was then coupled with the right fragment 6 to give the designed analogue 22 in 50% yield. The total number of steps is 25 for this analogue with 11 steps in the longest linear sequence, which compares favorably to the synthesis of meayamycin (28 total steps and 13 steps in the longest linear sequence).
Scheme 3.
Preparation of C12 analogues. a Conditions: (a) DIBALH (1.8 equiv), CH2Cl2, −78 °C; Ph3P=CHCO2Me (1.5 equiv), 45 °C, 87%. (b) H2, Pd/C (1 mol %), EtOAc, 23 °C, quant. (c) AcOH, 80 °C, 73%. (d) CH2=CHCH2MgCl (1.9 equiv), THF, −78 °C. (e) Et3SiH (10 equiv), BF3•OEt2 (4.0 equiv), CF3CH2OH (8.0 equiv), −78 °C, 43% (2 steps). (f) CH2Cl2/TFA (9:1), 23 °C; 19 (1.2 equiv), HATU (1.2 equiv), iPr2NEt (4.0 equiv), 23 °C, 84%. (g) methacrolein (10 equiv), Ru-1 (2 mol %), 23 °C, 60%. (h) Ph3PCH3Br (2.2 equiv), KOtBu (2.2 equiv), THF, 0 °C, 86%. (i) 6 (1.6 equiv), Ru-1 (0.10 equiv), benzoquinone (0.21 equiv), DCE, 35 °C, 50%. (j) NaBH4 (2.0 equiv), EtOH, 23 °C, 87%. (k) 1,1-diethoxy-3-butene (1.0 equiv), CSA (1 mol %), CH2Cl2, 0 °C, 67%. (l) CH2Cl2/TFA (9:1), 23 °C; 19 (1.2 equiv), HATU (1.2 equiv), iPr2NEt (4.0 equiv), 23 °C, 51%. (m) methacrolein (10 equiv), Ru-1 (2 mol %), 23 °C, 62%. (n) Ph3PCH3Br (2.2 equiv), KOtBu (2.2 equiv), THF, 0 °C, 87%. (o) 6 (1.6 equiv), Ru-1 (0.10 equiv), benzoquinone (0.22 equiv), DCE, 35 °C, 53%. DIBALH: diisobutylaluminum hydride. TFA: trifluoroacetic acid. HATU: O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate.
The acetal analogue synthesis was even more concise; treatment of the commercially available compound 23 with NaBH4 gave the known diol 24 in 87% yield (Scheme 3B).[32] This diol was reacted with 1,2-diethoxy-3-butene under acidic conditions to form the cyclic acetal 25 in 67% yield. After the same synthetic procedures as before, we were able to prepare the target analogue 28 in 15% yield for the 4 steps. The total number of steps is 21 for this analogue with only 11 steps in the longest linear sequence.
The antiproliferative activity of these two analogues was evaluated against MCF-7 cells (Table 1). The 12-desmethyl meayamycin 22 was less potent than meayamycin, but the GI50 is still in the respectable sub-nanomolar range (0.3 nm). The acetal analogue 28 was far less active, with the GI50 value at 2.5 nm. These data indicate the significance of hydrophobicity at the 12-position. The replacement of the A-ring with a cyclic acetal provided a means to probe the impact of substituents at positions 13 and 15. Thus, through similar synthetic schemes (Supporting Information), analogues 29 and 30 were prepared and evaluated. The additional methyl group at the 13 position in analogue 29 was detrimental to the biological activity. The replacement of the 15-methyl with a hydrogen atom in analogue 30 also had a negative impact with respect to the biological activity. All together, the substituents at positions 12, 13, and 15 are intricately responsible for the biological activity of FR901464. Although these analogues are more synthetically accessible, we tentatively decided to exclude these simplifications because a sufficient quantity of meayamycin could be produced for the purpose of this study using tissue-cultured cells.
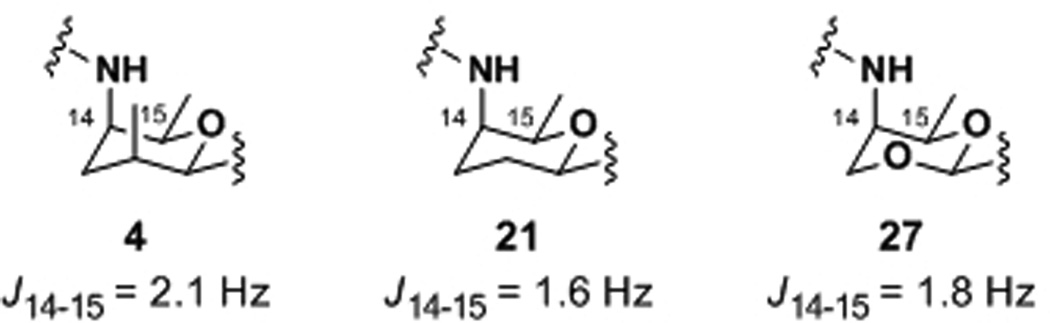
To understand if the loss in activity of these A-ring analogues was due to a conformational change or a loss of hydrophobicity, NMR experiments were undertaken for dienes 4, 21, and 27 in CD3OD (Figure 2). The J14–15 values were 2.1, 1.6, and 1.8 Hz for 4, 21, and 27, respectively, indicating similar chair-like conformers. Therefore, the loss in activities for 12-desmethyl meayamycin, 22, and 28 is mainly attributed to the loss of favorable hydrophobic interactions, and, to a lesser degree, a conformational change in the A-ring.
