Abstract
Background
Without stimulation, the human brain spontaneously produces highly organized, low-frequency fluctuations of neural activity in intrinsic connectivity networks (ICNs). Furthermore, without adequate explanatory nociceptive input, patients with somatoform pain disorder experience pain symptoms, thus implicating a central dysregulation of pain homeostasis. The present study aimed to test whether interactions among pain-related ICNs, such as the default mode network (DMN), cingular–insular network (CIN) and sensorimotor network (SMN), are altered in somatoform pain during resting conditions.
Methods
Patients with somatoform pain disorder and healthy controls underwent resting functional magnetic resonance imaging that lasted 370 seconds. Using a data-driven approach, the ICNs were isolated, and the functional network connectivity (FNC) was computed.
Results
Twenty-one patients and 19 controls enrolled in the study. Significant FNC (p < 0.05, corrected for false discovery rate) was detected between the CIN and SMN/anterior DMN, the anterior DMN and posterior DMN/SMN, and the posterior DMN and SMN. Interestingly, no group differences in FNC were detected.
Limitations
The most important limitation of this study was the relatively short resting state paradigm.
Conclusion
To our knowledge, our results demonstrated for the first time the resting FNC among pain-related ICNs. However, our results suggest that FNC signatures alone are not able to characterize the putative central dysfunction underpinning somatoform pain disorder.
Introduction
Somatoform pain disorder is a mental disorder characterized by chronic bodily complaints without sufficient explanatory peripheral pathology.1 Although the causes and mechanisms behind this mental disorder remain unclear, both functional and structural alterations in the limbic structures seem to correlate with this non-nociceptive chronic pain condition.2–4 Moreover, human brain imaging studies have revealed new roles that cortical neuronal networks play in chronic pain,5 including the unpleasant quality of pain.6 The current study expanded upon a new approach for testing one important facet of the network model to examine the intrinsic functional connectivity between networks active during resting state: the functional network connectivity (FNC).7
The human brain’s resting state is characterized by low-frequency fluctuations of spontaneous neural activity.8 Without stimulation, this activity is highly organized in several intrinsic connectivity networks (ICNs).9 Some of the ICNs appear to be pain-related, such as the default mode network (DMN), which comprises cortical midline structures and lateral parietal regions,10–12 the cingular-insular network (CIN), and the sensorimotor network (SMN).8,13–19 There is interplay among the regions within an ICN and among the ICNs themselves. As shown recently in individuals with schizophrenia, differences in internetwork communication regarding FNC could be a valid measure that reflects the deficiencies in cortical processing in patients with chronic psychiatric symptoms.20 Therefore, we aimed to test the practical relevance of FNC for chronic, medically unexplained pain. Specifically, given a central disconnection of pain-related neural systems, we hypothesized that alterations exist in the FNC between the DMN, CIN and SMN in patients with somatoform pain disorder.
Methods
This study was approved by the local ethics committee (Ethikkommission der Fakultaet fuer Medizin der Technischen Universitaet Muenchen) and conducted in accordance with the Declaration of Helsinki. We obtained written informed consent from all participants. Healthy controls were recruited from the general community. All patients had pain-predominant multi-somatoform disorder12,21 and were recruited from outpatient departments of neurology, internal medicine and pain treatment centres. Pain-predominant multisomatoform disorder, a medium–severe somatoform disorder, was primarily diagnosed by an experienced physician (M.N.-H.), who performed a modified Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), verifying the official criteria for somatoform and chronic pain disorder. We modified the interview to check for the presence of multisomatoform disorder according to the published criteria.22 The main feature of somatoform disorders is the repeated presentation of physical symptoms with persistent requests for medical examinations, despite repeated negative findings and reassurances by doctors that the symptoms have no physical basis. If any physical disorders are present, the disorders do not explain the nature and extent of the symptoms or the distress and preoccupation of the patient.23 Multisomatoform disorder is defined as “3 or more medically unexplained, currently bothersome physical symptoms plus a long (≥ 2 years) history of somatization.”22 It has been shown that, compared with mood and anxiety disorders, multisomatoform disorder is associated with comparable impairments in health-related quality of life, more self-reported disability days and clinic visits, and the highest level of provider frustration.22,24
In this context, as a precondition, the physical component summary (PCS) measure25 in our patient group was required to be 1 standard deviation [SD] or more below the population norm (i.e., ≤ 40, as measured by the SF-36), thus meeting the DSM-IV criterion B for significant distress or psychosocial impairment due to the somatoform pain in patients with pain disorder.1 The second precondition was that the score on the 15-item Patient Health Questionnaire (PHQ-15) had to be greater than 10, which represents medium somatic symptom severity. We used the German version of the Brief Pain Inventory26 to estimate the intensity of the participant’s pain. We excluded patients with insufficient cognitive abilities, severe chronic somatic diseases, unambiguous nociceptive pain (postsurgical or phantom limb pain), hypochondria, posttraumatic stress disorder (PTSD), a severe comorbid mental disorder that caused major social functioning impairment (e.g., schizophrenia or severe substance abuse), or insufficient German language skills. We assessed handedness using the Edinburgh Handedness Inventory.27
