Abstract
A major problem in medical research is to translate in vitro observations into the living organism. In this perspective, we discuss ongoing efforts to non-invasively image pancreatic islets/β-cells by techniques, such as magnetic resonance imaging and positron emission tomography, and present an experimental platform, which allows in vivo imaging of pancreatic β-cell mass and function longitudinally and at the single-cell level. Following transplantation of pancreatic islets into the anterior chamber of the eye of mice and rats, these islets are studied by functional microscopic imaging. This imaging platform can be utilized to address fundamental aspects of pancreatic islet cell biology in vivo in health and disease. These include the dynamics of pancreatic islet vascularization, islet cell innervation, signal-transduction, change in functional β-cell mass and immune responses. Moreover, we discuss the feasibility of studying human islet cell physiology and pathology in vivo as well as the potential of using the anterior chamber of the eye as a site for therapeutic transplantation in type 1 diabetes mellitus.
Keywords: diabetes mellitus, fluorescence microscopy, in vivo optical imaging, islets of Langerhans, pancreatic β-cell mass
The incidence of both forms of diabetes, i.e. type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), is increasing, T2DM reaching epidemic proportions. Genome-wide association studies revealed that more than 30 candidate genes are involved in the pathogenesis of T2DM, most of them potentially linked with pancreatic islet/β -cell function. This shifts the paradigm of diabetes being primarily due to insulin resistance of the classical insulin target tissues liver, muscle and fat towards a more central role of the β-cell in the pathogenesis of this disorder. Hence, it pinpoints the importance of an intact integrated signal-transduction for a proper function and survival of the β-cell. There is a growing body of evidence that the β-cell relies on multiple signals provided by the architecture of the islet of Langerhans for an appropriate function. To understand the dynamics of β-cell function and survival under normal conditions and the development of β-cell dysfunction in diabetes, these processes must be studied in the context of the intact islet of Langerhans in vivo where the capillary network is intact and the innervation is adequate. Here we present an experimental platform, which allows non-invasive imaging of pancreatic β-cell function and survival longitudinally with single-cell resolution.
The physiology of islets has been extensively investigated in vitro
There is a wealth of information about the physiology of rodent islets in vitro. A variety of techniques have been applied to isolated islets to record membrane electrical activity, changes in cytoplasmic free Ca2+ concentration, [Ca2+]i, mitochondrial membrane potential and hormone release. The main signal-transduction cascades have been dissected in detail and the different steps involved in the stimulus-secretion coupling have been elucidated for rodent β-cells. Glucose is effectively taken-up by the β-cell glucose transporters. It is metabolized in glycolysis and Krebs-cycle, which results in an increased ratio of ATP to ADP in the cytoplasm. This closes ATP-sensitive potassium channels (KATP channels) and leads to cell membrane depolarization, opening of voltage-gated Ca2+ channels, increase in [Ca2+]i and finally, insulin secretion. As a consequence of this chain of events, insulin secretion is tightly coupled to changes in the extracellular glucose concentration. Besides this KATP-channel-dependent ‘triggering’ pathway, glucose in addition acts through a so-called KATP-channel-independent ‘amplifying’ pathway to achieve maximal insulin exocytosis.
Methods for in vivo imaging of pancreatic islet function are scarce
In contrast to isolated islets in vitro, glucose reaches endocrine islet cells in the native pancreas through the microvasculature and this arrangement, for sure has an impact on islet cell function. In addition, native islets are innervated and surrounded by a dense basement membrane. Imaging islets in the living organism is challenging and unfortunately, non- or minimal-invasive technologies to monitor islet cell function and survival longitudinally, specifically in terms of molecular signal-transduction processes, have not been developed.
To study β-cell function in a relatively intact environment, pancreatic slices containing islets were used for electrophysiological recordings (Speier & Rupnik 2003). In this set-up, islets retain their structural integrity and are not subjected to enzymatic digestion, but are isolated from the organism. Islets have been imaged after transplantation under the kidney capsule, where it has been possible to visualize the vascularization process (Bertera et al. 2003, Nyqvist et al. 2005). However, the instability and inaccessibility of this transplantation site makes this approach difficult, in particular for functional and longitudinal microscopic studies.
