Abstract
Background
Prepulse inhibition (PPI) of the startle reflex is modulated by a complex neural network. Prepulse inhibition impairments are found at all stages of schizophrenia. Previous magnetic resonance imaging (MRI) studies suggest that brain correlates of PPI differ between patients with schizophrenia and healthy controls; however, these studies included only patients with chronic illness and medicated patients. Our aim was to examine the structural brain correlates of PPI in antipsychotic-naive patients with first-episode schizophrenia.
Methods
We performed acoustic PPI assessment and structural MRI (1.5 and 3 T) in men with first-episode schizophrenia and age-matched controls. Voxel-based morphometry was used to investigate the association between PPI and grey matter volumes.
Results
We included 27 patients and 38 controls in the study. Patients had lower PPI than controls. The brain areas in which PPI and grey matter volume correlated did not differ between the groups. Independent of group, PPI was significantly and positively associated with regional grey matter volume in the right superior parietal cortex. Prepulse inhibition and grey matter volume associations were also observed in the left rostral dorsal premotor cortex, the right presupplementary motor area and the anterior medial superior frontal gyrus bilaterally. Follow-up analyses suggested that the rostral dorsal premotor cortex and presupplementary motor area correlations were driven predominantly by the controls.
Limitations
We used 2 different MRI scanners, which might have limited our ability to find subcortical associations since interscanner consistency is low for subcortical regions.
Conclusion
The superior parietal cortex seems to be involved in the regulation of PPI in controls and antipsychotic-naive men with first-episode schizophrenia. Our observation that PPI deficits in schizophrenia may be related to the rostral dorsal premotor cortex and presupplementary motor area, brain areas involved in maintaining relevant sensory information and voluntary inhibition, warrants further study.
Introduction
Disturbances in attention and information processing are thought to play a pivotal role in the symptomatology of schizophrenia.1 Patients with schizophrenia are generally unable to focus their attention and are easily distracted by irrelevant stimuli.2 This incapacity to filter out irrelevant stimuli has been hypothesized to cause an overload of sensory information, which may underlie positive symptoms.1
An often-used measure of automatic, preconscious information processing is the prepulse inhibition (PPI) of the startle reflex paradigm. The PPI paradigm is based on an individual’s startle reflex (i.e., the response to a sudden and intense stimulus).3 In humans, the startle reflex is usually measured as the amplitude of the electromyographic response of the orbicularis oculi muscle. If the startle-eliciting stimulus is preceded (30–500 ms) by a weak stimulus (i.e., the prepulse), the startle response is usually reduced. This is called sensorimotor gating, or PPI.4
Deficient PPI is not only found in patients at all stages and states of schizophrenia,5–7 but also in those with schizotypal disorder,7 psychosis-prone individuals6 and clinically unaffected relatives of patients with schizophrenia.7 Consequently, this deficit is regarded as an endophenotype for schizophrenia related to neurodevelopmental and/or genetic factors.8
Animal studies have found PPI to be mediated by the brainstem and regulated by forebrain structures through a cortico–striatal–pallido–thalamic network (for example, see the reviews by Fendt and colleagues,9 Swerdlow and colleagues,10 and Geyer and colleagues11). Human studies investigating neural brain correlates of PPI are sparse, and conclusions drawn from their findings are constrained by methodological differences (e.g., auditory or tactile PPI paradigms, functional magnetic resonance imaging [fMRI] or structural MRI, inclusion or exclusion of women), methodological limitations12 and by discrepancies in the results across the studies.13–19 Nevertheless, PPI has generally been found to be associated with regions within the forebrain: the thalamus; striatum; globus pallidus; hippocampus; insula; and the temporal, parietal and frontal cortices.13–19
Many of the aforementioned brain regions supposedly involved in the regulation of PPI have been shown to be affected in patients with schizophrenia. Studies using MRI techniques with region-of-interest (ROI)-based volume measurements have, among others, found grey matter volume changes in the thalamus; basal ganglia; hippocampus; and the temporal, parietal and frontal cortices in medicated patients with chronic schizophrenia.20 These findings are generally corroborated by findings from studies using voxel-based morphometry.21–23 Similarly, although more restrained, grey matter volume changes have been reported in patients with early-episode schizophrenia24,25 and even in individuals at ultra-high risk for psychosis.26
Several studies have shown associations between behaviour and cognition and structural properties (e.g., grey matter volume in specific brain regions).27 The lower PPI in patients could be due to structural abnormalities (reflected in grey matter volume changes) in brain areas contributing to the regulation of PPI (i.e., a quantitative difference between patients and healthy controls), or it could be due to PPI being regulated by less effective areas or networks in patients than in controls (i.e., a qualitative difference). This latter hypothesis is, for instance, supported by studies showing impaired cognition in patients with schizophrenia to be related to abnormal brain networks.28,29
To our knowledge, only 2 studies have addressed the association between PPI and grey matter volume. One study included healthy men and women.18 The other study included medicated men with chronic schizophrenia.17 Although both studies found PPI to correlate with grey matter volume in areas within the cortico–striatal–pallido–thalamic network, none of the areas found in the study on healthy individuals were confirmed in the patient study. Unfortunately, it is not possible to determine whether the discrepancies between the results of these 2 studies were related to the disease, disease progress, sex differences or medication effects.
The aim of the present study was to examine the structural brain correlates of PPI in patients and healthy controls without the influence of sex, age, antipsychotic medication and chronicity. To this end, we included antipsychotic-naive men with first-episode schizophrenia and age-matched healthy controls. We expected PPI to be associated with grey matter volume in the striatum; globus pallidus; thalamus; hippocampus; and the insular, temporal, frontal and parietal cortices. Moreover, we expected these associations to differ in patients and healthy controls.
