Abstract
Alzheimer disease is the most prevalent form of dementia globally and is characterized premortem by a gradual memory loss and deterioration of higher cognitive functions and postmortem by neuritic plaques containing amyloid β peptide and neurofibrillary tangles containing phospho-tau protein. Glutamate is the most abundant neurotransmitter in the brain and is essential to memory formation through processes such as long-term potentiation and so might be pivotal to Alzheimer disease progression. This review discusses how the glutamatergic system is impaired in Alzheimer disease and how interactions of amyloid β and glutamate influence synaptic function, tau phosphorylation and neurodegeneration. Interestingly, glutamate not only influences amyloid β production, but also amyloid β can alter the levels of glutamate at the synapse, indicating that small changes in the concentrations of both molecules could influence Alzheimer disease progression. Finally, we describe how the glutamate receptor antagonist, memantine, has been used in the treatment of individuals with Alzheimer disease and discuss its effectiveness.
Introduction
In 2005 there were an estimated 24.3 million individuals with dementia worldwide, with Alzheimer disease accounting for more than 60% of these cases.1 The etiology of Alzheimer disease is not yet fully understood; however, genetic and environmental factors contribute to disease pathogenesis and progression. Linkage studies indicate that a small proportion of individuals with Alzheimer disease have an autosomal dominant pattern of inheritance and implicate 3 genes in early onset familial forms of Alzheimer disease: those for amyloid β precursor protein (APP), presenillin 1 (PSEN1) and PSEN2.2 A small proportion of early-onset Alzheimer disease cases are linked to APP, which is situated on chromosome 21. This gene is triplicated in Down syndrome, which invariably leads to the development of Alzheimer disease pathology, indicating that increased expression of APP could facilitate Alzheimer disease progression. Whereas mutations in this gene can also lead to the development of early-onset Alzheimer disease by altering/increasing the proteolytic cleavage of APP, most cases are linked to point mutations in the PSEN genes, especially PSEN1.2,3 Although these findings are critical in deducing the biological pathogenesis of Alzheimer disease, it must be remembered that mutations in these 3 genes are responsible for only 30%–50% of early-onset Alzheimer disease cases, which in turn only represents 6%–8% of all Alzheimer disease cases, suggesting that many other factors are involved in disease pathology.2,3 There are also genetic risk factors associated with late-onset Alzheimer disease, but in most instances there has been no genetic cause of the disease identified as yet. One such genetic risk factor is possession of 1 or 2 copies of the ɛ4 allele of the gene for apolipoprotein E, which has been shown to increase the probability of Alzheimer disease developing, whereas the presence of an ɛ2 allele appears to protect against the disease.2 A number of recent studies have shown that variants in some other genes, such as those for clusterin on chromosome 8,4,5 phosphatidylinositol-binding clathrin assembly protein on chromosome 11,4,5 complement receptor 1 on chromosome 1,5 bridging integrator 1 on chromosome 26 and sortilin-related receptor 1 on chromosome 11,7 may be associated with an enhanced risk for Alzheimer disease, but these susceptibility genes need to be validated in future studies.8,9 The lack of conclusive genetic linkage in most Alzheimer disease cases not only highlights the possible existence of unidentified genetic risk factors, but also the importance of environmental factors in the development of disease pathology. There is increasing evidence that lower educational and occupational attainment, female sex, low mental ability in early life and reduced mental and physical activity during late life increase the risk for the disease. Moreover, several epidemiological studies have shown that head trauma,10 stroke and heart disease,11 atherosclerosis, hypercholesterolemia, smoking, obesity and diabetes12 can also increase the risk for Alzheimer disease.13 Whether these are true causal risk factors driving the pathogenic process or whether they add to the clinically silent disease pathology remains to be established. However, among all these factors, age is considered to be by far the greatest risk factor for late-onset Alzheimer disease. Therefore, as the baby boomers enter their mid-60s, the prevalence of Alzheimer disease will increase rapidly, possibly by as many as 4.6 million new cases per year.1