Other emerging efforts to image β-cells in vivo include magnetic resonance imaging (MRI) and position emission tomography (PET), (reviewed in Paty et al. 2004, Souza et al. 2006, Lin et al. 2008, Moore 2009). Although MRI has been successfully applied to monitor the mass change of transplanted β-cells/islets in diabetic rats (Koblas et al. 2005), these methods are limited because they poorly discriminate between exocrine and endocrine tissues, thus failing to accurately measure β-cell mass. This is likely because of the low abundance of islets and the lack of contrast from the surrounding tissue in the pancreas. An additional major challenge is identifying markers that can be combined with imaging reporters that are specific to endocrine cells of the pancreas. While it is expected that these methods will eventually improve quantifying islet mass in vivo, they may not be useful to visualize subtle changes in apoptosis, vascularization or innervation. Even with the use of high-contrast materials or tissue-specific luminescence, these techniques are limited by high background signal from surrounding tissues, lack spatial resolution (<100 µm) and do not allow functional monitoring of islets.
Optical imaging techniques, including confocal microscopy and optical coherence tomography, have been recently applied to study the biology of islets in the pancreas or in transplanted islets (reviewed in (Lin et al. 2008, Virostko & Powers 2009). To perform these studies, islets and their associated structures, such as blood vessels and nerves, need to be labelled with probes that emit light or that affect light scattering. Using an early marker of apoptosis (annexin V) coupled to a fluorophore emitting near-infrared radiation, β-cell death can be detected in diabetic mice in vivo (Medarova et al. 2005). To image with single-cell resolution, however, the pancreas needs to be externalized. This approach has been used to investigate blood flow in pancreatic islets (Nyman et al. 2008) and has been proposed to investigate immune responses to islets in the pancreas (Martinic & von Herrath 2008). Longitudinal studies, however, are unlikely to be possible because the pancreas cannot be externalized repeatedly.
An alternative approach is to apply fluorometric imaging techniques that have been used for in vitro islet cell characterization, such as epifluorescence microscopy, confocal laser scanning microscopy (CLSM) and two-photon laser scanning microscopy (TPLSM), to islets grafted in a site that is readily accessible for imaging, sufficiently stable for longitudinal and functional studies, and in which islets are vascularized and innervated. This site is the anterior chamber of the eye (Speier et al. 2008a,b).
The anterior chamber of the eye as a natural ‘body-window’ for in vivo imaging
The anterior chamber of the eye can be used as a natural body-window to non-invasively image pancreatic β-cell function and survival longitudinally in the context of the intact islet under in vivo conditions (Speier et al. 2008a, b). The cornea is transparent and the grafts are easily vascularized and innervated because of the rich blood and nerve supply of the iris that forms the bed of the anterior chamber of the eye. The iris contains nerve fibres that have been shown to invade the intra-ocular grafts (Taylor et al. 1978). Therefore, the autonomic input to the grafted tissue can be modulated non-invasively.
The anterior chamber of the eye is a classical transplantation site used since the early 1870s (van Dooremaal 1873), when van Dooremaal made the observation that tumour cells injected into the anterior chamber of the eye formed progressively growing tumours. More than 60 years later, Sir Peter Medawar discovered that skin grafts survived for prolonged intervals in the eye. Since then, the anterior chamber of the eye has been widely used as a model system to study not only immune deviation but also growth and differentiation of a variety of tissues, including ovaries (Falck 1959), peripheral and central nervous tissues (Bickford-Wimer et al. 1987) and also pancreatic tissue grafts (Adeghate & Donath 1990).