Methods
Participants
We recruited 2 cohorts of participants, comprising medication-naive men with first-episode schizophrenia and age-matched controls each, for inclusion in the study. Cohort A was assessed from 1998 to 2002 and cohort B from 2005 to 2008. The inclusion criteria were identical, and the same measures were obtained from the 2 cohorts; however, the procedures differed as described in the MRI methods section that follows.
We recruited in- and outpatients with schizophrenia-like symptoms from hospitals around Copenhagen, Denmark. All patients were admitted to treatment for the first time and had never received any antipsychotic medication. A schizophrenia diagnosis according to International Classification of Diseases (ICD)-10 criteria was confirmed using the Schedule for Clinical Assessment in Neuropsychiatry (SCAN), version 2.0 or 2.1.30 Exclusion criteria were coercive measures, a history of mental retardation, organic brain damage or organic psychosis. We assessed psychopathology using the Positive and Negative Syndrome Scale (PANSS).31 Patients with a history of alcohol or cannabis abuse were not excluded, but urine samples were analyzed for the presence of the following drugs: amphetamines, opiates, cannabis, cocaine, methamphetamine and benzodiazepines. Healthy controls were excluded if they had any history of psychiatric disorder, somatic disease or a history of drug or alcohol abuse, or if their first-degree relatives had a history of psychiatric illness. Healthy controls were screened using the SCAN version 2.0 to exclude major psychopathology. Women were excluded from the current study because PPI fluctuates through the menstrual cycle.32
All participants underwent a comprehensive examination program, including psychopathological ratings, psychophysiological5,33,34 and neuropsychological examinations,35,36 single-photon emission computed tomography (SPECT)37 or positron emission tomography,38,39 structural MRI40,41 and fMRI.42 We report only on the combined PPI and structural MRI data.
The ethical committees of Copenhagen and Frederiksberg approved the study. We obtained written informed consent from all participants before inclusion and after oral and written explanation of the study aims and procedures. The individual appraisal of participants’ capacity to consent was done by trained psychiatrists and was based on formal contact with the patients during the introduction to the study and continually throughout the study.
MRI methods
High-resolution 3-dimensional (3-D) T1-weighted, sagittal magnetization-prepared rapid-gradient echo scans of the whole head were acquired in the unit of magnetic resonance imaging, University of Copenhagen, Hvidovre Hospital, Hvidovre, Denmark. Cohort A was examined using a 1.5 T Siemens Vision scanner. The T1-weighted sequence was as follows: echo time (TE) 4 ms, repetition time (TR) 9.7 ms, flip angle 12°, field of view (FOV) 250 mm, matrix 256 × 256, 0.98 × 0.98 × 1 mm voxels, 170 slices. Cohort B was examined on a 3.0 T Siemens Magnetom Trio scanner. The T1-weighted sequence parameters were as follows: TE 3.93 ms, TR 1540 ms, inversion time 800 ms, flip angle 9°, FOV 256 mm, matrix 256 × 256, 1 × 1 × 1 mm voxels, 192 slices.
Image processing
All acquired images were visually inspected to ensure sufficient quality. Images acquired on the 3 T scanner were corrected for spatial distortions owing to nonlinearity in the gradient system of the scanner.43 This step was not necessary for images acquired on the 1.5 T scanner. All images were processed using the VBM8 toolbox in SPM8 (http://dbm.neuro.uni-jena.de/vbm/; Wellcome Trust Centre for Neuroimaging) with default settings. The VBM8 toolbox incorporates a number of image processing techniques. The processing began with a segmentation algorithm based on a maximum a posteriori technique and a partial volume estimation method,44,45 including estimation of parameters for affine transformation to Montreal Neurological Institute (MNI) space. Furthermore, we applied 2 denoising methods: a spatially adaptive nonlocal means denoising filter46 and a Markov random field method.44 The T1-images were warped to the VBM8 template of 550 healthy adult controls using DARTEL through use of the calculated segmentations. Note that the VBM8 template itself also was created using DARTEL. Using the flow fields that parameterize the deformations, grey and white matter and cerebrospinal fluid images were warped into MNI space. Images were modulated with the Jacobian determinant of the applied deformation fields to correct for local volume changes following the high dimensional intersubject warping. We calculated Jacobian determinants from the nonaffine components of the applied deformation fields only, such that the resulting tissue maps were corrected for differences in head size. Finally, we smoothed the resulting warped and modulated tissue images using an 8 mm full-width at half-maximum Gaussian kernel. The quality of the images generated at each processing stage was visually checked.
PPI assessments
All participants were screened for hearing deficits with 40 dB(A) stimuli at 500, 1000 and 6000 Hz, and no hearing deficits were found. Participants were asked to not smoke or to drink any caffeinated beverages in the hour preceding the testing. Participants were seated in an armchair and instructed to sit still and fix their gaze at a point straight ahead of them. The eye-blink component of the acoustic startle response was measured by recording electromyographic activity from the right orbicularis oculi muscle. The acoustic stimuli were presented binaurally.
