Abstract
Background
Rodent studies have demonstrated that adolescent social isolation results in many behavioral perturbations, including increases in anxiety-like behaviors. Socially isolated rats have also been shown to self-administer greater amounts ethanol in some, but not all, studies. Here, we tested whether juvenile social isolation increases ethanol drinking using an intermittent procedure that engenders relatively high intake in normally reared animals. We also compared the behavioral phenotype of rats reared under social isolation or group housed conditions with adult rats housed under conditions commonly used in ethanol drinking studies.
Methods
Male Long Evans rats were procured immediately post-weaning and were group-housed for one week. Subjects were then randomly divided into two groups: socially isolated (SI) rats, housed individually for six weeks and group housed rats (GH, 4/cage). A third group were procured as young adults and were housed individually upon arrival for one week (standard housing condition, STD). Rats were then tested in aplusmaze and novelty assay and then all subjects were singly housed and ethanol drinking was assessed.
Results
SI rats displayed increased anxiety-like behaviors on the plus-maze, a greater locomotor response to a novel environment, and increased ethanol intake, relative to GH rats. STD rats exhibited an anxiety-like behavioral profile on the plus-maze that was similar to SI, and not GH, rats and also drank ethanol at levels comparable to SI subjects. In addition, anxiety-like behavior on the plus-maze correlated with intermittent ethanol intake in SI and GH rats.
Conclusions
These data further support the validity of the rodent juvenile social isolationmodel for studies directed at elucidating behavioral and neurobiological mechanisms linking anxiety and ethanol drinking. These findings further suggest that housing conditions commonly employed in rodent drinking studies may recapitulate the anxiety-like and ethanol drinking phenotype engendered by a juvenile social isolation procedure.
INTRODUCTION
Many studies have identified an important relationship between anxiety and alcohol use disorders (AUDs)(Ciccocioppo et al., 2006; Kushner et al., 2000). For example, epidemiological studies have demonstrated a significant comorbidity between anxiety disorders and AUDs(Chan et al., 2008; Kessler et al., 1997). Acute alcohol exposure decreases a wide range of anxiety-related behaviors (Koob, 2004; Kushner et al., 2000)and many studies have shown that withdrawal following chronic alcohol exposure is often associated with increases in anxiety-like behaviors (Kliethermes, 2005; Santucci et al., 2008), some of which can persist for several months after the last exposure to ethanol (Santucci et al., 2008). Numerous animal studies have also demonstrated that withdrawal promotes increased ethanol self-administration in dependent subjects and thatdependence-induced increases in ethanol intake are reduced by treatments that can attenuate withdrawal-induced anxiety (e.g. CRF antagonists; Chu et al., 2007; Finn et al., 2007). These, and other findings have led some to hypothesize that alcohol’s acute anxiolytic effects may play an integral role in the etiology of alcoholism(Breese et al., 2011; Fox et al., 2007).
Despite the clinical importance of understanding the link between anxiety and AUDs, much remains unknown about the neurobiological substrates that underlie this relationship. These efforts have been hampered, to some extent, by the lack of reliable animal models, particularly models that reveal a consistent relationship between initial levels of anxiety-like behaviors and subsequent ethanol drinking. Although some studies have shown that animals with elevated levels of anxiety-like behaviors also exhibit higher ethanol intake (Ciccocioppo et al., 2006; Spanagel et al., 1995), this relationship is not always observed (Fernandez-Teruel et al., 2002; Henniger et al., 2002). One model that shows promiseinvolves social isolation of rodents during adolescence. Adolescence represents a critical period of physiological, neural and behavioral maturation in humans and rodents, particularly with regard to stress and anxiety-like behaviors (Doremus-Fitzwater et al., 2010; Spear, 2009). Rats reared in isolation during the juvenile adolescent period (~ PD 28-70) exhibit a broad spectrum of enduring behavioral alterations relative to peers raised in groups(Hall, 1998; Veenema, 2009).Anxiety-like behaviors are particularly sensitive to juvenile isolation, with many studies reporting significant increases in a wide range of anxiety measures (Hall et al., 1998a; Hellemans et al., 2004; Lim et al., 2011; Wright et al., 1991). Importantly, we and others have also reported a significant increase in ethanol self-administration in rats that have been reared under juvenile social isolation conditions (Deehan et al., 2007; Hall et al., 1998b; Lodge and Lawrence, 2003; McCool and Chappell, 2009). However, this relationship has also not been observed with all drinking procedures (Ehlers et al., 2007; Fahlke et al., 1997)and the reasons for these disparate findings are not well understood. Here, we sought to reexamine the effect of juvenile social isolation on ethanol drinking using an intermittent self-administration procedure that has been shown to engender relatively high levels of ethanol intake, even in outbred rats reared under standard housing conditions used in many rodent home-cage ethanol drinking studies (Simms et al., 2008; Wise, 1973). To that end, we also included animals in this study that were procured as adults and maintained under housing conditions commonly employed by us (Chappell and Weiner, 2008) and many others (e.g. Czachowski and Delory, 2009; Li et al., 2010; Simms et al., 2008) to study rodent drinking behaviors. Our results confirm that juvenile social isolation results in significant increases in anxiety-like behavioras well as enduring increases in measures of ethanol intake. In addition, initial anxiety-like behavior was positively correlated with intermittent ethanol drinking in group housed and socially isolated rats. Interestingly, our data also reveal that adult rats raised under housing conditions commonly employed in ethanol self-administration studies recapitulate much of the anxiety-like and ethanol drinking phenotype of socially isolated rats.