Psychometric measurement
The occurrence of somatoform disorder was assessed according to a modified structured psychiatric interview based on the German version of the SCID-I.28 The SCID-I evaluates the present (i.e., the 4 weeks preceding the interview) and lifetime psychiatric status for major Axis I psychiatric disorders using criteria that correspond with the DSM-IV.1
The SF-36 is a multipurpose, short-form health survey comprising 36 questions.29 It yields an 8-scale profile of functional health and well-being scores, psychometrically based physical and mental health summary measures, and a preference-based health utility index. This questionnaire is a generic measure instead of one that targets a specific age, disease or treatment group. Accordingly, the SF-36 has been proven useful in surveys of general and specific population groups because it compares the relative burden of disease and differentiates the health benefits of a wide range of treatments.30 Its German translation has been validated in a variety of German health care settings.31,32 The PCS subscore of the SF-36 has been shown to be a valid and change-sensitive indicator of bodily function and quality of life;33 moreover, it addresses the major concerns of our patients more directly than the mental component summary.34
The PHQ-1535,36 is a brief, self-administered questionnaire that is useful in screening for somatization and monitoring the severity of somatic symptoms in clinical practice and research. Scores of 5, 10 and 15 represent the cutoff values for low, medium and high somatic symptom severity, respectively.
The Brief Pain Inventory (BPI)37 was developed by the Pain Research Group of the World Health Organization Collaborating Centre for Symptom Evaluation in Cancer Care to provide information on the intensity of pain (the sensory dimension) and degree to which pain interferes with function (the reactive dimension). The validity of the BPI has been demonstrated in both the German version26 and for measuring pain in patients without cancer.38 The BPI item scores for each patient are provided in Appendix, Table S1, available at cma.ca/jpn.
The Beck Depression Inventory (BDI-I)39,40 is a 21-item self-report instrument that measures cognitive and endogenous aspects of depression on a 4-point scale ranging from 0 to 3. The standard cutoffs are as follows: a total score of 0–9 indicates no depression, 10–18 indicates mild depression, 19–29 indicates moderate depression and a score of 30 or greater indicates severe depression. This questionnaire has undergone extensive reliability and validation studies.
According to the homepage of the publishing house Pearson Assessments,41 “the Symptom Checklist-90-R (SCL-90-R) instrument helps evaluate a broad range of psychological problems and symptoms of psychopathology. The instrument is also useful in measuring patient progress or treatment outcomes.” The 90 items of the German version of this checklist are scaled from 0 to 4 and are associated with problems that the patient has been experiencing during the last 7 days.42 The summarizing global severity index is a de facto standard for psychotherapy clinical practice and research, and it serves as a “symptom severity thermometer.” The 9 specific subscales of the SCL-90 (e.g., SOM: somatization) provide an overview of the spectrum of patient complaints.43
Functional MRI resting state paradigm
Participants were asked to stay awake but close their eyes and relax for 370 seconds. After the scanning session, participants were asked whether they had fallen asleep during the scan. Patients who responded positively or ambiguously were excluded from the study.
Data acquisition and fMRI procedures
Images were acquired using a 3 T Philips Achieva scanner with a standard 8-channel SENSE head coil. Thirty-two contiguous slices (no gap) were acquired with a steep angulation, such that the eyes were excluded, using a gradient echo-planar sequence with the following parameters: repetition time (TR) 2000 ms, echo time (TE) 35 ms, 82° flip angle, field of view (FOV) 220 mm, slice thickness 4 mm, 80 × 80 matrix, 2.75 × 2.75 mm voxel size, and SENSE factor 2. Anatomic images were obtained using a T1-weighted turbo gradient echo sequence with the following parameters: TR 9 ms, TE 4 ms, 8° flip angle, FOV 240 mm, 240 × 240 matrix, 1 mm isotropic voxel size, 170 slices and no gap.
Image processing and data analysis: preprocessing
The data analysis was performed using the SPM5 (Statistical Parametric Mapping software, Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk). We discarded the first 3 images of each run to allow for equilibration of the longitudinal magnetization. The preprocessing steps included
the realignment and unwarping of the images to correct for movement artifacts and related susceptibility artifacts,
a coregistration of the anatomic to the functional images,
the segmentation and normalization of the anatomic image to the standard stereotactic space (Montreal Neurological Institute [MNI]),4
the application of a normalization transformation to the functional images, and
the smoothing with a 8 mm Gaussian kernel for the group analysis.
