Abstract
Background
An emerging endophenotype of schizophrenia is the reduction of both power and phase locking of the 40 Hz auditory steady state response (ASSR), and there have been a number of reports linking increased γ activity with positive psychotic symptoms. Schizophrenia and, more specifically, positive psychotic symptoms have been closely linked to increased dopamine (DA) neurophysiology. Therefore, we gave dexamphetamine to healthy participants to determine the effect that increased DA transmission would have on the ASSR.
Methods
We administered 0.45 mg/kg of dexamphetamine orally in a double-blind placebo-controlled crossover study. Stimuli were 20 Hz and 40 Hz click trains presented in an auditory oddball-type stimulus format (probability of stimulus presentation: 0.2 for targets, 0.8 for nontargets).
Results
We included 44 healthy volunteers (18 women) in the study. Dexamphetamine significantly increased the 40 Hz power for both target and nontarget ASSR stimuli. Dexamphetamine did not significantly affect the 40 Hz phase-locking factor (PLF) or the 20 Hz power and PLF. Whereas there were significant effects of selective attention on power and PLF for 20 and 40 Hz ASSR, there were no significant interactions between dexamphetamine and selective attention.
Limitations
Dexampheta-mine releases both noradrenaline and DA with equal potency. Further research with selective dopaminergic and noradrenergic agents will better characterize the effects of monoamines on γ activity.
Conclusion
The results demonstrate a frequency-specific effect of dexamphetamine on the ASSR. This finding is consistent with previous research that has found an association between increased γ and positive symptoms of psychosis. However, this result also raises the possibility that previous 40 Hz ASSR findings in people with schizophrenia may be confounded by effects of antipsychotic medication. Possible neural mechanisms by which dexamphetamine specifically increases 40 Hz power are also discussed.
Australian and New Zealand Clinical Trials Registry number
ACTRN12608000610336.
Introduction
An emerging endophenotype of schizophrenia is the reduction of both power and phase locking of the 40 Hz auditory steady state response (ASSR).1–7 It is not just people with schizophrenia who display 40 Hz ASSR reductions. First-degree relatives8 and people with schizoaffective disorder,2 bipolar disorder9 or early onset psychosis10 and people presenting at first hospital admission for affective disorders and schizophrenia4 also demonstrate similar deficits. These event-related potentials (ERPs) are generated by the entrainment of neural activity to the frequency of the driving stimulus. In humans, this response has a maximum in the γ frequency range at about 40 Hz,11,12 indicating an optimal resonance frequency for neurophysiological processes.
ASSR mechanisms, dopamine and schizophrenia
The ASSR has been sourced primarily in the primary auditory cortex, and 40 Hz stimulation frequencies also activate cerebellar structures that are not activated during other stimulation frequencies.12–14 The generation of γ oscillations has been modelled in vitro to be the result of a network of GABAergic interneurons interacting with glutamatergic pyramidal neurons.15,16 Glutamatergic and GABAergic systems have both been implicated in the pathophysiology of schizophrenia;17,18 therefore, it seems logical that researchers would posit links between γ deficits in schizophrenia with both glutamatergic and GABAergic neurophysiology.1,3,19,20
Whereas many studies have focused their reasoning of 40 Hz ASSR deficits in schizophrenia as deficits in GABA-ergic and/or glutamatergic neurons, the current prevailing model for the positive symptoms of psychosis is the dopamine (DA) hyperactivity model.21 This is largely for 4 reasons. First, current therapeutic agents for treating schizophrenia and other psychoses are all without exception DA D2 receptor antagonists or DA D2 receptor partial agonists. The DA D2-like receptor binding potencies of antipsychotics are highly correlated with average clinical doses,22 although whether this relationship is owing to antipsychotic or adverse effects (e.g., extrapyramidal effects) remains unclear. Second, drugs that either increase DA transmission or act directly on DA receptors (e.g., amphetamine and apomorphine, respectively) can induce psychosis with paranoid features in healthy volunteers or in patients with Parkinson disease with no history of schizophrenia.23,24 Third, psychosis is precipitated by lower doses of stimulants in patients with schizophrenia than those required for otherwise healthy people.25 Fourth, patients with schizophrenia have higher basal levels of DA26 and show greater amphetamine-induced DA release than controls.26
Dopamine, glutamate and γ-aminobutyric acid (GABA) hypotheses are not mutually exclusive. Indeed, the primary action of DA in both the mesocorticolimbic and nigrostriatal pathways is a modulator of GABA efferent neurons that are primarily driven by glutamate afferents. Dopamine also appears to play a role in modulating the glutamate release at those GABAergic neurons. Dopamine and glutamate are coreleased from some neurons,27 DA receptors are found on glutamate and GABA neurons,28 and glutamate neurons innervate DA cells.29 As such, it is likely that all 3 systems play a role in schizophrenia and psychosis, but dopaminergic systems are far more localized compared with the ubiquity of glutamatergic and GABAergic neurons. This makes targeting the DA system for therapeutics a useful approach not just for the treatment of schizophrenia,29 but also for studying mechanisms underlying psychosis.