Materials and Methods
Animals
Subjects were male LongEvans rats sourced from a common research animal supplier (Harlan Laboratories, Indianapolis, IN). At the supplier, rats were maintained on a 12 hr. light/dark cycle, housed in non-transparent cages and minimally handled (weighed once each week; cages changed once each week). Food (TeklabGlobal 2018 rodent diet, Harlan, IN) and water were available ad libitum. Pups were housed with their mothers until weaning and were then housed 8/cage until they reached 100 g at which time the cage density was reduced to 6/cage(Harlan Laboratories, personal communication).Upon arrival in our laboratory, postweaninganimals were group housed (4/cage) for one week under housing and handling conditions similar to those employed by the supplier. Thus, rats were maintained on the same light/dark cycle, fed a very similar diet (Prolab RMH 3000, LabDiet) and cages were changed once each week. All animal care procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. Animals were housed under one of the following three experimental conditions: Group Housed (GH): rats arrived in the lab at postnatal day (PD) 21 and were housed in groups of 4-5 in largeplexiglass cages (33.0 cm X 59.7 cm; Nalgene, Rochester, NY) until PD 78, at which point they were individually housed in smaller rodent cages (25.4 cm X 45.7 cm) for the remainder of the study. A total of 16 GH rats were tested in these studies.
Socially Isolated (SI)
rats arrived in the lab at PD21, remained group housed (4-5/cage) in the large, plexiglass cages for one week and were then singly housed in the smaller cages for the remainder of the study. These rats received the same olfactory, visual, and auditory stimulation as GH rats, but were deprived of any physical contact and/or social interaction with their peers during this period. A total of 17 SI rats were tested in these studies.
Standard Adult Housed (STD)
rats arrived in the lab at PD 63 (to match the age of SI and GH rats five weeks into the juvenile housing manipulation) and were immediately single housed in the small plexiglass cages for the duration of the study. The housing conditions of this group were similar those that we and many others have employed in home-cage ethanol drinking studies.A total of 17 STD rats were tested in these studies. Throughout the adolescent housing period, GH and SI rats were only handled once/week. STD housed rats were also handled in a similar manner during the first week of acclimation. Cohorts of GH, SI, and STD rats were tested concurrently throughout these studies in two separate replications of the experimental procedures, separated by approximately 6 months.
Behavioral Testing
All behavioral testing was conducted between PD 72-78. Anxiety-like behavior was assessed using standard elevated plus-mazes (Med Associates, St. Albans, VT)and response to novelty was measured using locomotor activity chambers (see Supporting Information for methodological details of these tests).
Drinking Procedures
On PD 78, following the completion of behavioral testing, ethanol consumption was assessed using three home-cage drinking procedures. Note that all animals were single housed throughout each of these ethanol self-administration procedures.From PD 79-81, subjects were given access to a 10% ethanol solution (v/v) as their only liquid. Following this forced ethanol exposure regimen, home-cage drinking was assessed for 5 consecutive days (PD 82-86) using a standard two bottle choice procedure (10% ethanol (v/v); water). Finally, the effect of housing condition on ethanol self-administration was assessed using an intermittent home-cage drinking procedure which has previously been shown to engender relatively high levels of ethanol intake in male Long-Evans rats (Simms et al., 2008; Wise, 1973)(see Supporting Information for a more detailed description of the drinking procedures).
Blood Ethanol Determination
Following the completion of the four week intermittent drinking procedure, a final intermittent drinking session was conducted. Subjects were given access to ethanol and water for 30 minutes and then a 10 μl blood sample was collected from a tail snip. Blood ethanol concentrations were determined using a commercially available alcohol dehydrogenase/NADH enzymatic assay kit (Diagnostic Chemicals, Oxford, CT).
Statistics
Datasets were analysed using one- or two-way ANOVA followed by the Newman-Keuls post hoc test, as indicated in the text. In cases where datasets were not normally distributed, the Kruskal-WallisANOVA on ranks was conducted followed by the Dunn’s post hoc test. Correlations between antecedent behavioral measures and intermittent ethanol intake were only conducted on normally distributed datasets (Shapiro-Wilk). These analyses were performed using Pearson’s correlation test. The significance level for all statistical analyses was set at P < 0.05.
Results
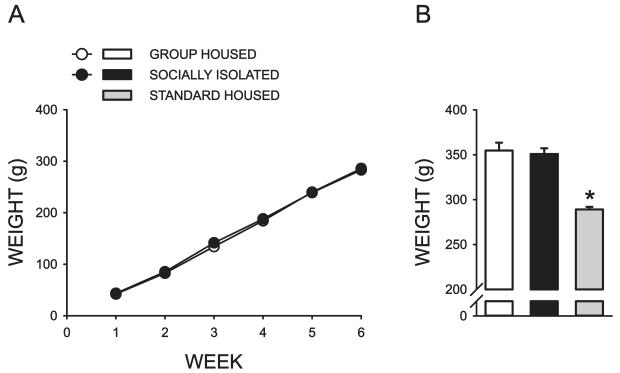
During the adolescent rearing manipulation, socially isolated (SI) and group housed (GH) rats were weighed once each week for six weeks. There was no effect of housing condition on body weight (F = 0.102, P = 0.752) but there was a significant effect of week (F = 2548.220, P < 0.001) (Fig 1A). No interaction between housing condition and week was detected (F = 0.940, P = 0.457). Standard housed rats were procured from the same commercial supplier as the SI and GH rats and were age-matched with the subjects in these other cohorts. When the body weight of all three groups was compared immediately prior to the onset of behavioral testing (PD70), a significant effect of housing condition was observed (F = 34.171, P < 0.001) and post hoc analysis revealed that STD housed rats weighed significantly less than SI and GH rats (P < 0.001)(Fig 1B). Subjects in all three cohorts gained weight at a comparable rate throughout the remainder of these studies. As a result, the body weight of STD rats was significantly less than that of SI and GH rats throughout the entire study.
