Abstract
Coronary arteries distal to chronic occlusion exhibit enhanced vasoconstriction and impaired relaxation when compared with nonoccluded arteries. In the present study, we tested the hypotheses that an increase in peak Ca2+ channel current density and/or increased Ca2+ sensitivity contributes to altered contractility in collateral-dependent coronary arteries. Ameroid occluders were surgically placed around the proximal left circumflex coronary artery (LCX) of female miniature swine. Segments of epicardial arteries (∼1 mm luminal diameter) were isolated from the LCX and nonoccluded left anterior descending (LAD) arteries 24 wks following Ameroid placement. Contractile responses to depolarization (10-100 mM KCl) were significantly enhanced in LCX compared with size-matched LAD arterial rings (EC50; LAD=41.7±2.3, LCX=34.3±2.7 mM). However, peak Ca2+ channel current was not altered in isolated smooth muscle cells from the LCX compared with LAD (−5.29±0.42 vs. −5.68±0.55 pA/pF, respectively). Furthermore, while half-maximal activation of Ca2+ channel current occurred at nearly the same membrane potential in LAD and LCX, half-maximal inactivation was shifted to a more positive membrane potential in LCX cells. Simultaneous measures of contractile tension and intracellular free Ca2+ (fura-2) levels in arterial rings revealed that significantly more tension was produced per unit change in fura-2 ratio in the LCX compared with the LAD in response to KCl-induced membrane depolarization, but not during receptor-agonist stimulation with endothelin-1. Taken together, our data indicate that coronary arteries distal to chronic occlusion display increased Ca2+ sensitivity in response to high KCl-induced membrane-depolarization, independent of changes in whole-cell peak Ca2+ channel current. Unaltered Ca2+ sensitivity in endothelin-stimulated arterial rings suggests more than one mechanism regulates Ca2+ sensitization in coronary smooth muscle.
Introduction
Collateral-dependent coronary arteries exhibit enhanced constriction and impaired relaxation responses when compared with control, nonoccluded arteries (7,8,13,20,22,28-32). These alterations in vasomotor responsiveness may have important implications in the regulation of blood flow to collateral-dependent myocardium (29). Indeed, episodes of myocardial ischemia in patients with chronic stable angina have been attributed to constriction of arteries distal to the site of stenosis (21). Previous studies have reported that the altered reactivity of coronary vasculature distal to chronic occlusion results from changes in both endothelial and smooth muscle function (7,8,13,20,22,28-32). Furthermore, alterations in vasomotor responsiveness of endothelium-denuded, collateral-dependent arterial rings are closely associated with altered smooth muscle intracellular free Ca2+ levels (13,22). The purpose of this study was to examine potential mechanisms related to smooth muscle function that may be responsible for the increased contractility observed in the collateral-dependent vasculature. We hypothesized that an increased peak Ca2+ channel current density and/or increased Ca2+ sensitivity in arterial smooth muscle distal to chronic occlusion would contribute to enhanced contractile activity of the collateral-dependent vasculature. We compared the Ca2+ sensitivity of collateral-dependent and nonoccluded coronary arteries by measuring simultaneous changes in contractile tension and intracellular free Ca2+ concentration (Cai) in response to high-KCl membrane depolarization and receptor-agonist stimulation with endothelin-1. Our results indicate that coronary arteries distal to chronic occlusion display increased Ca2+ sensitivity in response to high KCl-induced membrane-depolarization, independent of changes in whole-cell peak Ca2+ channel current. Unaltered Ca2+ sensitivity in endothelin-stimulated arterial rings suggests more than one mechanism regulates Ca2+ sensitization in coronary smooth muscle.
Methods
Experimental animals and surgical procedures
Animal protocols were approved by the University of Missouri Animal Care and Use Committee in accordance with the “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training”. Adult female Yucatan miniature swine (Charles River, Wilmington, MA). were surgically instrumented with Ameroid constrictors around the proximal left circumflex coronary (LCX) artery as described previously (7,13). Animals were preanesthetized with glycopyrrolate (0.004 mg·kg−1, im) and midazolam (0.5 mg·kg−1, im). Anesthesia was induced with ketamine (20 mg·kg−1, im) and maintained with 3% isoflurane and 97% O2 throughout aseptic surgery.
Preparation of coronary arteries
Twenty-four weeks following Ameroid placement, the animals were anesthetized with ketamine (30 mg·kg−1) and pentobarbital sodium (35 mg·kg−1). The hearts were removed and placed in ice cold Krebs bicarbonate buffer (0-4 °C) for isolation of the occluded LCX and the nonoccluded left anterior descending (LAD) coronary arteries (13). Visual inspection at the Ameroid occluder during dissection of the LCX artery indicated 100% occlusion in all animals used in this study.
With the aid of a dissection microscope, segments of the LCX and LAD arteries were trimmed of fat and connective tissue, cut into rings and measured with a calibrated Filar micrometer eyepiece (Hitschfel Instruments, St. Louis, MO) in a relaxed, nonpressurized state. Arterial rings (axial length 3.5-4.0 mm, luminal diameter ∼1 mm) used for evaluation of concentration-response relationships were prepared in Krebs buffer. Additional arterial rings (axial length ∼1.0 mm, luminal diameter ∼1 mm) used for fura-2 experiments (fura-2/acetoxymethyl ester; Molecular Probes, Eugene, OR) were denuded of endothelium by gently rubbing the luminal surface with a silk surgical suture. A segment of adventitia (∼1.0 mm2) was carefully removed from the arterial ring, creating a window that allowed direct excitation/emission of fura-2 in medial smooth muscle and concomitantly minimized autofluorescence of the adventitial connective tissue. Care was taken to ensure underlying smooth muscle was not damaged during removal of the adventitia. These arterial rings were incubated in fura-2/AM loading solution at 37°C for 2 h, followed by a 30-min rinse in sterile modified Eagle's minimal essential storage media.