Dopamine manipulation has also been found to affect γ activity. Preclinical studies have generally found that increased DA activity increased γ power,30 and reduced DA activity decreased power.31 This generality has also been found in human participants. Indirect increases in extracellular DA concentrations from genetic variability in DA transporter genes is associated with an increase in γ activity to target/attended stimuli.32
ASSR and attentional manipulations
It appears that DA modulation of γ activity in humans may be dependent on attention. Haloperidol administration in humans reduced γ amplitude to attended stimuli but not unattended stimuli.33 Initial attempts at modulating the ASSR with attention were unsuccessful, and concluding comments by Linden and colleagues34 remark that there is no evidence that the ASSR is affected by attention. It is not completely clear why this series of experiments failed to find an effect, but it may be owing to small sample size and the use of a more stringent α level than 0.05. More recent experiments have found attention manipulations to be successful in modulating the ASSR.35 In an auditory oddball-type paradigm, power and phase-locking factor (PLF) were larger at 40 Hz targets than 40 Hz frequent stimuli, but no differences in signal power or PLF were observed during 20 Hz stimulation.35 Interestingly, the P3 wave elicited from auditory odd-ball experiments, an indicator of attention and working memory,36 has been one of the most robust deficits found in patients with schizophrenia and psychosis more generally.37 We have recently shown that dexamphetamine, a DA, nor-adrenaline and, to a much lesser extent, serotonin releaser can similarly reduce the auditory P3 in healthy participants.38
As amphetamine administration can induce both the symptoms of psychosis and auditory ERP deficits, we investigated whether an increase in monoaminergic transmission would induce an ASSR deficit like that seen in people with schizophrenia or whether it would result in an attention-dependent increase of 40 Hz activity like the 40 Hz findings by Demiralp and colleagues32 and Ahveninen and colleagues.33 Therefore, we administered 0.45 mg/kg of the monoamine releasing agent dexamphetamine or placebo to healthy participants to determine the effect of increased monoamines on the 20 Hz and 40 Hz ASSR and whether these effects are modulated by selective attention.
Methods
Participants
We recruited healthy participants between the ages of 19 and 48 years mostly from within the School of Medicine and Pharmacology at the University of Western Australia. Participants were asked to abstain from alcohol for 24 hours before testing and from other recreational drugs for 7 days. Nicotine was permitted ad libitum to prevent withdrawal effects. Exclusion criteria were any self-reported psychiatric diagnosis, any cardiovascular disorder, epilepsy, a first-degree relative with schizophrenia and known hypersensitivity to amphetamines. Participants were also excluded if they were on any medication other than the contraceptive pill. After giving informed consent, all participants underwent a medical examination and were screened by the study psychiatrists (J.L. and R.I.) using an informal mental state exam covering appearance, speech, thought content, cognition and risk. Participants were asked to eat a light breakfast (not standardized) before coming in for testing. Reimbursement for time was $50 per day of participation in the study.
Procedure
The experiment was a double-blind, placebo-controlled, crossover trial of dexamphetamine sulfate administered orally (0.45 mg/kg). This dose was selected because it is at the upper end of amphetamine dosages used in human experiments while still being safe and ethical. Participants came into the centre for 2 testing sessions 1 week apart. Participants who received placebo or dexamphetamine at the first session received the opposite at the second session. Blood pressure and heart rate were measured with an Omron automatic sphygmomanometer at 0, 75, 130, 180 and 270 minutes postdose. Testing the ASSR began at 240 minutes postdose. The University of Western Australia Human Ethics Committee and the North Metropolitan Area Mental Health Services Human Research Ethics Committee approved our study protocol. The Australian and New Zealand Clinical Trials Registry number is ACTRN12608000610336.
Task
Participants sat upright at about 60 cm from a computer monitor and were asked to focus on a crosshairs on the monitor to minimize eye movements. Stimuli were 20 Hz and 40 Hz click trains of 500 ms duration with an inter-stimulus interval of 500 ms. Click trains were presented at 80 dB, and each individual click consisted of 1 ms of white noise with instantaneous rise–fall time. Stimuli were presented in an auditory oddball-type stimulus format (similar to that used by Skosnik and colleagues35), and the probability of stimulus presentation was 0.2 for targets and 0.8 for nontargets (i.e., within each set of 10 stimulus presentations, 2 target stimuli would be presented randomly among 8 standard stimuli). There were 2 blocks of 200 click trains in total, and within each block there were 40 target stimuli and 160 nontarget stimuli. Blocks were counterbalanced such that half the participants received 20 Hz click trains as target stimuli first with 40 Hz stimuli as standards and the other half received 40 Hz click trains as target stimuli first with 20 Hz stimuli as standards. To ensure that participants were attending to the sounds and counting the correct stimuli, we asked them to count in their heads the number of target click trains that occurred.
Recording and processing
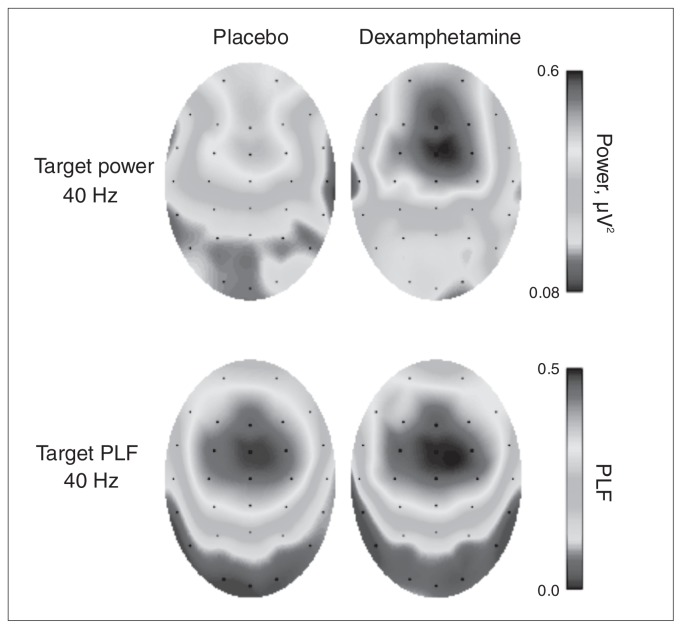
The electroencephalography scan was recorded with a Neuroscan 32-channel system according to the extended 10–20 system using an Ag/AgCl electrode cap that included vertical and horizontal electro-oculograms for artifact rejection. The reference was linked earlobes, and ground was at AFz. Impedance for all channels was kept below 6 k. The hardware bandpass filter was set between 0.05 Hz and 200 Hz, and the sampling rate was 1000 Hz. Electroencephalography data were treated for artifacts in Neuroscan version 4.3. This included automatic electro-oculogram artifact correction,39 artifact rejection (individual epochs that exceeded ± 90 μV) and further filtering (bandpass filtered between 14 Hz and 60 Hz to encompass the main frequencies of interest, 20 Hz and 40 Hz). The mean number of epochs was similar across drug conditions. For rare stimuli under placebo, the mean number of epochs included was 39.4 and for dexamphetamine was 39.3; for standard stimuli the mean number of epochs included was 157.2 for placebo and 157.8 for dexamphetamine; 40 Hz ASSR amplitude was largest at the midline electrode FCz (Fig. 1), therefore, this electrode was used for all analysis and figures.