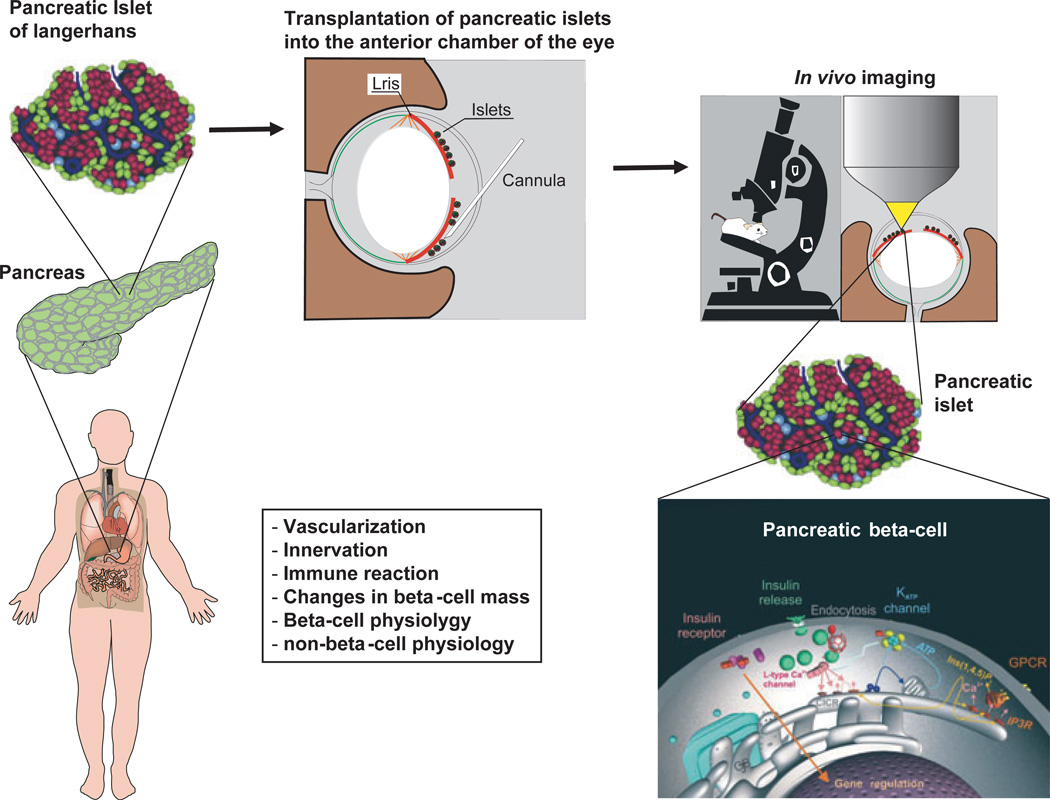
Utilizing the anterior chamber of the eye as an imaging platform will move in vitro islet studies into real-time in vivo islet physiology. Fluorescent tools and probes that have been used to image structure and function in isolated islets in vitro can now be applied to intra-ocular islet grafts. It is easy to envisage applications of this platform for studying the mechanisms of vascularization and innervation after transplantation, inflammatory responses, as well as physiological responses to changes in plasma glucose concentration or pharmacological intervention (Fig. 1).
Figure 1.
Schematic illustration of the in vivo imaging platform to study pancreatic islet/β-cell physiology and pathology. Following their isolation, pancreatic islets of Langerhans are transplanted into the anterior chamber of the eye, where they engraft on the iris, become vascularized and innervated. As the cornea represents a natural body-window, this transplantation site allows non-invasive, repetitive in vivo imaging of the islet with high resolution, i.e. at the single-cell level, and to study various aspects of islet/β-cell function and survival under normal as well as diabetic conditions.
Pancreatic islets following transplantation into the anterior chamber of the eye are functional
After transplantation of mouse islets into the anterior chamber of the eye, these islets engraft on the iris and are readily observed and imaged through the cornea. Immunohistochemical staining of engrafted islets shows that the proportion of insulin containing β-cells and glucagon-containing α-cells does not change after transplantation (Speier et al. 2008a,b). Moreover, comparing the histology of islets transplanted into the eye with islets in the pancreas reveals no changes in islet composition (Ilegems et al. 2010). In fact, electron micrographs show that fine structures such as fenestrae within the capillary endothelial cell layer are maintained. Engrafted islets are functional and contribute to glucose homeostasis of the recipient mouse. After transplantation of syngeneic islets (approx. 300 islet equivalents) into the anterior chamber of the eye of streptozotocin-induced diabetic C57BL/6 mice, all animals achieve normoglycaemia within 2 weeks (9.2 ± 2.2 days). Removal of the islet graft-bearing eye resulted in prompt return to hyperglycaemia (Speier et al. 2008a). Thus, engrafted islets are responsible for glycaemic control in the recipient mouse.
