Abstract
Background
Many studies using diffusion tensor imaging (DTI) have demonstrated impaired white matter integrity in patients with major depressive disorder (MDD), with significant results found in diverse brain regions. We sought to identify whether there are consistent changes of regional white matter integrity in patients with MDD, as shown by decreased fractional anisotropy in DTI.
Method
A systematic search strategy was used to identify relevant whole brain voxel-based DTI studies of patients with MDD in relation to comparison groups. Relevant databases were searched for studies published between January 1994 and February 2011 using combinations of the terms “DTI” or “diffusion tensor;” “whole brain” or “voxel-based;” and “depress*.” Using the studies that met our inclusion criteria, we performed a meta-analysis of the coordinates of decreased fractional anisotropy using the activation likelihood estimation (ALE) method, which detects 3-dimensional conjunctions of coordinates from multiple studies, weighted by sample size. We then used DTIquery software for fibre tracking to locate the fascicles involved in each region.
Results
We included 11 studies with a combined sample of 231 patients with MDD and 261 comparison participants, providing 50 coordinates of decreased fractional anisotropy. Our meta-analysis identified 4 consistent locations of decreased fractional anisotropy in patients with MDD: white matter in the right frontal lobe, right fusiform gyrus, left frontal lobe and right occipital lobe. Fibre tracking showed that the main fascicles involved were the right inferior longitudinal fasciculus, right inferior fronto-occipital fasciculus, right posterior thalamic radiation and interhemispheric fibres running through the genu and body of the corpus callosum.
Limitations
The number of studies included was relatively small, and the DTI data acquisition and analysis techniques were heterogeneous. The ALE method cannot handle studies with no significant group differences.
Conclusion
Voxel-based analysis of DTI studies of patients with MDD consistently identified decreased fractional anisotropy in the white matter fascicles connecting the prefrontal cortex within cortical (frontal, temporal and occipital lobes) and subcortical areas (amygdala and hippocampus). This is strong evidence for the involvement of these neural circuits in the pathology of MDD.
Introduction
Major depressive disorder (MDD) is a globally prevalent psychiatric disorder causing a great social burden. In hopes of identifying better treatment options, many attempts have been made to clarify pathological brain mechanisms. Using novel imaging techniques in vivo, several microstructural brain abnormalities have been reported.1 It is likely that most psychiatric disorders do not result from a deficit in a single brain area and that a network perspective is necessary to explain their complex etiology. Accordingly, studies of both functional and structural connectivity have become popular. Robust functional connectivity has been observed even in the absence of a direct anatomic link.2 However, given the surprisingly low within-subject test–retest reliability of functional connectivity, structural connectivity, which is a good predictor of functional connectivity, offers a more practical approach to characterizing brain abnormalities related to disease. White matter integrity reflects the structural connectivity that provides the basis for functional abnormality in the brain.3 It has been suggested that cortical–subcortical neural circuits play an important role in the pathogenesis of MDD; specifically, that microstructural changes in the white matter of cortical–subcortical circuits lead to a “disconnection syndrome” between cortical and subcortical regions.4 Consequently, white matter abnormalities have been proposed as potential biomarkers of MDD.
Diffusion tensor imaging (DTI), a novel magnetic resonance imaging (MRI) technique, can quantify the fibre orientation and integrity of white matter pathways within neural networks. It measures water diffusion using diffusion-weighted pulse sequences sensitive to microscopic random water motion.5 Diffusion is described as isotropic if water mobility is unrestricted and anisotropic if it is restricted in any direction. This is commonly quantified using fractional anisotropy: in general, greater fractional anisotropy indicates greater white matter anisotropy due to highly organized and myelinated bundles and may also be influenced by axon size and density, pathway geometry, and fibre intersections.6–8
To explore brain fractional anisotropy differences between 2 populations, measurements can be extracted globally using voxel-based analysis (VBA) or tract-based spatial statistics (TBSS) or locally in a priori defined regions using region-of-interest (ROI) analysis or tractography. A number of studies have demonstrated fractional anisotropy changes in patients with MDD. For example, studies of late-life depression have revealed frontal and temporal white matter abnormalities.9–12 In studies of first-episode depression, untreated young adults with MDD exhibited significantly lower fractional anisotropy in the frontal lobes.13,14 However, although most DTI studies of patients with MDD have identified decreases in fractional anisotropy, a variety of white matter regions have been implicated. In this situation, a powerful technique is the meta-analysis of findings from multiple studies to detect common regions of white matter abnormality. Here we used activation likelihood estimation (ALE) to locate common regional abnormalities in white matter microintegrity in patients with MDD revealed by published DTI studies. Additionally, fibre tracking was performed to demonstrate the fascicles passing through the identified regions.