Fig. 1.
Topographic maps of power (top) and phase-locking factor (PLF; bottom) during the 40 Hz target stimuli when participants were under placebo (left) and dexamphetamine (right). Both power and PLF values were averaged over the period between 100 and 500 ms poststimulus onset.
The 40 Hz ASSR was analyzed by convolution with complex Morlet wavelets (for example, see Tallon-Baudry and colleagues40). The wavelet family ratio f/σf was equal to 7. The energy normalization factor A was equal to σt−1* (2/π)1/2 to correspond with the amplitude of the ERP. For evoked power analysis, epochs were averaged from 300 ms prestimulus onset to 800 ms poststimulus onset and baseline corrected over the whole epoch. The averaged ERP then underwent wavelet transformation, and power was calculated by squaring the absolute value of the convolution to give a time–frequency power map. For the phase-locking analysis, each individual epoch underwent Morlet wavelet convolution. This was then unit normalized and averaged to indicate phase stability (range 0, non–phase locked, to 1, completely phase locked) over time and frequency. We averaged PLF and power values over 100 ms bins between 0 ms and 600 ms. We did not perform further baseline correction, as both power and PLF at FCz were close to 0 during the prestimulus period.
To determine whether unequal trial numbers for target versus standard stimuli would lead to differences in the signal-to-noise ratio between stimulus types and, therefore, possibly confound interpretations drawn from the analysis of stimulus type, we randomly resampled 40 standard trials 5000 times to give a normal distribution of power and PLF values (for example, see Skosnik and colleagues35). The average value of this distribution for each person was then compared with that participant’s fully sampled average. Just as in the study by Skosnik and colleagues,35 there was a high correlation between resampled and fully sampled values for power and PLF (r > 0.999, p < 0.001). When we entered the resampled values into the repeated-measures analysis of variance (ANOVA), there were no appreciable differences in the results (e.g., the F values for the main effect of drug for 40 Hz power at 40 Hz stimulation for fully sampled epochs were 11.46 and 11.41, respectively, for the resampled epochs). Therefore, we report only the fully sampled results.
Statistical analysis
For power and PLF analysis, a separate ANOVA was conducted for the 20 Hz and 40 Hz response to 20 Hz and 40 Hz stimulating frequencies as well as the 40 Hz harmonic to the 20 Hz stimulating frequency. The 40 Hz harmonic response to 20 Hz stimuli has been shown to be a relatively robust response to 20 Hz stimulation frequencies and correlates with hallucination history.4 Factor groupings for the ANOVA were drug order (placebo first and dexamphetamine first) × drug (dexamphetamine and placebo) × stimulus type (standard and target) × time (100 ms bins: 1–100, 101–200, 201–300, 301–400, 401–500 and 501–600 ms). Drug order was treated as a between-subject factor, all others were treated as within-subject factors.
All statistical analyses, figures and wavelet processing were performed using R version 2.13.1 with the “ez” and “car” packages for repeated-measure ANOVAs,41,42 and the “fields” and “plotrix” packages for figures.43,44 Repeated-measure ANOVAs were checked for violations of sphericity using the Mauchly test. Where significant departures occurred, we performed Greenhouse–Geisser corrections. Corrections for multiple comparisons were done using the Holm–Bonferroni method. The effect size reported is generalized eta squared, η2G. The effects of sex, smoking status and previous amphetamine use on the ASSR were analyzed as possible confounding variables. These analyses yielded no significant results and are not presented.
Results
Participants
We included 44 healthy participants aged 19 to 48 (mean 23.8) years in our study. Their demographic characteristics are summarized in Table 1. The mean participant weight was 75 kg, which gave an average dose of dexamphetamine sulfate of 33.7 mg. Twenty-three participants received placebo in the first session and dexamphetamine in the second session; the remaining 21 participants received placebo and dexamphetamine in the opposite order.
Table 1.
Participant demographic characteristics
| Characteristic | No. (%)* |
|---|---|
| Sex, female:male | 18:26 (41:59) |
| Drug order, pla–dex:dex–pla | 23:21 (52:48) |
| Smoke tobacco, yes:no | 7:37 (16:84) |
| Ever used amphetamines, yes:no | 23:21 (52:48) |
| Age, mean (range) yr | 23.8 (19–48) |
| Education, mean (range) yr | 14.4 (12–20) |
| Weight, mean (range) kg | 75.1 (50–107) |
dex = dexamphetamine; pla = placebo.
Unless otherwise indicated.
Autonomic measures
Systolic blood pressure, diastolic blood pressure and heart rate were analyzed using paired t tests before and after ASSR testing (180 and 270 min postdose) to show that dexamphetamine was still active at these times. Dexamphetamine significantly increased all 3 responses at both the 180 and 270 minute time points (all p < 0.001).
