Abstract
Objective
Mal de debarquement syndrome (MdDS) is a chronic disorder of imbalance characterized by a feeling of rocking and swaying. The disorder starts after prolonged exposure to passive motion such as from a boat or plane. All medical treatment is palliative and symptoms that persist beyond six months show low likelihood of remission. This pilot study explored the feasibility and tolerability of repetitive transcranial magnetic stimulation (rTMS) as potential treatment for MdDS.
Patients/Intervention
Ten subjects (8 women) with persistent MdDS lasting from 10 to 91 months were given one session each of four counterbalanced protocols: left 10Hz (high frequency), left 1Hz (low frequency), right 10Hz, and right 1Hz rTMS over the dorsolateral prefrontal cortex (DLPFC).
Main Outcome Measure
Reduction of rocking sensation reported on a visual analogue scale.
Results
1) Right-handers improved most with 10Hz stimulation over the left DLPFC while left-handers improved most with 10Hz stimulation over the right DLPFC; 2) Low frequency DLPFC stimulation was associated with symptom worsening in some subjects; 3) Duration of symptoms was negatively correlated with treatment response; 4) rTMS was well-tolerated in MdDS subjects, showing similar rates of headache (10 of 40 sessions) as for other studies; 5) Fatigue occurred after six sessions usually with low frequency stimulation.
Conclusion
rTMS was well-tolerated in subjects with MdDS with promising short-term symptom improvement. Future studies of rTMS in MdDS may consider sequential days of stimulation, longer post-rTMS observation periods, formal measurement of post-TMS fatigue, and randomization with a sham condition.
Keywords: mal de debarquement syndrome, DLPFC, rTMS, neuromodulation
INTRODUCTION
The feeling of rocking dizziness that occurs after one disembarks from a boat or plane is a common phenomenon that normally ceases within two days of returning to land (1). However, in some people, a sensation that they are still rocking on the boat persists for months or years. This disorder, termed mal de debarquement syndrome (MdDS) is unexplained by structural brain or inner ear pathology (2). Despite causing significant disability, therapy for persistent MdDS remains limited (3).
The stereotypical triggers that cause MdDS suggest that it is a disorder of maladaptive neuroplasticity that might be responsive to external neuromodulation. Repetitive transcranial magnetic stimulation (rTMS) is a method of neuromodulation in which a local magnetic field is applied over the scalp in order to induce an electrical current in the cortical structures underlying the coil. Low frequency rTMS (<=1Hz) induces local inhibition whereas high frequency rTMS (>=5Hz) induces local excitation (4). However, remote effects of rTMS can occur because of network connectivity and inter-hemispheric inhibition (5).
This pilot study investigated the following: 1] feasibility; 2] tolerability; 3] side effects; and 4] possible therapeutic effects of rTMS in MdDS. Four counterbalanced rTMS protocols were tested using dorsolateral prefrontal cortex (DLPFC) as an empiric target. DLPFC was chosen because our recent functional imaging data showed that patients with MdDS have relative hypermetabolism in the left entorhinal cortex and amygdala with relative hypometabolism in prefrontal cortex, including the area of the left DLPFC compared to controls (6). Spatial information received by the posterior parietal lobes projects to the DLPFC, making it an important area for cognitive control over spatial information processing, particularly in spatial working memory (7; 8). This role of the DLPFC is relevant to MdDS patients who, along with rocking dizziness, experience poor attention and significant intolerance to visual motion (9). We hypothesized that high frequency left DLPFC rTMS could temporarily reduce the symptoms of rocking, potentially mediated by its functional connectivity with the entorhinal cortex and posterior parietal lobe (10, 11). Right-sided low frequency stimulation may also be effective by reducing inter-hemispheric inhibition of the left DLPFC. Internal controls used were low frequency stimulation of the left DLPFC and high frequency stimulation of the right DLPFC.
METHODS
Subject Selection
Patients with a history of persistent MdDS meeting the following criteria were recruited through a University Neurology clinic: 1) A chronic perception of rocking dizziness that started after disembarking from sea, air, or land based travel; 2) Symptoms lasted at least six months; 3) Normal peripheral inner ear function testing with ENG/VNG and audiograms 4) Normal structural imaging with brain MRI; 5) No other cause for symptoms after evaluation by a neurologist.
rTMS safety assessments were performed to exclude any subject who had a high risk of seizures. Study procedures were completed according to Declaration of Helsinki guidelines and were approved by the Institutional Review Board of the University. Subjects signed written informed consent and completed the Edinburgh Handedness Inventory (12).