Connectivity analysis
We performed an independent component analysis (ICA) on all participants (patients and controls) using the group ICA from the fMRI toolbox (GIFT version 1.3h; http://icatb.sourceforge.net) developed for fMRI data analysis.44 Following the method of Jafri and colleagues,20 we additionally performed 2 separate group ICAs on patients and controls “to ensure that the resulting components had similar resting state fluctuations in the 2 groups, as in the resulting components attained from all [...] participants combined.”20 For group comparisons, however, a separate group ICA may not be optimal because it biases toward false-positive results of group differences.45 Therefore, we reported and used the data of the combined ICA for group comparisons.
First, the individual data sets were concatenated across time. This was followed by computing the subject-specific components and time courses. The toolbox performed the analysis in 3 stages: data reduction, application of the ICA algorithm and back reconstruction for each participant.44 In the initial step, the data from each participant underwent principal component analysis to reduce the computational complexity. Thus, most of the informational data content was preserved. After concatenating the resulting volumes, 29 independent sources were estimated using the GIFT dimensionality estimation tool based on the aggregated data. The final reduction was again achieved using principal component analysis according to the selected number of components. In the second stage of the analysis, we used the Infomax algorithm to run the ICA and a mask based on all participants. In the final stage of back reconstruction, the time courses and spatial maps were computed for each participant. The resulting mean spatial maps for each participant were transformed to z scores for display.44
Individual participant maps of the ICNs were entered into 1-sample t tests for 1-group analyses and 2-sample t tests for group comparison in SPM5. Results were thresholded at p = 0.05 and corrected for family-wise error with a cluster extent threshold of 50 voxels.
Functional network connectivity
The functional networks isolated by ICA are both spatially and temporally independent.44 However, temporal correlations can exist between the networks. To measure this functional network connectivity (FNC), we computed a constrained maximal lagged correlation using the FNC toolbox (http://mialab.mrn.org/software/#fnc).20 Next, the maximal lagged correlation was assessed between all pair-wise combinations of the 4 ICNs selected for the analysis, which led to 6 possible combinations.
We calculated the correlation between the 2 time courses using the following formula, where ρ is the correlation between 2 time courses, X is time course 1 (dimension T × 1 unit), Y is time course 2 (dimension T × 1 unit), T is the number of time points in the time course, io is the starting reference of the 2 original time courses, Δi is the noninteger change in time in seconds, Xio is X at the initial reference point io, Yio+Δi is Y shifted from the reference point io, ρΔi is the maximal lagged correlation and Δi is the lag between the time courses in seconds:20
The correlation and lag values were computed for all participants and then averaged for the controls and patients. The correlation value reflects the dependency between 2 resting state networks. Significant correlation combinations from the 6 possible combinations were separately extracted for both groups, which led to FNC maps for each group (t test, p < 0.05). In addition, corresponding to the significant correlation combinations, the averaged lag values, which represent the amount of delay between 2 correlated component time courses, were calculated for each group.20
Group difference
Significant differences in the FNC between patients and controls were calculated using a 2-sample t test (p < 0.05, corrected for false discovery rate).46 The lag values were compared between both groups (2-sample t test, p < 0.05, corrected for false discovery rate).
Correlation analysis
The FNC was correlated with the BDI and BPI scores (p < 0.05, corrected for multiple comparisons).
Results
In all, 19 healthy controls (mean age 48.79 [SD 12.25] yr; 12 women) and 21 outpatients (mean age 46.62 [SD 12.49] yr; 17 women) were involved in this study. All participants were native speakers of German and were of Caucasian origin. All participants were right-handed. Participant demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of healthy controls and patients with somatoform pain
| Group; mean (SD) [range]* | ||
|---|---|---|
|
|
||
| Characteristic | Controls | Patients |
| Age, yr | 48.79 (12.25) [24–64] | 46.62 (12.49) [22–68] |
| Sex, no. male:female | 7:12 | 4:17 |
| Medication, no. | ||
| Antidepressants | — | 10 |
| Analgesics/relaxants/NSAIDs | — | 10 |
| Anxiolytics | — | 1 |
| BDI score | 4.43 (4.70)† [0–16] | 17.84 (9.03)† [3–37] |
| BPI item (scale) | ||
| 1: Pain within the last week (yes/no) | 19 no† | 21 yes† |
| 2: Pain today (yes/no) | 19 no† | 21 yes† |
| 3: Pain at its worst during the last week (0–10) | — | 7 (2.25)† |
| 4: Pain at its least during the last week (0–10) | — | 4.21 (2.5)† |
| 5: Pain on the average (0–10) | — | 5.63 (2.1)† |
| 6: Pain right now (0–10) | — | 5.53 (2.9)† |
| 8: Pain relief by therapy (0–10) | — | 5.50 (2.8)† |
| 9: Impairment (0–10) | — | |
| 9A: General activity | — | 5.74 (2.6)† |
| 9B: Mood | — | 4.84 (2.9)† |
| 9C: Walking ability | — | 4.32 (3.1)† |
| 9D: Normal work | — | 5.37 (2.5)† |
| 9E: Relation with other people | — | 4 (2.6)† |
| 9F: Sleep | — | 4.89 (3.0)† |
| 9G: Enjoyment of life | — | 4.86 (2.8)† |
| SCL-90-R | ||
| Global severity index | 0.28 (0.28)† | 0.96 (0.56)† |
| Somatization | 0.34 (0.31)† | 1.4 (0.64)† |
Before the fMRI scan, the mean value of pain intensity among participants with somatoform pain disorder (item 5) using the BPI was 7 of 10 (SD 2.24). All of the patients with chronic pain but none of the controls experienced persistent somatoform pain throughout the scan (Table 1 and Appendix 1, Table S1).