Figure 2.
Coupling constants for A-ring analogues.
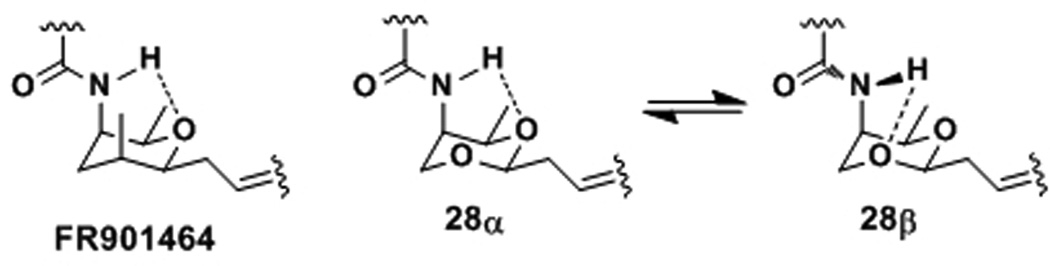
Why is compound 28 less potent than compound 22? The lower potency of 28 can be attributed to the additional conformer 28β (Figure 3). From 1H NMR experiments, the secondary N-H signal was very prominent in CDCl3, which did not diminish upon the addition of CD3OD. This indicates that there is strong H-bonding between the N-H and the oxygen atom on the A-ring (pyran or acetal). An additional reason for the lower potency of 28 may be because the additional oxygen is bound to a water molecule, which may need to be dissociated to enter the putative hydrophobic binding pocket.
Figure 3.
Rotamers of 28.
The 4'-position
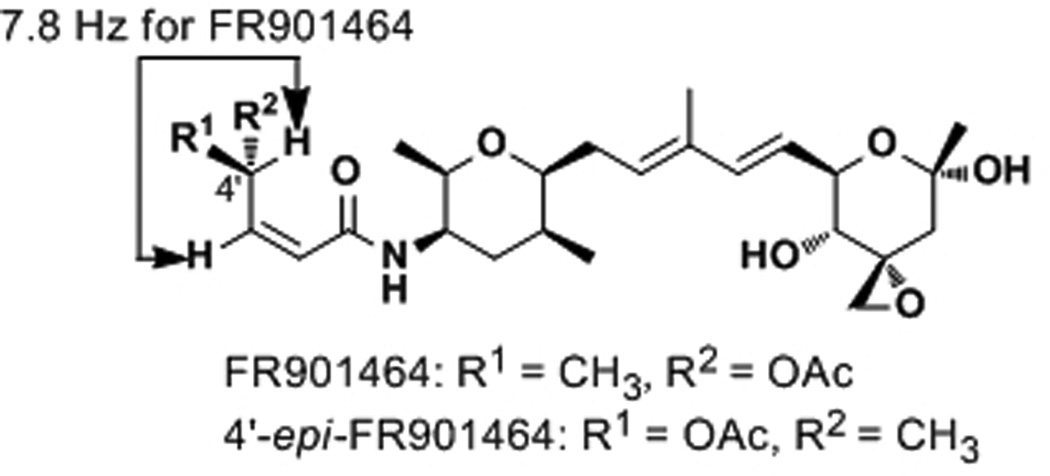
Due to a 1,3-allylic strain, the 4'-H is nearly in the same plane as the conjugated amide, as suggested by the 1H NMR spectrum of FR901464 (J3'–4' = 7.8 Hz, Figure 4). Inversion of the 4'-stereocenter places its methyl and acetoxy substituents in opposite faces of the plane defined by the conjugated amide. This analogue, 4'-epi-FR901464, was synthesized by the Jacobsen group and found to be 15 times less active than FR901464.[17] The reduced activity could be because either the acetoxy or 5'-methyl group is in the wrong orientation (or both groups). To probe this idea, we prepared 4'-desacetyl meayamycin, which began with the methanolysis of the previously prepared 4[21, 22] to give alcohol 31 in 82% yield (Scheme 4A). This alcohol was then subjected to olefin cross metathesis conditions with 6 and Ru-1 to give 4'-desacetyl meayamycin in 81% yield. The potency of 4'-desacetyl meayamycin dropped by 24-fold (GI50 = 0.48 nm; Table 1) as compared to meayamycin, further indicating the significance of the 4'-position.
Figure 4.
FR901464 and Its 4'-epi-Analogue.
Scheme 4.
Preparation of 4'-analogues. a Conditions: (a) K2CO3 (5.0 equiv), MeOH, 0 °C, 82%. (b) 6 (1.6 equiv), Ru-1 (0.10 equiv), benzoquinone (0.17 equiv), DCE, 32 °C, 81%. (c) nBuLi (2.8 equiv), HC≡CCH2OTHP (3.0 equiv), THF, −78 to 0 °C, 90%. (d) Ru-2 (2 mol %), HCO2Na (10 equiv), CTAB (0.2 equiv), H2O, 23 °C, 78%. (e) Ac2O (5.0 equiv), pyridine, 23 °C, 93%. (f) PPTS (0.2 equiv), MeOH, 23 °C, 84%; Na2Cr2O7 (1.5 equiv), acetone, H2SO4, 0 to 23 °C, 86%. (g) H2, Lindlar’s catalyst (1 mol %), quinoline (0.10 equiv) EtOH, 23 °C, 68%. (h) 34 (1 equiv), CH2Cl2/TFA (9:1), 23 °C; then 4'-epi-19 (1.2 equiv), HATU (1.2 equiv), iPr2NEt (4.0 equiv), MeCN, 23 °C, 71%. (i) methacrolein (10 equiv), Ru-1 (2 mol %), 23 °C, 62%. (j) Ph3PCH3Br (2.2 equiv), KOtBu (2.2 equiv), THF, 0 °C, 84%. (k) K2CO3 (2.0 equiv), MeOH, 0 °C, 86%. (l) 6 (1.5 equiv), Ru-1 (0.10 equiv), DCE, 35 °C, 34%. (m) CDI (1.5 equiv), CH2Cl2, 23 °C; morpholine (4.1 equiv), 23 °C, 80%. (n) 6 (1.6 equiv), Ru-1 (0.07 equiv), DCE, 42 °C, 44%. CDI: 1,1'-carbonyldiimidazole.