MRI
Subjects underwent high resolution structural MRI scans used for neuronavigation during rTMS sessions.
rTMS
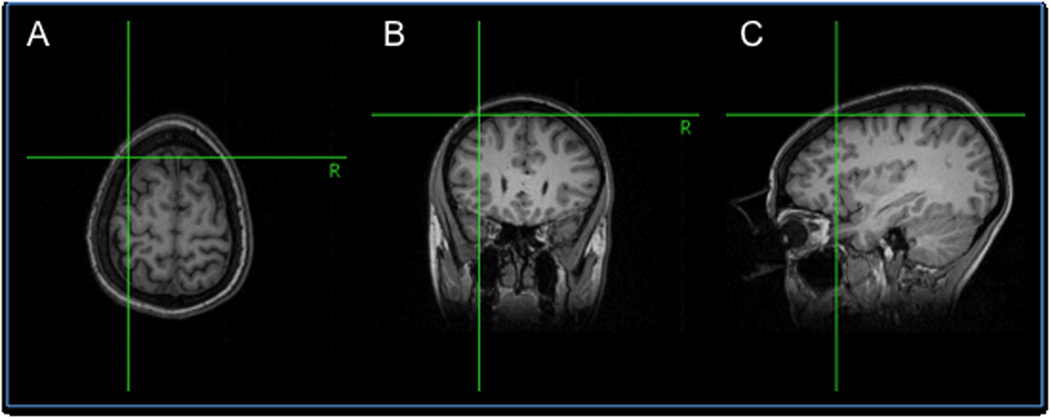
rTMS was performed with the Magstim Rapid2 stimulator with a figure-of-eight coil using the Brainsight® frameless stereotaxy system for neuronavigation. Motor thresholds (MT) were determined before each session and were defined as the percent intensity output of the stimulator that generated a 50µV motor evoked response in the first dorsal interossei in five out of 10 trials. The DLPFC was localized by a point 5.5cm anterior to the motor hotspot along the parasagittal plane with placement confirmed by neuronavigation (Figure 1).
Figure 1. Neuronavigation using Brainsight®.
Frameless stereotaxy neuronavigation used to verify position of TMS coil over the dorsolateral prefrontal cortex shown in transverse (A), coronal (B), and sagittal (C) planes.
For low-frequency stimulation, 110% of MT was used for 1800 continuous pulses at 1Hz (30minutes). For high-frequency stimulation, 100% MT was used for 1800 pulses (22.5minutes) delivered as 4 second trains at 10Hz (40 pulses) with a 26 second inter-train interval for a total of 45 trains. Each subject underwent the four rTMS conditions (left 10Hz, left 1Hz, right 10Hz, right 1Hz) with two to seven days between each session. Subjects were randomly assigned to start with either the right or left hemisphere.
Symptom reporting
Subjects reported the intensity of their rocking sensation on a 0–100 visual analogue scale (VAS) immediately before and during a 60-minute post-rTMS observation period. On this scale, the 0 end refers to no rocking sensation. The subjects were encouraged to report all other effects, but the scale was to be used only for the intensity of rocking.
RESULTS
Eight women and two men, ages 27–59, participated in the study. Triggers, symptom duration, and handedness are reported in Table 1.
TABLE 1. Clinical profile of subjects with MdDS who underwent rTMS.
The Edinburgh Handedness Inventory ranges from +100 to −100 with positive values representing right-handedness and negative values representing left-handedness.
| Subject number |
Sex | Hand Dominance |
Edinburgh score |
Age at onset |
Age at TMS |
Duration (months) |
Trigger |
|---|---|---|---|---|---|---|---|
| 1 | F | R | 100 | 29 | 32 | 41 | Airplane |
| 2 | M | R | 86 | 30 | 38 | 91 | Boat |
| 3 | F | R | 89 | 40 | 45 | 61 | Boat, Airplane |
| 4 | M | R | 33 | 42 | 47 | 60 | Airplane |
| 5 | F | R | 55 | 27 | 28 | 10 | Airplane |
| 6 | F | R | 83 | 59 | 60 | 12 | Train |
| 7 | F | R | 100 | 58 | 60 | 24 | Boat |
| 8 | F | L | −33 | 45 | 48 | 33 | Car |
| 9 | F | L | −100 | 54 | 57 | 36 | Boat |
| 10 | F | L | −44 | 42 | 44 | 27 | Airplane |
Change in rocking perception
Figure 2 shows the percent change in VAS at 5-minutes, 30-minutes, and 60-minutes after rTMS for each condition. In right-handed subjects, left 10Hz stimulation was the most effective in reducing the rocking sensation followed by right 1Hz stimulation. Left 1Hz stimulation was particularly associated with worsened symptoms. In the three left-handed subjects, right 10Hz stimulation was the most effective (Table 2). Two long-term events were seen. Subject #6 reported no rocking sensation for 2.5 days after left 10Hz stimulation and Subject #8 (left-handed) reported reduction in visual motion intolerance and oscillopsia for 3 weeks after right 10Hz stimulation.