KCl concentration-response relationships
Coronary rings (3.5-4.0 mm axial length) were mounted on two stainless steel wires passed through the vessel lumen. One wire was fixed to a force transducer (Grass FT03, Grass Instruments, Quincy, MA) and the other to a micrometer microdrive (Stoelting/Prior Microdrive, Wood Dale, IL) to allow precise changes in circumferential length of the vessel. The mounted arterial ring was lowered into a 20-ml tissue bath containing Krebs bicarbonate buffer at 37 ± 0.5 °C and aerated with 95% O2, 5% CO2. Coronary rings were progressively stretched to the maximum of the length-developed tension relationship (Lmax) in increments equal to 10% of the initial vessel outer diameter. After each stretch, contraction was elicited with exposure to high KCl (80 mM) and Lmax was defined as the circumferential length at which developed tension was <5% greater than the developed tension produced at the previous length. Arterial rings were allowed to equilibrate for 45-60 min at Lmax prior to subsequent evaluation of pharmacological responsiveness. Following equilibration, concentration-response relationships were determined by cumulative additions of increasing concentrations of KCl (10-100 mM). Complete exchange of the tissue bath was achieved for each increase in KCl concentration to maintain the equimolar substitution of KCl for NaCl. KCl concentrations were increased when the response to the previous concentration had stabilized. Isometric contraction responses were measured on a Grass polygraph recorder (Grass Instruments, Quincy, MA). Percent constriction was defined as the percentage increase in developed tension.
Simultaneous measures of intracellular free Ca2+ (Cai) and contractile tension
In additional studies, Cai and contractile tension were measured simultaneously in arterial rings (13,22). Endothelium-denuded arterial rings were mounted on two stainless steel wires; one fixed to a force transducer (Kulite Semiconductor Products Inc., Leonia, NJ) and the other attached to a lever driven by a digital micrometer to permit precise changes in circumferential length of the vessel. The mounted arterial ring was lowered into a heated superfusion chamber of a microfluorometry system (Nikon Diaphot, Nikon, Garden City, NY) and stretched to Lmax as determined by repeated exposure to high KCl (60 mM) at increasing vessel diameter. Excitation light from a 150 W xenon arc lamp was passed through a rotating filter wheel (50 ms intervals) containing alternating 340 and 380 nm interference filters. Fluorescence emission at 510 nm was synchronized with the appropriate excitation wavelength by a photodetector mounted on the filter wheel and was reflected to a photomultiplier tube with a dichroic mirror. Fluorescence was analyzed with an analog fluorescence signal processor and an analog-to-digital converter. The change in fluorescence ratio (F340/F380) was representative of relative Cai following off-line subtraction of vessel autofluorescence. Autofluorescence was determined at the end of each experiment by superfusion in physiological saline solution (PSS) containing 2 mM MnCl2, followed by bathing the vessel in PSS containing 2 mM MnCl2 plus ionomycin (10 μM) to quench intracellular fura-2. Fluorescence and contractile data were sampled every 5 s. Data acquisition and transformations were performed using AxoBASIC 1.0 software (Axon Instruments, Foster City, CA) customized for multichannel data acquisition.
Smooth muscle cell dissociation
All electrophysiology experiments were performed using freshly dispersed smooth muscle cells dissociated from vessels on the day following animal sacrifice. Previous comparison of first and second day voltage clamp experiments has demonstrated no detrimental effect of overnight storage on whole-cell Ca2+ channel currents. Segments of LCX and LAD coronary arteries were cut longitudinally and pinned lumen side up in low-Ca2+ (0.1 mM) physiological buffer containing 294 U/ml collagenase, 5 U/ml elastase, 2 mg/ml bovine serum albumin, 1 mg/ml soybean trypsin inhibitor, and 0.4 mg/ml DNase I. Cells were enzymatically dissociated by incubation in a 37 °C water bath for 1 h. The enzyme solution was then replaced with enzyme-free low-Ca2+ solution and isolated single cells were obtained by repeatedly directing a stream of low-Ca2+ solution over the artery via fire-polished Pasteur pipette. Isolated cells were maintained in low-Ca2+ solution at 4 °C until use (0-4 h).
Whole-cell voltage clamp
Whole-cell Ca2+ channel currents were obtained from single cells using standard whole-cell voltage clamp techniques as used routinely (2,10). Cells were initially superfused with PSS containing (in mM): 138 NaCl, 5 KCl, 0.1 CaCl2, 1 MgCl2, 10 glucose, 20 HEPES, pH 7.4 during gigaseal formation. Following whole cell configuration, the superfusate was switched to PSS with tetraethylammonium chloride (TEACl) substituted for NaCl and 10 mM Ba2+ as the charge carrier. Heat-polished glass pipettes (2-5 MΩ) were filled with a solution containing (in mM): 120 CsCl, 10 TEACl, 1 MgCl2, 20 HEPES, 5 Na2ATP, 0.5 Tris-GTP, 10 EGTA, pH 7.1 with KOH. Ionic currents were amplified with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA). Currents were low-pass filtered with a cutoff frequency of 1000 Hz, digitized at 2.5 kHz and stored on computer. Leak subtraction was not performed. Current densities (pA/pF) were obtained for each cell by normalization of whole cell current to cell capacitance to account for differences in cell membrane surface area. Capacity currents were measured for each cell during 10 ms pulses from a holding potential of −80 mV to a test potential of −75 mV. Data acquisition and analysis were accomplished using pClamp 8.0 software (Axon Instruments). Cells were continuously perfused under gravity flow at room temperature (22-25 °C).