Methods
Data sources
We used a systematic search strategy to identify relevant studies. We performed online searches of the PubMed, MEDLINE, EMBASE, Cochrane Library, China Hospital Knowledge Database, Chinese Biomedical Literature Database, Index to Science and Technical Proceedings, China Conference Paper Database, National Technical Information Service and System for Information on Grey Literature for studies published between January 1994 and February 2011. Our search used systematic combinations of the following keywords: “DTI” or “diffusion tensor;” and “whole brain” or “voxel based;” and “depress*.” Additional studies were identified by manually searching the references of each article found in the electronic search.
Study selection
We assessed all studies yielded by our search for potential suitability. The abstracts were all written in English; if the articles were written in other languages, they were translated into English for assessment. Studies were included if they had been published as a peer-reviewed paper before February 2011, if they compared a group of patients with MDD with a healthy control group, if they used DTI with at least 6 directions, if they investigated fractional anisotropy differences in the whole brain (or whole white matter) and if they reported the 3-dimensional coordinates of changes in stereo-tactic space. Studies were excluded if they were case reports or reviews, if it was impossible to obtain the 3-dimensional coordinates in stereotactic space, if the data also contributed to another included publication and if the participants had comorbid conditions (e.g., Parkinson disease, mania or traumatic brain injury) or did not completely meet the diagnostic criteria for MDD. Particular care was taken to exclude multiple publications of data derived from the same patients or overlapping patient samples. We tried our best to contact authors for more information when necessary. Two of us (Y.L. and X.Q.H.) performed the searches and extracted the data independently; if agreement was not obtained, one of us (Q.Y.G.) mediated.
Data extraction
For quality assessment, we extracted the age, sex, diagnosis, medication, magnetic field strength, acquisition voxel size, number of diffusion directions and type of analysis from each included study. For the meta-analysis, we used the Lancaster transform in GingerALE15 to convert coordinates reported in Montreal Neurological Institute (MNI) stereotactic space to Talairach coordinates.16 Talairach coordinates that had been generated by the Brett transform applied to MNI coordinates were retransformed to Talairach space using the Lancaster transform.17
Meta-analysis
We used GingerALE version 2.0.4 to perform ALE meta-analysis on sets of coordinates extracted from the database in Talairach or MNI space. GingerALE is a coordinate-based meta-analysis method, supported within the BrainMap software environment, which is useful for identifying consistent regions of activation across a collection of studies. The ALE technique treats the reported foci not as points, but as spatial probability distributions centred on the given coordinates.18 In its revised version, the algorithm allows the weighting of studies in the meta-analysis on the basis of sample size. It also provides a random-effects model, rather than a fixed-effects model, that considers the spatial relationship between the individual foci reported for each study. Consequently, it gives more weight to the spatial conjunction of different foci from different studies than to different foci from a single study.19 In ALE, coordinates are modelled with a Gaussian function to accommodate spatial uncertainty and then analyzed to ascertain where coordinates converge.15 We set the false discovery rate at q < 0.05 and the minimum volume as 80 mm3 (see the GingerALE forum at www.brainmap.org/forum/).
Since GingerALE reports the results on grey matter, we used DTI Editor software to localize the correct regions for the reported coordinates. The resulting maps were registered to the standard template, and the reported coordinates were calculated to match the standard maps. We then localized the correct region using the corrected coordinates.
Sensitivity analysis was performed by reanalysis using GingerALE software after excluding the articles that used TBSS analysis.20–22
Fibre tracking
White matter tracts passing through clusters of voxels showing significant fractional anisotropy differences were mapped by reference to an atlas of human white matter anatomy23 and explored using DTIquery software. DTIquery is a novel set of interaction techniques that makes it easier to explore and interpret white matter pathways. It allows placement and interactive manipulation of box-shaped ROIs to display pathways passing through specific anatomic areas. Queries can be further restricted by numerical path properties such as length, mean fractional anisotropy and mean curvature.24 We used precomputed pathways from DTI data on a healthy 35-year-old man (the standard set provided by the software), mapped using streamlines tracking techniques and filtered by tract length and ROI.