From this result, it was not clear whether this reduced activity was due to the loss of the acetyl group itself or possibly placing the methyl group in the α-face by virtue of the intramolecular hydrogen bonding between the 4'-hydroxy group and the amide group. Therefore, we also prepared 4'-epi-desacetyl meayamycin according to Scheme 4B. The enantiomer of acid 19 (i.e., epi-19) was prepared by the following route: N-acetyl morpholine was coupled to an in situ prepared lithium acetylide to give 32 in 90% yield.[33] This ynone was reduced with HCO2Na and Noyori’s catalyst,[34] Ru-2, to give an alcohol, which was acetylated to give 33 in 73% yield for the two steps. This material was then exposed to acidic methanol followed by the Jones’ reagent to give an ynoic acid in 72%. The ynoic acid was hydrogenated in the presence of Lindlar’s catalyst to give 4'-epi-19 in 68% yield. Acid 4'-epi-19 was then used to prepare 4'-epi-desacetyl meayamycin by the same synthetic route previously outlined.[21, 22] This analogue was found to be one order of magnitude less active (GI50 = 4.7 nm, Table 1) than 4'-desacetyl meayamycin, indicating that the acetyl group is important for the biological activity.
The correlation between the loss of the acetyl group and activity was a concern to us because esterases might hydrolyze the ester group in tissue culture and in vivo. Consequently, we measured the half-lives of meayamycin in mouse and human sera at 10 µm. As we expected, meayamycin was hydrolyzed to its desacetyl analogue (t1/2 = 2 h in mouse serum, 9 h in human serum), which was then converted to uncharacterized products. We hypothesized that if we prolong the half-life of meayamycin in sera, effective concentrations of this compound might be higher during the assays, thus improving the potency. To test this hypothesis, we replaced the acetyl group with a morpholine-based carbamate (meayamycin B) to enhance stability under biological conditions as carbamates are known to be more stable than esters under biological conditions.[35]
The synthesis of this analogue was accomplished by the reaction of 31 with carbonyldiimidazole (CDI) followed by morpholine to give 36 in 80% yield (Scheme 4C). This carbamate was then coupled to 6 in the presence of Ru-1 to give meayamycin B in 44% yield. The half-life of this carbamate analogue was 13 h in mouse serum (Figure 5), significantly longer than that of meayamycin, presumably due to the esterase-resistant nature of this compound. Encouraged by this result, the antiproliferative activity of this new analogue was measured; it was gratifying to find that meayamycin B was even more potent than extremely potent meayamycin in MCF-7 cells (Table 2). With this result, we tested meayamycin[36] and meayamycin B with various cancer cell lines. MCF-7 and MDA-MB-231, both human breast cancer cell lines, were the most responsive. PC3, HCT-116 and H1299 were also very responsive, exhibiting GI50 values in a low picomolar range. A549 and DU145 cells were less responsive, but the GI50 values were still sub-nanomolar. On the average, meayamycin B is 3.1 ± 1.9 times more potent than meayamycin in these seven cancer cell lines.
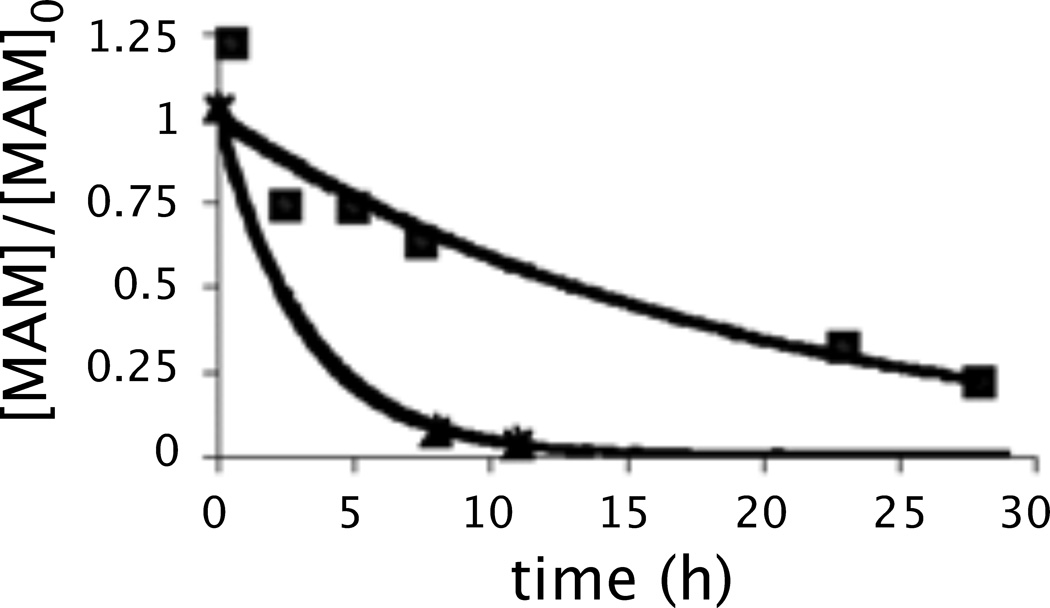
Figure 5.