Solutions
Krebs buffer contained (in mM): 131.5 NaCl, 5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11.2 glucose, 13.5 NaHCO3 and 0.025 EDTA. Vessels used for fura-2 experiments were stored and rinsed in sterile modified Eagle's minimal essential storage media which contained (in mM): 135 NaCl, 5 KCl, 0.34 NaH2PO4, 1 MgCl2, 2 CaCl2, 10 glucose, 2.6 NaHCO3, 0.44 KH2PO4 and 20 HEPES and (vol:vol) 0.02 amino acids, 0.01 vitamins, 0.002 phenol red, 0.01 penicillin/streptomycin and 2% horse serum, pH to 7.2 with NaOH at 23 °C and passed through a sterile filter to a final pH of 7.4. Fura-2/AM loading solution contained 10 μM fura-2/AM, 0.5% cremophor, and 5% bovine serum albumin. The loading solution was vortexed for 1 min and then sonicated for an additional 1 min to increase solubilization of fura-2/AM. Drugs were obtained from Sigma Chemical (St. Louis, MO) unless otherwise noted. Smooth muscle cell dispersion chemicals were obtained from Worthington Chemicals (Freehold, NJ), endothelin-1 from Peninsula Laboratories (Belmont, CA) and ionomycin from Calbiochem (La Jolla, CA).
Statistical Analysis
Dimensional characteristics of coronary arterial rings were compared using Student's t-tests. KCl concentration-response relationships and Ca2+ channel current-voltage (I-V) relationships were analyzed using repeated measures analysis of variance (RM ANOVA) and the Student Newman-Keuls correction for multiple comparisons when appropriate. The concentration of KCl causing 50% of the maximal contractile response was designated EC50 and was calculated by nonlinear regression analysis. The individual EC50 values were averaged for LAD and LCX and compared using Student's t-test. Ca2+ sensitivity data are presented as grams of tension as a function of fluorescence ratio and were fit by nonlinear regression analysis to a four parameter sigmoidal equation (SigmaPlot 8.0, SPSS) with tension produced at 60mM KCl set as maximal tension and EC50 values calculated. EC50 values for LAD and LCX were compared using Student's t test. Student's t-tests were used to compare half-maximal activation and inactivation values of whole-cell Ca2+ currents. For all analyses, a P value ≤ 0.05 was considered significant. Data are presented as mean ± SE, and n values in parentheses reflect the number of animals for arterial ring studies and the number of animals and cells for voltage clamp studies. When more than one vascular ring from a single coronary artery was used in identical protocols, the responses from these rings were averaged before data analyses were conducted.
Results
Coronary vessel dimensions and characteristics
Dimensions of coronary arterial rings are divided into those used for KCl concentration-response relationships (Table 1) and those used for simultaneous Cai and contractile tension experiments (Table 2). No significant differences in dimensional characteristics were observed between nonoccluded LAD and collateral-dependent LCX arterial rings used in the concentration-response relationships (Table 1) or in LAD and LCX rings used for combined tension and fura-2 experiments (Table 2).
Table. Dimensional characteristics of coronary artery rings.
| Outer Diameter, mm | Lumen Diameter, mm | Wall Thickness, mm | Axial Length, mm | |
|---|---|---|---|---|
| KCl concentration-response experiments | ||||
| LAD (n=9) | 1.85 ± 0.05 | 1.25 ± 0.05 | 0.23 ± 0.02 | 3.90 ± 0.11 |
| LCX (n=9) | 1.70 ± 0.15 | 1.07 ± 0.12 | 0.22 ± 0.02 | 3.85 ± 0.10 |
|
| ||||
| Combined tension and fura-2 experiments | ||||
| LAD (n=10) | 1.61 ± 0.08 | 0.98 ± 0.09 | 0.30 ± 0.03 | |
| LCX (n=10) | 1.70 ± 0.13 | 1.00 ± 0.10 | 0.29 ± 0.03 | |
Values are mean ± SE. LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery. Numbers in parentheses indicates number of arterial rings studied. No significant differences exist.
KCl concentration-response relationships
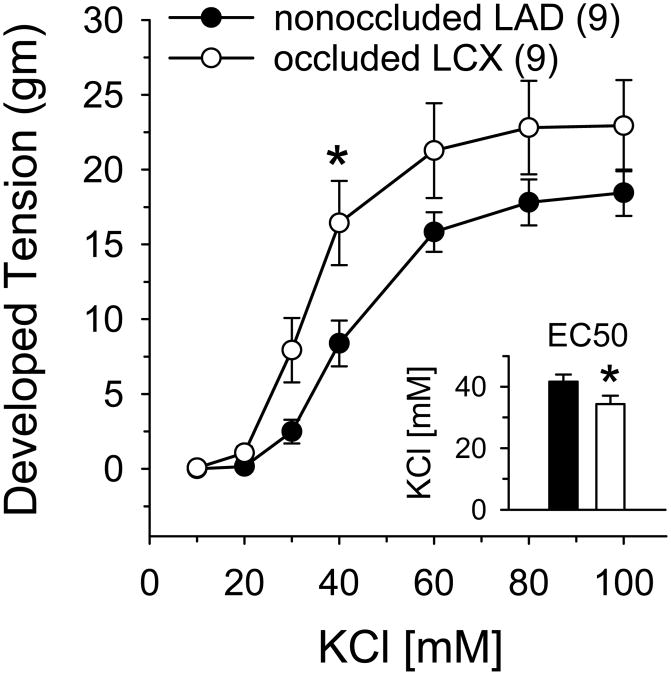
We compared steady-state contractile responses of the LAD and LCX to increasing concentrations of KCl (10-100 mM). Contraction to KCl was significantly enhanced in the collateral-dependent LCX compared with the nonoccluded LAD (Figure 1). Furthermore, as illustrated in Figure 1 inset, EC50 was attained at a significantly lower KCl concentration in LCX compared with LAD rings (34.3±2.7 vs. 41.7±2.3 mM, respectively) indicating that constriction to KCl was significantly enhanced in the collateral-dependent LCX compared with the nonoccluded LAD.