Results
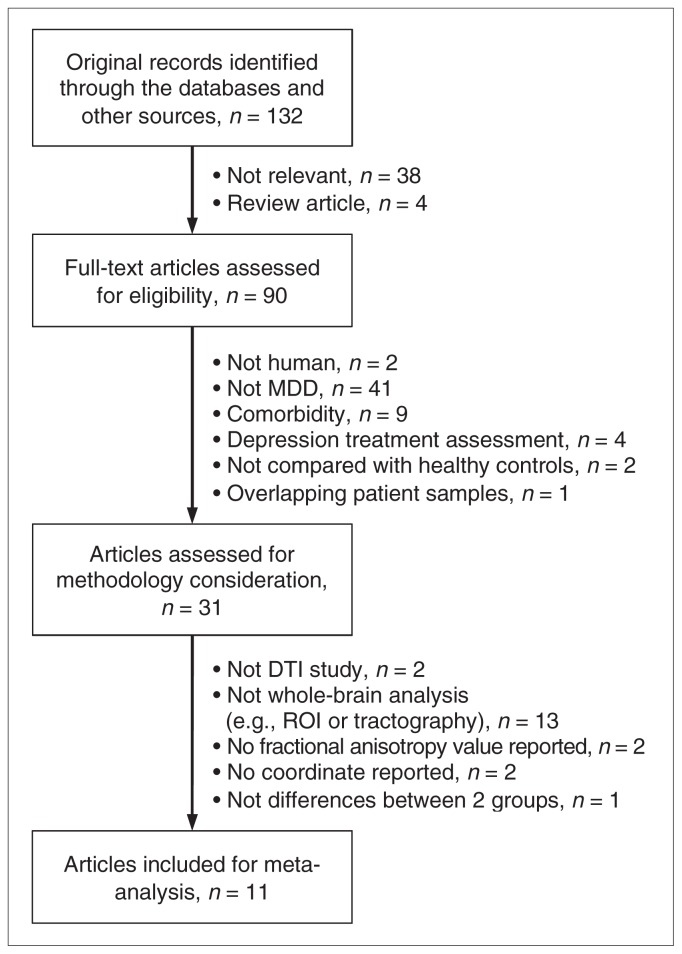
Our initial literature search yielded 132 original articles, and 11 were eligible for inclusion in the meta-analysis (Tables 1 and 2). They included a combined total of 231 patients with MDD and 261 controls and provided 50 coordinates of decreased fractional anisotropy. The flow of studies and reasons for exclusion are shown in Figure 1. There was no statistical difference in age and sex between the 2 groups: the mean age was 33.5 years in the patient group versus 34.1 years in the control group, there were 149 (65%) female patients versus 153 (59%) female controls.
Table 1.
Summary of studies included in the meta-analysis of diffusion tensor imaging studies of patients with MDD
| Controls | Patients | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Study | No. (female) | Age, mean (SD) yr | No. (female) | Age, mean (SD) yr | Diagnosis | Medication |
| Versace et al.20 | 24 (15) | 28 (9) | 16 (12) | 33 (10) | Recurrent MDD | Antidepressants |
| Blood et al.25 | 22 (12) | 35 (12) | 22 (12) | 36 (12) | MDD | Antidepressants |
| Liu et al.26 | 16 (12) | 30 | 16 (12) | 30 | MDD* | Antidepressants† |
| Ma et al.14 | 14 (12) | 27 (7) | 14 (12) | 29 (8) | First-episode MDD | Medication naive |
| Abe et al.27 | 42 (20) | 48 (13) | 21 (10) | 48 (14) | MDD | Not stated |
| Steele et al.28 | 14 (7) | 43 | 15 (11) | 46 | Recurrent MDD | Multiple medications‡ |
| Wu et al.29 | 21 (12) | 30 (8) | 23 (13) | 31 (9) | SSDE of MDD | Medication-naive |
| Zou et al.30 | 45 (30) | 31 (10) | 45 (30) | 32 (9) | Non–late onset MDD | Antidepressants |
| Ouyang et al.31 | 18 (9) | 27.0 (6.8) | 18 (9) | 27.4 (6.4) | MDD | Medication-naive |
| Zhu et al.21 | 25 (14) | 20.3 (1.7) | 25 (14) | 20.6 (1.9) | MDD* | Medication-naive |
| Kieseppä et al.22 | 20 (10) | 48.4 (0.3) | 16 (14) | 42.0 (11.6) | MDD | Medication-naive |
MDD = major depressive disorder; SD = standard devistion; SSDE = single shirt-duration episode.
The sample included only young patients (aged 21 to 35 years) with MDD.
2 patients took antidepressants.
Includes anticonvulsant mood stabilizers, antidepressants, antipsychotics, benzodiazepines and lithium treatment.
Table 2.