Degradation of meayamycin (triangles) and meayamycin B (squares) in mouse serum at 37 °C. y-axis: The relative quantity of meayamycin (or meayamycin B) compared to the initial meayamycin (or meayamycin B) quantity. The initial meayamycin concentration was 10 µm. Meayamycin: y = e−0.310x (R2 = 0.997). Meayamycin B: y = e−0.053x (R2 = 0.954). MAM: meayamycin or meayamycin B.
Table 2.
GI50 Values of Meayamycin and Meayamycin B in pma
| Cell line | Meayamycin | Meayamycin B |
|---|---|---|
| MCF-7 | 20 ± 8.9 | 8 ± 2 |
| MDA-MB-231 | 71 ± 55 | 15 ± 1.4 |
| A549 | 258 ± 162 | 175 ± 77 |
| DU145 | 1230 ± 697 | 211 ± 23 |
| HCT116 | 157 ± 33 | 4 ± 2 |
| H1299 | 841 ± 271 | 146 ± 30 |
| PC3 | 196 ± 42 | 101 ± 4.0 |
Each experiment consists of at least twelve data points (twelve different concentrations).
After the synthesis and biological evaluation of meayamycin B were complete in our laboratory, the Webb group published similar carbamate analogues with great loss of activity.[12] In retrospect, we were fortunate that we chose morpholine for water solubility, the size of which appeared to be adequately small to fit in an unknown binding pocket unlike Webb's larger carbamate groups.
For in vivo studies, the most ideal vehicle is saline; therefore, the solubility of drugs in saline is a crucial parameter. Although meayamycin was not entirely soluble in saline at 0.1 mgmL−1, meayamycin B was easily soluble at this concentration (presumably soluble at higher concentrations), showing an additional benefit for meayamycin B.[37]
C2′–C3′ Olefin on the side chain
Next, we asked whether the C2'–C3' trans isomer was the actual active species after the potential isomerization of the cis enamide to its trans form. To address this question, we synthesized analogue 41 according to Scheme 5. A key step is the deliberate isomerization of the cis acid 19 to the activated trans acid derivative during the amide-bond-forming reaction. Specifically, we exploited the facile cis-trans isomerization of the HATU-activated acid 19 prior to the addition of amine 34 to yield a ≈1:1 cis:trans mixture as illustrated in Scheme 5A. Analogue 41 was assembled in three additional steps as illustrated in the scheme. The GI50 of this analogue was 3 orders of magnitude higher than that of the corresponding cis compound, indicating that the cis enamide is the active species.
Scheme 5.
Preparation of C2'-C3' analogues 41 and 48. a Conditions: (a) CH2Cl2/TFA (9:1), 23 °C; 19 (1.7 equiv), HATU (1.3 equiv), iPr2NEt (5.0 equiv), MeCN, 23 °C, 43%. (b) K2CO3 (2.5 equiv), MeOH, 0 °C to 23 °C, 75%. (c) CDI (1.2 equiv), CH2Cl2, 23 °C; morpholine (2–5 equiv), 23 °C, 97% (38) or 94% (43). (d) methacrolein (13–20 equiv), Ru-1 (2 mol%), CH2Cl2 (39) or PhH (46), 23 °C or 50 °C, 74% (39) or 63% (46). (e) Ph3PCH3Br (2.4 equiv), KOtBu (2.3 equiv), THF, 0 °C, 50% (40) or 76% (47). (f) 6 (1.3 equiv), Ru-1 (0.07–0.2 equiv), benzoquinone (0.2–0.5 equiv), DCE, 42–50 °C, 46% (41) or 36% (48). (g) DIBALH (1.1 equiv), CH2Cl2, −78 °C; Ph3P=CHCO2Me (1.5 equiv), 23 °C. (h) H2, Pd/C (2 mol%), EtOAc, 23 °C. (i) NaOH (2.1 equiv), MeOH/H2O (1:1.1), 23 °C, 46% (over 3 steps). (j) 34, CH2Cl2/TFA (9:1), 23 °C; 44 (1.3 equiv), HATU (1.2 equiv), iPr2NEt (3.4 equiv), 23 °C, 66%.
Considering that an α,β-unsaturated amide could serve as an electrophile,[38] we asked whether the side chain was an electrophile. To answer this question, analogue 48 was designed and synthesized according to Scheme 5B. The GI50 of this side chain analogue was found to be 19 nm with MCF-7 cells (Table 1) (93 nm with HCT-116). The antiproliferative activity of two analogues 41 and 48 implies that the cis C2′–C3′ enamide is not an electrophile but rather a structural requirement.