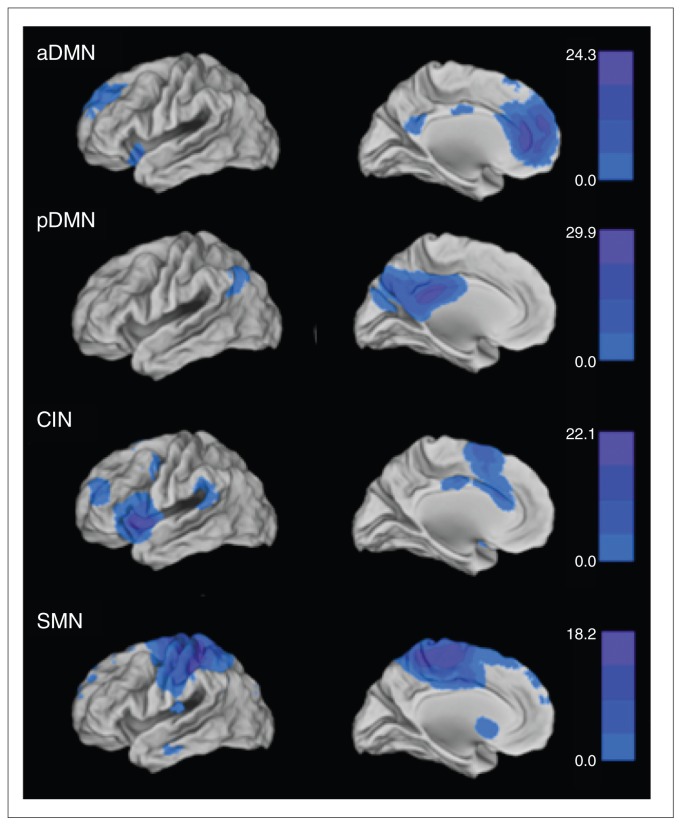
In accordance with published results, we identified the following pain-related networks by visual inspection (Fig. 1 and Table 2):
Fig. 1.
Intrinsic connectivity networks (ICNs) of the entire participant group (19 healthy controls and 21 patients with somatoform pain): anterior default mode network (aDMN), posterior default mode network (pDMN), cingular-insular network (CIN) and sensorimotor network (SMN). For illustration purposes, the spatial maps of the patients and controls were concatenated into SPM5 and thresholded at p < 0.05, corrected for family-wise error; the colour bars represent t values.
Table 2.
Intrinsic connectivity networks*
| Network | Region | MNI coordinate† | Cluster size, voxels | t value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| Anterior default mode network | Left anterior cingular cortex | −2 | 46 | 6 | 7559 | 24.33 |
| Left gyrus frontalis inferior, pars orbitalis | −34 | 18 | −20 | 328 | 10.34 | |
| Left precuneus | −6 | −54 | 24 | 180 | 10.26 | |
| Right gyrus frontalis inferior, pars orbitalis | 38 | 24 | −16 | 379 | 10.20 | |
| Left middle cingular cortex | 0 | −14 | 36 | 115 | 9.89 | |
| Right precuneus | 6 | −52 | 24 | 30 | 7.52 | |
| Right thalamus | 4 | −16 | 6 | 49 | 7.03 | |
| Left gyrus parahippocampalis | −22 | −28 | −14 | 8 | 6.38 | |
| Posterior default mode network | Right posterior cingular cortex | 6 | −42 | 26 | 7846 | 29.88 |
| Left gyrus angularis | −42 | −62 | 40 | 686 | 10.17 | |
| Right gyrus angularis | 38 | −58 | 38 | 423 | 7.69 | |
| Left gyrus temporalis medius | −54 | −10 | −18 | 3 | 6.20 | |
| Cingular–insular network | Left insula | −40 | 16 | −6 | 2940 | 22.08 |
| Right supplementary motor area | 2 | 12 | 64 | 2642 | 17.01 | |
| Right gyrus frontalis inferior, pars orbitalis | 40 | 24 | −12 | 2046 | 16.39 | |
| Left gyrus frontalis medius | −36 | 52 | 18 | 765 | 10.63 | |
| — | −2 | −16 | −44 | 211 | 10.56 | |
| Left gyrus supramarginalis | −60 | −42 | 24 | 295 | 8.97 | |
| Left precentral gyrus | −40 | −2 | 54 | 242 | 8.93 | |
| Right gyrus supramarginalis | 62 | −40 | 26 | 150 | 8.06 | |
| Left gyrus frontals inferior, pars opercularis | −52 | 14 | 32 | 41 | 7.37 | |
| Right gyrus frontalis medius | 30 | 50 | 22 | 72 | 7.03 | |
| Right precentral gyrus | 46 | 6 | 48 | 19 | 6.89 | |
| Right gyrus temporalis medius | 52 | −22 | −12 | 12 | 6.21 | |
| Sensorimotor network | Right precentral gyrus | 24 | −16 | 70 | 16580 | 18.19 |
| Right insula | 34 | −24 | 14 | 48 | 8.19 | |
| — | −2 | 10 | −4 | 16 | 6.82 | |
| Right gyrus temporalis inferior | 52 | −66 | −6 | 3 | 5.96 | |
MNI = Montreal Neurological Institute.