Figure 1.
Concentration-dependent contractile responses to KCl in collateral-dependent LCX and nonoccluded LAD arterial rings. KCl-induced constriction was significantly enhanced in LCX versus LAD arteries. The concentration of KCl yielding 50% maximum contraction (EC50; inset) was significantly lower in LCX compared with LAD arterial rings. Values are means ± SE of the number of animals in parentheses; *P≤0.05 versus LAD.
Ca2+ channel current density and kinetics
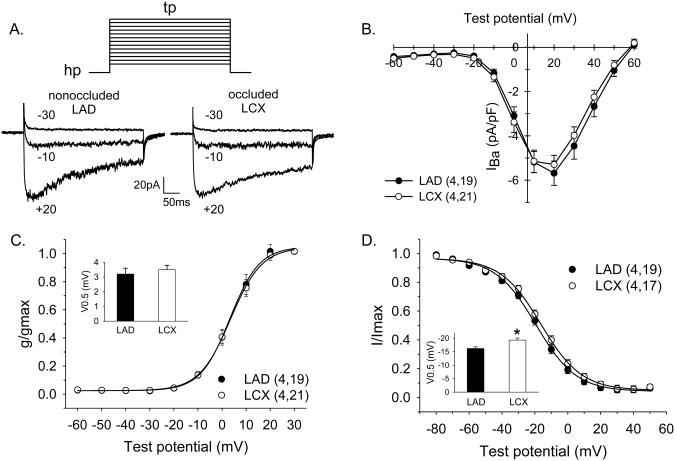
Ca2+ channel currents were elicited by 400-ms step-depolarizations to potentials ranging from −60 to +60 mV from a holding potential of −80 mV. Representative traces for currents from both LAD and LCX cells at selected test potentials are included in Figure 2A. I-V relationships for smooth muscle cells isolated from the collateral-dependent LCX and the nonoccluded LAD are presented in Figure 2B. Current is plotted as peak inward current and is normalized to cell membrane capacitance (pA/pF). Cell capacitance (17.5±1.3 vs. 16.3±1.5 pF) was not significantly different between smooth muscle cells isolated from LCX (n=4 animals, 21 cells) and LAD (n=4 animals, 19 cells), respectively. These data demonstrate that peak Ca2+ channel current was not altered by chronic coronary occlusion (−5.68±0.55 vs. −5.29±0.42 pA/pF, LAD vs. LCX, respectively). As shown in Figure 2C, the test potential at which inward current attained half-maximal activation was not significantly different between cells of the LAD and LCX (3.2±0.4 and 3.5±0.3 mV, respectively; inset). The slope of the activation curves, which provides an indication of the sensitivity of the current to voltage, was also not significantly different between cells from LAD and LCX (6.1±0.4 and 6.5±03 mV, respectively). Steady-state inactivation of inward current was measured using 5-sec conditioning pulses (−80 to +50 mV) in 10 mV increments from a holding potential of −80 mV, followed by a 600-ms test potential of +20 mV. As shown in Figure 2D, the test potential at which Ca2+ channel currents attained half-maximal inactivation was significantly shifted to a more positive membrane potential in cells from LCX when compared with LAD (−16.2±0.6 vs. −19.3±0.8 mV, respectively; inset). This rightward shift in Ca2+ channel current inactivation results in a 3% increase in channel availability in LCX cells at a membrane potential of −40 mV (Figure 2D). Slope values for inactivation curves were not different between LAD and LCX cells (−12.2±0.7 and −12.3±0.6 mV, respectively).
Figure 2.
Effect of chronic coronary occlusion on whole-cell voltage-gated Ca2+ channel currents in isolated smooth muscle cells. A. Representative current traces of inward Ca2+ channel current in cells from LAD and LCX arteries at selected test potentials. Currents were elicited by 400-ms step depolarizations to potentials of −60 to +60 mV in 10 mV increments from a holding potential of −80 mV with 10 mM external Ba2+ as charge carrier. B. I-V relationships obtained by plotting peak inward current as a function of the indicated step potential. C. Voltage-dependent activation data were fit to a Boltzmann distribution equation. Half-maximal activation (V0.5; inset) was not significantly different between cells of the LAD and LCX. D. Voltage-dependent inactivation of the inward current. Steady-state inactivation of inward current was measured using 5-sec conditioning pulses (−70 to +40 mV) in 10 mV increments from a holding potential of −80 mV, followed by a 600-ms test potential of +20 mV. Data were fit to a Boltzmann distribution equation. Currents attained half-maximal inactivation (V0.5; inset) at a significantly more positive membrane potential in cells from collateral-dependent LCX when compared with nonoccluded LAD. Values in B, C and D, are means ± SE. Numbers in parentheses are number of animals, cells.