Effects of dexamphetamine on the ASSR
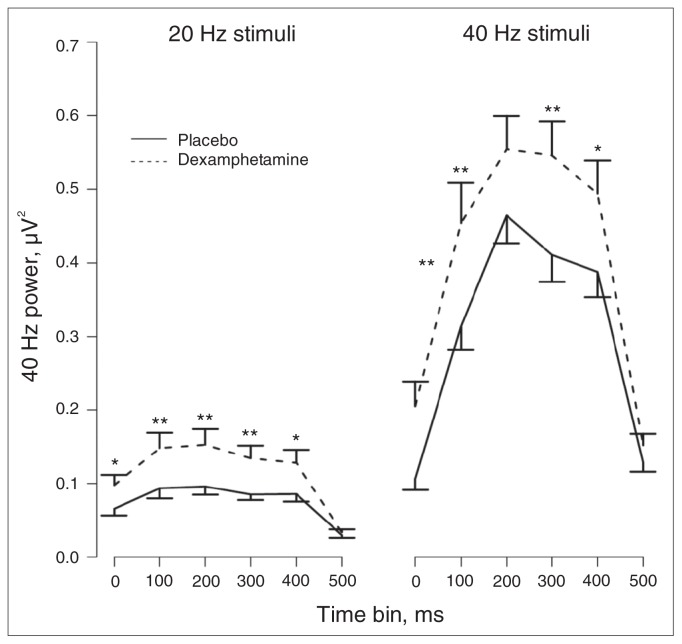
The time–frequency plots of ASSR power and PLF for each drug × stimulus type × stimulation frequency condition are presented in Appendix 1 (Figs. S1 and S2, available online at www.cma.ca/jpn). The figures show robust entrainment effects during stimulation periods and a predominant 40 Hz component for all conditions. Table 2 presents the ANOVA results for 40 Hz power and PLF for the main effect of drug, the interactions between drug and stimulus type and between drug and time, and the 3-way interaction between drug, stimulus type and time for 20 Hz and 40 Hz stimuli. As can be seen in Table 2, for both 20 Hz and 40 Hz stimuli there were significant main effects of drug and significant drug × time interactions for 40 Hz power. The main effects indicated a general increase in 40 Hz power by dexamphetamine, whereas the interactions indicate that this effect varied over time. Figure 2 illustrates the drug × time interactions for 40 Hz power. Dexamphetamine increased 40 Hz power at all time bins for the 40 Hz stimuli except for the 201–300 ms and the 501–600 ms time bins. A similar effect was shown for the 20 Hz stimuli, where 40 Hz power for all but the last time bin (501–600 ms) were significantly increased by dexamphetamine. There were no effects of dexamphetamine on 40 Hz PLF (Table 2).
Table 2.
Dexamphetamine effects on 20 Hz and 40 Hz power and phase-locking factor to 20 Hz and 40 Hz stimuli and the 40 Hz harmonic to 20 Hz stimuli
| Auditory response; comparison | 20 Hz stimuli | 40 Hz stimuli | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| F | p value | η2G | F | p value | η2G | |
| 20 Hz power | ||||||
| Drug, F1,42 | 0.87 | 0.36 | 0.002 | |||
| Drug × type, F1,42 | 0.13 | 0.72 | 0.001 | |||
| Drug × time, F5,210 | 0.52 | 0.76 | 0.001 | |||
| Drug × type × time, F5,210 | 0.89 | 0.49 | 0.002 | |||
| 20 Hz PLF | ||||||
| Drug, F1,42 | 0.00 | 0.96 | 0.000 | |||
| Drug ×type, F1,42 | 0.18 | 0.68 | 0.000 | |||
| Drug × time, F5,210 | 1.79 | 0.14 | 0.004 | |||
| Drug × type × time, F5,210 | 0.88 | 0.47 | 0.002 | |||
| 40 Hz power | ||||||
| Drug, F1,42 | 11.97 | 0.001 | 0.039* | 11.43 | 0.002 | 0.037* |
| Drug × type, F1,42 | 1.32 | 0.26 | 0.002 | 2.77 | 0.10 | 0.002 |
| Drug × time, F5,210 | 6.06 | < 0.001 | 0.008† | 4.14 | 0.006 | 0.006* |
| Drug × type × time, F5,210 | 0.56 | 0.66 | 0.001 | 1.08 | 0.36 | 0.001 |
| 40 Hz PLF | ||||||
| Drug, F1,42 | 1.44 | 0.24 | 0.004 | 0.97 | 0.33 | 0.003 |
| Drug × type, F1,42 | 1.91 | 0.17 | 0.002 | 3.34 | 0.07 | 0.001 |
| Drug × time, F5,210 | 1.84 | 0.11 | 0.003 | 1.98 | 0.11 | 0.003 |
| Drug × type × time, F5,210 | 0.38 | 0.86 | 0.000 | 0.55 | 0.69 | 0.001 |
PLF = phase-locking factor.
p < 0.01.
p < 0.001.
Fig. 2.
The effects of dexamphetamine on 40 Hz power over time at electrode FCz. Both standard and rare stimuli are pooled in the analysis as there was no drug × stimulus type interaction. Displayed are the means and standard error of the mean. Dexamphetamine significantly increased 40 Hz power for both stimulation frequencies for all time bins except for the last 50–600 ms time bin. Holm-corrected p values: *p < 0.05, **p < 0.01; n = 44.
There was no effect of dexamphetamine on 20 Hz power or PLF for 20 Hz stimuli. There were no interactions between drug and stimulus type or between drug and time, nor was there a 3-way interaction between drug, stimulus type and time (Table 2). To determine whether there was a differential effect of dexamphetamine on 20 Hz versus 40 Hz power and to provide a more direct comparison of this effect, we conducted a drug (placebo, dexamphetamine) × frequency response (20 Hz response to 20 Hz stimuli, 40 Hz response to 40 Hz stimuli) repeated-measures ANOVA on the power measures for these frequencies. There was a significant drug × frequency response interaction (F1,42 = 8.23, p = 0.006, η2G = 0.009), indicating that dexamphetamine significantly increased 40 Hz power but not 20 Hz power.