Figure 1.
Effect of rearing condition on body weight. A. Average weekly body weight of group housed (GH) and socially isolated (SI) rats during the six week housing manipulation (N= 16 GH, 17 SI; error bars fall within symbols). B. Body weight of group housed, socially isolated, and age-matched standard housed ( N = 17) rats assessed on postnatal day 70, prior to the onset of behavioral testing. (*, significant difference relative to GH and SI rats, P < 0.001).
Effect of Rearing Condition on Anxiety-Like Behavior
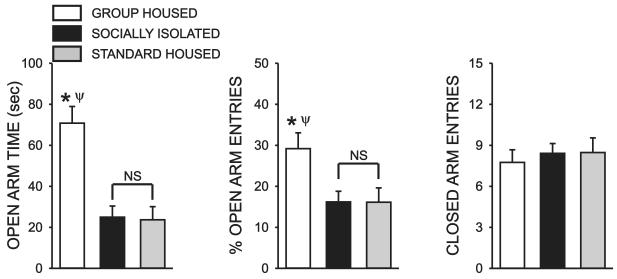
To determine the effect of adolescent social isolation on anxiety-like behavior, SI, GH, and STD rats were tested on an elevated plus-maze at PD 72. There was a significant overall effect of housing condition on time spent in the open arms, a well-validated measure of anxiety-like behavior (H = 18.538, P < 0.001) (Fig 2A). Post hoc analysis revealed that GH rats spent significantly more time exploring the open arms than both SI (Q = 3.567, P < 0.05) and STD (Q = 3.891, P < 0.05) rats. SI and STD rats did not differ in the amount of time that they spent on the open arms of the maze (Q = 0.329, N.S.)(Fig 2). There was also an overall effect of housing condition on open arm entries (H = 8.533, P < 0.02) and, again, post hoc analysis revealed that GH rats had a higher percentage of open arm entries than either SI (Q = 2.557, P < 0.05) or STD subjects (Q = 2.511, P < 0.05)(Fig 2). In contrast, no effect of housing condition on the number ofclosed arm entries (F = 0.147, P = 0.864), a measure of non-specific locomotor activity, was observed (Fig 2).
Figure 2.
Effect of rearing condition on anxiety-like behavior assessed in the elevated plus-maze. Bar graph illustrating the effect of adolescent rearing condition on mean (± SEM) open arm time , % open arm entries and the number of closed arm entries in the plus-maze for group housed (GH), socially isolated (SI) and age-matched standard housed (STD) rats. The test was conducted on PD 72 for all groups. (N==16 GH, 17 SI, 17 STD; *, significant difference between GH and SI rats; Ψ, significant difference between GH and STD rats (P < 0.05); NS, no significant difference).
Effect of Rearing Condition on Response to Novelty
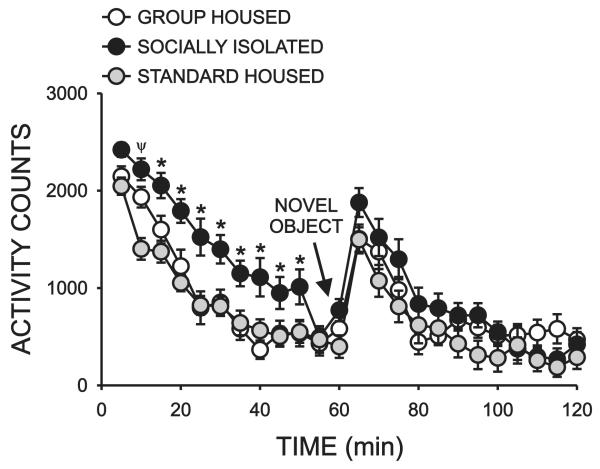
Response to novelty was assessed between PD 74-78. Subjects were placed in a novel environment for 60 minutes and locomotor activity was measured in five minute bins. Following this period, a novel object was introduced into the center of the activity arena and locomotor activity was measured for an additional 60 min. A significant overall effect of housing condition (F = 6.631, P < 0.003) and time (F = 61.356, P < 0.001) were detected as well as a significant interaction between these factors (F = 2.804, P < 0.001)(Fig 3). Post-hoc analysis revealed a significant difference in the locomotor response of SI rats to the novel environment, relative to subjects in the GH (P < 0.002) and STD (P < 0.002) cohorts. Specifically, SI rats exhibited significantly greater locomotor activity during the time bins between 15-50 min (*, P< 0.05). A significant difference in locomotor activity between SI and STD rats was also seen between 5-10 min (Ψ, P < 0.05). No significant effect of rearing condition on peak response to the novel objectwas observed (65 min time bin).
Figure 3.
Effect of rearing condition on response to novelty. Timecourse illustrating the effect of adolescent rearing condition on mean locomotor response (± SEM) to a novel environment and a novel object, plotted in five minute bins. The test was conducted between PD 74-78 for all groups. (N = 16 GH, 17 SI, 17 STD; *, significant difference between SI rats and both GH and STD rats; Ψ, significant difference between SI and STD rats, P < 0.05).