The anterior chamber of the eye: a transplantation site to study dynamics of vascularization
Both revascularization and reinnervation of islets in the process of engraftment are of vital importance for islet cell function and survival (Korsgren et al. 1993, Adeghate 2002, Andersson et al. 2004, Brissova & Powers 2008, Richards et al. 2010). The vascularization of transplanted islets in the anterior chamber of the eye can be investigated by using angiographic imaging. Syngeneic mouse islets that express green fluorescent protein in their pancreatic β-cells [rat insulin promoter (RIP)-GFP mice (Speier et al. 2008a)] are transplanted into the eyes of C57Bl/6 mice. Because islets can be readily observed and retrieved, it is feasible to image repeatedly the same group of islets. To visualize blood vessels, the fluorescent dye rhodamine dextran is injected into the tail vein. Blood vessels can be seen contacting grafted islets <1 week after transplantation. The vascularization pattern becomes more complex with time and 10 days after transplantation smaller vessels branching from larger vessels in grafted islets can be seen. Tie2-GFP mice, that express GFP in their endothelial cells can be used as recipients to explore the vascularization process non-invasively. Longitudinal experiments demonstrate that the vascularization process reaches a plateau after 4 weeks, showing a vasculature density of the islets equivalent to that found in the pancreas.
Hence, this platform will enable researchers to image angiogenesis at high resolution in real-time in a mammalian model. This platform will also allow studies of the microcirculation within the pancreatic islet under normal and diabetic conditions and thereby reveal possible islet vascular complications associated with the disorder.
The anterior chamber of the eye: a transplantation site to study dynamics and nature of innervation
An important task in islet cell biology as well as pathology is to understand the dynamics, nature and origin of innervation of the various endocrine cells. When transplanted into the anterior chamber of the eye, tissues become innervated by the autonomic nerve supply of the iris (Rodriguez-Diaz et al. 2009). The use of mice expressing GFP specifically in neurones [e.g. B6; 129S-Mapttm1(EGFP)Klt or ChATBAC-eGFP (Tallini et al. 2006)] as recipients of the grafts allows for non-invasive visualization of the innervation process and thereby the possibility to follow elongating nerve axons. With retrograde tracing from the eye, one will be able to investigate which brain areas project to the various endocrine cells.
By using the in vivo imaging platform, it is thus possible to follow in real-time innervation and nature of innervation of the various islet cells subsequent to transplantation. Not only can one investigate how this process is affected in diabetes but also how important this innervation is for overall signal-transduction patterns and thereby the function and survival of the various endocrine cells.
The anterior chamber of the eye: a transplantation site to study signal-transduction in pancreatic β-cells in vivo
To fully understand the molecular mechanisms that lead to β-cell dysfunction in diabetes and to develop strategies strategies aiming at preventing β-cell dysfunction, signal-transduction pathways in β-cells have to be investigated under natural conditions in the islet in vivo. Hence, a potential strategy to achieve this is based on online monitoring/imaging of key events in β-cell signal-transduction in pancreatic islets that are transplanted into the anterior chamber of the eye to allow non-invasive in vivo monitoring using biosensors that reflect (1) glucose metabolism/ATP production, (2) [Ca2+]i, (3) exocytosis, (4) stimulus-induced insulin gene transcription, (5) total β-cell mass, (6) apoptosis and (7) regeneration. Biosensors to be used in these experiments are genetically engineered proteins that carry fluorescent probes, which allow imaging of cellular signalling events by measuring with spatio-temporal resolution fluorescence resonance energy transfer (FRET) between suitably labelled interacting partners as well as the fluorescence intensities of signalling components per se. Various genetically engineered Ca2+-biosensors are available and have been successfully used in vitro and in vivo (reviewed in (Kotlikoff 2007). Biosensors reflecting insulin gene expression (insulin promoter-driven GFP) and apoptosis (C-DEVD-Y) have been extensively tested in in vitro studies (Leibiger et al. 1998, 2001, Kohler et al. 2003, Uhles et al. 2007). Biosensors can be specifically expressed in β-cells by employing the insulin promoter. A strategy will be to use virus-based expression vectors for transduction of islets in vitro prior to their transplantation into the eye.