Simultaneous contractile tension and Cai
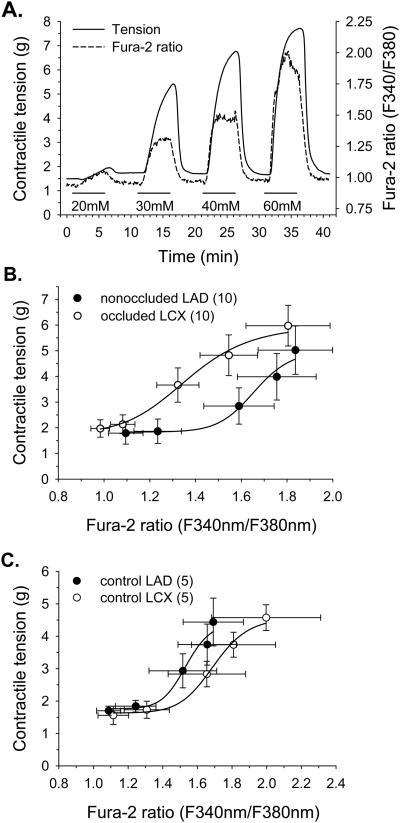
In additional experiments designed to further elucidate potential cellular mechanisms underlying alterations in KCl responsiveness resulting from chronic coronary occlusion, we simultaneously measured KCl-induced changes in contractile tension and Cai in collateral-dependent LCX and nonoccluded LAD arteries. Figure 3A represents our experimental protocol in which endothelium-denuded arterial rings were constricted with increasing concentrations of KCl and both contractile response and Cai data were obtained. Evaluation of grams of tension as a function of fura-2 ratio (Figure 3B) revealed that significantly more tension was produced per unit change in fura-2 ratio (EC50, LAD=1.66±0.13, LCX=1.36±0.07 fluorescence ratio units) indicating that Ca2+ sensitivity was significantly enhanced in the collateral-dependent LCX compared with the nonoccluded LAD. Additional control experiments examining Ca2+ sensitivity in nonoccluded animals demonstrated no regional differences in Ca2+ sensitivity (Figure 3C; EC50, LAD=1.54±0.17, LCX=1.70±0.05 fluorescence ratio units) and established that the nonoccluded LAD from chronically occluded hearts displayed Ca2+ sensitivity similar to that observed in vessels from nonoccluded hearts.
Figure 3.
Comparison of Ca2+-sensitivity of collateral-dependent LCX and nonoccluded LAD arterial rings during KCl-induced membrane depolarization. A. Representative traces showing simultaneous measures of tension and Cai during exposure to noncumulative, increasing concentrations of KCl. Coronary arterial rings were superfused with KCl for 5 min, followed by 5-min recovery in normal PSS and subsequent exposure to a higher KCl concentration. B. Evaluation of tension as a function of Cai (fura-2 ratio) revealed that the collateral-dependent LCX developed significantly more tension per unit change in fura-2 ratio compared with the nonoccluded LAD (EC50, LAD=1.66±0.13, LCX=1.36±0.07 fura-2 ratio units). C. Control experiments examining Ca2+ sensitivity in nonoccluded animals demonstrated no significant regional differences (LAD vs. LCX) in tension development as a function of fura-2 ratio in response to increasing KCl concentrations (EC50, LAD=1.54±0.17, LCX=1.70±0.05 fura-2 ratio units). Values are means ± SE of the number of animals in parentheses.
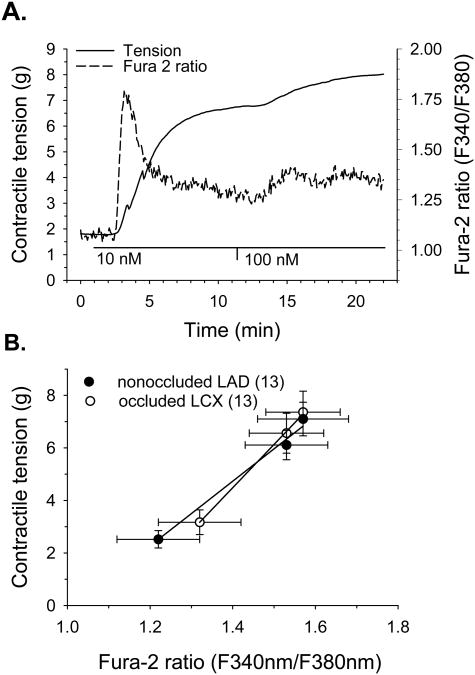
We also evaluated contractile tension and Cai simultaneously in collateral-dependent LCX and nonoccluded LAD arteries in response to receptor-agonist stimulation with endothelin-1. Figure 4A represents our experimental protocol in which endothelium-denuded arterial rings were constricted with 10 and 100nM endothelin-1 and contractile responses and Cai were measured. Evaluation of grams of tension as a function of fura-2 ratio (Figure 4B) revealed similar changes in tension per unit change in fura-2 ratio in both LAD and LCX in response to endothelin-1.
Figure 4.
Comparison of Ca2+-sensitivity of collateral-dependent LCX and nonoccluded LAD arterial rings during receptor-agonist stimulation with endothelin-1. A. Representative traces showing simultaneous measures of tension and Cai during exposure to the cumulative addition of 10 and 100 nM endothelin-1. Coronary arterial rings were superfused with normal PSS for 1 min with subsequent exposure to each 10 and 100 nM endothelin-1 for 10 min. B. Evaluation of tension as a function of Cai (fura-2 ratio) revealed no difference in tension development per unit change in fura-2 ratio in the collateral-dependent LCX compared with the nonoccluded LAD.