We used 2 slightly different PPI paradigms, which have been described in detail elsewhere.5,33,34 Briefly, we used paradigm A5,34 to assess all participants from cohort A. It consisted of 73 trials of pulse alone and prepulse–pulse trials. Pulse alone (PA) stimuli were 40 ms of 116 dB(A) broad frequency spectrum noise. Prepulse–pulse trials were PA trials preceded by a prepulse stimulus (20 ms, 85 dB(A) broad frequency spectrum noise). The prepulse stimulus was presented with a 30, 60 or 120 ms stimulus onset asynchrony (SOA) before the PA stimulus. The first trial was a PA trial, which was followed by 6 blocks of 12 trials. Each block consisted of 3 PA trials and 3 of each prepulse–pulse trial type (PPI30, SOA = 30 ms; PPI60, SOA = 60 ms; PPI120, SOA = 120 ms) presented in a pseudorandomized order. We used paradigm B33 to assess all participants from cohort B. It consisted of 66 trials presented in 3 experimental blocks. Blocks 1 and 3 consisted of 8 PA trials (20 ms broad frequency spectrum noise of 115 dB(A)). Block 2 consisted of 10 PA trials and 40 prepulse–pulse trials. The prepulse stimuli had an intensity of 76 or 85 dB(A) and consisted of 20 ms of broad frequency spectrum noise. The SOAs were either 60 or 120 ms. Each of the 4 prepulse–pulse combinations contained 10 trials and were presented in a pseudorandomized order. In both paradigms, PPI was expressed as [1 – (mean startle amplitude to prepulse–pulse trials)/(mean startle amplitude to PA trials)] × 100%.
Common to both paradigms was the PPI60 and PPI120 with a 85 dB(A) prepulse. However, only the PPI120/85 dB(A) trial type was used in the PPI and MRI analysis, since this trial type consistently elicits higher PPI than the PPI60/85 dB(A) trial type (for example, see Mackeprang and colleagues,5 Aggernaes and colleagues,33 Braff and colleagues47). Furthermore, numerous studies have found PPI120/85 dB(A) to be lower in patients with schizophrenia than in healthy controls.5,48,49
We combined PPI derived from paradigms A and B with MRI scans acquired on the 1.5 and 3 T scanners, respectively.
Statistical analysis
We used SPSS for Windows, version 18.0, for statistical analysis of demographic and clinical variables and PPI. The α level for significance was set at p < 0.05, 2-tailed. Age, PPI120 and PANSS scores were normally distributed (Kolmogorov–Smirnov test). Group differences in age were tested using independent-sample Student t tests. Differences in handedness were tested with the Fisher exact test. The same tests were performed to assess differences between participants in cohorts A and B. To investigate differences in PPI between groups (patients, healthy controls) and PPI paradigms, we performed an analysis of variance with PPI120 as a dependent factor and group and paradigm/cohort as between-subject factors. We analyzed correlations between PPI and psychopathology with Pearson correlation tests.
Associations between PPI and grey matter volumes
We performed the voxel-wise statistical analyses in SPM8. The α level for significance was set at p < 0.05, family-wise error (FWE)-corrected. To avoid type II errors, we also considered voxels surviving a significance level of p < 0.001, uncorrected for multiple comparisons with an extent threshold, empirically determined by SPM8 to be of interest. Liebermann and Cunningham50 recommended a combined intensity and cluster size threshold since it has been demonstrated to provide a good balance between risk of type I and type II errors. Only voxels with more than 10% grey matter volume were included to diminish possible partial volume effects at the borders between grey and white matter and between grey matter and cerebrospinal fluid.
Since we hypothesized PPI to be associated with grey matter in the striatum; thalamus; hippocampus; and insular, frontal, temporal and parietal cortices, we applied a mask only including these regions to restrict our statistical search area and thereby the amount of multiple comparisons. The mask was created with the MARINA software (Masks for Region of INterest Analysis, version 0.6.1, Bertram Walter Bender Institute of Neuroimaging, University of Giessen).51
We used flexible factorial design to test for PPI120 and grey matter volume associations. Group and scanner type (and thus paradigm type and cohort) were included as between-subject factors, and PPI120, age and scanner drift (number of days passed since the first scan of the study) were included as covariates. An interaction was set for group and PPI120. Four statistical parametric maps were generated: 1 for the positive correlation between PPI120 and grey matter volume in both patients and controls, 1 for the negative correlation between PPI120 and grey matter volume in both patients and controls, and 2 for the interactions between patients and controls. With respect to the interactions between patients and controls, we expected that any effects would be found in areas where PPI and grey matter volume were associated in healthy controls. Therefore, when testing for interaction effects, we restricted our statistical search area to those brain regions where controls exhibited PPI120–grey matter volume correlations (p < 0.05, no extent threshold).
Planned follow-up single-group analyses were performed to examine PPI120–grey matter volume correlations separately for patients and controls. The design matrices included PPI120, scanner type, age and scanner drift as covariates. For each group, 2 statistical parametric maps were generated: 1 for the positive correlation and 1 for the negative correlation between PPI120 and grey matter volume.
Correlations between volumetric measures of the associated brain areas and PPI
Grey matter volume estimates were extracted from the 5 clusters of voxels that showed PPI120–grey matter volume correlations in patients and controls by integrating the grey matter intensities within each cluster. The grey matter volume estimates used regression analysis to examine how much of the total variance in PPI (dependent factor) could be explained by the joint grey matter volume estimates. Finally, we calculated correlations between PPI120 and grey matter volume estimates for the individual anatomic areas separately using Pearson correlations.
To address a possible scanner effect, we used flexible factorial design to test for a main effect of scanner and scanner × PPI interaction effects. Group and scanner type were included as between-subject factors, and PPI120, age and scanner drift were included as covariates. An interaction was set for scanner and PPI120. Besides addressing possible scanner effects, the aforementioned analyses were also sensitive for possible anatomic differences between cohorts.