Effect of Rearing Condition on Ethanol Self-Administration
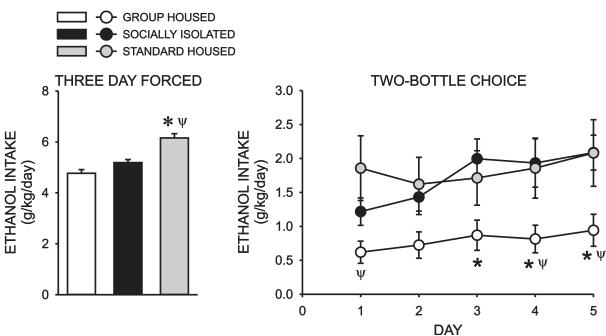
Immediately following the completion of the behavioral testing, all subjects were singly housed under identical conditions for the remainder of the study. A significant overall effect of housing condition on intake during the three day forced ethanol exposure regimen was detected (H = 19.704, P < 0.001) and posthoc analysis revealed that STD rats drank significantly more ethanol than GH (Q = 4.419, P < 0.05) and SI (Q = 2.541, P < 0.05) rats (Fig 4A). No significant difference in forced ethanol intake was observed between GH and SI rats (Q = 1.917, P > 0.05).There was also an overall effect of housing condition (F = 3.753, P < 0.03) and day (F = 4.262, P < 0.003) on ethanol intake during the continuous access two-bottle choice procedure, with no significant interaction between these factors (F = 1.302, P < 0.245)(Fig 4B).Post-Hoc analysis revealed that GH rats drank significantly less ethanol than either SI (P < 0.02) or STD (P < 0.04) rats. No significant differences in ethanol intake were observed between SI and STD rats (P = 0.821). Although the ethanol preference ratio of GH rats trended lower than that of the other two cohorts, the overall effect of housing condition on ethanol preference fell just below the level of statistical significance (F = 3.042, P = 0.057)(Data not shown).
Figure 4.
Effect of rearing condition on forced and two-bottle choice continuous access ethanol self-administration. A. Bar graph illustrating the effect of rearing condition on daily ethanol intake during a three day forced exposure procedure in which 10% ethanol was the only solution available. (*, significant difference between STD and GH rats; Ψ, significant difference between STD and SI rats; P < 0.05). B. Average daily ethanol intake during a five day, two-bottle choice drinking assay (10% ethanol/water) conducted immediately following the three day forced procedure. *, significant difference between GH and SI rats; Ψ, significant difference between GH and STD rats; P < 0.05).
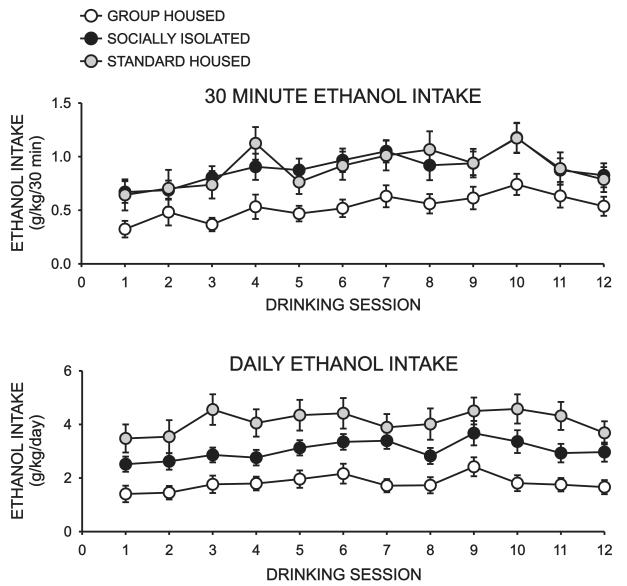
As observed in prior studies, during the intermittent drinking procedure, subjects in all cohorts consumed approximately 25% of their daily ethanol intake during the first 30 min. of each ethanol drinking session. There was an overall effect of both housing condition (F = 10.001, P < 0.001) and drinking session (F= 5.888, P < 0.001) on 30 min. ethanol intake with no significant interaction between these factors. Post-hoc analysis revealed that GH rats had significantly lower levels of ethanol intake than SI (P < 0.02) or STD (P < 0.001) rats. There were no significant differences in 30 min. ethanol intake between SI and STD rats. A significant overall effect of both housing condition (F = 10.327, P < 0.001) and drinking session (F = 6.820, P < 0.001) were also observed for daily ethanol intake with no significant interaction between these factors (F = 0.859, P = 0.651). Post hoc comparisons revealed that GH rats had significantly lower levels of daily ethanol intake than either SI (P < 0.02) or STD (P < 0.001) rats. Moreover, SI rats drank significantly less than STD rats during the intermittent drinking procedure (P < 0.04).
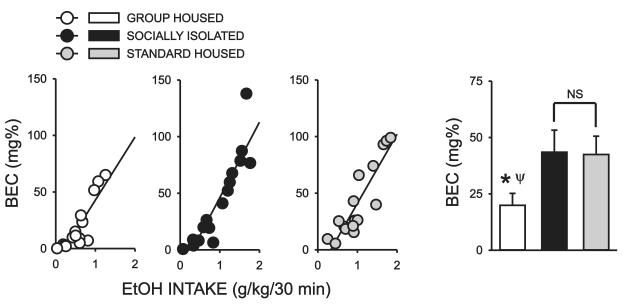
Blood ethanol determinations were made immediately following the first 30 minutes of a final ethanol drinking session. Blood ethanol levels were strongly correlated with 30 min. ethanol intake for subjects in all three cohorts (Fig 6)(GH: r2= 0.76, F=45.29, P<0.0001; SI: r2=0.82, F=64.25, P<0.0001; STD: r2=0.80, F=56.33, P<0.0001). There was a significant overall effect of housing condition on BEC (H = 6.423, P < 0.05) and post hoc analysis revealed that GH rats had significantly lower BEC values than subjects in either SI or STD groups (P < 0.05). No differences in BEC levels were detected between SI and STD rats.