Discussion
These studies are the first to document that Ca2+ sensitivity is significantly enhanced in coronary arterial rings distal to chronic occlusion compared with control, nonoccluded arteries and provide a mechanism for the increased contractility commonly observed in collateral-dependent vasculature. Increased Ca2+ sensitivity was revealed in response to KCl-induced membrane depolarization but not receptor-agonist stimulation with endothelin-1. These data suggest that more than one mechanism for Ca2+ sensitization of the contractile apparatus in vascular smooth muscle as indicated previously (3,6,25,26,33,34). Contrary to our alternative hypothesis, we also provide evidence that whole-cell peak Ca2+ channel currents are not altered in isolated smooth muscle cells from the collateral-dependent LCX compared with the nonoccluded LAD. These data indicate that peak Ca2+ influx during a brief depolarization is not altered by chronic occlusion. Furthermore, although half-maximal activation of Ca2+ current occurred at nearly the same membrane potential in LAD and LCX smooth muscle cells, half- maximal inactivation was shifted to a more positive membrane potential in LCX cells indicating Ca2+ channels from these cells are less likely to inactivate at any given membrane potential and thus, potentially allow greater Ca2+ influx during sustained depolarization.
Our observation of an increased high KCl-induced Ca2+ sensitivity of the contractile apparatus in the endothelium-denuded collateral-dependent LCX artery may be due to numerous adaptations related to a reduced blood flow to the region distal to chronic occlusion including ischemia and/or changes in mechanical stimuli such as, decreases in distending pressure and altered pulsatile nature of the blood flow, as previously proposed (30). However, studies which have examined ischemia-associated perturbations in smooth muscle or reductions in arteriolar transmural pressure have typically documented a reduction in smooth muscle Ca2+ sensitivity (19,27,37). These findings provide evidence against ischemia or alterations in transmural pressure as direct contributors to the enhanced Ca2+ sensitivity of the collateral-dependent LCX. However, the chronic nature of the reduced blood flow and associated stimuli in our model of occlusion may result in adaptations of Ca2+ sensitivity that differ from the acute adaptations observed in other studies.
Although we report that peak Ca2+ channel currents were not altered in smooth muscle cells distal to chronic occlusion, our finding that Ca2+ channels from these cells exhibit decreased voltage-dependent inactivation suggests that during sustained depolarizations, Ca2+ channels in LCX smooth muscle cells likely allow more Ca2+ influx than in cells from the nonoccluded LAD. Although small, the ∼3 mV shift in voltage-dependent inactivation in LCX cells has great potential for impacting Ca2+ influx. For example, under physiological conditions, this shift results in a 3% increase in channel availability at a membrane potential of −40 mV. The resultant increase in Ca2+ influx through Ca2+ channels of the LCX cells would translate to a potential ∼15 μM/s increase in Cai in the absence of Ca2+ buffering. This calculation is based on a unitary current amplitude of −0.18 pA or ∼500,000 ions/s at −40 mV for Ca2+ channels (24), an estimated arterial smooth muscle cell volume of ∼1 pL (1), and the estimation that one arterial smooth muscle cell contains ∼5000 Ca2+ channels (18). As Cai increases in coronary smooth muscle are typically in the nM range, this represents a potentially large increase in Ca2+ influx into LCX smooth muscle and emphasizes the importance of Ca2+ regulatory processes (e.g., negative-feedback and buffering) in maintaining Ca2+ homeostasis. Despite this potential for greater Ca2+ channel availability, our data indicate that the global Ca2+ concentration (i.e., steady-state fura-2 signal; Figure 3B) in response to high KCl was not enhanced in the LCX compared with LAD suggesting that the increased Ca2+ influx must be compensated by another mechanism, such as Ca2+-dependent inactivation of the Ca2+ channels or increased Ca2+ extrusion. We have previously concluded that Ca2+ extrusion by the Na/Ca exchanger and sarcolemmal Ca2+-ATPase are not enhanced in smooth muscle from the LCX compared with LAD (12) suggesting other compensatory mechanisms. Furthermore, use of external Ba2+ as charge carrier in our voltage clamp experiments precludes comparison of Ca2+-dependent inactivation in cells from LCX and LAD. On the other hand, we cannot disregard a potential increase in subsarcolemmal Ca2+ levels, undetectable by fura-2, in the LCX smooth muscle cells.
Previous studies have reported that altered subsarcolemmal Ca2+ concentrations, which are distinct from global Ca2+ regulation, influence cellular regulatory processes and may alter cellular contractile states (4,5,9,17,23,35). The presence of an increased Ca2+ concentration in a restricted subdomain of the plasma membrane might provide a link between the shift in inactivation and enhanced Ca2+ sensitivity observed in the collateral-dependent LCX. Previous studies have demonstrated that local increases in intracellular Ca2+ subsequent to KCl-induced membrane depolarization produce translocation and activation of PKC (14). Furthermore, KCl-induced Ca2+ influx also has been shown to stimulate the RhoA/Rho kinase signaling pathway (16,25,36). In turn, both PKC and Rho kinase have been reported to augment Ca2+ sensitivity of the contractile proteins through enhanced myosin light chain phosphorylation. Thus, the shift in Ca2+ channel current inactivation observed in the LCX cells and the potential resultant increased subsarcolemmal Ca2+ concentrations may produce Ca2+ sensitization via activation of a Ca2+-dependent PKC, Rho kinase or other biochemical pathways recognized to increase Ca2+ sensitivity (34). Interestingly, increased activity of Rho kinase has been implicated in both increased contractility of vascular smooth muscle and coronary artery spasm in patients with vasospastic angina (15). Alternatively, our data using endothelin-1 (Figure 4) indicates that receptor-agonist stimulation-induced Ca2+ sensitivity was not altered by chronic occlusion. Previous studies have indicated that KCl-induced membrane depolarization and receptor-agonist stimulation may increase Ca2+ sensitivity via distinct pathways (25). Sakurada et al. (25) report that high KCl-mediated membrane depolarization induces Rho kinase-dependent Ca2+ sensitization, which is completely Ca2+-dependent. These investigators propose that in addition to Ca2+-dependent Rho kinase activation, receptor agonists likely also activate non-Ca2+-dependent G protein coupled pathways of Ca2+ sensitization, which likely are not stimulated by KCl. Based on these statements, the disparity in our Ca2+ sensitivity data with KCl and endothelin-1 suggests chronic occlusion may alter only the mechanism by which membrane depolarization increases Ca2+ sensitivity. Use of selective inhibitors of the RhoA/Rho kinase and other biochemical pathways recognized to increase Ca2+ sensitivity (e.g., PKC) in future experiments could further our understanding of the mechanisms contributing to enhanced Ca2+ sensitization in collateral-dependent coronary vasculature.