p < 0.05, corrected for family wise error.
Determined using the Wake Forest University Pickatlas (http://fmri.wfubmc.edu/software/PickAtlas).
the anterior default mode network (aDMN), which consists of the cortical midline structures, such as the medial pre-frontal cortex and precuneus;15–17,47
the posterior default mode network (pDMN), which consists of the lateral parietal regions and precuneus;15–17,47
the CIN, which consists of both the insular and cingular cortex;13,19 and
the SMN, which consists of the pre- and postcentral gyrus.14
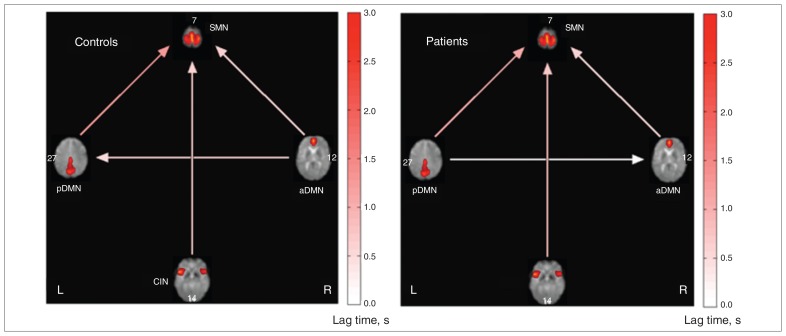
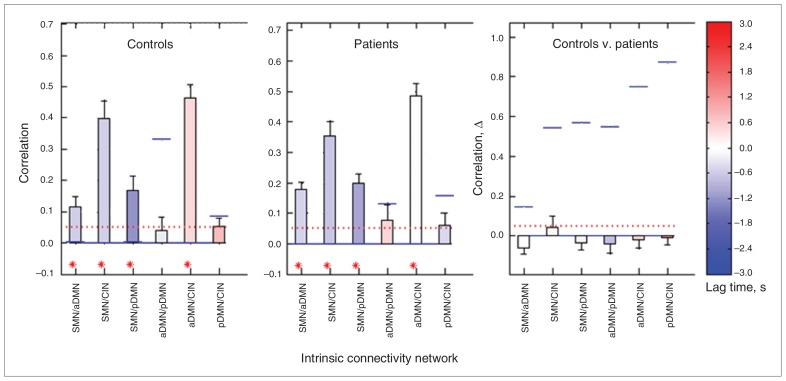
The FNCs of the patients with chronic pain and the control group are shown in Figure 2. Both groups showed a significant FNC between the CIN and SMN, the aDMN and pDMN/SMN, and the pDMN and SMN. No significant differences in FNCs were found between groups (Fig. 3). No significant correlation was found between the FNC and BDI or BPI scores (p < 0.05, corrected for multiple comparisons).
Fig. 2.
Functional network connectivity (FNC) between the anterior default mode network (aDMN), posterior default mode network (pDMN), sensorimotor network (SMN) and cingular–insular network (CIN) in the control group (left) and patient group (right). Arrows represent a significant correlation between components (p < 0.05, corrected for false discovery rate). The lag time between the connected networks is shown by the direction of each arrow. An arrow that connects the CIN and SMN (pointing toward the latter) signifies that the time course of the SMN is delayed with respect to the CIN. However, no significant group differences were detected (p < 0.05, corrected for false discovery rate).
Fig. 3.
Correlation and lag values between intrinsic connectivity networks (ICNs) of the controls (left) and patients (middle) and a group comparison (right). The numbers on the abscissa represent the 6 possible combinations between the ICNs. The ordinates show the correlation coefficient describing the functional network connectivity (FNC) of each combination for the controls and patients and the difference in the correlation coefficient (correlation Δ) between the controls and patients. The red-dotted horizontal line shows the user p value threshold (p < 0.05, corrected for false discovery rate). Blue horizontal lines show correlation p values of each test. The colour of the bars represents the lag time in seconds. In controls and patients, significant FNC was detected between the SMN/aDMN, SMN/CIN, SMN/pDMN and aDMN/pDMN but not the aDMN/CIN or pDMN/CIN. Compared with the control group, the FNC of patients was nonsignificantly lower between the SMN/CIN and nonsignificantly higher between all the other ICNs. aDMN = anterior default mode network; CIN = cingular–insular network; pDMN = posterior default mode network; SMN = sensorimotor network.