The relationship between power and PLF was examined by averaging across stimulus types and over the time period between 100 ms and 500 ms and entered into correlational analysis. There were significant correlations between power and PLF for the 40 Hz response during 20 Hz and 40 Hz stimulation under placebo (20 Hz stimulation, r = 0.68, 95% confidence interval [CI] 0.48–0.81; 40 Hz stimulation, r = 0.675, 95% CI 0.47–0.81) and dexamphetamine (20 Hz stimulation, r = 0.59, 95% CI 0.35–0.75; 40 Hz stimulation, r = 0.68, 95% CI 0.34–0.75). For the 20 Hz response during 20 Hz stimulation there were also significant correlations between power and PLF under placebo (r = 0.66, 95% CI 0.47–0.81) and dexamphetamine (r = 0.76, 95% CI 0.60–0.86). Furthermore, as indicated by the 95% CIs, dexamphetamine did not significantly alter this relationship.
Effects of stimulus type on the ASSR
Table 3 presents the ANOVA results for the main effect of stimulus type and the interaction between stimulus type and time for 20 Hz and 40 Hz power and PLF for both stimulation frequencies. As seen in Table 3, there was a significant interaction between stimulus type and time for 40 Hz power and PLF at 40 Hz stimuli. The largest increases in PLF and power by target stimuli were during the 301–400 ms and 401–500 ms range, with lesser effects surrounding these times (Appendix 1, Figure S3). Target stimuli increased 20 Hz and 40 Hz PLF and power for both the 20 Hz and 40 Hz stimulation frequencies (Table 3; Appendix 1, Figs. S1 and S2).
Table 3.
The effects of stimulus type on the 20 Hz and 40 Hz auditory steady state response
| Auditory response; comparison | 20 Hz stimuli | 40 Hz stimuli | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| F | p value | η2G | F | p value | η2G | |
| 20 Hz power | ||||||
| Type, F1,42 | 21.87 | < 0.001 | 0.042* | |||
| Type × time, F5,210 | 0.52 | 0.63 | 0.001 | |||
| 20 Hz PLF | ||||||
| Type, F1,42 | 42.14 | < 0.001 | 0.101* | |||
| Type × time, F5,210 | 0.23 | 0.91 | 0.001 | |||
| 40 Hz power | ||||||
| Type, F1,42 | 9.42 | 0.004 | 0.005† | 28.83 | < 0.001 | 0.025* |
| Type × time, F5,210 | 0.31 | 0.87 | 0.000 | 11.54 | < 0.001 | 0.010* |
| 40 Hz PLF | ||||||
| Type, F1,42 | 28.66 | < 0.001 | 0.030* | 32.01 | < 0.001 | 0.027* |
| Type × time, F5,210 | 1.86 | 0.12 | 0.003 | 8.09 | < 0.001 | 0.009* |
PLF = phase-locking factor.
p < 0.001.
p < 0.01.
Discussion
Dexamphetamine administration increased 40 Hz power to target and nontarget stimuli to both 20 Hz and 40 Hz stimulation rates. Despite this, we found no difference between dexamphetamine and placebo on 40 Hz PLF. This contrasts the 40 Hz ASSR findings in patients with schizophrenia who consistently display reduced 40 Hz power and reduced 40 Hz PLF (for example, see Kwon and colleagues1 as well as other studies cited in our introduction). Therefore acute increases in monoamine (including DA) transmission are not likely to be a direct cause of the 40 Hz ASSR deficit in individuals with schizophrenia. However, there may be a link between DA, the 40 Hz ASSR and the positive symptoms of schizophrenia. Nested within the reduced 40 Hz ASSR in individuals with schizophrenia, an increase in 40 Hz ASSR activity has been associated with increased positive symptoms, particularly auditory hallucinations.4,5
It should be noted that the relationship between positive symptoms and ASSR γ findings reported by Spencer and colleagues4,5 has been driven by the PLF. However, as they note in their discussions, the correlation between γ PLF and hallucinations was found on a measure reflecting the lifetime history of hallucinations in patients who were not hallucinating during the study. Actively hallucinating patients have demonstrated high amplitude β/γ oscillations in the appropriate sensory cortex,45,46 and there have been other studies indicating positive correlations between γ and positive symptoms.47,48 The contrast between the finding by Spencer and colleagues5 of a correlation between γ PLF and history of hallucinations and those from studies that reported increased amplitude in patients actively experiencing hallucinations may suggest that the γ PLF reflects a proneness to hallucinatory experiences when there is ineffective dopaminergic antagonism to dampen γ oscillations.5 It may be that increased DA transmission increases the excitability of neurons responsible for high-frequency oscillations in the primary auditory cortex, the major ASSR generator. When this is coupled with other neurophysiological deficits that are evident in schizophrenia, such as alterations in GABAergic and glutamatergic transmission leading to deficits in neural timing processes, as indexed by PLF reductions in schizophrenia, it could lead to increased susceptibility to hallucinations. Our participants were healthy, nonpsychotic, nonpsychiatric participants. Therefore, the underlying deficit that may cause PLF disruptions was not there. However, we found increased power after dexamphetamine administration, indicating that modulations of DA activity can significantly affect high-frequency power.