Alzheimer disease is characterized by 3 distinct major neuropathological abnormalities: intracellular neurofibrillary tangles, extracellular plaques and neuronal loss.14,15 Neurofibrillary tangles are intracellular aggregates of the hyperphos-phorylated microtubule-associated protein, tau. In normal healthy neurons, tau stabilizes microtubules that form the cy-toskeleton of the cell by a process involving phosphorylation and dephosphorylation of the protein.16–18 Phosphorylated tau is unable to bind microtubules and instead polymerizes with other tau molecules, forming straight filaments that subsequently form paired helical filaments. This may cause a failure of neuronal transport that eventually leads to cell death.17,18 Tangles are particularly abundant in the entorhinal cortex; hippocampus; amygdala; association cortices of the frontal, temporal and parietal lobes; and certain subcortical nuclei that project into these areas.15,19 Interestingly, the number and distribution of cortical tangles correlates positively with cognitive deficits and serves as a good marker of disease progression.15,19
Extracellular plaques are formed by the aggregation of amyloid β, a short peptide of 39–42 amino acids in length. This peptide is produced by the sequential cleavage of APP, which exists in the brain predominantly in 3 different isoforms. The shortest isoform, APP695, is mostly expressed by neurons, whereas the 2 longer isoforms, APP751 and APP770, are expressed predominantly in glial cells, such as astroyctes.20–23 At extracellular sites within the brain parenchyma, amyloid β forms compact deposits, often seeded by aggregation of the longer amyloid β1–42 peptide, that are associated with dystrophic neurites, activated microglia and reactive astrocytes.15,23 Extracellular amyloid β–containing plaques are apparent in most areas of the brain in Alzheimer disease individuals and are associated with neuronal loss in areas such as the entorhinal cortex, hippocampus and association cortices.19 The number of plaques present in the brain does not correlate with the severity of dementia, but a clinical correlation between elevated levels of total amyloid β peptide in the brain and cognitive decline has been reported.24 There is also a growing consensus that amyloid β peptide increases tau phosphorylation and initiates multiple pathways that lead to neuronal cell death.25 These pathways include increased oxidative stress,26 altered Ca2+ homeostasis27,28 and excitotoxicity,14,29 which trigger apoptotic dependent and independent cell death mechanisms. The significance of amyloid β peptide in disease pathology has been highlighted by the fact that a number of small compounds that inhibit amyloid β formation are currently undergoing clinical testing as potential therapies for Alzheimer disease.30 Additionally, there is strong experimental data from animal studies to suggest that active amyloid β immunotherapy has disease modifying potential in individuals with Alzheimer disease. Although the first clinical trials with AN1792 (from Elan/Wyeth), a synthetic amyloid β1–42 peptide along with QS21 as adjuvant, were halted prematurely when subacute menin-goencephalitis developed in 6% of the vaccinated individuals, the drug was effective in clearing amyloid β plaques and improving performance on some neuropsychological tests. However, autopsy studies of some individuals revealed no association between vaccination with AN1792 and onset of severe dementia or survival, even among individuals whose brains were virtually devoid of plaques.31,32 Other clinical trials with passive immunotherapy confirmed the occasional appearance of microhemorrhage and the occurrence of vasogenic edema in some individuals, particularly those with apolipoprotein E ɛ4 genotypes.31,33 Nonetheless, several ongoing clinical trials with different amyloid β fragments and with humanized amyloid β antibodies are likely to indicate not only the importance of amyloid β in Alzheimer disease pathogenesis, but also whether antiamyloid β therapy can modify the disease pathology.