Similarly, expressing these biosensors under the control of the glucagon or somatostatin promoter will allow real-time imaging of signal-transduction in pancreatic α- and δ-cells respectively.
The anterior chamber of the eye: a transplantation site to study changes in pancreatic β-cell mass
To investigate the feasibility of non-invasive imaging of β-cell death, we transplant islets from RIP-GFP mice (expressing GFP in their β-cells under control of the RIP) into the anterior chamber of the eye and, after complete engraftment and vascularization, we monitor cell death following intravenously administered annexin V (which binds to the surface of early apoptotic cells) conjugated to allophycocyanin (APC). Transplanted RIP-GFP islets imaged in mice with regular blood glucose levels display normal morphology and absence of annexin V-APC labelling. β-cell death in mice transplanted with RIP-GFP islets is induced by intravenous administration of alloxan. After 24 h, substantial loss of GFP fluorescence and structural changes in the reflection of the islet grafts are observed, indicating loss of β-cells. Administration of annexin V-APC at this time point results in strong labelling of islet grafts. High magnification imaging reveals that most annexin V-APC labelling is found in graft regions devoid of GFP fluorescence. These data illustrate that β-cell death can be imaged non-invasively and longitudinally in real-time under in vivo conditions in islets engrafted in the eye (Speier et al. 2008a). These experiments also demonstrate that engrafted islets in the anterior chamber of the eye can be used as visible representatives of their natural counterparts in the pancreas. This allows monitoring longitudinally, the gain in β-cell mass in the ob/ob mouse as well as the loss of β-cells in response to autoimmune destruction in the T1DM BB-rat.
The anterior chamber of the eye: a transplantation site to study immune reactions at the cellular level
Development of T1DM and its treatment by islet transplantation covers two fundamental aspects in immunology research, namely autoimmune destruction of cells (T1DM) and allograft rejection following transplantation (islet transplantation in T1DM). The here-presented experimental platform allows for the first time monitoring, in real-time, the respective immune responses in vivo at the cellular level (Abdulreda et al. 2010). In a model of allograft transplantation, DBA/2 mouse islets are transplanted into diabetic C57BL/6 recipients without immune-suppression, which leads to intra-ocular graft loss. When C57BL/6(B6.129P2-Cxcr6tm1Litt/J) mice expressing GFP in T-lymphocytes are used as recipients, T-lymphocytes can be tracked to show that increasing cellular infiltration correlates with loss of islet morphology and function. Collectively, intra-ocular islet allografts are rejected via a T-cell-mediated destructive process resulting in hyperglycaemia. These results show that real-time, repetitive live imaging of immune cell infiltration and islet morphology is feasible in the anterior chamber of the eye. The ability of monitoring individual islets non-invasively in the same animal over time is an invaluable advantage of this model compared with conventional transplantation sites. Note-worthy is that these data clearly illustrate that the immune privilege, which is transiently provided by this transplantation site is finally broken following vascularization of the graft.
The anterior chamber of the eye: a transplantation site to study human pancreatic islet cell physiology as well as cell pathology
It is clear from our own as well as other studies (Brissova et al. 2005, Cabrera et al. 2006) that the human pancreatic islet is different from rodent islets in terms of both structure and function. Therefore, it is of utmost importance to clarify signal-transduction processes in human islets under normal conditions and why these do not function properly in diabetes. To be able to integrate the complex processes involved in the living organism, a systems biology approach is needed. In this context, the application of the in vivo imaging platform will be mandatory. An interesting challenge for the success of these experiments is to develop a humanized mouse model (Caicedo et al. 2009, Rodriguez-Diaz et al. 2009). For this purpose human islets are transplanted into immunodeficient nude mice, whose endogenous β-cells have been chemically destroyed, and subjected to our imaging platform. Similar to rodent islets, the dynamics of human islet vascularization can be monitored longitudinally. Moreover, it will be possible to study the function of human islet cells in an in vivo setting, which will then allow relating human islet cell function to glucose homeostasis in the organism.