Our finding of increased 40 Hz activity induced by dexamphetamine also raises the possibility that the deficit seen in individuals with schizophrenia may be an effect of medication. All antipsychotics are DA D2 antagonists or partial agonists that can lead to cell loss,49 possibly impairing the neurocircuitry for generating γ oscillations. At therapeutic doses for psychosis, these drugs have functionally opposite effects to the DA transporter reverser dexamphetamine. Evidence that the deficit may not be due to medication effects is the finding of similar 40 Hz ASSR reductions in nonmedicated patients3 and in relatives of people with schizophrenia.8 There are confounding issues in the inferences drawn from these studies involving nonmedicated patients. The first is an issue of power; the number of nonmedicated patients recruited for these studies is small, with the largest sample so far being 9 unmedicated patients.3 The second is that these patients may be from a different population than chronically medicated patients and, therefore, are not representative of individuals with schizophrenia as a whole.
It is unknown how neural adaptations to chronic medication in patients compound the 40 Hz ASSR findings. Studies that have examined medication effects have found inconsistent results. Hong and colleagues8 found increased 40 Hz ASSR with atypical antipsychotics, notably clozapine. However, the initial study by Kwon and colleagues,1 and a much larger study with 100 participants conducted by Light and colleagues,3 found no differences between medication groups. Hong and colleagues8 suggested that the increased 40 Hz ASSR from atypical antipsychotics may be because the patients in the study were on clozapine, which can stimulate glutamate and DA release. As shown in the present study, an increase in DA release can lead to an increase in the strength of the 40 Hz ASSR, possibly explaining the results with clozapine compared with other antipsychotic drugs.
Frequency-specific effect of dexamphetamine
Dexamphetamine significantly increased 40 Hz power but not 20 Hz power and did not affect the PLF for either frequency. The increase in 40 Hz power occurred despite there being no significant difference between placebo and dexamphetamine on the relationship between PLF and power. However, only 46% of the variance was common between PLF and power after dexamphetamine treatment, so the correlation is not so high that there cannot be independent factors affecting the 2 measures differently. An increase in power of the 40 Hz ASSR without a concomitant increase in PLF or 20 Hz power suggests that dexamphetamine is increasing the 40 Hz signal in a frequency-dependent manner by recruiting more neurons to fire synchronously. There is direct innervation of the primary auditory cortex by monoamine neurons.50 Further, an increase in synchronization between these neurons has been demonstrated when the primary auditory cortex is activated via the ventral tegmental area, the region of the cell bodies of the major DA projections to cortical structures.51 Possible mechanisms behind the frequency-specific effect comes from in vitro work in hippocampal cells (CA1) by Ito and Schuman.52 The authors demonstrate a frequency-dependent role of DA on pyramidal neuron efficiency. During low-frequency stimulation, DA depressed excitatory inputs onto pyramidal neurons and GABAergic interneurons via presynaptic inhibition. During high-frequency stimulation, on the other hand, DA facilitated the steady state current that was blocked by GABAA and GABAB receptor antagonists, suggesting that DA facilitation of excitatory drive depends on disinhibition of neurons.
Dexamphetamine and selective attention
We replicated the main effects of selective attention on the 40 Hz ASSR as described by Skosnik and colleagues.35 This included enhanced power and phase locking for target 40 Hz stimuli compared with standard stimuli. However, Skosnik and colleagues found no effect of stimulus type on power or PLF for the 20 Hz ASSR, whereas we did. This may be due to increased power in our study gained from a larger sample size (present study, n = 44 v. Skosnik and colleagues, n = 15). Therefore, in addition to the effects of selective attention on the 40 Hz ASSR found by Skosnik and colleagues, selective attention also enhances 20 Hz ASSR power and PLF given sufficient power to detect the effect.
As mentioned by Krishnan and colleagues,6 most studies on the ASSR in individuals with schizophrenia have not controlled for effects of attention. Krishnan and colleagues replicated the ASSR deficit in individuals with schizophrenia while participants directed their attention toward the visual modality (i.e., while ignoring the ASSR stimuli). This suggests that the ASSR reduction in schizophrenia is unlikely to be due to the well-known deficits in attention.6 This is contrasted by some of the previous research that has suggested attention-dependent effects of DA on γ.32,33 This research suggests that the enhancement of the 40 Hz ASSR by dexamphetamine may be through increased attention to the stimuli. In support, amphetamine administration can increase selective attention and is an effective pharmacotherapy for attention-deficit/hyperactivity disorder.53 However, there was little evidence that dexamphetamine selectively influenced the target stimulus, as would be expected if the drug was enhancing selective attention. Whereas the lack of a significant drug × attention interaction in the present study may be due to insufficient power, the effect sizes were very small (PLF η2G = 0.001; power η2G = 0.002). A larger dose of dexamphetamine may prove necessary to establish a selective attention effect on this task.
Previous research has suggested that ASSR deficits may be due to effects on arousal.54 The amplitude and phase of the ASSR was demonstrated to be larger during a low arousal state than a high arousal state.54 The authors assumed that the attention modulation in their study was sparse owing to the study design and the instruction to participants not to pay attention to the stimulation. Despite this, the study failed to appropriately control for attention. In the high arousal condition, participants read a book while sitting up; this is an active attention control away from the stimulus. In the low arousal condition, participants reclined with their eyes closed and without a distractor; this is an inactive attention control, likely having the effect of making the auditory stimuli more salient and therefore less easy to ignore. A similar design (playing a visual electronic game v. listening to repetitive clicks) found effects attributed to attention that were later confirmed to be largely attentional in a more balanced attention condition.55 As there are strong effects of attention on the ASSR, it seems more likely that attention differences accounted for the differences in arousal state in their study. Further, a primary effect of dexamphetamine is to increase arousal, yet dexamphetamine administration increased the 40 Hz ASSR. This is not congruent with the proposed effect of arousal on the ASSR. Finally, it is not entirely clear that a unitary concept of arousal has much real utility.56
Limitations
There are 3 factors that limit correlation between the findings in the present study and those in studies of individuals with schizophrenia. First, dexamphetamine is equally effective at increasing synaptic concentrations of noradrenaline (compared with DA) and, therefore, may be a possible alternative for the effects of dexamphetamine on 40 Hz power. Noradrenaline has been shown to increase57 and decrease58 γ activity. Separating the actions of DA and noradrenaline is difficult given that they have some affinity for each other’s receptors and can be physiologically effective on each other’s receptors. While not conclusive, we have pointed out the evidence that DA is responsible for the effects on 40 Hz power previously. Briefly, ventral tegmental area activation has been shown to increase primary auditory cortical synchronization,51 DA has demonstrated a frequency-dependent role on pyramidal neurons,52 and there is the link between positive psychotic symptoms and γ activity.4,5 More pharmacological studies targeting specific DA or noradrenaline post-synaptic receptors would better elucidate the effects of these monoamines on γ activity.