Degenerating neurons and synapses in the brains of patients with Alzheimer disease are usually located within regions that project to or from areas displaying high densities of plaques and tangles. Severely affected regions include the hippocampus, entorhinal cortex, amygdala, neocortex and some subcortical areas, such as basal forebrain cholinergic neurons, serotonergic neurons of the dorsal raphe and nor-adrenergic neurons of the locus coeruleus.15,34 There is evidence that glutamatergic neurons located in the hippocampus and in the frontal, temporal and parietal cortex are severely affected, whereas similar neurons in the motor and sensory cortex are relatively spared.19,35 Since the hippocampus and cortex are essential for learning and memory, it is possible that degeneration of glutamatergic neurons could be an early event in the pathogenesis of the disease. A recent study investigating changes in the glutamatergic system has shown a greater correlation between reduced glutamatergic presynaptic bouton density and cognitive deficits than that for tangle or amyloid β burden, as determined by comparing mini mental status examination scores of healthy individuals to those of individuals with mild or severe Alzheimer disease.36 Interestingly, the study also found that there was an increase in glutamatergic synapses in individuals with mild cognitive impairment, suggesting that there may be an initial compensatory event occurring in the brains of such individuals that decays with disease progression. This phenomenon has already been observed in the cholinergic system, where there is an increase in choline acetyltransferase activity in individuals with mild cognitive impairment and a subsequent reduction in the enzyme activity as Alzheimer disease worsens.37,38 Studies from animal models of Alzheimer disease have shown that upregulation of cholinergic presynaptic boutons occurs before the involvement of glutamatergic terminals, thus raising the possibility that a compromised cholinergic system may affect the functioning/survival of glutamatergic neurons in the brain.39 Indeed, pyramidal neurons of the cortex that use glutamate as their primary transmitter are known to possess both cholinergic and glutamatergic receptors and receive inputs from the basal forebrain cholinergic neurons.35 There is evidence that cholinesterase inhibitors can promote the release of glutamate from pyramidal neurons, possibly by increasing cortical acetylcholine levels and subsequent activation of the cholinergic receptors.40 In addition, it has been demonstrated that uncoupling of the postsynaptic muscarinic M1 receptor from G-proteins can correlate with the loss of N-methyl-d-aspartate (NMDA) receptor density and protein kinase C (PKC) activity in the postmortem frontal cortex of individuals with Alzheimer disease.41 These results provide a neurochemical basis of interactions between cholinergic and glutamatergic systems and their potential implications in triggering pathological abnormalities in Alzheimer disease. However, further investigations using complementary approaches are needed to evaluate other components of their functions during aging and the progression of Alzheimer disease. This review gives an overview of the interactions between the central glutamate system amyloid β peptides and tau protein and their relevance in the development of Alzheimer disease pathology.
Neuropathological changes in the glutamate system in Alzheimer disease
Glutamate is the major excitatory neurotransmitter in the central nervous system and is known to be involved in a variety of functions, including synaptic transmission, neuronal growth and differentiation, synaptic plasticity and learning and memory.14,35 The glutamatergic neurons are situated in areas that are known to be affected in Alzheimer disease, and initial damage begins with the pyramidal neurons in layers III and V of the neocortex42,43 and glutamate innervated cortical and hippocampal neurons.35
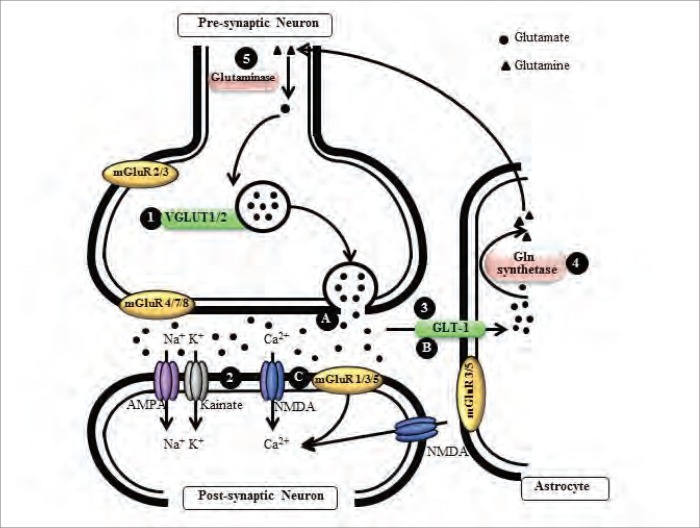
The role of the glutamatergic system is to convert nerve impulses into a chemical stimulus by controlling the concentration of glutamate at the synapse (Fig. 1). In presynaptic neurons, the vesicular glutamate transporters, VGLUT1 and VGLUT2, maintain the level of glutamate stored in vesicles,44 and when a neuron is depolarized, glutamate is released into the synaptic cleft where it binds glutamate receptors on pre-and postsynaptic neurons.45–47 There are 2 families of glutamate receptors located at the plasma membrane of neurons, ionotropic (iGluR) and metabotropic (mGluR) glutamate receptors. The iGluR family is further divided into 3 classes of receptor, which are based on specific agonists and/or permeability to different cations; NMDA receptors (NR1, NR2A–D and NR3A–B) are predominantly Ca2+ ion permeable, whereas α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA; GluR1–4) and kainate (KA; GluR5–7, KA1–2) receptors are predominantly permeable to Na+ and K+ ions.45 Currently, there are 8 metabotropic G-protein coupled glutamate receptors, which are classified on the basis of their structure and physiologic function into 3 distinct groups. Group 1 receptors (mGluRs 1 and 5) are coupled to phospholipase C through Gq and G11 proteins, whereas group 2 (mGluRs 2 and 3) and group 3 (mGluRs 4 and 6–8) receptors are coupled with adenylyl cyclase through Gi and Go proteins (Tables 1 and 2).45 There is evidence that almost every step in the signalling pathways associated with mGluRs requires or is modulated by Ca2+. For example, the efficacy of mGluR signalling is known to be regulated by physiologically relevant changes in extracellular Ca2+, such that Ca2+ depletion in the synaptic cleft can lead to substantial inhibition of postsynaptic mGluR function. There is also increasing experimental data to suggest that Group 1 mGluRs can mobilize intracellular Ca2+ not only through inositol 1,4,5-P3-sensitive Ca2+ stores, but also through the ryanodine-sensitive Ca2+ stores.48,49
Fig. 1.