Our finding that the porcine collateral-dependent LCX demonstrated enhanced contractile responsiveness to KCl compared with the nonoccluded LAD is in contrast to a previous study in the chronically occluded dog heart, which documented that collateral-dependent and nonoccluded arterial rings responded similarly to increasing KCl concentrations (22). These contradictory findings support previous data documenting differences between species (pig vs. dog) in adaptive responses to vasoactive agents following chronic coronary occlusion (11). These species differences in the adaptive response of the coronary circulation to chronic occlusion may be a product of the inherent differences in the collateral circulations of the dog and pig (26).
In conclusion, this study provides the first evidence of enhanced myofilament Ca2+ sensitivity in the collateral-dependent artery of chronically occluded hearts. Enhanced Ca2+ sensitization was evident with KCl-induced membrane depolarization, however, receptor-agonist stimulation-induced Ca2+ sensitivity was not altered by chronic occlusion suggesting that chronic occlusion may alter only the mechanism by which membrane depolarization increases Ca2+ sensitivity. Moreover, although peak Ca2+ channel current density and half-maximal activation of Ca2+ channel current was not altered by chronic occlusion, the collateral-dependent artery did display a rightward shift in half-maximal inactivation of Ca2+ channel current, potentially allowing increased subsarcolemmal Ca2+ concentrations that may augment Ca2+ sensitivity through a Ca2+-dependent mechanism. These data provide a mechanism for the enhanced vasoreactivity commonly observed in vasculature distal to chronic occlusion that may result in persistent ischemia of the collateral-dependent region.
Acknowledgments
The authors gratefully acknowledge the technical and surgical expertise of Millie Mattox and the technical contributions of Cathy Galle. These studies were supported by research funds from the National Institutes of Health, Program Project PO1-HL52490 and HL64931.
References
- 1.Aaronson PI, Bolton TB, Lang RJ, MacKenzie I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal-calcium and high-barium solutions. J Physiol (Lond) 1988;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowles DK, Hu Q, Laughlin MH, Sturek M. Heterogeneity of L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol. 1997;273:H2083–H2089. doi: 10.1152/ajpheart.1997.273.4.H2083. [DOI] [PubMed] [Google Scholar]
- 3.Eto M, Kitazawa T, Yazawa M, Mukai H, Ono Y, Brautigan DL. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C α and δ isoforms. J Biol Chem. 2001;276:29072–29078. doi: 10.1074/jbc.M103206200. [DOI] [PubMed] [Google Scholar]
- 4.Fay FS. Calcium sparks in vascular smooth muscle: relaxation regulators. Science. 1995;270:588–590. doi: 10.1126/science.270.5236.588. [DOI] [PubMed] [Google Scholar]
- 5.Ganitkevich VY, Isenberg G. Dissociation of subsarcolemmal from global cytosolic [Ca2+] in myocytes from guinea-pig coronary artery. J Physiol (Lond) 1996;490:305–318. doi: 10.1113/jphysiol.1996.sp021145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins-ras-family or trimeric proteins or both-in Ca2+ sensitization of smooth muscle. Proc Natl Acad Sci USA. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol. 1999;87:1948–1956. doi: 10.1152/jappl.1999.87.5.1948. [DOI] [PubMed] [Google Scholar]
- 8.Griffin KL, Woodman CR, Price EM, Laughlin MH, Parker JL. Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation. 2001;104:1393–1398. doi: 10.1161/hc3601.094274. [DOI] [PubMed] [Google Scholar]
- 9.Guia A, Wan X, Courtemanche M, Leblanc N. Local Ca2+ entry through L-type Ca2+ channels activates Ca2+-dependent K+ channels in rabbit coronary myocytes. Circ Res. 1999;84:1032–1042. doi: 10.1161/01.res.84.9.1032. [DOI] [PubMed] [Google Scholar]
- 10.Heaps CL, Bowles DK, Sturek M, Laughlin MH, Parker JL. Enhanced L-type Ca2+ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2+ accumulation. J Physiol (Lond) 2000;528:435–445. doi: 10.1111/j.1469-7793.2000.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaps CL, Rapps JA, Sawani AM, Laughlin MH, Parker JL. α-adrenergic vasoconstrictor responses in canine and porcine models of chronic coronary occlusion. FASEB J. 1998;12:A405. [Google Scholar]
- 12.Heaps CL, Sturek M, Price EM, Laughlin MH, Parker JL. Sarcoplasmic reticulum Ca2+ uptake is impaired in coronary smooth muscle distal to coronary occlusion. Am J Physiol Heart Circ Physiol. 2001;281:H223–H231. doi: 10.1152/ajpheart.2001.281.1.H223. [DOI] [PubMed] [Google Scholar]
- 13.Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol. 2000;278:H1984–H1992. doi: 10.1152/ajpheart.2000.278.6.H1984. [DOI] [PubMed] [Google Scholar]