Results
Demographic and clinical characteristics of participants
In all, 32 medication-naive men with first-episode schizophrenia and 42 healthy men underwent a PPI assessment and structural MRI (scanning to PPI assessment interval was mean 13.7 [range 0–118] d; 2 patients and 4 healthy controls had an interval of more than 1 month). After assessment, we excluded 2 patients and 1 control because they were non-responders on the PPI paradigm (startle amplitude < 10 μV); 1 patient and 2 controls because they had outlying PPI values (standard deviation [SD] > 2.5 from the mean); and 2 patients and 1 control because of artifacts on their MRI scans. Thus, 27 patients and 38 healthy controls were included in the final analysis. Of these, 11 patients and 12 controls were in cohort A, and 16 patients and 26 controls were in cohort B. Demographic and clinical characteristics of participants are listed in Table 1.
Table 1.
Demographic and clinical characteristics and prepulse inhibition of medication-naive men with first-episode schizophrenia and age-matched healthy controls*
| Patients with schizophrenia | Controls | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | Cohort A† | Cohort B† | p value | Cohort A† | Cohort B† | p value |
| Methodological features | ||||||
| No. participants | 11 | 16 | — | 12 | 26 | — |
| Scanner type | 1.5 T | 3 T | — | 1.5 T | 3 T | — |
| PPI paradigm‡ | A | B | — | A | B | — |
| Demographic and clinical data | ||||||
| Age, mean (SD) yr | 29.3 (1.9) | 25.7 (1.2) | 0.19 | 28.4 (5.1) | 26.6 (5.3) | 0.34 |
| Handedness, right/left | 9/2 | 16/0 | 0.16 | 9/3 | 25/1 | 0.09 |
| Current substance abuse, yes/no§ | 3/7 | 2/14 | 0.37 | 0/12 | 0/9 | — |
| Benzodiazepine, yes/no¶ | 1/10 | 5/11 | 0.18 | 0/12 | 0/9 | — |
| Antipsychotics, yes/no** | 0/11 | 1/15 | 0.62 | 0/12 | 0/9 | — |
| Smoking status, yes/no†† | 3/6 | 10/4 | Unknown | 3/23 | ||
| Psychopathology PANSS, mean (SD) score | ||||||
| Positive | 21.1 (4.1) | 19.9 (4.1) | 0.46 | — | — | — |
| Negative | 21.4 (4.5) | 21.7 (5.1) | 0.87 | — | — | — |
| General | 29.9 (4.6) | 40.0 (8.4) | 0.021 | — | — | — |
| Total | 72.4 (10.8) | 81.6 (14.2) | 0.08 | — | — | — |
| Percentage PPI, mean (SD) | ||||||
| PPI120/85 dB(A) | 62.1 (7.0) | 60.9 (6.4) | 0.91 | 75.0 (6.8) | 71.4 (3.6) | 0.61 |
ICD = International Classification of Diseases; PANSS = Positive and Negative Syndrome Scale;31 PPI = prepulse inhibition; SD = standard deviation.
No significant differences were observed between the 2 patient populations (except for PANSS general score) or between the 2 healthy control populations.
We recruited 2 cohorts of participants, each comprising medication-naive men with first-episode schizophrenia and age-matched. Cohort A was assessed from 1998 to 2002 using a 1.5 T scanner and cohort B was assessed from 2005 to 2008 using a 3 T scanner. The 2 cohorts did not differ significantly with regard to the ratio of patients and controls (Fisher exact test, p = 0.6).
Paradigm A has been described elsewhere by Mackeprang and colleagues5 and Hammer and colleagues;34 paradigm B has been described elsewhere by Aggernaes and colleagues.33
Substance abuse as determined by ICD-10.
Prescription of benzodiazepine.
Treatment with antipsychotics.
Smoking status was not available for all participants.
Patients and controls in the 2 cohorts did not differ significantly in age or handedness. Moreover, the patients in cohorts A and B did not differ in benzodiazepine prescribed or substance abuse. None of the participants tested positive on urine screening for substance use, and none had clinically important brain pathology. The 2 patient cohorts did not differ on PANSS negative and positive scores; however, the PANSS general score was significantly lower in cohort A than B (29.9 v. 40.0, respectively; Table 1). Finally, most likely as a result of their lower PANSS general score, there was a trend for a lower PANSS total score among patients from cohort A compared with those from cohort B (72.4 v. 81.6, respectively; Table 1).
Prepulse inhibition
We found a significant effect of group, indicating that patients had significantly lower PPI than healthy controls (F1,61 = 4.06, p = 0.048). We did not observe an effect of paradigm/cohort (F1,61 = 0.17, p = 0.68), nor did we find an interaction between paradigm/cohort and group (F1,61 = 0.04, p = 0.84). No significant correlations were found between PANSS positive, negative, general or total scores and PPI120 (all p > 0.16).