Figure 2. Change in VAS for four rTMS conditions.
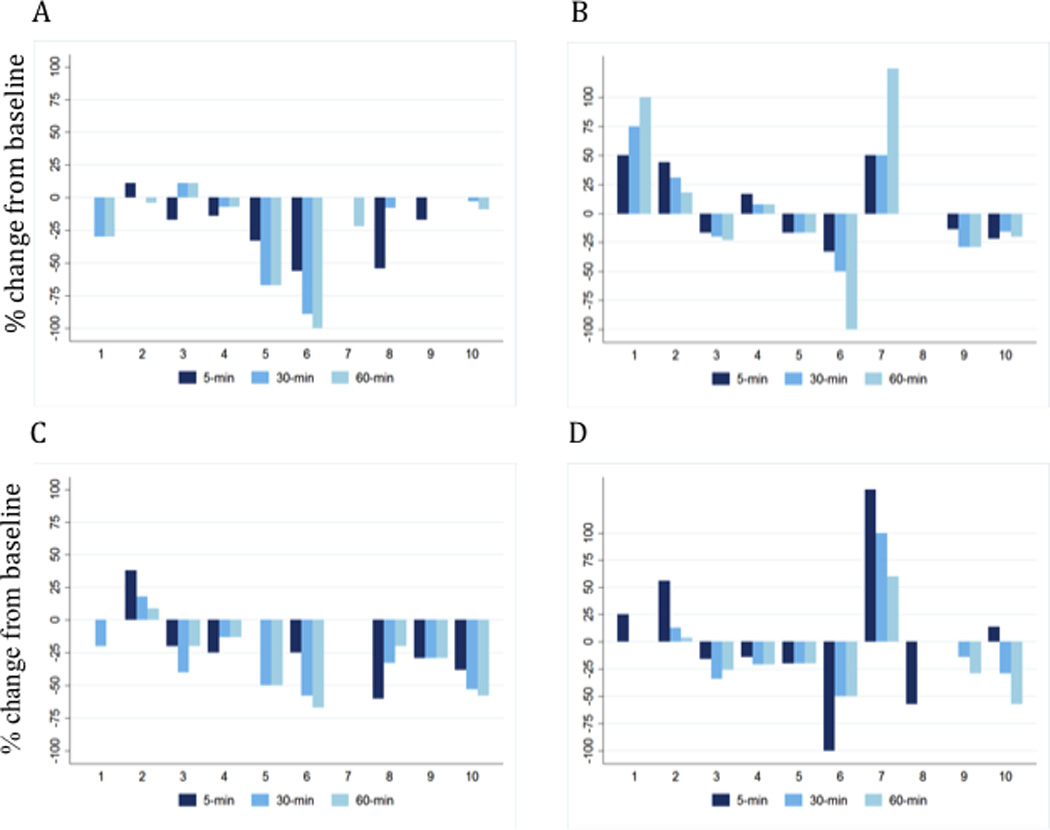
Percentage change in visual analogue scale (0–100) from baseline at 5-minutes, 30-minutes, and 60-minutes post for A) High frequency left hemisphere (10Hz); B) Low frequency left hemisphere; C) High frequency right hemisphere (10Hz); and D) Low frequency right hemisphere (1Hz). Numbers from 1 to 10 on the x-axis represent subjects from Table 1. Negative deflections represent a decrease in rocking; positive deflections, an increase in rocking.
TABLE 2. Percentage change in VAS after rTMS.