Discussion
The present study shows how pain-related ICNs are interconnected during the resting state using a reasonably sized group of clinically well-classified participants. Using a data-driven approach, we isolated the CIN, SMN and DMN. According to previous studies, an anterior and posterior subsystem of the DMN could be identified.47,48 The aDMN is associated with cognitive control of emotions and self-referential processing, whereas the pDMN is related to mnestic functions.49–53 The CIN subserves affective reactions, and the SMN underpins sensory-discriminative processing.18,19 The SMN strongly interacts with the CIN, aDMN and pDMN. These interactions suggest that sensory-discriminative processing is highly related to affective processing, self-referential thoughts and memory functions. Furthermore, the SMN lags the time course of the other ICNs by seconds. Emotional and cognitive processing appear to precede the activity of the sensorimotor system during the resting state. This may explain the influence of the inner world, with its various subjective states, such as anxiety, sadness and individual predictions about the future on the perception of the outer world via sensory systems.54–56 Because our analysis does not provide insight into causality, our results encourage further research on the putative effects of DMN and CIN activity on the SMN.
Contrary to our hypothesis, the present study shows that somatoform pain does not lead to significantly disturbed FNC among pain-associated networks during the resting state. This finding is remarkable because chronic pain has been shown to be a strong disruptor of intranetwork functional connectivity within the somatosensory, affective and cognitive neural systems.13–15,17 Notably, our patients subjectively experienced severe ongoing pain, as their pain intensity rating using the BPI was 7 of 10. In comparison, in cancer-induced bone pain, for example, which is the most common cause of pain in cancer patients, the median average pain rating based on the BPI has been reported to be 4 of 10.57 One may speculate several explanations for this finding. Evidence for an important role of resting FNC in central nervous system disorders stems from research on schizophrenia, which is widely known to be characterized by bizarre inner processes, such as hallucinations, delusions and disorganized thoughts.20 One important characteristic of schizophrenia is the patient’s disability to distinguish between inner experiences caused by psychotic states and outer reality. Somatoform pain, however, is not associated with a disturbed sense of reality or personality. Thus, disturbed FNC may reflect highly disorganized states of consciousness rather than symptoms, such as ongoing non-nociceptive pain.
Furthermore, as external triggers, such as aversive emotional experiences, are considered to be relevant in the etiology of somatoform pain disorder, one may speculate that significant differences in FNC are not elicited during rest but in response to stimulation. For example, noxious heat led to higher blood oxygen–level dependent signalling in the insula and parahippocampal gyrus, while medial prefrontal cortex activity was reduced.58 Reduced insula and amygdala activity was observed during emotional empathy, indicating disturbed emotional processing.59
However, fibromyalgia, which most closely resembles somatoform pain disorders in many aspects, displays a characteristic connectivity pattern during rest, as recently shown by Cifre and colleagues.60 They found that functional connectivity of the anterior cingulate, insula and somatosensory regions with amygdala and basal ganglia was enhanced, whereas the interplay between somatosensory and default mode regions was reduced. In our study, however, a non-significantly higher FNC between the CIN and SMN was observed in controls, whereas the FNC of the aDMN/pDMN, aDMN/SMN, and pDMN/SMN was nonsignificantly higher in patients with somatoform pain. For this reason, the lack of differences between controls and patients in terms of FNC may mirror methodological issues rather than etiological characteristics of different psychiatric and psychosomatic entities.
Limitations
An important limitation of the current study was medication. Antidepressants and analgesics were being taken by more than half of our patients. It is of note that despite ethical reasons, it was nearly impossible to convince patients with somatoform pain to interrupt their (psychotropic) medication in this intentionally naturalistic study. As the patients of Cifre and colleagues60 did not undergo a drug washout, we cannot exclude the possibility that medication influenced our results. Moreover, regarding the poor health status of our patients, our resting paradigm lasting 370 seconds was relatively short. Other studies used rest sessions of about 10 minutes.13,60 However, given that patients with somatoform pain normally complain about long recumbency in the scanner, one may argue that a longer paradigm may have enhanced patient pain and led to false-positive results.
Given the high comorbidity of somatoform pain with affective disorders61 and their influence on brain function,58,62 depressive symptoms may have influenced our results. Several studies have indicated an important role of functional connectivity in depressive symptoms. For example, functional connectivity within the DMN was enhanced in our study, which has been correlated with stronger self-referential processes in depressed patients.63–65 Northoff and colleagues66 found meta-analytic evidence that not only intranetwork connectivity, but also disturbed interplay between several brain systems, may be the neural underpinning of this disease. In our study, however, no significant effect of depression on FNC was observed.