Associations between PPI and grey matter volume
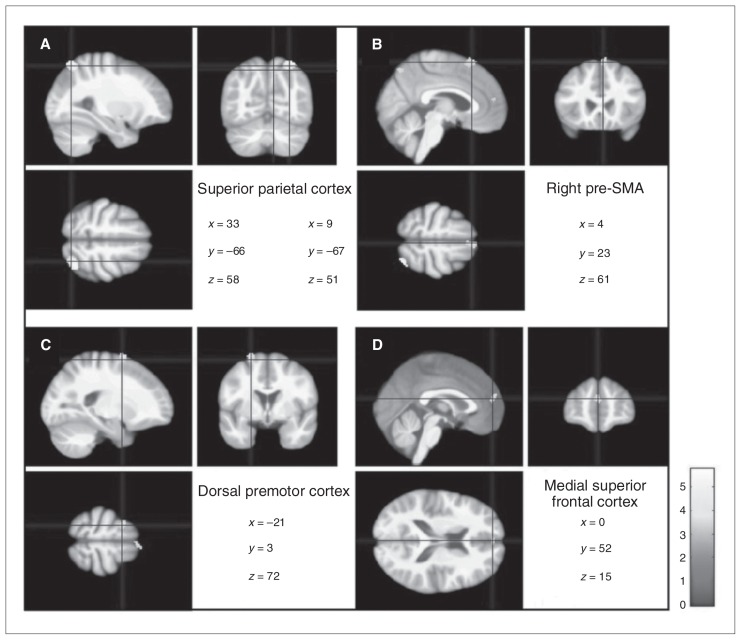
Results of the voxel-wise analysis are presented in Table 2. There was no significant interaction between group and PPI120, indicating that patients and controls did not differ with respect to PPI120–grey matter volume associations. However, independent of diagnosis, PPI120 was significantly and positively associated with grey matter volume in a cluster in the right superior parietal lobe (p = 0.006, FWE-corrected). Moreover, although not significant at an FWE-corrected level, clusters of interest (i.e., voxel level significance of p < 0.001, extent threshold 88 voxels) were observed in the right superior parietal lobe, the right presupplementary motor area, the left rostral dorsal premotor cortex and the anterior medial superior frontal lobe bilaterally (Fig. 1).
Table 2.
Structural brain correlates of prepulse inhibition with a stimulus onset asynchrony of 120 ms in medication-naive men with first-episode schizophrenia and healthy controls*
| Anatomic area | Lobe | Side | Controls and patients, n = 65† | Controls, n = 38‡ | Patients, n = 27§ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||
| MNI coordinates | t value | Cluster size | MNI coordinates | t value | Cluster size | MNI coordinates | t value | Cluster size | |||||||||
|
|
|
|
|||||||||||||||
| x | y | z | x | y | z | x | y | z | |||||||||
| Superior (7A) | Parietal | R | 33 | −66 | 58 | 5.8¶ | 589 | 33 | −60 | 57 | 4.9 | 140 | 30 | −66 | 60 | 5.6 | 220 |
| Parietal | R | 9 | −67 | 51 | 3.8 | 116 | |||||||||||
| Pre-SMA | Frontal | R | 4 | 23 | 61 | 3.9 | 321 | 4 | 24 | 64 | 5.11 | 508 | |||||
| rPMd | Frontal | L | −21 | 3 | 72 | 4.3 | 237 | −21 | 3 | 67 | 3.77 | 110 | |||||
| Medial superior | Frontal | R/L | 0 | 52 | 15 | 3.9 | 122 | ||||||||||
L = left; MNI = Montreal Neurological Institute; R = right; rPMd = rostral dorsal premotor cortex; SMA = supplementary motor area.
Statistical parametric maps were thresholded at p < 0.001 with an extent threshold, empirically determined by SPM8. Only positive correlations were found. All analyses were corrected for scanner type, age and scanner drift.
Extent threshold of kE = 88 voxels.
Extent threshold of kE = 75 voxels.
Extent threshold of kE = 81 voxels.
p = 0.006, family-wise error–corrected.
Fig. 1.
Structural brain correlates of prepulse inhibition (PPI) with a stimulus onset asynchrony of 120 ms (PPI120) in patients with schizophrenia and healthy controls. Statistical parametric maps of the positive correlations between grey matter volume and PPI120 independent of diagnosis (p < 0.001, extended threshold kE = 88) show the (A) right superior parietal cortex, (B) right presupplementary motor area, (C) left rostral dorsal premotor cortex and (D) bilateral anterior medial superior parietal cortex. Neurological (right is right) convention is used. Results are projected on an average of all DARTEL warped magnetization-prepared rapid-gradient echo images.
Planned follow-up analyses revealed that in healthy controls, when analyzed separately, PPI120 was positively associated with grey matter volume in the right superior parietal lobe, presupplementary motor area and the left rostral dorsal premotor cortex. Separate analyses of patients showed that PPI was positively associated with grey matter volume in the right superior parietal lobe only. Further lowering the voxel-level threshold to 0.01 (no extent threshold) revealed additional positive associations between PPI120 and bilateral anterior medial superior frontal grey matter in patients and healthy controls. No negative associations were observed in the analyses.
In addition, both data sets were analyzed separately (independent of group, p < 0.005, uncorrected). All associations found in the analyses of the combined data set were confirmed. Details are presented in Appendix 1, Table S1, available at cma.ca/jpn.
Grey matter volume estimates and their correlations with PPI
The cumulated grey matter volume estimates of the areas that were positively associated with PPI120 in the main analysis — the right superior parietal cortex, right presupplementary motor area, left rostral dorsal premotor cortex and bilateral anterior medial superior frontal lobe — explained 67% of the variance in PPI among healthy controls (R2 = 0.67, F4,33 = 16.83, p < 0.001) and 47% of the variance in PPI among patients (R2 = 0.47, F4,22 = 4.85, p = 0.006).
The Pearson correlation for PPI120 and grey matter volume estimates in the right superior parietal cortex in patients was 0.67 (p < 0.001). The Pearson correlations for PPI120 and grey matter volume estimates in the right superior parietal cortex, right presupplementary motor area and rostral dorsal premotor cortex in healthy controls were 0.75 (p < 0.001), 0.62 (p < 0.001) and 0.45 (p = 0.005), respectively.