Figure 6.
Effect of rearing condition on blood ethanol levels. Scatter plots illustrating the strong correlation between 30 min. ethanol intake and blood ethanol levels determined immediately following the first 30 minutes of this ethanol drinking session for all three cohorts. Bar graph summarizes average blood ethanol levels obtained after the first 30 min. of this drinking session for GH, SI and STD rats. *, significant difference between GH and SI; Ψ, significant difference between GH and STD; P < 0.05).
Relationship Between Initial Behavioral Responses and Intermittent Ethanol Self-Administration
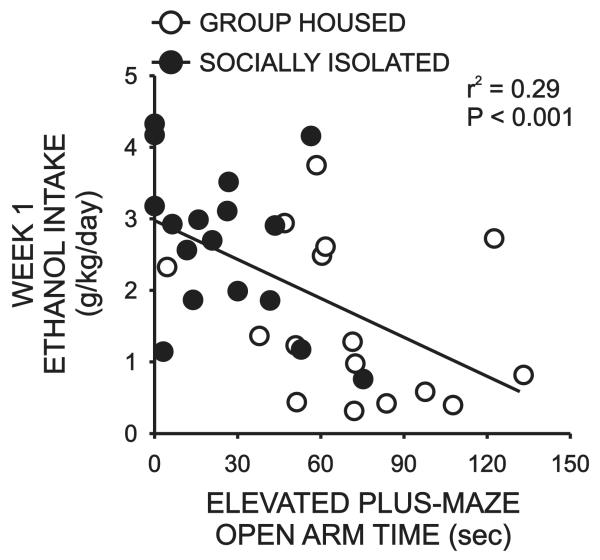
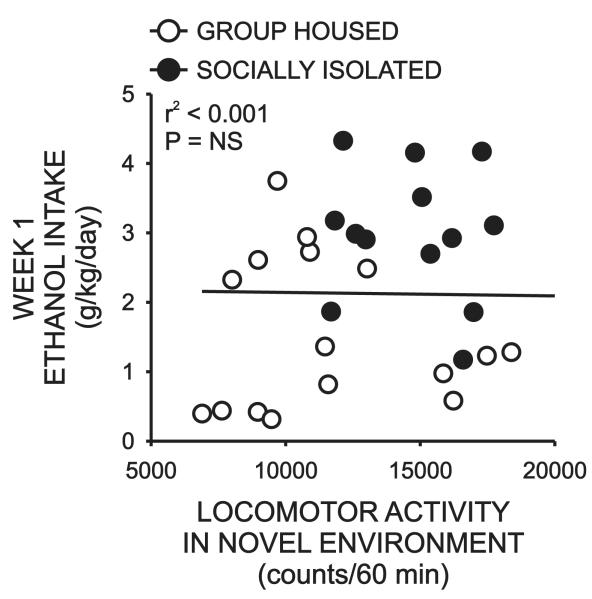
Finally, we sought to determine if there were any relationships between individual differences in initial behavioral measures and ethanol intake during the first week of the intermittent ethanol self-administration procedure.Although time spent on the open arms of the plus-maze did not correlate with ethanol intake in any of the cohorts examined alone, when data from the SI and GH cohorts were combined, a significant negative correlation emerged (r2 = 0.29, P < 0.001)(Fig 7). In other words, animals with low levels if anxiety-like behavior (greater time on open arms) drank less that animals with high levels of anxiety-like behavior (less time on open arms).This relationship remained significant when STD subjects were included in the analysis (r2 = 0.15, P < 0.005, data not shown) however the strength of the correlation was actually decreased by the inclusion of this cohort. We did not perform a correlational analysis of these measures within the STD group itself as this dataset was not normally distributed. Despite the fact that there were also significant differences in locomotor activity between SI and GH rats, no correlation was observed between this measure and intermittent ethanol intake for these subjects (r2< 0.001, P = 0.9108)(Fig 8).
Figure 7.
Effect of rearing condition on the relationship between initial levels of anxiety-like behavior (open arm time on the elevated plus-maze) and daily ethanol intake during the first week of intermittent ethanol self-administration.
Figure 8.
Effect of rearing condition on the relationship between initial levels of locomotor activity in a novel environment and daily ethanol intake during the first week of intermittent ethanol self-administration.
Discussion
The results of this study confirm those of a number of prior reports, that rats raised in isolation during adolescence display increased anxiety-like behavior in adulthood as well as a greater locomotor response to a novel environment,relative to rats grouped housed during this same period. Adolescent social isolation also resulted in significant increasesin multiple measures of ethanol intake as well as higher blood ethanol levels. Notably, age-matched adult rats maintained under housing conditions commonly used in home-cage ethanol drinking studiesseemed to recapitulatethe anxiety-like and ethanol-drinking phenotype of socially isolated (SI), and not group housed (GH) rats.