Scanning methods and decreased fractional anisotropy regions of the studies included in this meta-analysis of diffusion tensor imaging studies of patients with MDD
| Methods | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Study | Field, T | Acquisition voxel, mm3 | No. of directions | No. of coordinates | Type of analysis | Coordinate system | Area of significantly decreased fractional anisotropy |
| Versace et al.20 | 3 | Not stated | 6 | 1 | TBSS | MNI | Left inferior longitudinal fasciculi |
| Blood et al.25 | 3 | 1 × 1 × 1 | 6 | 12 | VBA | MNI | Right anterior cingulate cortex Dorsolateral prefrontal cortex |
| Liu et al.26 | 1.5 | Not stated | 12 | 13 | VBA | MNI | Middle temporal gyrus Middle frontal gyrus Fusiform gyrus et al. |
| Ma et al.14 | 1.5 | 1.9 × 1.9 × 4 | 13 | 4 | VBA | Talairach | Right middle frontal gyrus Left lateral occipitotemporal gyrus Right parietal lobe Right angular gyri of parietal lobe |
| Abe et al.27 | 1.5 | Not stated | 6 | 2 | VBA | MNI | Right anterior cingulate cortex Left frontal white matter |
| Steele et al.28 | 1.5 | 1.9 × 1.9 × 4 | 6 | 2 | VBA | MNI | Right lateral temporal cortex Left uncinate fasciulus |
| Wu et al.29 | 1.5 | 2 × 2 × 2 | 13 | 3 | VBA | MNI | Right superior longitudinal fasciculus Right middle frontal white matter Left inferior parietal lobe |
| Zou et al.30 | 3 | 1.9 × 1.9 × 3 | 15 | 2 | VBA | Talairach | Left internal capsule Left superior longitudinal fasciculus |
| Ouyang et al.31 | 1.5 | 2 × 2 × 2 | 13 | 6 | VBA | MNI | Bilateral medial frontal gyri Right subgyral frontal Temporal lobes Left middle frontal and cingulate gyri |
| Zhu et al.21 | 1.5 | 1 × 1 × 1 | 13 | 3 | TBSS | MNI | Left anterior limb of internal capsule Right parahippocampal gyrus Left posterior cingulate cortex |
| Kieseppä et al.22 | 1.5 | Not stated | 12 | 2 | TBSS | MNI | Left sagittal stratum Right anterior cingulum Right corpus callosum |
MDD = major depressive disorder; MNI = Montreal Neurological Institute; TBSS = tract-based spatial statistics; VBA = voxel-based analysis.
Fig. 1.
Flow chart showing the results of the search and reasons for exclusion of studies. DTI = diffusion tensor imaging; MDD = major depressive disorder; ROI = region of interest.
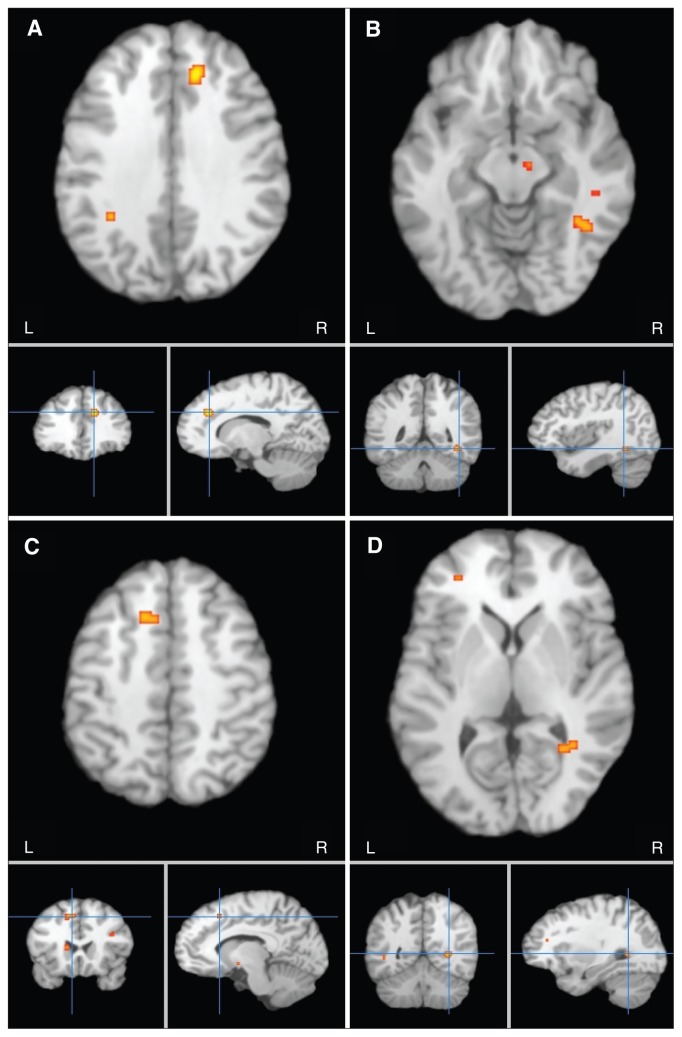
Meta-analysis of the reported coordinates identified 4 regions of decreased fractional anisotropy in patients compared with controls (Fig. 2 and Table 3).
Fig. 2.
Meta-analytic maps of fractional anisotropy reductions in patients with major depressive disorder; decreases in fractional anisotropy were localized in the (A) white matter of the right frontal lobe, (B) right fusiform gyrus, (C) left frontal gyrus and the (D) right occipital lobe.
Table 3.