Percentage change in VAS scores are given at 5-minutes, 30-minutes, and 60-minutes after rTMS was completed indicating immediate, middle, and late effects of rTMS. Other effects such as headache, fatigue, cognitive changes, and mood changes are individually noted. The overall best and worst overall parameters are indicated.. Subjects are listed in the same order as in Table 1.
| Subject | Group | 5-min | 30-min | 60-min | Average | Other effects | Most overall benefit |
Least overall benefit |
|---|---|---|---|---|---|---|---|---|
| 1 | Left high | 0 | −30 | −30 | −20 | Improved mood, more energetic rest of day | Left high | Left low |
| Left low | 50 | 75 | 100 | 75 | Less balance, sore neck, head pressure | |||
| Right high | 0 | −20 | 0 | −7 | Some head pressure | |||
| Right low | 25 | 0 | 0 | 8 | Fatigue, more emotional ×10min post-rTMS | |||
| 2 | Left high | 11 | 0 | −4 | 2 | Less visual intolerance, pictures more pleasant | Left high | Left low |
| Left low | 44 | 31 | 18 | 31 | Headache | |||
| Right high | 38 | 18 | 9 | 21 | Apathy, slowed thinking, fatigue, headache | |||
| Right low | 56 | 13 | 4 | 24 | Fatigue, "emotionally draining" | |||
| 3 | Left high | −17 | 11 | 11 | 2 | More energetic ×20min, headache | Right low | Left high |
| Left low | −17 | −20 | −23 | −20 | None | |||
| Right high | −20 | −40 | −20 | −27 | Headache | |||
| Right low | −16 | −34 | −26 | −25 | Mild nausea, metallic taste | |||
| 4 | Left high | −14 | −7 | −7 | −10 | Headache | Right low | Left low |
| Left low | 17 | 8 | 8 | 11 | Fatigue, headache | |||
| Right high | −25 | −13 | −13 | −17 | None | |||
| Right low | −14 | −21 | −21 | −19 | Tinnitus decreased | |||
| 5 | Left high | −33 | −67 | −67 | −56 | More energy, mood improved | Left high | Left low |
| Left low | −17 | −17 | −17 | −17 | None | |||
| Right high | 0 | −50 | −50 | −33 | Fatigue | |||
| Right low | −20 | −20 | −20 | −20 | None | |||
| 6 | Left high | −56 | −89 | −100 | −81 | No rocking × 2.5days | Left high | Right low |
| Left low | −33 | −50 | −100 | −61 | No rocking × 6hours | |||
| Right high | −25 | −58 | −67 | −50 | Rocking changed directions | |||
| Right low | −100 | −50 | −50 | −67 | Legs tired, head "full," more room movement | |||
| 7 | Left high | 0 | 0 | −22 | −7 | Delayed onset headache | Left high | Left low |
| Left low | 50 | 50 | 125 | 75 | None | |||
| Right high | 0 | 0 | 0 | 0 | None | |||
| Right low | 140 | 100 | 60 | 100 | None | |||
| 8 | Left high | −54 | −8 | 0 | −21 | Uncomfortable "rolling" feeling, headache | Right high | Left high |
| Left low | 0 | 0 | 0 | 0 | None | |||
| Right high | −60 | −33 | −20 | −38 | 3 week decreased visual motion intolerance | |||
| Right low | −57 | 0 | 0 | −19 | Tinnitus decreased | |||
| 9 | Left high | −17 | 0 | 0 | −6 | None | Right high | Left high |
| Left low | −14 | −29 | −29 | −24 | None | |||
| Right high | −29 | −29 | −29 | −29 | None | |||
| Right low | 0 | −14 | −29 | −14 | Fatigue, rocking changed direction | |||
| 10 | Left high | 0 | −3 | −9 | −4 | Disoriented, edgy, heightened senses | Right high | Left high |
| Left low | −22 | −16 | −20 | −19 | Heightened senses, photophobic | |||
| Right high | −38 | −53 | −58 | −49 | Feel calmer, less "brain fog," more alert | |||
| Right low | 14 | −29 | −57 | −24 | Less lightheaded, less "brain fog" | |||
Figure 3 shows the negative relationship (R2=−0.5509) between the duration of illness and the percent change in VAS at 60-minutes post-rTMS. Sixty-minutes was chosen because any improvement noted was sustained at 60-minutes in all subjects and seven subjects showed the maximum improvement at this time point.
Figure 3. Correlation between treatment effect at 60-minutes and duration of illness.