Second, the manipulations in the current experiment reflect acute changes in monoamine transmission rather than the chronic and periodic DA hyperactivity associated with schizophrenia.
Third, our participants did not display or report any psychotic-like symptoms, nor did we assess these symptoms directly. However, we have shown increased illusory experiences on the rubber hand illusion in a subset of participants enrolled in the present study.59 This illusion is reported to be increased in people with schizophrenia and has been shown to correlate with positive symptoms.60 Future experiments using pharmacological models of psychosis would benefit from the administration of an instrument measuring psychosis or psychosis-like experience.
Conclusion
Dexamphetamine selectively increased the 40 Hz power for both target and nontarget ASSR stimuli but did not affect 40 Hz PLF or 20 Hz power and PLF. Further, whereas we found main effects of selective attention for both the 20 Hz and 40 Hz ASSR, there were no significant drug × stimulus type interactions. This suggests a frequency-specific effect of dexamphetamine on the 40 Hz ASSR that may be related to the in vitro findings by Ito and Schuman.52 Specifically, dexamphetamine may be increasing 40 Hz ASSR power via increasing firing efficiency of excitatory pyramidal neurons by a GABA-dependent dampening of the inhibition of the pyramidal neurons. These findings may also have clinical relevance with respect to effects of antipsychotic medications on the ASSR and the relationship between DA, positive psychotic symptoms and 40 Hz ASSR as reported by Spencer and colleagues.4,5
Acknowledgements
M.A. Albrecht is the recipient of a Clinical Neurophysiology supplementary scholarship from the Department of Neurophysiology, North Metropolitan Area Health Service —Mental Health and the School of Medicine and Pharmacology of the University of Western Australia. This research was funded by a National Health and Medical Research Council grant (ID: 254619) awarded to M.T. Martin-Iverson.
Footnotes
Competing interests: As above for M.A. Albrecht and M.T. Martin-Iverson. None declared for G. Price, J. Lee and R. Iyyalol.
Contributors: All authors helped design the article, acquired the data, reviewed the article and approved its publication. M.A. Albrecht, G. Price and M.T. Martin-Iverson analyzed the data. M.A. Albrecht and M.T. Martin-Iverson wrote the article.
References
- 1.Kwon JS, O’Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–5. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner CA, Sporns O, Lysaker PH, et al. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–40. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- 3.Light GA, Hsu JL, Hsieh MH, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–40. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Spencer KM, Salisbury DF, Shenton ME, et al. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–75. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer KM, Niznikiewicz M, Nestor P, et al. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan GP, Hetrick WP, Brenner CA, et al. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–9. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maharajh K, Teale P, Rojas DC, et al. Fluctuation of gamma-band phase synchronization within the auditory cortex in schizophrenia. Clin Neurophysiol. 2010;121:542–8. doi: 10.1016/j.clinph.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong LE, Summerfelt A, McMahon R, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell BF, Hetrick WP, Vohs JL, et al. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport. 2004;15:1369–72. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- 10.Wilson TW, Hernandez OO, Asherin RM, et al. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18:371. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galambos R, Makeig S, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981;78:2643–7. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastor MA, Artieda J, Arbizu J, et al. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22:10501–6. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mäkelä JP, Hari R. Evidence for cortical origin of the 40 Hz auditory evoked response in man. Electroencephalogr Clin Neurophysiol. 1987;66:539–46. doi: 10.1016/0013-4694(87)90101-5. [DOI] [PubMed] [Google Scholar]
- 14.Herdman AT, Lins O, Van Roon P, et al. Intracerebral sources of human auditory steady-state responses. Brain Topogr. 2002;15:69–86. doi: 10.1023/a:1021470822922. [DOI] [PubMed] [Google Scholar]
- 15.Traub RD, Whittington MA, Stanford IM, et al. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–4. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- 16.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 17.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. discussion 82–137. [DOI] [PubMed] [Google Scholar]
- 18.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 19.Woo TU, Spencer KM, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–89. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–44. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III — the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–9. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 23.Angrist BM, Gershon S. The phenomenology of experimentally induced amphetamine psychosis. Biol Psychiatry. 1970;2:95–107. [PubMed] [Google Scholar]
- 24.Frankel JP, Lees AJ, Kempster PA, et al. Subcutaneous apomorphine in the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1990;53:96–101. doi: 10.1136/jnnp.53.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman JA, Kane JM, Alvir J. Provocative tests with psycho-stimulant drugs in schizophrenia. Psychopharmacology (Berl) 1987;91:415–33. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- 26.Abi-Dargham A, van de Giessen E, Slifstein M, et al. Baseline and amphetamine-stimulated dopamine activity are related in drug-naïve schizophrenic subjects. Biol Psychiatry. 2009;65:1091–3. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Trudeau LE. Glutamate co-transmission as an emerging concept in monoamine neuron function. J Psychiatry Neurosci. 2004;29:296–310. [PMC free article] [PubMed] [Google Scholar]