Behavioral Effect of Adolescent Housing Condition
Numerous studies have demonstrated that rats deprived of social interaction during adolescence exhibit a wide range of behavioral alterations when tested in adulthood, many of which recapitulate behaviors seen in children that have been exposed to early life stress. For example, rats raised in single cages during adolescence exhibit increases in anxiety-like behaviors(Hall et al., 1998a; Hellemans et al., 2004; Lim et al., 2011; Wright et al., 1991), hyperactivity in a novel environment (Hall et al., 1998a; Hellemans et al., 2004),deficits in sensory gating (Heidbreder et al., 2000; McCool and Chappell, 2009)and cognitive function(Hedges and Woon, 2011), relative to rats raised in groups and/or in enriched environments. Our data confirm that, with adolescent social interaction as the sole dependent variable, rats deprived of physical contact with their peers during adolescence exhibit significant increases in anxiety-like behavior, assessed on the plus-maze, when compared with rats housed in the same vivarium, but in groups of four.
We also examined response to novelty by assessing locomotoractivity in a novel environment as well as the locomotorresponse to a novel object.As in other studies(Hall et al., 1997; Hellemans et al., 2004; Lapiz et al., 2000), SI rats exhibited a significant increase in locomotor activity during the first 60 minutes in the novel environment, relative to GH subjects, consistent with an increased behavioral responsivity to an unfamiliar environment.Although this effect was not apparent for the first ten minutes of the test and thedifference in activity dissipated after 60 minutes. Moreover, following the one hour acclimation, SI and GH rats showed a similar locomotor response upon the introduction of an immovable novel object. These findings suggest that adolescent social isolation may have a greater effect on response to novelty where there is a significant stress component (i.e. exposure to into an inescapable novel environment)(Robinet, 1998)than on novelty responding in a context that may be less susceptible to the influence of stress and more related to exploration (animals had already acclimated to the new test cage for 60 min. prior to the introduction of the novel object and they could choose to explore or ignore the object).
Since many rodent ethanol self-administration studies employ single housing conditions, often for extended time periods(Anacker and Ryabinin, 2010; Camarini et al., 2010; Samson and Czachowski, 2003), we sought to examine the behavioral phenotype of singly housed rats purchased to match the age of SI and GH rats, five weeks into the juvenile housing manipulation. Surprisingly, STD rats weighed significantly less than the other two cohorts upon arrival. Although STD subjectsgained weight at a rate comparable to that of SI and GH rats, their weight remained lower than these other two cohorts throughout the duration of this study. Moreover, the behavioral profile of STD rats on the plus-maze was very similar to that of SI rats. Therefore, rats obtained as adults from a standard commercial supplier and then housed under conditions commonly used in rodent ethanol drinking studies display at least some of the behavioral characteristics of animals that have been reared under impoverished conditions. It should be noted that STD rats were only given one week of acclimation prior to testingto mimic protocols frequently used in ethanol self-administration studies. Future studies will be needed to determine if longer acclimation periods can mitigate the anxiogenic behavioral profileobserved in STD rats.
Effects of Adolescent Housing Condition on Ethanol Self-Administration
To examinethe effect of housing condition on ethanol self-administration,subjects were first given three days of continuous access to a 10% ethanol solution as their only source of fluid and this was immediately followed by a five day two-bottle choice procedure (10% ethanol/water). The three day single-bottle procedure encourages the consumption of pharmacologically relevant amounts of ethanol, as the ethanol solution provides the only source of water to the animals. However, forced ethanol consumption does not always correlate with measures of voluntary ethanol drinking (Chappell and Weiner, 2008).In contrast, two-bottle choice procedures are frequently used as a simple measure of ethanol’s reinforcing effects, as intake in these assays often correlates with ethanol seeking behaviors (Green and Grahame, 2008), although see (Chappell and Weiner, 2008; Samson and Czachowski, 2003). As in our prior study(McCool and Chappell, 2009), no differences in forced ethanol consumption were noted between SI and GH rats, suggesting that adolescent social isolation does not exert non-specific effects on overall fluid consumption. However, in the two-bottle choice procedure, SI rats drank significantly more ethanol than GH rats, with a trend toward a significant increase in ethanol preference. These findings are generally consistent with our initial study and further suggest that high levels of antecedent anxiety-like behavior may promote increased ethanol self-administration.
STD rats drank more ethanol than either GH or SI rats in the forced consumption assay and also drank ethanol at levels comparable to SI rats in the two-bottle choice procedure. If increased levels of anxiety-like behavior promote ethanol drinking, it may not be surprising that STD rats drank as much ethanol as SI subjects asboth of these groups exhibited comparable levels of anxiety like behavior on the plus-maze. However, the observation that SI and STD rats drank similar amounts of ethanol raises the question of whether adolescent social isolation promotes ethanol intake or if group housing lowers ethanol consumption? While this question cannot be conclusively addressed from this studyit should be noted that measures of ethanol intake and preference in SI and STD rats were similar to those observed in a prior study that we conducted with STD Long Evans rats (Chappell and Weiner, 2008)and are also in good agreement with earlier studies conducted with this rodent strain in this same laboratory by Samson and colleagues(for review see: Samson and Czachowski, 2003).