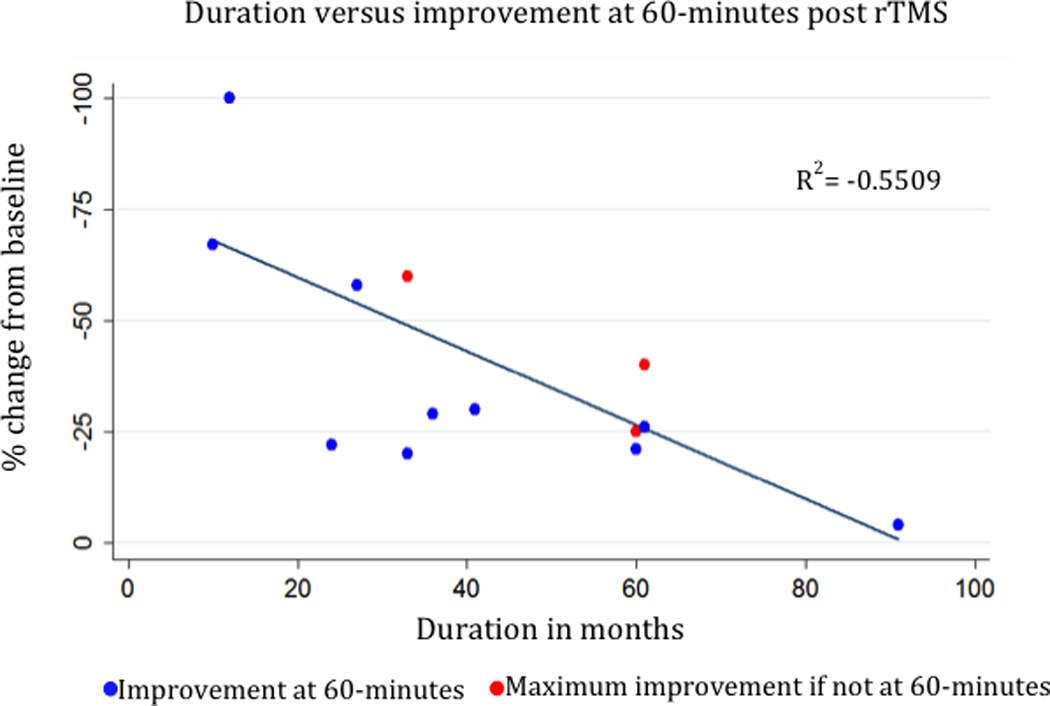
Correlation between duration of MdDS symptoms and the maximum treatment response at 60-minutes. Blue points represent the score at 60-minutes. The red points represent the three instances when the maximum change was not at 60-minutes. The regression line only includes data at 60-minutes. If maximum percentage change using all time points is used, then R2=−0.5529.
Side effects
Headache occurred in 10 of 40 sessions; it was usually mild and located only at the site of stimulation. Severe headache lasting three or more days occurred in one subject after 10Hz stimulation and in another after 1Hz stimulation. The first subject had a history of migraine with aura; the second already had ongoing neck pain. There were six reports of post-rTMS fatigue, three that occurred after right 1Hz, one after left 1Hz, and two after right 10Hz stimulation. Three subjects reported feeling more energetic after left10Hz stimulation lasting from 20-minutes to the rest of the day and three reported improved mood lasting from 60-minutes to the rest of the day. Negative cognitive effects occurred with right hemisphere stimulation in some right-handed subjects, but with left hemisphere stimulation in left-handers. Three subjects reported a change in the direction of the motion, even if the rocking did not change in intensity. No subjects reported hearing loss or tinnitus. There were no seizures. Other than the two long headaches, all negative side effects resolved within one day.
DISCUSSION
Treatment options need to be explored for MdDS, which remains incurable if symptoms do not remit in a short period (9). This pilot study revealed some important considerations for future clinical trials using rTMS in MdDS. rTMS was overall very well-tolerated in subjects with MdDS with stimulation site discomfort being within the range of what is generally reported for rTMS sessions (13). The two subjects who developed severe headache had particular risk factors, which should be considered in future screening. Fatigue was common enough to warrant formal assessment in future studies.
Subjects who had longer duration of symptoms generally had less symptom reduction with rTMS, a phenomenon that has been observed in other disorders (14) (15). However, sequential days of rTMS treatment may still be potentially beneficial for these subjects.