Supplementary analyses
Effect of scanner and scanner × PPI interactions
We did not find a significant main effect of scanner (p < 0.05, FWE-corrected); however, using a liberal voxel-level threshold (p < 0.01, uncorrected, no extent threshold) showed a possible scanner effect in various regions: the cerebellum, primary motor area, nucleus caudate and other subcortical regions. These regions did not overlap with brain areas showing PPI–grey matter volume correlations (Appendix 1, Figs. S1–S4). Likewise, we did not find a significant scanner × PPI interaction effect (p < 0.05, FWE-corrected); however, using a liberal voxel treshold (p < 0.01 uncorrected, no extent threshold) showed a possible scanner × PPI interaction in various regions. Again, these regions did not overlap with the brain areas showing PPI–grey matter volume correlations (Appendix 1, Figs. S5–S8).
Effect of substance abuse
Five patients reported current substance abuse. Excluding these 5 patients did not significantly change the PPI results or the PPI–grey matter volume correlation results.
Effect of extended scanning to PPI assessment interval
Excluding the 6 participants with a scanning to PPI assessment interval of more than 1 month did not significantly change the PPI–grey matter volume correlation results.
Discussion
To our knowledge, this is the first study in which the association between grey matter volume and PPI of the startle reflex was investigated in antipsychotic-naive men with first-episode schizophrenia and healthy age-matched controls. Independent of group, PPI120 correlated significantly and positively with grey matter volume in the right superior parietal cortex and also positively with 3 areas in the frontal cortex: the right presupplementary motor area, left rostral dorsal premotor cortex and bilateral anterior medial superior frontal gyrus. Follow-up analyses could not confirm the presence of presupplementary motor area and rostral dorsal premotor cortex correlations in the patient group even when using a very liberal threshold of significance. The latter suggests that these frontal correlations may have been driven predominantly by the healthy controls, whereas the correlation within the superior medial frontal gyrus was driven by both patients and controls. We did not observe any brain structure–PPI correlates distinct among patients.
Our finding of an association between PPI and grey matter volume in the cortical regions is in line with results from animal studies9–11 and human psychophysiological studies that indicate top–down cortical modulation of PPI. The latter is indicated since levels of PPI increase when cortical-emanated functions (e.g., attention, emotion) are activated during a PPI paradigm.52,53
To our knowledge, only 2 MRI studies have previously reported on structural brain correlates of PPI.17,18 Kumari and colleagues18 examined healthy individuals only and found PPI–grey matter volume correlations in the striatum, hippocampus and inferior frontal gyrus. The present study did not confirm correlates in any of these 3 brain areas. In a later study, Kumari and colleagues17 examined structural MRI in 42 chronically ill and medicated male patients with schizophrenia and, in contrast to the present study, found PPI to be associated with frontal cortical areas (i.e., left dorsolateral and right medial prefrontal cortex, right middle frontal cortex), but not the parietal cortex. Discrepancies between the findings of these 2 studies and the present study are most likely rooted in demographic, clinical (including medication) and methodological factors. Kumari and colleagues18 included both healthy men and women, whereas only men were included in the present study. Sex differences are found not only in levels of PPI (where men usually score higher than women), but also in brain structure and brain activation during cognitive tasks.54–57 Moreover, the level of PPI appears to fluctuate during the female menstrual cycle.32 Furthermore, in contrast to Kumari and colleagues,17 we investigated antipsychotic-naive patients with first-episode schizophrenia, thereby diminishing possible effects of disease chronicity, hospital admissions and medication. Finally, we used a high-dimensional inter-subject warping method and adjusted for differences in brain sizes, thereby diminishing inter-subject anatomic variability.
Although future studies are needed to confirm our findings, the superior parietal cortex is known to be involved in detecting or ignoring irrelevant stimuli.58 The presupplementary motor area and rostral dorsal premotor cortex are a part of the frontal premotor areas, but they are primarily involved in the more cognitive aspects of motor control (e.g., in the maintenance of relevant sensory information).59 The functions of the area we found in the anterior medial superior frontal gyrus are not well described; however, midline–frontal areas in general are found to be involved in voluntary response inhibition.60
Although functions of relevance for schizophrenia (e.g., feeling of self-agency, mirror neuron properties) seem to originate from the presupplementary motor area and rostral dorsal premotor cortex, only a few studies on schizophrenia have focused on these structures. However, the volume,61 integrity62 and functions63 emanating from the rostral dorsal premotor cortex and the presupplementary motor area have been found to be altered in patients with schizophrenia compared with healthy controls.
The grey matter volume of the 4 cortical areas (2 clusters in the parietal area and 3 clusters in the frontal areas) that we found to be associated with PPI explained 47% and 67% of the variance in PPI in antipsychotic-naive patients and healthy controls, respectively. Likewise, Kumari and colleagues17 found that brain regions associated with PPI explained about 40% of the variance in PPI in medicated patients with chronic schizophrenia and 62% of the variance in PPI in healthy men and women.18 These observations led them to suggest that there may be a stronger structure–function correspondence in healthy people than in patients with schizophrenia.17
Limitations
The present study has some limitations. Although the number of antipsychotic-naive patients studied was considerable given the difficulties of recruiting such a patient group, we cannot rule out that we did not have enough statistical power to observe any group differences. In addition, 2 different MRI scanners were used. Analyses testing for possible scanner effects, and thus implicitly also testing for possible anatomic differences between cohorts, revealed that brain areas showing a main effect of scanner or a scanner × PPI interaction effect, which was only apparent using liberal levels of significance, did not overlap with brain areas showing PPI–grey matter volume correlations. It is therefore unlikely that the use of 2 different scanners has affected our findings. Nevertheless, the use of 2 different scanners might especially have limited our ability to find possible associations between PPI and subcortical regions. Indeed, interscanner consistency has frequently been found to be low for subcortical regions (e.g., thalamus, basal ganglia) but high for cortical regions.64,65 Finally, the fact that PPI was derived from 2 somewhat different PPI paradigms could have affected the PPI–grey matter volume associations by introducing variability to the data. However, we used paradigm type (A or B) as a covariate in all analyses, and PPI levels in the 2 paradigms did not differ significantly. Significantly more patients than controls were smokers, and although we tried to avoid the acute effect of nicotine by requesting that participants not smoke in the hour before the PPI assessment, we cannot rule out that some of the differences found between patients and healthy controls were due to differences in smoking status. However, because smoking is found to increase PPI, and since PPI was lower in the group that smoked the most (i.e., the patients), we think it unlikely that observed group differences resulted from the effects of nicotine.