4-Hydroxy group
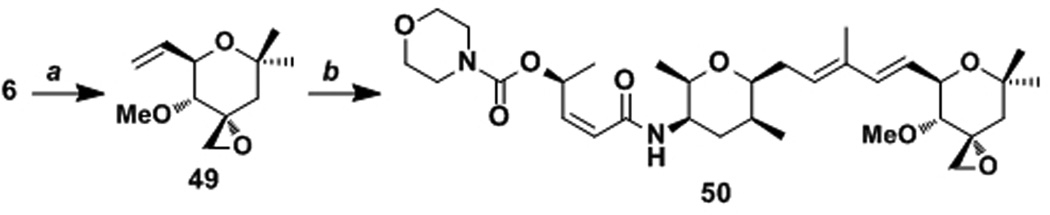
4-O-Alkyl FR901464 analogues were prepared and evaluated by others.[39] However, it was not clear to us whether the loss of activity was due to the large alkyl groups or the loss of a hydrogen bond donor. To minimize the additional sterics, we prepared the 4-O-methyl analogue 50 (Scheme 6). This analogue, meayamycin C, showed respectable potency with the GI50 of 0.38 nm. The diminished activity may be explained by the loss of a hydrogen bond donor or the additional steric bulk. Nonetheless, this analogue may prove to be an important one because in a separate study we observed glucuronidation of meayamycin B as a major metabolite in mice, for which this hydroxy group is the only site. Detailed pharmacokinetic studies of meayamycin B and meayamycin C will be reported by our laboratory in due course. It is noteworthy that the olefin cross metathesis of 36 with 49 was reproducibly higher yielding that that with 6 (Scheme 4C). This difference proved to be crucial in the preparation of cyclopropyl analogues (see below).
Scheme 6.
Preparation of C4-OMe analogue 50. a Conditions: (a) MeI (5.0 equiv), Ag2O (2.0 equiv), DMF, 23 °C, 87%. (b) 36 (1.0 equiv), 49 (1.5 equiv), Ru-1 (7 mol%), benzoquinone (0.2 equiv), DCE, 42 °C, 57%.
1,3-Diene moiety
We previously observed relatively facile isomerization of the 1,3-diene moiety under acidic conditions.[22] The same might occur in live cancer cells, raising a question of whether the trans, trans-diene moiety is actually the biologically active species. To examine the significance of the diene moiety, we synthesized cyclopropyl analogues 59 and 60 (Scheme 7).
Scheme 7.
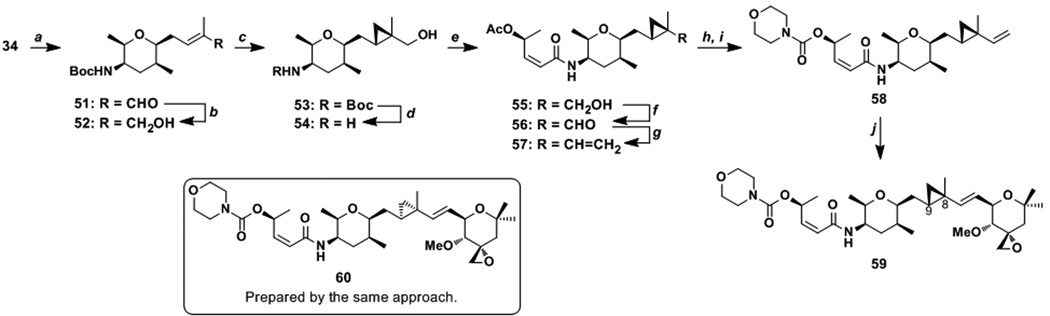
Preparation of cyclopropyl analogues. a Conditions: (a) methacrolein (20 equiv), Ru-1 (3 mol%), 23 °C, 84%. (b) DIBALH (2.0 equiv), CH2Cl2, −78 °C, 98%. (c) (S,S)-dioxaborolane (2.0 equiv), ZnEt2 (5.0 equiv), CH2I2 (7.0 equiv), CH2Cl2, 23 °C, 70%, d.r. = 89:11. (d) CH2Cl2/TFA (8:2), 0 to 23 °C, quant. (e) 19 (1.2 equiv), HATU (1.0 equiv), iPr2NEt (4.0 equiv), MeCN, 23 °C, 48%. (f) Dess-Martin periodinane (2.0 equiv), CH2Cl2, 0 °C, 70%. (g) Ph3PCH3Br (6.0 equiv), KOtBu (5.0 equiv), THF, 0 °C, 80%. (h) 1. K2CO3 (2.5 equiv), THF, 0 °C. (i) CDI (2.5 equiv), CH2Cl2, 23 °C; morpholine (5.0 equiv), 23 °C, 94% (over 2 steps). (j) 49 (1.7 equiv), Ru-1 (0.1 equiv), benzoquinone (0.2 equiv), toluene, 70 °C, 30%.
Compound 34 was first cross-coupled with methacrolein in the presence of Ru-1 in 84% yield. This aldehyde was converted to alcohol 52, which was stereoselectively cyclopropanated by Charette's method[40] to form 53 in 70% yield with a diastereoselective ratio of 89:11. We also prepared the epimer at the C8 and C9 position in 88% with a diastereoselective ratio 87:13 (Supporting Information). The allylic cyclopropyl group, after the conversion of the hydroxy group of 53 to an olefin, was not compatible with acidic conditions for the Boc removal (data not shown). These failures prompted us to remove the Boc group from 53 with TFA before the derivatization of the hydroxy group. The resulting amine 54 was condensed with carboxylic acid 19 to form 55 in 48% yield for the two steps. The oxidation-olefination sequence gave alkene 57 in 56% yield for the two steps. Further transformations yielded alkene 58. Interestingly, this alkene did not undergo cross olefin metathesis with alkene 6 but did so with alkene 49 to form 59 in 30% yield. By a similar sequence, diastereomer 60 was prepared (Supporting Information).