Clusters of fractional anisotropy reductions in patients with major depressive disorder relative to healthy controls
| Talairach coordinate | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| No. | Volume, mm3 | x | y | z | Label | Fibre tracking |
| 1 | 416 | 14 | 32 | 30 | Right frontal white matter | Genu of the corpus callosum |
| 2 | 224 | 38 | −50 | −10 | Right fusiform gyrus white matter | Right inferior longitudinal fasciculus Right inferior fronto-occipital fasciculus Right posterior thalamic radiation |
| 3 | 224 | −12 | 18 | 44 | Left frontal white matter | Body of the corpus callosum |
| 4 | 200 | 28 | −56 | 2 | Right temporal lobe white matter | Right inferior fronto-occipital fasciculus |
Region 1 was located in the right frontal white matter (Talairach coordinates x, y, z = 14, 32, 30; cluster size 416 mm3). The ROI applied in DTIquery was a bounding box of 7.5 × 7.5 × 7.5 mm3 centred on the coordinates of maximum sum-rank. The main tracts passing through this region were the inter-hemispheric fibres running through the genu of the corpus callosum, shown as the yellow tracts on the first row in Figure 3.
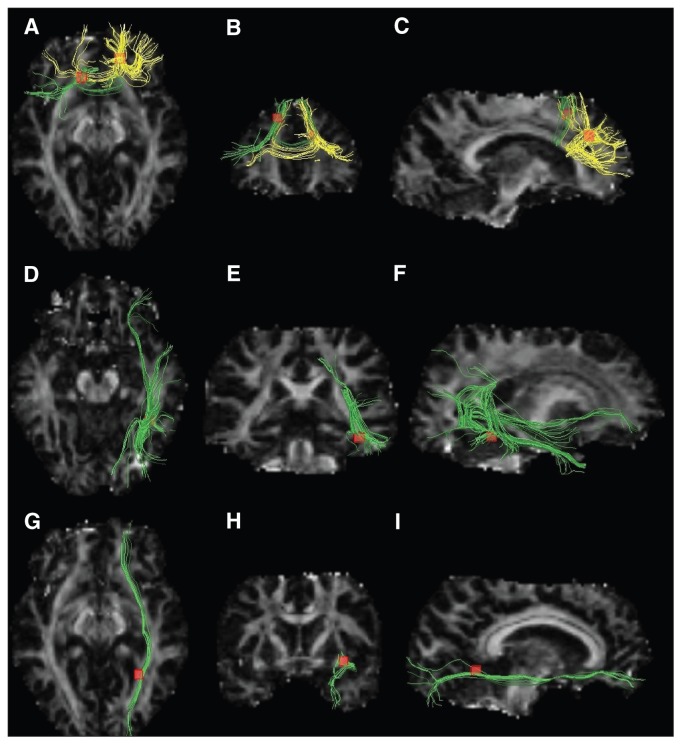
Fig. 3.
White mater diffusion tensor tracts traversing decreased fractional anisotropy clusters. Images on the left (A, D, G) are seen from above, medial images (B, E, H) are seen from behind and images on the right (C, F, I) are seen from the right. Fascicles traversing the right and left frontal white matter regions (yellow and green fibres, respectively, in the first row) were the genu and the body of the corpus callosum (A, B, C). Fascicles traversing the right fusiform gyrus white matter region were the right inferior longitudinal fasciculus, right interior fronto-occipital fasciculus and right posterior thalamic radiation (green fibres in the second row; D, E, F). Fascicles traversing the right occipital white matter region (yellow and green fibres, respectively, in the first row) were the right inferior fronto-occipital fasciculus (green tracts in the third row; G, H, I).
Region 2 was located in the white matter under the right fusiform gyrus of the temporal lobe (Talairach coordinates x, y, z = 38, −50, −10; cluster size 224 mm3). White matter tracts traversing this region were visualized using a 6.1 × 6.1 × 6.1 mm3 bounding box. The main tracts were the right inferior longitudinal fasciculus, right inferior fronto-occipital fasciculus and right posterior thalamic radiation, as shown on the second row in Figure 3.
Region 3 was in the left frontal white matter (Talairach coordinates x, y, z = −12, 18, 44; cluster size 224 mm3). White matter tracts traversing this region were the interhemispheric fibres passing through the body of the corpus callosum, shown as the green tracts on the first row in Figure 3, using a 6.1 × 6.1 × 6.1 mm3 bounding box.
Region 4 was in the right occipital lobe white matter (Talairach coordinates x, y, z = 28, −56, 2; cluster size 200 mm3). White matter tracts traversing this region were the right inferior fronto-occipital fasciculus, shown on the third row in Figure 3, using a 5.8 × 5.8 × 5.8 mm3 bounding box.
For sensitivity testing, we excluded 3 articles that used TBSS analysis20–22 and then reanalyzed using GingerALE software. The result was consistent with the original meta-analysis of all 11 articles.