Conclusion
The present data indicate that the superior parietal cortex is involved in the regulation of PPI in both antipsychotic-naive men with first-episode schizophrenia and healthy age-matched controls. Although the patients showed significantly lower levels of PPI than the controls, no significant group differences were found in the associations between grey matter volume and PPI. Our data did, however, suggest that the right presupplementary motor area and left rostral dorsal motor cortex grey matter values were more strongly associated with PPI in healthy controls than in patients. This could indicate a possible link between dysfunctions within the frontal premotor areas and PPI deficits in patients with schizophrenia.
Acknowledgements
This study was sponsored by The Danish Council for Independent Research | Medical Sciences, Gerhart Linds Foundation, Lundbeck Foundation, The Mental Health Service of the Capital Region of Denmark and Psykiatrisk Grundforskningsfond at Copenhagen University.
Footnotes
Competing interests: B. Glenthøj declares having received institutional grant support as listed above. None declared for T.B. Hammer, B. Oranje, A. Skimminge, B. Aggernæs, B.H. Ebdrup and W. Baaré.
Contributors: T.B. Hammer, B. Oranje B. Aggernaes, B.H. Ebdrup, B Glenthøj and W. Barré designed the study. T.B. Hammer, B. Oranje, B. Aggernaes and W. Barré acquired and analysed the data. B.H. Ebdrup participated in data acquisition, and A. Skimminge and G. Glenthøj participated in data analysis. T.B. Hammer and B. Oranje wrote the article, which all authors reviewed and approved for publication.
References
- 1.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–8. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 2.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–16. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 3.Landis C, Hunt WA. The startle pattern. New York (NY): Farrar & Rinehart Inc.; 1939. [Google Scholar]
- 4.Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–48. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 5.Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naive schizophrenic patients. Biol Psychiatry. 2002;52:863–73. doi: 10.1016/s0006-3223(02)01409-9. [DOI] [PubMed] [Google Scholar]
- 6.Quednow BB, Frommann I, Berning J, et al. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64:766–73. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 8.Turetsky BI, Calkins ME, Light GA, et al. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–24. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 11.Geyer MA, Krebs-Thomson K, Braff DL, et al. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Du Y, Li N, et al. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci Biobehav Rev. 2009;33:1157–67. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Kumari V, Antonova E, Geyer MA, et al. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:463–77. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- 14.Postma P, Gray JA, Sharma T, et al. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology (Berl) 2006;184:589–99. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- 15.Goldman MB, Heidinger L, Kulkarni K, et al. Changes in the amplitude and timing of the hemodynamic response associated with prepulse inhibition of acoustic startle. Neuroimage. 2006;32:1375–84. doi: 10.1016/j.neuroimage.2006.04.228. [DOI] [PubMed] [Google Scholar]
- 16.Campbell LE, Hughes M, Budd TW, et al. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur J Neurosci. 2007;26:2327–33. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumari V, Fannon D, Geyer MA, et al. Cortical grey matter volume and sensorimotor gating in schizophrenia. Cortex. 2008;44:1206–14. doi: 10.1016/j.cortex.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumari V, Antonova E, Zachariah E, et al. Structural brain correlates of prepulse inhibition of the acoustic startle response in healthy humans. Neuroimage. 2005;26:1052–8. doi: 10.1016/j.neuroimage.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Kumari V, Gray JA, Geyer MA, et al. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Res. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 20.Shenton ME, Dickey CC, Frumin M, et al. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honea R, Crow TJ, Passingham D, et al. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–45. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 22.Ellison-Wright I, Glahn DC, Laird AR, et al. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fornito A, Yucel M, Patti J, et al. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–13. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Steen RG, Mull C, McClure R, et al. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–8. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 25.Vita A, De PL. Hippocampal and amygdala volume reductions in first-episode schizophrenia. Br J Psychiatry. 2007;190:271. doi: 10.1192/bjp.190.3.271. [DOI] [PubMed] [Google Scholar]
- 26.Mechelli A, Riecher-Rossler A, Meisenzahl EM, et al. Neuro-anatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489–95. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- 27.Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011;12:231–42. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- 28.Meda SA, Stevens MC, Folley BS, et al. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PLoS ONE. 2009;4:e7911. doi: 10.1371/journal.pone.0007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson-Yeo W, Allen P, Benetti S, et al. Dysconnectivity in schizophrenia: Where are we now? Neurosci Biobehav Rev. 2011;35:1110–24. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–93. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 31.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 32.Jovanovic T, Szilagyi S, Chakravorty S, et al. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41:401–6. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 33.Aggernaes B, Glenthoj BY, Ebdrup BH, et al. Sensorimotor gating and habituation in antipsychotic-naive, first-episode schizophrenia patients before and after 6 months’ treatment with quetiapine. Int J Neuropsychopharmacol. 2010;13:1383–95. doi: 10.1017/S1461145710000787. [DOI] [PubMed] [Google Scholar]