The GI50 values of analogues 59 and 60 with MCF-7 cells were found to be 15 and 10 nm, respectively (Table 1). The potencies of these analogues were approximately one order of magnitude lower than meayamycin C but were still respectable. These results imply that the C8–C9 olefin of FR901464 remains trans as an active species in live cells. In order to fully understand the significance of the C8–C9 olefin, cyclopropyl analogues that would mimic the C8–C9 cis geometry must be prepared and evaluated. Nonetheless, the similar activity of 59 and 60 compared to meayamycin C implies that the C8–C9 olefin would remain trans as the active FR901464 species in cells.
Summary
The C3-spiroepoxide (Figure 6) was shown to be crucial for the retention of the antiproliferative activity, indicating that a covalent bond is presumably formed between target protein(s) and FR901464. The C12 position prefers to be hydrophobic, and C4'-acetate is important but the ester is labile, forming a less potent alcohol (4'-desacetyl meayamycin). The C2’–C3’ side chain olefin needs to be cis to be active. The replacement of one of the 13-H with a methyl group diminished biological activity. Replacement of the 15-methyl group with a hydrogen atom was also somewhat detrimental. The C8–C9 olefin remains trans as the active species of FR901464. Masking the 4-hydroxy group with a methyl group reduced the biological activity. Finally, the development of the more biologically stable and more saline-soluble C4'-carbamate analogue, meayamycin B, was shown to be significantly more potent than meayamycin in seven cancer cell lines. FR901464 binds SF3b and inhibits pre-mRNA splicing, which could be very exciting because the meayamycins should also inhibit the same process and exhibit anticancer activity. Since there is no approved antitumor drug that acts by inhibiting pre-mRNA splicing, the mode of action, potency, and water solubility of meayamycin B might further trigger interest in both oncology and RNA biology.
Figure 6.
Summary of this work.
Experimental Section
Materials and Methods for the Growth Inhibition Assays
Materials
FR901464 and its analogues were dissolved in dimethyl sulfoxide (DMSO) as 10 mm stocks and stored at −20 °C. For the experiments, aliquots were thawed at room temperature and dilutions were prepared in RPMI 1640 medium containing 2% DMSO at 2× the desired concentration prior to addition to the cells.
Cell Culture
MCF-7, A549, DU145, HCT116, MDA-MB-231, H1299, and PC3 cells were grown at 37 °C in an atmosphere containing 5% carbon dioxide in Corning cell culture dishes (150 mm) in RPMI-1640 cell culture medium containing 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin-streptomycin solution (Invitrogen).
Growth Inhibition Assay
Cells were plated in 96 well plates at an initial density of 2,000 or 4,000 cells per well in 100 µL of medium and were incubated for 24–48 h (depending on cell line) prior to compound addition (plating either 2,000 or 4,000 cells/well led to similar GI50 values). Serial two-fold dilutions were used in this experiment for the indicated ranges. The compounds were added to the cells at 2× the desired concentration in 100-µL cell culture medium (containing 2% DMSO). The cells were then incubated for an additional 5 to 10 days. Cell proliferation was measured using the commercial 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) dye reduction assay with phenazine methosulfate (PMS) as the electron acceptor (20 µL per well). The absorbance (at 490 nm minus that at 630 nm) was measured by a Spectromax M5 plate reader (Molecular Devices) or Modulus II Microplate Multimode Reader (Turner BioSystem). Evaluation of FR901464 analogues was performed in quadruplicate at each concentration, and the final numbers were averaged. Microsoft Excel and GraphPad Prism 5 were used to construct dose-response curves and calculating GI50. Growth inhibition is calculated as defined by the National Cancer Institute [GI50 = 100 × (T − T0)/(C − T0); T0 = cell density at time zero; T = cell density of the test well after period of exposure to test compound; C = cell density of the vehicle treated].41 Vincritsine or cisplatin were used as controls.
Procedure for the half-life determination for the decomposition of meayamycin and B in mouse serum.
To a 1 dram glass vial was added mouse serum (1.0 mL), rhodamine B (10 mm in DMSO, 2.0 µL), and DMSO (7.0 µL) under an open atmosphere and warmed to 37 °C. To the mixture was added meayamycin or meayamycin B (10 mm in DMSO, 1.0 µL), and the resulting solution was sealed with a cap, vortexed for 15 s, and then placed in a 37 °C incubator. The decomposition was monitored by HPLC at the indicated times. The resulting data were normalized by dividing the ratios of meayamycin or meayamycin B/rhodamine B by the ratio of the first data point. HPLC monitoring was performed on a Varian Pursuit XRs 5 C18 column, 250 × 10.0 cm, 2.5 mL/min, 30% MeOH in H2O (containing 1% HCO2H) to 100% MeOH linear gradient elution from 0.5 to 22 min, monitored at 230 nm (for meayamycins) and 550 nm (for rhodamine B).
Acknowledgements
We thank Professors Billy W. Day and Andreas Vogt (University of Pittsburgh) for helpful discussions. We thank Professor Stephen G. Weber (University of Pittsburgh) for allowing us to use his Spectromax M5 plate reader. B.J.A. and S.O. are recipients of a Graduate Excellence Fellowship from the University of Pittsburgh. N.L.C. is thankful for the Arnold and Mabel Beckman Scholar Award and the Averill Scholarship. We thank Dr. Damodaran Krishnan and Dr. John Williams for assisting with NMR and mass spectroscopic (NIH grant S10RR017977-01) analyses, respectively. This work was supported by the US National Cancer Institute (R01 CA120792).