- 14.Maasch C, Wagner S, Lindschau C, Alexander G, Buchner K, Gollasch M, Luft FC, Haller H. Protein kinase Cα targeting is regulated by temporal and spatial changes in intracellular free calcium concentration [Ca2+ ]i. FASEB J. 2000;14:1653–1663. doi: 10.1096/fj.14.11.1653. [DOI] [PubMed] [Google Scholar]
- 15.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 16.Mita M, Yanagihara H, Hishinuma S, Saito M, Walsh MP. Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem J. 2002;364:431–440. doi: 10.1042/BJ20020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 18.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 19.Perkins WJ, Lorenz RR, Bogoger M, Warner DO, Cremo CR, Jones KA. A novel mechanism by which hydrogen peroxide decreases calcium sensitivity in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2003;284:L324–L332. doi: 10.1152/ajplung.00159.2002. [DOI] [PubMed] [Google Scholar]
- 20.Peters KG, Marcus ML, Harrison DG. Vasopressin and the mature coronary collateral circulation. Circulation. 1989;79:1324–1331. doi: 10.1161/01.cir.79.6.1324. [DOI] [PubMed] [Google Scholar]
- 21.Pupita G, Maseri A, Galassi AR, Gavrielides S, Davies G, Crea F. Myocardial ischemia caused by distal coronary artery constriction in stable angina pectoris. N Engl J Med. 1990;323:514–520. doi: 10.1056/NEJM199008233230804. [DOI] [PubMed] [Google Scholar]
- 22.Rapps JA, Sturek M, Jones AW, Parker JL. Altered reactivity of coronary arteries located distal to a chronic occlusion. Am J Physiol Heart Circ Physiol. 1997;273:H1879–H1887. doi: 10.1152/ajpheart.1997.273.4.H1879. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen H, Barrett P, Zawalich W, Isales C, Stein P, Smallwood J, McCarthy R, Bollag W. Cycling of Ca2+ across the plasma membrane as a mechanism for generating a Ca2+ signal for cell activation. Ann NY Acad Sci. 1989;568:73–80. doi: 10.1111/j.1749-6632.1989.tb12492.x. [DOI] [PubMed] [Google Scholar]
- 24.Rubart M, Patlak JB, Nelson MT. Ca2+ currents in cerebral artery smooth muscle cells of rat at physiological Ca2+ concentrations. J Gen Physiol. 1996;107:459–472. doi: 10.1085/jgp.107.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakurada S, Takuwa N, Sugimoyo N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- 26.Schaper W. The Collateral Circulation of the Heart. New York: Elsevier; Comparative arteriography of the collateral circulation; pp. 1971pp. 29–50. [Google Scholar]
- 27.Schubert R, Kalentchuk VU, Krien U. Rho kinase inhibition partly weakens myogenic reactivity in rat small arteries by changing calcium sensitivity. Am J Physiol Heart Circ Physiol. 2002;283:H2288–H2295. doi: 10.1152/ajpheart.00549.2002. [DOI] [PubMed] [Google Scholar]
- 28.Sellke FW, Kagaya Y, Johnson RG, Sharique R, Schoen FJ, Grossman W, Weintraub RM. Endothelial modulation of porcine coronary microcirculation perfused via immature collaterals. Am J Physiol Heart Circ Physiol. 1992;262:H1669–H1675. doi: 10.1152/ajpheart.1992.262.6.H1669. [DOI] [PubMed] [Google Scholar]
- 29.Sellke FW, Li J, Stamler JS, Lopez JJ, Thomas KA, Simons M. Angiogenesis induced by acidic fibroblast growth factor as an alternative method of revascularization for chronic myocardial ischemia. Surgery. 1996;120:182–188. doi: 10.1016/s0039-6060(96)80286-8. [DOI] [PubMed] [Google Scholar]
- 30.Sellke FW, Quillen JE, Brooks LA, Harrison DG. Endothelial modulation of the coronary vasculature in vessels perfused via mature collaterals. Circulation. 1990;81:1938–1947. doi: 10.1161/01.cir.81.6.1938. [DOI] [PubMed] [Google Scholar]
- 31.Sellke FW, Wang SY, Friedman M, Dai HB, Harada K-I, Lopez JJ, Simons M. β-adrenergic modulation of the collateral-dependent coronary microcirculation. J Surg Res. 1995;59:185–190. doi: 10.1006/jsre.1995.1152. [DOI] [PubMed] [Google Scholar]
- 32.Sellke FW, Wang SY, Stamler A, Lopez JJ, Li J, Simons M. Enhanced microvascular relaxations to VEGF and bFGF in chronically ischemic porcine myocardium. Am J Physiol Heart Circ Physiol. 1996;271:H713–H720. doi: 10.1152/ajpheart.1996.271.2.H713. [DOI] [PubMed] [Google Scholar]
- 33.Shirasawa Y, Rutland TJ, Young JL, Dean DA, Joseph BN. Modulation of protein kinase C (PKC)-mediated contraction and the possible role of PKCε in rat mesenteric arteries. Frontiers in Bioscience. 2003;8:a133–a138. doi: 10.2741/1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 35.Stehno-Bittel L, Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol (Lond) 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urban NH, Berg KM, Ratz PH. K+ depolarization induces RhoA kinase translocation to caveolae and Ca2+ sensitization of arterial muscle. Am J Physiol Cell Physiol. 2003;285:C1377–C1385. doi: 10.1152/ajpcell.00501.2002. [DOI] [PubMed] [Google Scholar]
- 37.Yeon DS, Kim JS, Ahn DS, Kwon SC, Kang BS, Morgan KG, Lee YH. Role of protein kinase C- or RhoA-induced Ca2+ sensitization in stretch-induced myogenic tone. Cardiovasc Res. 2002;53:431–438. doi: 10.1016/s0008-6363(01)00496-5. [DOI] [PubMed] [Google Scholar]