The glutamate cycle. Glutamate is the most abundant neurotransmitter in the brain, and the levels of the amino acid are maintained in vesicles in the presynaptic neurons by 2 glutamate transporters, (1) VGLUT1 and 2. When the synapse is activated, glutamate is released into the synaptic cleft and activates 2 families of glutamate receptors, (2) ionotropic (iGluRs; N-methyl-d-aspartate [NMDA], α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] and kainate) and metabotropic (mGluRs) receptors. N-methyl-d-aspartate receptors only become fully active after synchronised AMPA receptor activation and depolarization of the postsynaptic membrane. Glutamate receptor activation stimulates further downstream cellular pathways. (3) Glutamate is removed from the synaptic cleft by another group of glutamate transporters, which are mostly expressed in astrocytes, GLT-1 and GLAST in mice or EAAT1 and 2 in humans. Within astrocytes, (4) glutamate is converted to glutamine by glutamine synthetase and (5) returned to the presynaptic terminals where glutaminase converts glutamine back to glutamate. Many aspects of the glutamate cycle are affected in Alzheimer disease, implicating glutamate in disease progression. Amyloid β–related peptides have been shown to (A) increase glutamate release, (B) inhibit clearance of glutamate by astrocytes and (C) affect glutamate receptor activity.
Table 1.
Classification of the ionotropic glutamate receptors
| Receptor agonist | Subunit | Composition | Ion permeability |
|---|---|---|---|
| NMDA | NR1A–H NR2A–D NR3A–B |
The functional protein contains a dimer of NR1 or NR2, with or without an NR3 subunit | Ca2+, Na+ |
| AMPA | GLUR1–4 | Heterotetrameric protein containing a dimer of GluR2 and a dimer of either GluR1, 3 or 4 | Na+, K+ |
| Kainate | GLUR5–7 KA 1–2 |
Heterotetrameric protein containing dimers of GluR5–7 with or without KA 1–2 | Na+, K+ |
AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA = N-methyl-d-aspartate.
Table 2.
Classification of the metabotropic glutamate receptors
| Group | G protein | Second messenger | Subtype localization | |
|---|---|---|---|---|
| 1 | Gq/G11 | Phospholipase C | mGluR 1 | Postsynaptic neurons |
| Ca2+ | mGluR 5 | Postsynaptic neurons, reactive astrocytes and cultured microglia | ||
| 2 | Gi/Go | Adenylyl cyclase | mGluR 2 | Pre- and postsynaptic neurons and cultured microglia |
| mGluR 3 | Pre- and postsynaptic neurons, reactive astrocytes and cultured astrocytes | |||
| 3 | Gi/Go | Adenylyl cyclase | mGluR 4 | Presynaptic neurons, cultured astrocytes and microglia |
| mGluR 5 | Presynaptic neurons, reactive astrocytes and cultured microglia | |||
| mGluR 6 | Retina only | |||
| mGluR 7 | Presynaptic neurons | |||
| mGluR 8 | Presynaptic neurons and cultured microglia |
mGluR = metabotropic glutamate receptor.