References
- 1.Capdeville R, Buchdunger E, Zimmermann J, Matter A. Nat. Rev. Drug Disc. 2002;1:493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- 2.Kamb A, Wee S, Lengauer C. Nat. Rev. Drug Disc. 2007;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 3.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 4.a) Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]; b) Schiff PB, Horwitz SB. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 6.a) Adams J. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]; b) Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. J. Clin. Oncol. 2005;23:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 7.a) Nakajima H, Hori Y, Terano H, Okuhara M, Manda T, Matsumoto S, Shimomura K. J. Antibiot. 1996;49:1204–1211. doi: 10.7164/antibiotics.49.1204. [DOI] [PubMed] [Google Scholar]; b) Nakajima H, Sato B, Fujita T, Takase S, Terano H, Okuhara M. J. Antibiot. 1996;49:1196–1203. doi: 10.7164/antibiotics.49.1196. [DOI] [PubMed] [Google Scholar]; c) Nakajima H, Takase S, Terano H, Tanaka H. J. Antibiot. 1997;50:96–99. doi: 10.7164/antibiotics.50.96. [DOI] [PubMed] [Google Scholar]
- 8.Kaida D, Motoyoshi H, Tashiro E, Nojima T, Hagiwara M, Ishigami K, Watanabe H, Kitahara T, Yoshida T, Nakajima H, Tani T, Horinouchi S, Yoshida M. Nat. Chem. Biol. 2007;3:576–583. doi: 10.1038/nchembio.2007.18. [DOI] [PubMed] [Google Scholar]
- 9.a) Das BK, Xia L, Palandjian L, Gozani O, Chyung Y, Reed R. Mol. Cell. Biol. 1999;19:6796–6802. doi: 10.1128/mcb.19.10.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Krämer A, Grüter P, Gröning K, Kastner B. J. Cell Biol. 1999;145:1355–1368. doi: 10.1083/jcb.145.7.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Will CL, Schneider C, MacMillan AM, Katopodis NF, Neubauer G, Wilm M, Lührmann R, Query CC. EMBO J. 2001;20:4536–4546. doi: 10.1093/emboj/20.16.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Will CL, Urlaub H, Achsel T, Gentzel M, Wilm M, Lührmann R. EMBO J. 2002;21:4978–4988. doi: 10.1093/emboj/cdf480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou ZL, Licklider LJ, Gygi SP, Reed R. Nature. 2002;419:182–185. doi: 10.1038/nature01031. [DOI] [PubMed] [Google Scholar]
- 11.Licatalosi DD, Darnell RB. Nat. Rev. Genet. 2010;11:75–87. doi: 10.1038/nrg2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagisetti C, Pourpak A, Goronga T, Jiang Q, Cui X, Hyle J, Lahti JM, Morris SW, Webb TR. J. Med. Chem. 2009;52:6979–6990. doi: 10.1021/jm901215m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Sakurai M, Kohno J, Nishio M, Yamamoto K, Okuda T, Kawano K, Nakanishi N. J. Antibiot. 2001;54:628–634. doi: 10.7164/antibiotics.54.628. [DOI] [PubMed] [Google Scholar]; b) Sakai Y, Tsujita T, Akiyama T, Yoshida T, Mizukami T, Akinaga S, Horinouchi S, Yoshida M, Yoshida T. J. Antibiot. 2002;55:863–872. doi: 10.7164/antibiotics.55.863. [DOI] [PubMed] [Google Scholar]; c) Sakai Y, Yoshida T, Ochiai K, Uosaki Y, Saitoh Y, Tanaka F, Akiyama T, Akinaga S, Mizukami T. J. Antibiot. 2002;55:855–860. doi: 10.7164/antibiotics.55.855. [DOI] [PubMed] [Google Scholar]
- 14.a) Sakai T, Asai N, Okuda A, Kawamura N, Mizui Y. J. Antibiot. 2004;57:180–187. doi: 10.7164/antibiotics.57.180. [DOI] [PubMed] [Google Scholar]; b Sakai T, Sameshima T, Matsufuji M, Kawamura N, Dobashi K, Mizui Y. J. Antibiot. 2004;57:173–179. doi: 10.7164/antibiotics.57.173. [DOI] [PubMed] [Google Scholar]
- 15.Kotake Y, Niijima J, Nagai M, Okano K, Sakai T, Yoshida M, Tsuchida T, Nakashima T, Dobashi K, Mizui Y, Shimizu H, Uenaka T, Iwata M, Asada M, Yoshimatsu K. Clin. Cancer Res. 2003;9:6208s. [Google Scholar]
- 16.Thompson CF, Jamison TF, Jacobsen EN. J. Am. Chem. Soc. 2000;122:10482–10483. [Google Scholar]
- 17.Thompson CF, Jamison TF, Jacobsen EN. J. Am. Chem. Soc. 2001;123:9974–9983. doi: 10.1021/ja016615t. [DOI] [PubMed] [Google Scholar]
- 18.Horigome M, Motoyoshi H, Watanabe H, Kitahara T. Tetrahedron Lett. 2001;42:8207–8210. [Google Scholar]
- 19.Motoyoshi H, Horigome M, Watanabe H, Kitahara T. Tetrahedron. 2006;62:1378–1389. [Google Scholar]
- 20.a) Albert BJ, Koide K. Org Lett. 2004;6:3655–3658. doi: 10.1021/ol049160w. [DOI] [PubMed] [Google Scholar]; b) Koide K, Albert BJ. J. Synth. Org. Chem., Jpn. 2007;65:119–126. [Google Scholar]
- 21.Albert BJ, Sivaramakrishnan A, Naka T, Koide K. J. Am. Chem. Soc. 2006;128:2792–2793. doi: 10.1021/ja058216u. [DOI] [PubMed] [Google Scholar]
- 22.Albert BJ, Sivaramakrishnan A, Naka T, Czaicki NL, Koide K. J. Am. Chem. Soc. 2007;129:2648–2659. doi: 10.1021/ja067870m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motoyoshi H, Horigome M, Ishigami K, Yoshida T, Horinouchi S, Yoshida M, Watanabe H, Kitahara T. Biosci. Biotech. Bioch. 2004;68:2178–2182. doi: 10.1271/bbb.68.2178. [DOI] [PubMed] [Google Scholar]
- 24.Furukawa H, Koide K, Takao K, Kobayashi S. Chem. Pharm. Bull. 1998;46:1244–1247. [Google Scholar]
- 25.A part of these results was also included in the patent; See Koide K, Albert BJ, Sivaramakrishnan A. US 2008096879 A1 20080424 and WO 2009031999 A1 20090312 .