Conclusion
To our knowledge, our results demonstrate for the first time resting FNC between pain-related ICNs and its association with somatoform pain disorder. In contrast to our hypothesis, the resting FNC approach may not sufficiently explain the putative central dysfunction of pain homeostasis in chronic non-nociceptive pain. Our negative results encourage further research on the effect of chronic pain and affective disorders on the FNC of the human brain.
Acknowledgements
This work was supported by a KKF fund (Klinikum rechts der Isar, Technische Universitaet Muenchen, Germany) awarded to M. Noll-Hussong and A.M. Wohlschlaeger and a grant awarded to M. Noll-Hussong from the Dr. Ing. Leonhard-Lorenz Foundation (Technische Universitaet Muenchen, Germany).
Footnotes
Competing interests: None declared for A. Otti and C. Zimmer. H. Guendel declares receiving consultancy fees, payment for expert testimony and payment for lectures from MAN, Océ and AUDI, and a grant from the German Federal Ministry of Education and Research (grant 01EL0815). P. Henningsen declares having received lecture sponsorship from Lilly and book royalties from Cambridge University Press. A.M. Wohlschlaeger declares having received support through her institution from German Federal Ministry of Education and Research grant 01EV0710. As above for M. Noll-Hussong; he also declares having received travel support from the German Academic Exchange Service DAAD).
Contributors: A. Otti conducted the research, analyzed data, and wrote the paper. H. Guendel designed the research and wrote the paper. P. Henningsen and C. Zimmer designed the research. A.M. Wohlschlaeger designed and performed the research. M. Noll-Hussong designed and conducted the research, analyzed the data, and wrote the paper. All authors have approved the final article.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Press; 2000. text revised. [Google Scholar]
- 2.Browning M, Fletcher P, Sharpe M. Can neuroimaging help us to understand and classify somatoform disorders? A systematic and critical review. Psychosom Med. 2011;73:173–84. doi: 10.1097/PSY.0b013e31820824f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Campayo J, Fayed N, Serrano-Blanco A, et al. Brain dysfunction behind functional symptoms: neuroimaging and somatoform, conversive, and dissociative disorders. Curr Opin Psychiatry. 2009;22:224–31. doi: 10.1097/YCO.0b013e3283252d43. [DOI] [PubMed] [Google Scholar]
- 4.Borkum JM. Chronic headaches and the neurobiology of somatization. Curr Pain Headache Rep. 2010;14:55–61. doi: 10.1007/s11916-009-0084-z. [DOI] [PubMed] [Google Scholar]
- 5.Balenzuela P, Chernomoretz A, Fraiman D, et al. Modular organization of brain resting state networks in chronic back pain patients. Front Neuroinform. 2010;4:116. doi: 10.3389/fninf.2010.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31:199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Sakoglu U, Upadhyay J, Chin CL, et al. Paradigm shift in translational neuroimaging of CNS disorders. Biochem Pharmacol. 2011;81:1374–87. doi: 10.1016/j.bcp.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagliazucchi E, Balenzuela P, Fraiman D, et al. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napadow V, LaCount L, Park K, et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otti A, Guendel H, Läer L, et al. I know the pain you feel — how the human brain’s default mode predicts our resonance to another’s suffering. Neuroscience. 2010;169:143–8. doi: 10.1016/j.neuroscience.2010.04.072. [DOI] [PubMed] [Google Scholar]
- 13.Malinen S, Vartiainen N, Hlushchuk Y, et al. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A. 2010;107:6493–7. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cauda F, Sacco K, D’Agata F, et al. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci. 2009;10:138. doi: 10.1186/1471-2202-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauda F, Sacco K, Duca S, et al. Altered resting state in diabetic neuropathic pain. PLoS ONE. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantini D, Caulo M, Ferretti A, et al. Noxious somatosensory stimulation affects the default mode of brain function: evidence from functional MR imaging. Radiology. 2009;253:797–804. doi: 10.1148/radiol.2533090602. [DOI] [PubMed] [Google Scholar]
- 17.Baliki MN, Geha PY, Apkarian AV, et al. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 19.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafri MJ, Pearlson GD, Stevens M, et al. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noll-Hussong M, Otti A, Laeer L, et al. Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychosom Res. 2010;68:483–7. doi: 10.1016/j.jpsychores.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, deGruy FV, III, et al. Multisomatoform disorder. An alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Arch Gen Psychiatry. 1997;54:352–8. doi: 10.1001/archpsyc.1997.01830160080011. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO) International classification of diseases and related health problems. 10th rev. Geneva: WHO; 2005. [Google Scholar]
- 24.Jackson JL, Kroenke K. Prevalence, impact, and prognosis of multi-somatoform disorder in primary care: a 5-year follow-up study. Psychosom Med. 2008;70:430–4. doi: 10.1097/PSY.0b013e31816aa0ee. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE., Jr . SF-36 physical & mental health summary scales: a user’s manual. Lincoln (RI): Quality Metric Inc.; 1997. [Google Scholar]