Discussion
This meta-analysis identified 4 consistent locations of decreased fractional anisotropy in patients with MDD: the right frontal white matter, the white matter under the right fusiform gyrus of the temporal lobe, the left frontal white matter and the white matter under the right occipital lobe. We discuss the roles of some of these structures and their possible importance in MDD.
To assess study quality, we extracted the number of participants in the control group and their ages; and the number of participants in the MDD group and their ages, diagnoses and medications (Table 1). All the included studies were case–control studies, and they all matched participant groups for age and sex. All patients met the standard criteria for depression of the DSM-IV. For the index of depression severity, 8 of the 11 studies used the Hamilton Rating Scale for Depression;32 the other 3 used the Beck Depression Inventory,14,22 the Beck Anxiety Inventory22 and the Chinese version of the Center for Epidemiologic Studies Depression Scale.21 All the included studies used at least 6 directions for DTI scanning (the minimum requirement for a qualitative DTI calculation) and also applied whole-brain analysis; studies that did not use whole-brain analysis were excluded to avoid bias. However, we cannot rule out that some potential missing confounders might result in bias.
The corpus callosum connects the left and right cerebral hemispheres and is the largest fibre bundle of the human brain. It is divided into an anterior portion (genu) connecting the prefrontal and orbitofrontal regions, a central part (body) connecting precentral frontal regions and parietal lobes, and a posterior portion connecting the occipital lobes (splenium) and temporal lobes (tapetum). The corpus callosum allows information transfer between hemispheres and is involved in several motor, perceptual and cognitive functions.33–35 The fibre bundles belonging to the central part of the corpus callosum recognized by the present study were the only region in the left hemisphere found by meta-analysis to have decreased fractional anisotropy. This may suggest a role for functional impairment of frontal lobe information transfer between hemispheres.
The fusiform gyrus is part of the temporal lobe in Brodmann area 37. Its functions are processing of colour information, face and body recognition, word recognition and within-category identification. It has been proposed that the right hemisphere “fusiform face area” acts as an isolated processing system for faces. One study has suggested that the role of the fusiform gyrus and prefrontal cortex in memory processing may contribute to cognitive vulnerability to depression.36 Right fusiform activation in response to happy faces is reduced in patients with MDD compared with healthy controls,37 and modulation of activity in this face-processing sensory area has been suggested to contribute to changes in the attentional salience of such emotional stimuli.38 Our finding of disrupted white matter integrity under this gyrus provides structural evidence of impairment in the neural circuit responsible for face recognition in patients with MDD from a brain connectivity point of view.
The inferior longitudinal fasciculus is an associative bundle connecting the occipital and temporal lobes. The long fibres connect visual areas to the amygdala and hippocampus,39 the main components of the limbic system related to emotional behaviour. The inferior longitudinal fasciculus is involved in face recognition,40 visual perception,41 reading,42 visual memory43 and other functions related to language.44 The inferior fronto-occipital fasciculus is a ventral associative bundle connecting the ventral occipital lobe and the orbitofrontal cortex.45 Its occipital component runs parallel to the inferior longitudinal fasciculus. On approaching the anterior temporal lobe, the fibres of the inferior fronto-occipital fasciculus gather together and enter the external capsule dorsally to the fibres of the uncinate fasciculus. The functions of the inferior fronto-occipital fasciculus are poorly understood, although it is possible that it is involved in reading,42,44 attention44 and visual processing.40,46
The thalamic radiations are 2-way fibre connections between the thalamus and cerebral cortex, grouped into 4 (anterior, superior, posterior and inferior) fan-like radiations from the thalamus to most regions of the cortex, forming a major part of the internal capsule and corona radiata. The posterior thalamic radiation joins the occipital and posterior parietal cortex with the posterior thalamus, including the pulvinar, and includes the optic radiation from the lateral geniculate body. It occupies the retrolenticular part of the internal capsule and includes fibres passing in both directions between the occipital lobe and the thalamus, in particular those connecting the lateral geniculate nucleus with the primary visual area, and the lateral posterior and pulvinar nuclei with the association areas of the occipital lobe. All aforementioned fascicles gave further evidence of the pathophysiological role of the occipital lobe, which may be related to dysfunctional facial recognition in patients with MDD, as shown by other functional MRI studies.