The anterior chamber of the eye: a novel clinical transplantation site for the treatment of T1DM
The observation that pancreatic islets engraft in the anterior chamber of the eye and are able to maintain glucose homeostasis in mice that are rendered diabetic by chemically destroying their own β-cells raises the fundamental question as to whether the anterior chamber of the eye represents a novel clinical transplantation site for the treatment of patients with T1DM.
The eye is an interesting novel potential site for clinical transplantation because of the immune privilege properties, the simple procedure, the easy non-invasive monitoring of the graft, the possibility of delivering local immunosuppression and the potential for systemic tolerization through anterior chamber-associated immune deviation as discussed in Stein-Streilein & Streilein (2002). Notably, if needed, immunosuppressive drugs may reach efficacy at lower levels by local application into the eye compared with systemic application. Contrary to the intrahepatic portal system, which is currently the site of choice for therapeutic islet transplantation, vascularization in the eye is rapid and effective and there is no inflammation after blood contact, thus limiting massive islet loss associated with the high vulnerability of islets in the immediate post-transplantation period.
To test the anterior chamber of the eye as a suitable transplantation site in non-human primates, we performed a pilot study where we transplanted two times 18 000 islet equivalents into the eye of a streptozotocin-diabetic baboon in combination with anti-CD154 monotherapy (Perez et al. 2011). The ophthalmological examination showed no inflammation, no immune response and no sympathetic ophthalmia. Over the monitored period of time, i.e. 357 days, blood glucose homeostasis improved, insulin C-peptide levels (evidence for insulin production) in the aqueous humor and in the circulation increased, and the HbA1c values (measure of long-term glucose regulation) decreased. Intra-ocular islet grafts thus manifestly contributed to glucose homeostasis and improved glycaemic control in this diabetic baboon.
Conclusions
A major problem in medical research in health and disease of today is to translate in vitro observations into the in vivo situation. While efforts are made to improve the specificity and resolution for imaging β-cell mass by PET and MRI, these techniques will not have the possibility provided by high-resolution optical imaging, i.e. to study β-cell biology and pathology at the single-cell level. The herein-described imaging platform allows non-invasive, longitudinal in vivo imaging of cell function and survival at single-cell resolution in a multicellular environment. Because the pancreatic islet is a micro-organ consisting of several endocrine and non-endocrine cell types where the function of each cell type is affected by cell–cell interactions, autocrine, endocrine and paracrine feedback loops as well as humoral and neural factors, this imaging platform in combination with the existing vast variety of transgenic (knock-out, knock-in, etc.) animal models will allow studies of islet cell biology in health and disease in a broad spectrum of disciplines of life sciences. Although no obvious differences in islet architecture, vascularization and innervation between engrafted islets in the anterior chamber of the eye and endogenous islets in the pancreas have been observed, other signalling input provided by, for example, the exocrine pancreas are missing. Thus, future work will have to reveal additional strengths and limitations of the presented imaging platform.
Acknowledgments
This study was supported by grants DK-58508, DK-075487 (to A.C.), and DK-084321 (to A.C.) from the US National Institutes of Health, by funds from Karolinska Institutet and by grants from the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Diabetes Association, the Berth von Kantzows Foundation, the Knut and Alice Wallenberg Foundation, VIBRANT (FP7-228933-2), Skandia Insurance Company, Ltd., Strategic Research Program in Diabetes at Karolinska Institutet, the World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (CR31-2008-000-10105-0), and the Family Erling-Persson Foundation.
Footnotes
Conflict of interest
P-OB is co-founder of BioCrine AB, IBL is consultant to BioCrine AB.