Unlike iGluRs, which are predominantly situated in the postsynaptic membrane to mediate fast excitatory transmission, mGluRs are located in various membrane compartments of both neuronal and glial cells in the brain.48 The mGluRs 1, 3, 5 and 7 are widely distributed throughout the brain, whereas mGluRs 2, 4 and 8 are localized in specific brain regions. The only mGluR not found in the brain is mGluR 6, which is expressed in the retina.48,50 Whereas most mGluRs in the adult rat are found in neuronal cells, mGluR 3 is localized extensively in glial cells in the brain. There is also evidence that mGluRs 3 and 5 are expressed in reactive astrocytes; that mGluRs 3–5 are localized in cultured astrocytes; and that mGluRs 2, 4, 5 and 8 are apparent in cultured microglia.48,50 At the subcellular level, Group 1 mGluRs are mostly localized in somatodendritic domains of neurons, especially in perisynaptic regions, whereas Group 2 mGluRs are evident in the somatodendritic compartment and axonal domains. The Group 3 mGluRs, with the exception of mGluR 6, are present predominantly in the presynaptic active zone of axon terminals.48 Functionally, there is convincing evidence that Group 1 mGluRs mediate presynaptic inhibitory effects, that Group 2 mGluRs are activated by the spillover of glutamate from distant synapses and that Group 3 mGluRs may function as autoreceptors. The mechanisms of presynaptic inhibition by Group 2 and Group 3 mGluRs possibly involve suppression of presynaptic voltage-dependent Ca2+ channels, activation of K+ channels and direct inhibition of regulated exocytosis.48,51 Following synapse activation, the majority of glutamate is cleared from the synaptic cleft by astrocytes, which express high levels of the glutamate transporters GLAST and GLT-1 in rats.52–54 The human homologues are the excitatory amino acid transporters 1 and 2 (EAAT1 and 2).52,55 In astrocytes glutamate is converted to glutamine by the enzyme glutamine synthetase and transported back to neurons, where glutamine is converted back to glutamate by glutaminase47 and then returned to vesicles in the presynaptic membrane by VGLUT1 and 2. This cycle and transamination of cytosolic aspartate are essential for maintaining glutamate levels in presynaptic terminals (Fig. 1).52
Deficiencies in many stages of the glutamate cycle have been shown to occur in Alzheimer disease, leading to increased concentrations of glutamate around the neurons of the synapse (Table 3). There have been a number of studies that noted reduced levels of VGLUT1 and 2 in the prefrontal cortex of individuals with Alzheimer disease, with VGLUT1 showing a much greater reduction than VGLUT2.56,57 More recent studies using synaptosome-enriched fractions also showed a trend for reduced levels of VGLUT1 and 2 in the parietal cortex of individuals with Alzheimer disease compared with controls, but they did not reach significant levels.58 Whether this could be due to the region of the brain examined or the relatively small number of samples used in the study remains to be established. Nevertheless, this study showed that amyloid β peptide accumulates more in VGLUT1/2-containing terminals than in non-VGLUT terminals, thus suggesting that these peptides may preferentially affect presynaptic glutamatergic terminals in the cortex of individuals with Alzheimer disease.58 The gene expression and protein level of EAAT1 and EAAT2, on the other hand, have also been shown to be reduced in the cortex and hippocampus of individuals with Alzheimer disease,59,60,71 suggesting that glutamate clearance from the synaptic cleft is attenuated. In one study, it has been demonstrated that reduced levels of EAAT2, produced mainly by astrocytes, inversely correlates with APP751/770 mRNA levels, thus indicating that abnormal functioning and/or processing of APP may be involved in regulating EAAT2 levels/functions.72 Changes in glutamate transporter expression have been identified at an early stage of Alzheimer disease, indicating that dysfunction in glutamate transport might be an early event in the disease pathology.71 Concurrent with reduced glutamate transporters there is a reduction in glutamine synthetase in Alzheimer disease,61 thus preventing the transport of glutamate back to presynaptic neurons. High levels of glutamate in astrocytes lead to the risk of glutamate being returned to the synaptic cleft if mitochondrial membranes are depolarized, a process that is thought to occur in Alzheimer disease because of reduced energy supplies.73,74 Taken together, these changes in glutamate transport and conversion to glutamine can lead to increased concentrations of glutamate at the synapse, which can subsequently trigger excitotoxic cell death via NMDA receptor–mediated increase of intracellular Ca2+ concentrations.75,76 Elevated Ca2+ levels lead to neuronal death through a number of known pathways, including increased activation of calpain,77 release of reactive oxygen species and depolarization of the mitochondrial membrane, which leads to reduced energy metabolism and cytochrome c release.27 This ultimately results in neuronal loss through apoptotic and necrotic pathways.76
Table 3.