- 34.Hammer TB, Oranje B, Fagerlund B, et al. Stability of prepulse mminhibition and habituation of the startle reflex in schizophrenia: a 6-year follow-up study of initially antipsychotic-naive, first-episode schizophrenia patients. Int J Neuropsychopharmacol. 2011;14:913–25. doi: 10.1017/S1461145711000034. [DOI] [PubMed] [Google Scholar]
- 35.Fagerlund B, Mackeprang T, Gade A, et al. Effects of low-dose risperi-done and low-dose zuclopenthixol on cognitive functions in first-episode drug-naive schizophrenic patients. CNS Spectr. 2004;9:364–74. doi: 10.1017/s1092852900009354. [DOI] [PubMed] [Google Scholar]
- 36.Andersen R, Fagerlund B, Rasmussen H, et al. Cognitive effects of six months of treatment with quetiapine in antipsychotic-naive first-episode schizophrenia. Psychiatry Res. 2011;187:49–54. doi: 10.1016/j.psychres.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Glenthoj BY, Mackeprang T, Svarer C, et al. Frontal dopamine D(2/3) receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol Psychiatry. 2006;60:621–9. doi: 10.1016/j.biopsych.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen H, Erritzoe D, Andersen R, et al. Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 2010;67:9–16. doi: 10.1001/archgenpsychiatry.2009.176. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen H, Ebdrup BH, Erritzoe D, et al. Serotonin2A receptor blockade and clinical effect in first-episode schizophrenia patients treated with quetiapine. Psychopharmacology (Berl) 2011;213:583–92. doi: 10.1007/s00213-010-1941-5. [DOI] [PubMed] [Google Scholar]
- 40.Ebdrup BH, Glenthoj B, Rasmussen H, et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci. 2010;35:95–104. doi: 10.1503/jpn.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glenthoj A, Glenthoj BY, Mackeprang T, et al. Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res. 2007;154:199–208. doi: 10.1016/j.pscychresns.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Nejad AB, Ebdrup BH, Siebner HR, et al. Impaired temporoparietal deactivation with working memory load in antipsychotic-naive patients with first-episode schizophrenia. World J Biol Psychiatry. 2011;12:271–81. doi: 10.3109/15622975.2010.556199. [DOI] [PubMed] [Google Scholar]
- 43.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 44.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–86. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- 45.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Manjón JV, Coupe P, Marti-Bonmati L, et al. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- 47.Braff D, Stone C, Callaway E, et al. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 48.Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–8. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- 49.Swerdlow NR, Light GA, Cadenhead KS, et al. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–35. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- 50.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walter B, Blecker C, Kirsch P, et al. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses [abstract]. Available on CD-Rom in NeuroImage; Presented at the 9th International Conference on Functional Mapping of the Human Brain; June 19–22, 2003; New York, NY. [Google Scholar]
- 52.Hazlett EA, Dawson ME, Schell AM, et al. Attentional stages of information processing during a continuous performance test: a startle modification analysis. Psychophysiology. 2001;38:669–77. [PubMed] [Google Scholar]
- 53.Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–97. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 54.Luders E, Toga AW. Sex differences in brain anatomy. Prog Brain Res. 2010;186:3–12. doi: 10.1016/B978-0-444-53630-3.00001-4. [DOI] [PubMed] [Google Scholar]
- 55.Hines M. Sex-related variation in human behavior and the brain. Trends Cogn Sci. 2010;14:448–56. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumari V, Aasen I, Papadopoulos A, et al. A comparison of pre-pulse inhibition in pre- and postmenopausal women and age-matched men. Neuropsychopharmacology. 2008;33:2610–8. doi: 10.1038/sj.npp.1301670. [DOI] [PubMed] [Google Scholar]
- 57.Paus T. Sex differences in the human brain: a developmental perspective. Prog Brain Res. 2010;186:13–28. doi: 10.1016/B978-0-444-53630-3.00002-6. [DOI] [PubMed] [Google Scholar]
- 58.Kanai R, Dong MY, Bahrami B, et al. Distractibility in daily life is reflected in the structure and function of human parietal cortex. J Neurosci. 2011;31:6620–6. doi: 10.1523/JNEUROSCI.5864-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 60.Yamanaka K, Yamamoto Y. Single-trial EEG power and phase dynamics associated with voluntary response inhibition. J Cogn Neurosci. 2010;22:714–27. doi: 10.1162/jocn.2009.21258. [DOI] [PubMed] [Google Scholar]
- 61.Exner C, Weniger G, Schmidt-Samoa C, et al. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr Res. 2006;84:386–96. doi: 10.1016/j.schres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 62.Yoon JH, Minzenberg MJ, Ursu S, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006–14. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc. 2010;16:1064–76. doi: 10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- 64.Schaufelberger MS, Duran FL, Lappin JM, et al. Grey matter abnormalities in Brazilians with first-episode psychosis. Br J Psychiatry Suppl. 2007;51:s117–22. doi: 10.1192/bjp.191.51.s117. [DOI] [PubMed] [Google Scholar]
- 65.Stonnington CM, Tan G, Kloppel S, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer’ s disease. Neuroimage. 2008;39:1180–5. doi: 10.1016/j.neuroimage.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]