References
- Abdulreda MH, Faleo G, Molano RD, Molina J, Tan Y, Echeverria OA, Zahr E, Rodriguez-Diaz R, Edlund PK, Leibiger I, et al. Highly dynamic cytotoxic T-lymphocytes and pancreatic islet alograft rejection. Diabetes. 2010;59:A39. [Google Scholar]
- Adeghate E. Pancreatic tissue grafts are reinnervated by neuro-peptidergic and cholinergic nerves within five days of transplantation. Transpl Immunol. 2002;10:73–80. doi: 10.1016/s0966-3274(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Adeghate E, Donath T. Morphological findings in long-term pancreatic tissue transplants in the anterior eye chamber of rats. Pancreas. 1990;5:298–305. doi: 10.1097/00006676-199005000-00009. [DOI] [PubMed] [Google Scholar]
- Andersson A, Carlsson PO, Carlsson C, Olsson R, Nordin A, Johansson M, Palm F, Tyrberg B, Kallskog O, Tillmar L, Welsh N, Mattsson G, Jansson L. Promoting islet cell function after transplantation. Cell Biochem Biophys. 2004;40:55–64. doi: 10.1385/cbb:40:3:55. [DOI] [PubMed] [Google Scholar]
- Bertera S, Geng X, Tawadrous Z, Bottino R, Balamurugan AN, Rudert WA, Drain P, Watkins SC, Trucco M. Body window-enabled in vivo multicolor imaging of transplanted mouse islets expressing an insulin-timer fusion protein. Bio Techniques. 2003;35:718–722. doi: 10.2144/03354st01. [DOI] [PubMed] [Google Scholar]
- Bickford-Wimer P, Granholm AC, Bygdeman M, Hoffer B, Olson L, Seiger A, Stromberg I. Human fetal cerebellar and cortical tissue transplanted to the anterior eye chamber of athymic rats: electrophysiological and structural studies. Proc Natl Acad Sci USA. 1987;84:5957–5961. doi: 10.1073/pnas.84.16.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes. 2008;57:2269–2271. doi: 10.2337/db08-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Fowler MJ, Nicholson WE, Chu A, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Rodriguez R, Molano RD, Ricordi C, Berggren PO, Pileggi A. Noninvasive, live imaging studies of human islet cell biology in the anterior chamber of the eye. Xenotransplantation. 2009;16:415–416. [Google Scholar]
- van Dooremaal JC. Die Entwicklung der in fremden Grund versetzten lebenden Gewebe. Von Graefes Arch Ophtal. 1873;19:359–373. [Google Scholar]
- Falck B. Site of production of oestrogen in rat ovary as studied in micro-transplants. Acta Physiol Scand Suppl. 1959;47:1–101. doi: 10.1111/j.1748-1716.1960.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Ilegems E, Bahow A, Sharma A, Dicker A, Speier S, Karlsson-Edlund P, Kohler M, Leibiger IB, Berggren PO. Islets transplanted into the anterior chamber of the eye serve as a mirror of in situ pancreatic islets. Diabetologia. 2010;53:S195–S475. [Google Scholar]
- Koblas T, Girman P, Berkova Z, Jirak D, Kriz J, Dovolilova E, Zacharovova K, Hajek M, Saudek F. Magnetic resonance imaging of intrahepatically transplanted islets using paramagnetic beads. Transplant Proc. 2005;37:3493–3495. doi: 10.1016/j.transproceed.2005.09.142. [DOI] [PubMed] [Google Scholar]
- Kohler M, Zaitsev SV, Zaitseva II, Leibiger B, Leibiger IB, Turunen M, Kapelioukh IL, Bakkman L, Appelskog IB, de Monvel JB, Imreh G, Berggren PO. On-line monitoring of apoptosis in insulin-secreting cells. Diabetes. 2003;52:2943–2950. doi: 10.2337/diabetes.52.12.2943. [DOI] [PubMed] [Google Scholar]
- Korsgren O, Jansson L, Andersson A, Sundler F. Reinnervation of transplanted pancreatic islets. A comparison among islets implanted into the kidney, spleen, and liver. Transplantation. 1993;56:138–143. [PubMed] [Google Scholar]
- Kotlikoff MI. Genetically encoded Ca2+ indicators: using genetics and molecular design to understand complex physiology. J Physiol. 2007;578:55–67. doi: 10.1113/jphysiol.2006.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibiger IB, Leibiger B, Moede T, Berggren PO. Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 s6 kinase and CaM kinase pathways. Mol Cell. 1998;1:933–938. doi: 10.1016/s1097-2765(00)80093-3. [DOI] [PubMed] [Google Scholar]