- 28.Muly EC, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci. 1998;18:10553–65. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sesack SR, Carr DB, Omelchenko N, et al. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 30.Brown P, Kupsch A, Magill PJ, et al. Oscillatory local field potentials recorded from the subthalamic nucleus of the alert rat. Exp Neurol. 2002;177:581–5. doi: 10.1006/exnr.2002.7984. [DOI] [PubMed] [Google Scholar]
- 31.Lee K-M, Ahn T-B, Jeon BS, et al. Change in phase synchronization of local field potentials in anesthetized rats after chronic dopamine depletion. Neurosci Res. 2004;49:179–84. doi: 10.1016/j.neures.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Demiralp T, Herrmann CS, Erdal ME, et al. DRD4 and DAT1 polymorphisms modulate human gamma band responses. Cereb Cortex. 2007;17:1007–19. doi: 10.1093/cercor/bhl011. [DOI] [PubMed] [Google Scholar]
- 33.Ahveninen J, Kahkonen S, Tiitinen H, et al. Suppression of transient 40-Hz auditory response by haloperidol suggests modulation of human selective attention by dopamine D2 receptors. Neurosci Lett. 2000;292:29–32. doi: 10.1016/s0304-3940(00)01429-4. [DOI] [PubMed] [Google Scholar]
- 34.Linden RD, Picton TW, Hamel G, et al. Human auditory steady-state evoked potentials during selective attention. Electroencephalogr Clin Neurophysiol. 1987;66:145–59. doi: 10.1016/0013-4694(87)90184-2. [DOI] [PubMed] [Google Scholar]
- 35.Skosnik PD, Krishnan GP, O’Donnell BF. The effect of selective attention on the gamma-band auditory steady-state response. Neurosci Lett. 2007;420:223–8. doi: 10.1016/j.neulet.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 36.Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–77. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- 37.Bramon E, McDonald C, Croft RJ, et al. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27:960–8. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Albrecht MA, Martin-Iverson MT, Price G, et al. Dexamphetamine-induced reduction of P3a and P3b in healthy participants. J Psychopharmacol. 2011;25:1623–31. doi: 10.1177/0269881110376686. [DOI] [PubMed] [Google Scholar]
- 39.Semlitsch HV, Anderer P, Schuster P, et al. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 40.Tallon-Baudry C, Bertrand O, Delpuech C, et al. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–9. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox J, Weisberg S. An R companion to applied regression. 2nd ed. Thousand Oaks (CA): Sage; 2011. [accessed 2012 Apr. 26]. Available: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. [Google Scholar]
- 42.Lawrence MA. ez: Easy analysis and visualization of factorial experiments. 2011. [accessed 2010 July 22]. Available: http://CRAN.R-project.org/package=ez.
- 43.Furrer R, Nychka D, Sain S.fields: Tools for spatial data 2011Available: http://CRAN.R-project.org/package=fieldssaccessed 2012 Apr. 26
- 44.Lemon J. Plotrix: a package in the red light district of R. R-News. 2006;64:8–12. [Google Scholar]
- 45.Baldeweg T, Spence S, Hirsch SR, et al. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet. 1998;352:620–1. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- 46.Ropohl A, Sperling W, Elstner S, et al. Cortical activity associated with auditory hallucinations. Neuroreport. 2004;15:523–6. doi: 10.1097/00001756-200403010-00028. [DOI] [PubMed] [Google Scholar]
- 47.Gordon E, Williams LM, Haig AR, et al. Symptom profile and ‘gamma’ processing in schizophrenia. Cogn Neuropsychiatry. 2001;6:7–19. [Google Scholar]
- 48.Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–93. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho B-C, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–37. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell MJ, Lewis DA, Foote SL, et al. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta-hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987;261:209–20. doi: 10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- 51.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 52.Ito HT, Schuman EM. Frequency-dependent gating of synaptic transmission and plasticity by dopamine. Front Neural Circuits. 2007;1:1. doi: 10.3389/neuro.04.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillberg C, Melander H, von Knorring A-L, et al. Long-term stimulant treatment of children with attention-deficit hyperactivity disorder symptoms: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54:857–64. doi: 10.1001/archpsyc.1997.01830210105014. [DOI] [PubMed] [Google Scholar]
- 54.Griskova I, Morup M, Parnas J, et al. The amplitude and phase precision of 40 Hz auditory steady-state response depend on the level of arousal. Exp Brain Res. 2007;183:133–8. doi: 10.1007/s00221-007-1111-0. [DOI] [PubMed] [Google Scholar]
- 55.Scholes KE, Martin-Iverson MT. Alterations to pre-pulse inhibition (PPI) in chronic cannabis users are secondary to sustained attention deficits. Psychopharmacology (Berl) 2009;207:469–84. doi: 10.1007/s00213-009-1679-0. [DOI] [PubMed] [Google Scholar]
- 56.Robbins TW. Arousal systems and attentional processes. Biol Psychol. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- 57.Cape EG, Jones BE. Differential modulation of high-frequency γ-electroencephalogram activity and sleep–wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–66. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown RAM, Walling SG, Milway JS, et al. Locus ceruleus activation suppresses feed forward interneurons and reduces beta-gamma electroencephalogram frequencies while it enhances theta frequencies in rat dentate gyrus. J Neurosci. 2005;25:1985–91. doi: 10.1523/JNEUROSCI.4307-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albrecht MA, Martin-Iverson MT, Price G, et al. Dexamphetamine effects on separate constructs in the rubber hand illusion test. Psychopharmacology Berl. 2011;217:39–50. doi: 10.1007/s00213-011-2255-y. [DOI] [PubMed] [Google Scholar]
- 60.Peled A, Ritsner M, Hirschmann S, et al. Touch feel illusion in schizophrenic patients. Biol Psychiatry. 2000;48:1105–8. doi: 10.1016/s0006-3223(00)00947-1. [DOI] [PubMed] [Google Scholar]