Although two-bottle choice procedures are frequently used to assess ethanol self-administration in rats, intake using these models with outbred ratsis rather modest and it can be difficult to generate pharmacologically meaningful blood ethanol levels with these protocols(Samson and Czachowski, 2003). Therefore, we also employed an intermittent two-bottle choice procedure that results in a robust escalation of ethanol intake,even in outbred, commercially procured animals(Simms et al., 2008). Both SI and STD rats escalated their ethanol intake to almost 1 g/kg within the first 30 min of drinking sessions, with some subjects reaching intake levels as high as 8 g/kg per day. In contrast, GH rats did not escalate beyond an average of 0.5 g/kg in the initial 30 min of ethanol availability and achieved significantly lower BECs than either SI or STD rats.
These data also support our prior findings, using a limited access operant procedure,in which the adolescent isolation increased consummatory measures of ethanol drinking in Long Evans rats (McCool and Chappell, 2009). However, again, based on the observation that STD rats drank ethanol at levels comparable to SI rats, our findings may be interpreted as evidence that group housing blocks the escalation in ethanol intake that is normally promoted by the intermittent model. One caveat to consider is that cohorts were subjected to three distinct ethanol self-administration procedures. It is possible that factors such as stress associated with the three day forced ethanol drinking procedure may have contributed to some of the subsequent differences in voluntary ethanol self-administration. Nevertheless, given that SI and STD rats also displayed comparablelevels of anxiety-like behavior on the plus-maze in this study, prior to any ethanol exposure, perhaps the better question may be whether commercially procured animals housed under conditions frequently used in home-cage ethanol drinking studies can be considered “normal”? Many factors, in addition to the number of cage mates, can impact experimental results. For example, the commercial source of rodent strains can significantly influence the results of diverse studies ranging from an analysis of motor, sensory and autonomic outcomes associated with a compression model of spinal cord injury (Lonjon et al., 2009)to the induction and long-term consequences of status epilepticus(Langer et al., 2011). Of direct relevance to this study, rats of the same strain, but ordered from different suppliers, have been reported to differ markedly in their initial levels of anxiety-like behaviors(Rex et al., 1996)as well as voluntary ethanol drinking measures(Palm et al., 2011). Allelic variations that can arise when breeding outbred strains of rats may have contributed to some of the disparate findings noted above. Such factors are not likely relevant here as all cohorts were obtained from the same supplier. There are, however, many other differences related to housing conditions that may contribute to within-strain differences in experimental results such as those observed between in this study (e.g. diet, frequency of cage-cleaning and animal handling, density of animals/cage). Unfortunately, many of these factors are not readily available from suppliers and are often go unreported in publications. While we controlled for many of these factors, using a diet, cage-changing schedule and animal handling frequency similar to those employed by ourcommercial supplier, there were likely many additional variables that we were either unaware of, or could not readily control for. For example, while GH and STD rats were both group housed during adolescence (4-6/cage), there were extreme differences in the density of cages/room. GH subjects were housed in a relatively small vivarium (15 × 30 ft) that contained between 80-120 animals. In contrast, while at the commercial supplier, STD rats were housed in large, industrial barrier facilities that contained thousands of animals (Harlan Laboratories, personal communication). Our observations, that age-matched STD rats weighed significantly less than GH rats, and thatlarge and enduring differences in anxiety-like behavior and ethanol drinking measures were observed between these groups, suggest that early life housing conditions can profoundly influence physiological and behavioral outcomes in adulthood, at least for male, Long Evans rats.
Thus, the question of which cohort should be considered “normal” may really depend on the experimental questions being asked. For studies directed at characterizing the relationship between antecedent anxiety-like behavior and ethanol drinking, our findings suggest that commercially sourced adult rats may not be a suitable model, as the anxiety-like behavior of these animals on the elevated plus-maze wasvery similar to that of SI rats. In fact, the distribution of open arm times for both STD and SI subjects were clustered within a relatively narrow window, near the upper limit of anxiogenic behavior on the plus-maze. In contrast, animals purchased from a commercial supplier, immediately post-weaning, and then group housed during adolescence, exhibited a much broader range of anxiety-like responses on the plus-maze and may, thus, better reflect the spectrum of anxiety-like behaviors expected in “normal” LongEvans rats. In addition, a significant relationship between initial anxiety-like behavior and ethanol drinking, similar to that observed in humans (Chan et al., 2008; Kessler et al., 1997; Kushner et al., 2000), could only be detected in datasets that included GH subjects. Clearly, additional studies will be needed to compare the behavioral profile of STD rats with that of SI and GH animals across a broader range of anxiety-like measures. However, given that seemingly minor environmental factors can significantly influence at least some measures of anxiety-like behavior in rats, and the fact that commercial laboratory animal suppliers do not typically provide detailed information on the rearing conditions of their animals, it would seem that GH subjects may represent a preferable control group in studies examining the complex relationship between anxiety and ethanol drinking.
In summary, our data confirm prior findings that rats reared under conditions in which they are deprived of social contact during adolescence exhibit increased levels of anxiety-like behavior and novelty responding in adulthood, relative to animals raised in small groups during this period. Socially isolated rats also consume significantly more ethanol and obtain higher blood ethanol levels in home-cage voluntary drinking procedures. Our data further suggest that rats obtained from a commercial supplier as young adults and then housed under conditions commonly used in home-cage ethanol self-administration studies display an anxiety-like and ethanol drinking profile similar to that of rats that have been socially isolated during adolescence. Together, these findings provide further evidence that studies with juvenile group housed and socially isolated rats may provide a useful model with which to study behavioral and neurobiological substrates linking early life stress and vulnerability to alcoholism. These data also suggest that commercially sourced adult rats may not be suitable for studies examining the relationship between anxiety and ethanol drinking as these animals seem to share an anxiety-like behavioral profile similar to that of rats that have been exposed to chronic early life stress.