Alterations in the glutamate system in brains of people with Alzheimer disease
| Component of the glutamate system | Alteration in the brain |
|---|---|
| VGLUT1 | Reduced protein levels before cell loss and onset of pathology56–58 |
| VGLUT2 | Reduced protein levels before cell loss and onset of pathology56–58 |
| GLAST/EAAT1 | Reduced protein at early clinical stages59,60 |
| GLT-1/EAAT2 | Reduced protein levels at early clinical stages59,60 |
| Glutamine synthetase | Reduced protein levels61 |
| NMDA receptor | Increased protein levels in mild cognitive impairment36 |
| NR1 | Reduced protein levels62 |
| NR2A–B | Reduced protein levels63 |
| NR2C–D | Unaffected63 |
| NR3 | Not assessed |
| AMPA receptor | Variable results indicate that there is initial increase at very early stages before a reduction in GluR1 and increased GluR2/3 at later stages64–66 |
| GLUR1–3 | |
| GLUR4 | Not assessed |
| Kainate receptor | Reduced receptor binding67 |
| GLUR5–7 | Reduced expression68 |
| Metabotropic receptor | |
| mGluR 1 | Reduced protein levels67,69 |
| mGluR 2 | Increased protein levels70 |
| mGluRs 3–7 | Not assessed |
AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; mGluR = metabotropic glutamate receptor; NMDA = N-methyl-d-aspartate.
Alterations in the levels of iGluRs remain inconclusive. Some reports suggest that there is an increase in NMDA-sensitive glutamate binding in the striatum of individuals with Alzheimer disease,78 whereas other studies indicate that there are no changes in the level of NMDA receptors in the affected areas of the brain in individuals with Alzheimer disease.79 However, most reports suggest that there is a decrease in protein and mRNA levels of these receptors, at least in later stages of the disease.62,63,80,81 Discrepancies in these results could be explained by differences in the severity of the disease, as there is evidence of increased synaptic activity in individuals with mild cognitive impairment.62,81 Autoradiography studies have shown reduced NMDA receptor binding in the CA1 and CA3 regions of the hippocampus, supporting the idea that there are fewer NMDA receptors in these areas of the brain. Western blot analysis and immunohistochemical evidence have also shown changes in the levels of AMPA receptors. These results suggest that there is an initial increase in GluR1 and GluR2/3 levels in membrane preparations taken from the neocortex of individuals with mild cognitive impairment, followed by a reduction in receptor levels in individuals in the early stages of Alzheimer disease, but the levels of GluR2/3 increase in severe cases. A transcriptome analysis of synaptoneurosomes prepared from Alzheimer disease and control brains also showed an increase in the levels of mRNA encoding GluR2 in the Alzheimer disease samples compared with the controls.64 Detailed studies of brains affected by Alzheimer disease have shown that the reductions in AMPA receptors are well correlated to areas of the brain that are affected, but there is substantial variation in AMPA receptor levels among individuals with Alzheimer disease.65,66 These researchers showed GluR1 and GluR2/3 loss was restricted to the subiculum and CA1 layer of the hippocampus, which indicates that changes in AMPA receptor levels are likely to be very region-specific. The KA receptors have received less attention than the other classes of iGluRs, and it remains unclear whether there are alterations in the number of these receptors in Alzheimer disease. Autoradiography studies suggest little change in KA binding in areas of the brain that remain unaffected in Alzheimer disease, but there is reduced KA binding in the hippocampus of individuals with Alzheimer disease compared with controls.67 Immunohistochemical staining using an antibody directed against GluR5/6/7 also showed some reduction in the levels of the KA receptors in the CA1 region of the hippocampus from individuals with Alzheimer disease compared with controls, but this was most likely due to neuronal loss in this area rather than alterations in the levels of the receptor.68
As for mGluRs, radioligand binding assays showed a general reduction in the levels of mGluRs in the cortex and hippocampus of individuals with Alzheimer disease.67 Western blot analysis confirmed a reduction in mGluR 1 levels in the CA1 region and subiculum of the hippocampus, but other regions of the hippocampus, such as the CA3 region, that are less susceptible to damage in Alzheimer disease were unaffected.67,69 Conversely, increased levels of mGluR 2 are found in the hippocampus of individuals with Alzheimer disease, and this correlates well with elevated phosphorylated tau levels.67,70 This increased mGluR 2 expression may result in increased intracellular Ca2+, which may in turn activate tau kinases. It is therefore probable that alterations in mGluR expression are involved in Alzheimer disease pathogenesis.