Cortical regions, including the anterior cingulate, orbitofrontal and prefrontal cortices are decreased in volume in patients with MDD,47 and frontal–subcortical circuits are thought to play a key role in the regulation of motor, cognitive and motivational processes.4 Five anatomically and functionally discrete parallel loops connect specific areas of the prefrontal cortex and interconnect with functionally related brain areas.48 Among them, structural and functional abnormalities within neural systems, including frontal–subcortical circuits, are associated with the neurobiology of depression.48,49 White matter abnormality can be seen as the substantial basis of the network dysfunction. In particular, it has been hypothesized that microstructural changes in the white matter of frontal–subcortical circuits lead to a “disconnection syndrome” between frontal and subcortical regions.9,13,50 Converging evidence from postmortem and in vivo studies suggests that frontolimbic dysfunction plays an important role in the pathophysiology of MDD,51–53 whereas executive dysfunction, associated with compromised integrity of frontal structures and their subcortical connections, has also been reported in late-life depression.54 Recently, a thorough review of the neurocircuitry involved in depression combining functional and structural imaging with lesion evidence and histological material postulated that a system that links the medial prefrontal cortex and other related cortical areas to subcortical areas, such as the amygdala, the ventral striatum and pallidum, the medial thalamus and the hypothalamus, is centrally involved in mood disorders.55 The present meta-analysis gives strong evidence of a structural basis for the involvement of this neurocircuitry.
Considering the results from our meta-analysis that point to white matter impairment in multiple neural fascicules, we suggest that depression may indeed be considered a “disconnection syndrome.”
Limitations
This meta-analysis used a relatively robust approach for identifying consistent regional changes between studies. Voxel-based analysis avoids a priori bias in the identification of regional white matter abnormalities by analyzing whole-brain white matter. However, the approach has potential limitations. As the number of studies included in this meta-analysis was small, we should interpret the results with caution. For example, patient characteristics, including illness duration, drugs and onset time, may contribute to the diversity of results, but we were not able to perform separate meta-analyses for clinical variables. Given our relatively strict criteria for participant selection, our results should be representative of patients with MDD. Moreover, study results may be heterogeneous in terms of data acquisition and analysis techniques. The accuracy of the diffusion tensor and thus the reliability of the data derived from it are influenced by the signal-to-noise ratio, image resolution, image distortion due to magnetic susceptibility effects and motion artifacts. An on-site study with a very large sample size would be required to minimize this kind of technical artifact. Lastly, the ALE technique cannot incorporate information about negative findings and thus may be susceptible to publication bias.
Conclusion
Numerous DTI studies have identified white matter abnormalities in patients with MDD from a voxel-based perspective. By performing a meta-analysis, we located the most consistent positive findings in patients with MDD within the white matter of right frontal white matter, right fusiform gyrus, left frontal and right occipital lobe. The decrease of white matter fractional anisotropy is suggestive of loss of integrity of white matter fibre tracts linking the prefrontal lobe with the cortex in the temporal lobe, occipital lobe and subcortical areas, such as the amygdala and hippocampus. Although limitations arose from grouping heterogeneous studies, common white matter abnormalities that provide evidence for the disconnection in patients with MDD could be detected regardless of age, sex and medication status. Our results demonstrate that some regions identified in individual studies may not meet the threshold in the aggregate analysis. Thus we should interpret results from any single study with a small sample size with caution. Future studies with much larger sample sizes may be more robust in delineating disease-specific brain connectivity characteristics in patients with MDD.
Acknowledgements
This study was supported by National Natural Science Foundation (Grant Nos. 81171488 and 81030027) and the National Key Technologies R&D Program of China (Program No: 2012BAI01B03). We would also like to thank Dr. Jun Li for her suggestions regarding statistical methodology.
Footnotes
Competing interests: None declared.
Contributors: Y. Liao, X. Huang and Q. Gong designed the study and wrote the article. Y. Liao, X. Huang, Q. Wu, C. Yang and M. Du acquired the data, which Y. Liao, X. Huang, Q. Wu, C. Yang, W. Kuang, S. Lui, Q. Yue, R.C.K. Chan and G.J. Kemp analyzed. All authors reviewed the article and approved its publication.