Supplementary Material
Figure 5.
Effect of rearing condition on intermittent ethanol self-administration. Graphs represent summaries of 30 min. and 24 hr. (daily) ethanol intake assessed on 12 consecutive drinking sessions conducted over a four week period. An overall effect of housing condition and drinking day, with no interaction between these variables, was observed for both 30 min. and 24 hr. intake.Post hoc analysis revealed that GH rats drank significantly less than SI (P<0.02) and STD rats (P<0.001) for both drinking measures. No differences in 30 min intake were observed between SI and STD rats but SI rats did exhibit signfiicantly lower daily ethanol intake than STD rats (P<0.04).
Acknowledgements
This work was supported by National Institutes of Health Grants AA 17056, AA 17531, and AA 10422.
References
- Anacker AM, Ryabinin AE. Biological contribution to social influences on alcohol drinking: evidence from animal models. Int J Environ Res Public Health. 2010;7:473–493. doi: 10.3390/ijerph7020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R, Pautassi RM, Mendez M, Quadros IM, Souza-Formigoni ML, Boerngen-Lacerda R. Behavioral and neurochemical studies in distinct animal models of ethanol’s motivational effects. Curr Drug Abuse Rev. 2010;3:205–221. doi: 10.2174/1874473711003040205. [DOI] [PubMed] [Google Scholar]
- Chan YF, Dennis ML, Funk RR. Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. J Subst Abuse Treat. 2008;34:14–24. doi: 10.1016/j.jsat.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Weiner JL. Relationship between ethanol’s acute locomotor effects and ethanol self-administration in male Long-Evans rats. Alcohol Clin Exp Res. 2008;32:2088–2099. doi: 10.1111/j.1530-0277.2008.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 2009;204:335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, Jr., Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcohol Clin Exp Res. 2007;31:1692–1698. doi: 10.1111/j.1530-0277.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–119. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ. Effects of early weaning and social isolation on subsequent alcohol intake in rats. Alcohol. 1997;14:175–180. doi: 10.1016/s0741-8329(96)00141-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Driscoll P, Gil L, Aguilar R, Tobena A, Escorihuela RM. Enduring effects of environmental enrichment on novelty seeking, saccharin and ethanol intake in two rat lines (RHA/Verh and RLA/Verh) differing in incentive-seeking behavior. Pharmacol Biochem Behav. 2002;73:225–231. doi: 10.1016/s0091-3057(02)00784-0. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998a;139:203–209. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998b;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing of rats on behavioural responses to food and environmental novelty. Physiol Behav. 1997;62:281–290. doi: 10.1016/s0031-9384(97)00115-7. [DOI] [PubMed] [Google Scholar]
- Hedges DW, Woon FL. Early-life stress and cognitive outcome. Psychopharmacology (Berl) 2011;214:121–130. doi: 10.1007/s00213-010-2090-6. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Henniger MS, Spanagel R, Wigger A, Landgraf R, Holter SM. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology. 2002;26:729–736. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, C B. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clinical Psychology Review. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Langer M, Brandt C, Loscher W. Marked strain and substrain differences in induction of status epilepticus and subsequent development of neurodegeneration, epilepsy, and behavioral alterations in rats strain and substrain differences in an epilepsy model in rats. Epilepsy Res. 2011;96:207–224. doi: 10.1016/j.eplepsyres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Mateo Y, Parker T, Marsden C. Effects of noradrenaline depletion in the brain on response on novelty in isolation-reared rats. Psychopharmacology (Berl) 2000;152:312–320. doi: 10.1007/s002130000534. [DOI] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. Region-specific induction of FosB/DeltaFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin Exp Res. 2010;34:1742–1750. doi: 10.1111/j.1530-0277.2010.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AL, Taylor DA, Malone DT. Isolation rearing in rats: effect on expression of synaptic, myelin and GABA-related immunoreactivity and its utility for drug screening via the subchronic parenteral route. Brain Res. 2011;1381:52–65. doi: 10.1016/j.brainres.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The effect of isolation rearing on volitional ethanol consumption and central CCK/dopamine systems in Fawn-Hooded rats. Behav Brain Res. 2003;141:113–122. doi: 10.1016/s0166-4328(02)00328-5. [DOI] [PubMed] [Google Scholar]
- Lonjon N, Prieto M, Haton H, Brochner CB, Bauchet L, Costalat V, Privat A, Gaviria M, Perrin FE. Minimum information about animal experiments: supplier is also important. J Neurosci Res. 2009;87:403–407. doi: 10.1002/jnr.21871. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S, Roman E, Nylander I. Differences in voluntary ethanol consumption in Wistar rats from five different suppliers. Alcohol. 2011;45:607–614. doi: 10.1016/j.alcohol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Rex A, Sondern U, Voigt JP, Franck S, Fink H. Strain differences in fear-motivated behavior of rats. Pharmacol Biochem Behav. 1996;54:107–111. doi: 10.1016/0091-3057(95)02128-0. [DOI] [PubMed] [Google Scholar]
- Robinet PM, Rowlett JK, Bardo MT. Individual differences in novelty-induced activity and the rewarding effects of novelty and amphetamine in rats. Behavioral Processes. 1998;44:1–9. doi: 10.1016/s0376-6357(98)00022-9. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Cortes C, Bettica A, Cortes F. Chronic ethanol consumption in rats produces residual increases in anxiety 4 months after withdrawal. Behav Brain Res. 2008;188:24–31. doi: 10.1016/j.bbr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.