- 26.Michrowska A, Bujok R, Harutyunyan S, Sashuk V, Dolgonos G, Grela K. J. Am. Chem. Soc. 2004;126:9318–9325. doi: 10.1021/ja048794v. [DOI] [PubMed] [Google Scholar]
- 27.a) Albert BJ, Koide K. ChemBioChem. 2007;8:1912–1915. doi: 10.1002/cbic.200700365. [DOI] [PubMed] [Google Scholar]; b) Weerapana E, Simon GM, Cravatt BF. Nat. Chem. Biol. 2008;4:405–407. doi: 10.1038/nchembio.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernet B, Piantini U, Vasella A. Carbohydr. Res. 1990;204:11–25. [Google Scholar]
- 29.a) Wender PA, De Brabander J, Harran PG, Jimenez JM, Koehler MFT, Lippa B, Park CM, Shiozaki M. J. Am. Chem. Soc. 1998;120:4534–4535. [Google Scholar]; b) Wender PA, Baryza JL. Org. Lett. 2005;7:1177–1180. doi: 10.1021/ol0501931. [DOI] [PubMed] [Google Scholar]; c) Wender PA, Horan JC. Org. Lett. 2006;8:4581–4584. doi: 10.1021/ol0618149. [DOI] [PubMed] [Google Scholar]; d) Smith AB, Razler TM, Meis RM, Pettit GR. Org. Lett. 2006;8:797–799. doi: 10.1021/ol060014v. [DOI] [PubMed] [Google Scholar]
- 30.Smith AB, Liu ZQ, Hogan AML, Dalisay DS, Molinski TF. Org. Lett. 2009;11:3766–3769. doi: 10.1021/ol9014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garner P, Park JM. J. Org. Chem. 1987;52:2361–2364. [Google Scholar]
- 32.Saito K, Saijo S, Kotera K, Date T. Chem. Pharm. Bull. 1985;33:1342. [Google Scholar]; Nakamura Y, Hirai M, Tamotsu K, Yonezawa Y, Shin CG. Bull. Chem. Soc. Jpn. 1995;68:1369–1376. [Google Scholar]
- 33.Jackson MM, Leverett C, Toczko JF, Roberts JC. J. Org. Chem. 2002;67:5032–5035. doi: 10.1021/jo025682i. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura K, Hashiguchi S, Ikariya T, Noyori R. J. Am. Chem. Soc. 1997;119:8738–8739. [Google Scholar]
- 35.a) Huang TL, Székács A, Uematsu T, Kuwano E, Parkinson A, Hammock BD. Pharm. Res. 1993;10:639–648. doi: 10.1023/a:1018987111362. [DOI] [PubMed] [Google Scholar]; b) Ghosh AK, Kincaid JF, Cho WH, Walters DE, Krishnan K, Hussain KA, Koo Y, Cho H, Rudall C, Holland L, Buthod J. Bioorg. Med. Chem. Lett. 1998;8:687–690. doi: 10.1016/s0960-894x(98)00098-5. [DOI] [PubMed] [Google Scholar]
- 36.Albert BJ, McPherson PA, O'Brien K, Czaicki NL, DeStefino V, Osman S, Li MS, Day BW, Grabowski PJ, Moore MJ, Vogt A, Koide K. Mol. Cancer Ther. 2009;8:2308–2318. doi: 10.1158/1535-7163.MCT-09-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.With this solubility at 0.1 mgmL−1 in saline, it is possible to reach the maximum tolerated dosage of FR901464 (1 mgkg−1).
- 38.a) Groll M, Schellenberg B, Bachmann AS, Archer CR, Huber R, Powell TK, Lindow S, Kaiser M, Dudler R. Nature. 2008;452:755. doi: 10.1038/nature06782. [DOI] [PubMed] [Google Scholar]; b) Clerc J, Groll M, Illich DJ, Bachmann AS, Huber R, Schellenberg B, Dudler R, Kaiser M. Proc. Natl. Adad. Sci. U. S. A. 2009;106:6507–6512. doi: 10.1073/pnas.0901982106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imamura Y, Ohtsu Y, Tanaka H, Hatakeyama M, Manabe T, Kawaguchi H, Handa H, Takahashi T. Heterocycles. 2004;64:51–56. [Google Scholar]
- 40.Charette AB, Marcoux JF. Synlett. 1995:1197–1207. [Google Scholar]
- 41. http://www.dtp.nci.nih.gov/branches/btb/ivclsp.html.