References
- 1.Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Curr Opin Psychiatry. 2006;19:25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- 2.Koch MA, Norris DG, Hund-Georgiadis M. An investigation of functional and anatomical connectivity using magnetic resonance imaging. Neuroimage. 2002;16:241–50. doi: 10.1006/nimg.2001.1052. [DOI] [PubMed] [Google Scholar]
- 3.Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–40. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66:814–23. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Taylor WD, Hsu E, Krishnan K, et al. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol Psychiatry. 2004;55:201–7. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 7.Mamata H, Mamata Y, Westin CF, et al. High-resolution line scan diffusion tensor MR imaging of white matter fiber tract anatomy. AJNR Am J Neuroradiol. 2002;23:67–75. [PMC free article] [PubMed] [Google Scholar]
- 8.Shimony JS, McKinstry RC, Akbudak E, et al. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212:770–84. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Kiosses D, Choi S, et al. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–32. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 10.Taylor WD, MacFall JR, Payne ME, et al. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–6. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 11.Nobuhara K, Okugawa G, Sugimoto T, et al. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–2. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Huang X, Hong N, et al. White matter microstructural abnormalities in late-life depression. Int Psychogeriatr. 2007;19:757–66. doi: 10.1017/S1041610207004875. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Ma N, Li Z, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–8. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 14.Ma N, Li L, Shu N, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164:823–6. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- 15.Laird AR, Eickhoff SB, Kurth F, et al. ALE meta-analysis work-flows via the BrainMap database: progress towards a probabilistic functional brain atlas. Front Neuroinform. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York (NY): Thieme Medical Publishers; 1988. [Google Scholar]
- 17.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Turkeltaub PE, Eden GF, Jones KM, et al. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 19.Eickhoff SB, Laird AR, Grefkes C, et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Versace A, Almeida JRC, Quevedo K, et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry. 2010;68:560–7. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Wang X, Xiao J, et al. Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res. 2011;1369:223–9. doi: 10.1016/j.brainres.2010.10.104. [DOI] [PubMed] [Google Scholar]
- 22.Kieseppä T, Eerola M, Mäntylä R, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010;120:240–4. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Mori S, Wakana S, Van Zijl PCM, et al. MRI atlas of human white matter. Amsterdam (The Netherlands): Elsevier; 2005. [Google Scholar]
- 24.Akers D, Sherbondy A, Mackenzie R, et al. Exploration of the brain’s white matter pathways with dynamic queries. Proceedings of the conference on visualization 2004; 2004 Oct. 10–15; Austin (TX). Washington (DC): IEEE Computer Society; 2004. [Google Scholar]
- 25.Blood AJ, Iosifescu DV, Makris N, et al. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS ONE. 2010;5:e13945. doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Wang Y, Liu H, et al. [Diffusion tensor imaging and resting state functional magnetic resonance imaging on young patients with major depressive disorder]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:25–31. doi: 10.3969/j.issn.1672-7347.2010.01.004. [Article in Chinese] [DOI] [PubMed] [Google Scholar]
- 27.Abe O, Yamasue H, Kasai K, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 2010;181:64–70. doi: 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Steele JD, Bastin M, Wardlaw J, et al. Possible structural abnormality of the brainstem in unipolar depressive illness: a transcranial ultrasound and diffusion tensor magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 2005;76:1510–5. doi: 10.1136/jnnp.2004.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu F, Tang Y, Xu K, et al. Whiter matter abnormalities in medication-naïve subjects with a single short-duration episode of major depressive disorder. Psychiatry Res. 2011;191:80–3. doi: 10.1016/j.pscychresns.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou K, Huang X, Li T, et al. Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:525–30. [PMC free article] [PubMed] [Google Scholar]
- 31.Ouyang X, Tao H, Liu H, et al. White matter integrity deficit in treatment-naïve adult patients with major depressive disorder. East Asian Arch Psychiatry. 2011;21:5–9. [PubMed] [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glickstein M, Berlucchi G. Classical disconnection studies of the corpus callosum. Cortex. 2008;44:914–27. doi: 10.1016/j.cortex.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Doron KW, Gazzaniga MS. Neuroimaging techniques offer new perspectives on callosal transfer and interhemispheric communication. Cortex. 2008;44:1023–9. doi: 10.1016/j.cortex.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Balsamo M, Trojano L, Giamundo A, et al. Left hand tactile agnosia after posterior callosal lesion. Cortex. 2008;44:1030–6. doi: 10.1016/j.cortex.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 36.van Wingen GA, van Eijndhoven P, Cremers HR, et al. Neural state and trait bases of mood-incongruent memory formation and retrieval in first-episode major depression. J Psychiatr Res. 2010;44:527–34. doi: 10.1016/j.jpsychires.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Surguladze S, Brammer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–9. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 38.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Catani M, Jones DK, Donato R. Occipito-temporal connections in the human brain. Brain. 2003;126:2093. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 40.Fox CJ, Iaria G, Barton JJS. Disconnection in prosopagnosia and face processing. Cortex. 2008;44:996–1009. doi: 10.1016/j.cortex.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Ffytche DH. The hodology of hallucinations. Cortex. 2008;44:1067–83. doi: 10.1016/j.cortex.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Epelbaum S, Pinel P, Gaillard R, et al. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44:962–74. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Ross ED. Sensory-specific amnesia and hypoemotionality in humans and monkeys: gateway for developing a hodology of memory. Cortex. 2008;44:1010–22. doi: 10.1016/j.cortex.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–61. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catani M. From hodology to function. Brain. 2007;130:602–5. doi: 10.1093/brain/awm008. [DOI] [PubMed] [Google Scholar]
- 46.Rudrauf D, Mehta S, Grabowski TJ. Disconnection’s renaissance takes shape: formal incorporation in group-level lesion studies. Cortex. 2008;44:1084–96. doi: 10.1016/j.cortex.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 47.aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180:305–13. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 49.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 50.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuro-imaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 52.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–54. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 53.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–52. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 54.Lesser IM, Boone K, Mehringer C, et al. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153:1280–7. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- 55.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsycho-pharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]