Finally, the effect of even one session of rTMS may last longer than the typical effects seen with motor excitability changes, which is generally about 15–20 minutes (4). This suggests that rTMS does not simply suppress the perception of rocking but may be introducing a periodic stimulus that is desynchronizing an abnormal rhythm that gradually dampens with time.
This pilot study had no formal sham condition but used physiologically counterbalanced stimuli as internal controls. Although high frequency left DLPFC stimulation gave the most positive effects and inhibiting DLPFC with low frequency stimulation gave the worst effects, there was variability in responses. The suppression of rocking perception did not clearly follow the trend of side effects. As DLPFC is a highly interconnected area, different networks connected with the DLPFC may be differentially affected by rTMS in some patients, thus affecting motion perception, fatigue, mood, and visual motion intolerance to variable degrees. For example, in some subjects, DLPFC stimulation may have affected connectivity to the posterior-parietal network more than the cortico-limbic network.
Although we did not expect handedness to be relevant to MdDS physiology a priori, the pattern was consistent within the small number of subjects. The effect of handedness was previously seen in perfusion studies after vestibular caloric irrigation, showing that the non-dominant hemisphere activates the greatest with ipsilateral caloric stimulation (16).
Future trials should include a formal sham condition and include multiple days of treatment and possibly assess functional connectivity as a marker of treatment response. These pilot data show that rTMS may be a viable treatment option for MdDS, an otherwise incurable disorder, and would thus warrant further studies.
ACKNOWLEDGEMENTS
The authors thank the subjects and their families for participating in the study.
FUNDING/ DISCLOSURES
This project was funded by NIH/NIDCD grant R03 DC010451, UCLA GCRC grant M01- RR000865, MdDS Balance Foundation Early Career Award. The funding sources had no influence over the design, implementation, or interpretation of the study. This was not an industry-sponsored study.
Role of each author:
Dr. Cha was responsible for the design and implementation of the study, interpreting the data and writing up the first draft of the manuscript.
Ms. Cui was responsible for the implementation of the study and editing the manuscript.
Dr. Baloh was responsible for interpreting the data and editing the final draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statistical analysis was performed by Dr. Cha.
REFERENCES
- 1.Gordon CR, Spitzer O, Shupak A, Doweck I. Survey of mal de debarquement. British Medical Journal. 1992;304(6826):544–544. doi: 10.1136/bmj.304.6826.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha YH. Mal de debarquement. Semin Neurol. 2009 Nov;29(5):520–527. doi: 10.1055/s-0029-1241038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macke A, Leporte A, Clark BC. Social, societal, and economic burden of mal de debarquement syndrome. J Neurol. 2012 Jan 10; doi: 10.1007/s00415-011-6349-6. [DOI] [PubMed] [Google Scholar]
- 4.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007 Jul 19;55(2):187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain's intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences. 2011;108(52):21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha YH. Left entorhinal cortex and amygdala hypermetabolism in mal de debarquement syndrome; Apr 24, 2012; 64th Annual American Academy of Neurology, Poster P02.248. [Google Scholar]
- 7.Grimault S, Robitaille N, Grova C, Lina JM, Dubarry AS, Jolicoeur P. Oscillatory activity in parietal and dorsolateral prefrontal cortex during retention in visual short-term memory: additive effects of spatial attention and memory load. Hum Brain Mapp. 2009 Oct;30(10):3378–3392. doi: 10.1002/hbm.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diwadkar VA, Carpenter PA, Just MA. Collaborative activity between parietal and dorso-lateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. Neuroimage. 2000 Jul;12(1):85–99. doi: 10.1006/nimg.2000.0586. [DOI] [PubMed] [Google Scholar]
- 9.Cha YH, Brodsky J, Ishiyama G, Sabatti C, Baloh RW. Clinical features and associated syndromes of mal de debarquement. J Neurol. 2008 Jul;255(7):1038–1044. doi: 10.1007/s00415-008-0837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988 Nov;8(11):4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984 Jul;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- 12.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 13.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009 Dec;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Ridder D, Verstraeten E, Van der Kelen K, De Mulder G, Sunaert S, Verlooy J, et al. Transcranial magnetic stimulation for tinnitus: influence of tinnitus duration on stimulation parameter choice and maximal tinnitus suppression. Otol Neurotol. 2005 Jul;26(4):616–619. doi: 10.1097/01.mao.0000178146.91139.3c. [DOI] [PubMed] [Google Scholar]
- 15.Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009 Jan;34(2):522–534. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 16.Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003 Sep;13(9):994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]