- 26.Radbruch L, Loick G, Kiencke P, et al. Validation of the German version of the Brief Pain Inventory. J Pain Symptom Manage. 1999;18:180–7. doi: 10.1016/s0885-3924(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 27.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 28.Wittchen HU, Wunderlich U, Gruschwitz S, et al. Strukturiertes klinisches Interview für DSM-IV, Achse I (SKID) Göttingen: Hogrefe Verlag; 1997. [Google Scholar]
- 29.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res. 2004;13:283–98. doi: 10.1023/b:qure.0000018472.46236.05. [DOI] [PubMed] [Google Scholar]
- 31.Bullinger M. German translation and psychometric testing of the SF-36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. Soc Sci Med. 1995;41:1359–66. doi: 10.1016/0277-9536(95)00115-n. [DOI] [PubMed] [Google Scholar]
- 32.Keller SD, Ware JE, Jr, Gandek B, et al. Testing the equivalence of translations of widely used response choice labels: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:933–44. doi: 10.1016/s0895-4356(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 33.Riddle DL, Lee KT, Stratford PW. Use of SF-36 and SF-12 health status measures: a quantitative comparison for groups versus individual patients. Med Care. 2001;39:867–78. doi: 10.1097/00005650-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Sattel H, Lahmann C, Gundel H, et al. Brief psychodynamic interpersonal psychotherapy for patients with multisomatoform disorder: randomised controlled trial. Br J Psychiatry. 2012;200:60–7. doi: 10.1192/bjp.bp.111.093526. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–66. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345–59. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 38.Keller S, Bann CM, Dodd SL, et al. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–18. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 40.Hautzinger M. [The Beck Depression Inventory in clinical practice]. Nervenarzt. 1991;62:689–96. [Article in German] [PubMed] [Google Scholar]
- 41.Symptom Checklist-90-Revised (SCL-90-R®) product page. Pearson [website of Pearson] [accessed 2012 May 17]. Available: http://psychcorp.pearsonassessments.com/HAIWEB/Cultures/en-us/Productdetail.htm?Pid=PAg514.
- 42.Schmitz N, Hartkamp N, Kiuse J, et al. The Symptom Check-List-90-R (SCL-90-R): a German validation study. Qual Life Res. 2000;9:185–93. doi: 10.1023/a:1008931926181. [DOI] [PubMed] [Google Scholar]
- 43.Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: a step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–9. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- 44.Calhoun VD, Adali T, Pearlson GD, et al. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 47.Damoiseaux JS, Beckmann CF, Arigita EJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 48.Mantini D, Perrucci MG, Del Gratta C, et al. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–5. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 50.D’Argembeau A, Collette F, Van der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25:616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 51.Schneider F, Bermpohl F, Heinzel A, et al. The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience. 2008;157:120–31. doi: 10.1016/j.neuroscience.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Wagner AD, Shannon BJ, Kahn I, et al. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Gusnard DA, Akbudak E, Shulman GL, et al. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vancleef LM, Peters ML. The influence of perceived control and self-efficacy on the sensory evaluation of experimentally induced pain. J Behav Ther Exp Psychiatry. 2011;42:511–7. doi: 10.1016/j.jbtep.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Coen SJ, Kano M, Farmer AD, et al. Neuroticism influences brain activity during the experience of visceral pain. Gastroenterology. 2011;141:909–17. e1. doi: 10.1053/j.gastro.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364:1235–43. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laird BJ, Walley J, Murray GD, et al. Characterization of cancer-induced bone pain: an exploratory study. Support Care Cancer. 2011;19:1393–401. doi: 10.1007/s00520-010-0961-3. [DOI] [PubMed] [Google Scholar]
- 58.Gündel H, Valet M, Sorg C, et al. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain. 2008;137:413–21. doi: 10.1016/j.pain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 59.de Greck M, Scheidt L, Bolter AF, et al. Altered brain activity during emotional empathy in somatoform disorder. Hum Brain Mapp. 2011 Oct. 13; doi: 10.1002/hbm.21392. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cifre I, Sitges C, Fraiman D, et al. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74:55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- 61.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65:528–33. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 62.Stein DJ, Muller J. Cognitive-affective neuroscience of somatization disorder and functional somatic syndromes: reconceptualizing the triad of depression-anxiety-somatic symptoms. CNS Spectr. 2008;13:379–84. doi: 10.1017/s1092852900016540. [DOI] [PubMed] [Google Scholar]
- 63.Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 65.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Northoff G, Wiebking C, Feinberg T, et al. The ‘resting-state hypothesis’ of major depressive disorder-a translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev. 2011;35:1929–45. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]