- Leibiger B, Leibiger IB, Moede T, Kemper S, Kulkarni RN, Kahn CR, de Vargas LM, Berggren PO. Selective signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol Cell. 2001;7:559–570. doi: 10.1016/s1097-2765(01)00203-9. [DOI] [PubMed] [Google Scholar]
- Lin M, Lubag A, McGuire MJ, Seliounine SY, Tsyganov EN, Antich PP, Sherry AD, Brown KC, Sun X. Advances in molecular imaging of pancreatic beta cells. Front Biosci. 2008;13:4558–4575. doi: 10.2741/3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinic MM, von Herrath MG. Real-time imaging of the pancreas during development of diabetes. Immunol Rev. 2008;221:200–213. doi: 10.1111/j.1600-065X.2008.00581.x. [DOI] [PubMed] [Google Scholar]
- Medarova Z, Bonner-Weir S, Lipes M, Moore A. Imaging beta-cell death with a near-infrared probe. Diabetes. 2005;54:1780–1788. doi: 10.2337/diabetes.54.6.1780. [DOI] [PubMed] [Google Scholar]
- Moore A. Advances in beta-cell imaging. Eur J Radiol. 2009;70:254–257. doi: 10.1016/j.ejrad.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest. 2008;118:3790–3797. doi: 10.1172/JCI36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyqvist D, Kohler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54:2287–2293. doi: 10.2337/diabetes.54.8.2287. [DOI] [PubMed] [Google Scholar]
- Paty BW, Bonner-Weir S, Laughlin MR, McEwan AJ, Shapiro AM. Toward development of imaging modalities for islets after transplantation: insights from the National Institutes of Health Workshop on Beta Cell Imaging. Transplantation. 2004;77:1133–1137. doi: 10.1097/01.tp.0000113231.90613.0e. [DOI] [PubMed] [Google Scholar]
- Perez VL, Caicedo A, Berman DM, Arrieta E, Abdulreda MH, Rodriguez-Diaz R, Pileggi A, Hernandez E, Dubovy SR, Parel JM, Ricordi C, Kenyon NM, Kenyon NS, Berggren PO. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: a study in a baboon model of diabetes. Diabetologia. 2011;54:1121–1126. doi: 10.1007/s00125-011-2091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev. 2010;31:343–363. doi: 10.1210/er.2009-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Gans I, Ricordi C, Caicedo A, Berggren PO. The human islet innervation is unique. Diabetes. 2009;58:A425. [Google Scholar]
- Souza F, Freeby M, Hultman K, Simpson N, Herron A, Witkowsky P, Liu E, Maffei A, Harris PE. Current progress in non-invasive imaging of beta cell mass of the endocrine pancreas. Curr Med Chem. 2006;13:2761–2773. doi: 10.2174/092986706778521940. [DOI] [PubMed] [Google Scholar]
- Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Arch. 2003;446:553–558. doi: 10.1007/s00424-003-1097-9. [DOI] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, Ricordi C, Leibiger IB, Caicedo A, Berggren PO. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008a;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Kohler M, Caicedo A, Leibiger IB, Berggren PO. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protocols. 2008b;3:1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21:123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- Tallini YN, Shui B, Greene KS, Deng KY, Doran R, Fisher PJ, Zipfel W, Kotlikoff MI. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiol Genomics. 2006;27:391–397. doi: 10.1152/physiolgenomics.00092.2006. [DOI] [PubMed] [Google Scholar]
- Taylor D, Seiger A, Freedman R, Olson L, Hoffer B. Electrophysiological analysis reinnervation of transplants in the anterior chamber of the eye by the autonomic ground plexus of the iris. Proc Natl Acad Sci USA. 1978;75:1009–1012. doi: 10.1073/pnas.75.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhles S, Moede T, Leibiger B, Berggren PO, Leibiger IB. Selective gene activation by spatial segregation of insulin receptor B signaling. FASEB J. 2007;21:1609–1621. doi: 10.1096/fj.06-7589com. [DOI] [PubMed] [Google Scholar]
- Virostko J, Powers AC. Molecular imaging of the pancreas in small animal models. Gastroenterology. 2009;136:407–409. doi: 10.1053/j.gastro.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]