Glutamatergic receptors and APP processing
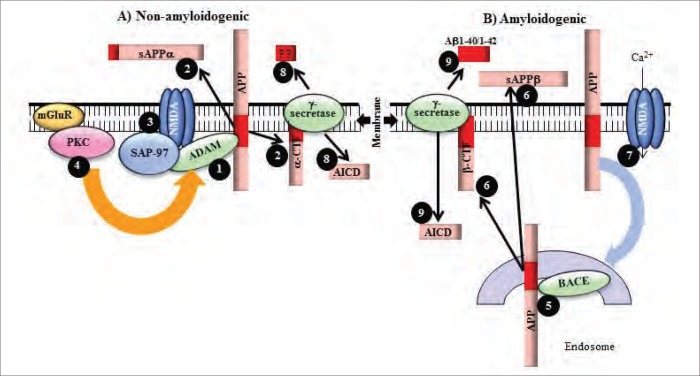
The APP is a type 1 integral membrane protein with a long N-terminal extracellular region, a single membrane-spanning domain and a short cytoplasmic C-terminal. The mature APP is known to be proteolytically processed via 2 alternative pathways (Fig. 2). Most APP, following transport to the cell surface, is known to be cleaved by an α-secretase, which is thought to be a member of the ADAM (a distintegrin and metalloprotease) family.20,22,82 Currently the best candidates for the α-secretase are ADAM10 and ADAM17.82 This pathway precludes the formation of amyloid β as the α-cleavage site is within the peptide sequence and produces an 89 amino acid C terminal fragment (α-CTF) and soluble APPα. The second, the amyloidogenic pathway, is initiated by β-site APP cleaving enzyme and produces a C-terminal fragment containing 99 amino acids (β-CTF) and soluble APPβ. The α-CTF and β-CTF are then cleaved by γ-secretase, a tetrameric complex containing PS1 or PS2 and anterior pharynx defective 1, presenilin enhancer 2 and nicastrin, to form P3 or amyloid β peptide, respectively, and the APP intracellular domain.20,22,83 The physiologic role of APP remains elusive, but it has been suggested to be involved in a variety of functions, including cell adhesion, regulation of cell/cell or cell/matrix interactions and mediation of neurite outgrowth.83,84 Low nanomolar and picomolar concentrations of amyloid β1–40 are also thought to increase cell viability and may also play a role in synaptic control.85 However, prolonged exposure and higher micromolar concentrations of the peptide are known to induce cell death.23,84 Relative activities of the 2 alternative APP processing pathways can be influenced by a variety of factors, including the stimulation of receptors for neurotransmitters/neuromodulators, such as acetylcholine, serotonin, glutamate, estrogen, neuropeptides and growth factors.20,22,86 The role of glutamate in APP processing has received a lot of attention as the activation of both iGluRs and mGluRs has been shown to differentially regulate the amyloidogenic and non-amyloidogenic pathways.22
Fig. 2.
Effects of glutamate receptor activation on APP processing. The APP is cleaved via (A) nonamyloidogenic and (B) amyloidogenic pathways. (A) The nonamyloidogenic pathway precludes amyloid β production due to (1) α-secretase cleavage, by ADAM10 or ADAM17, of APP within the amyloid β domain, which (2) results in production of soluble APPα and α-CTF. (3) Short-term N-methyl-d-aspartate (NMDA) receptor activation increases the trafficking of ADAM10 to the cell membrane via interactions with SAP-97 and (4) metabotropic glutamate receptor activation of ADAM17 via PKC, thus increasing nonamyloidogenic APP processing. (B) The main β-secretase, BACE1, (5) initiates the production of amyloid β peptide, which occurs partly within the late-endosomes, by (6) cleaving APP into soluble APPβ and β-CTF. Prolonged NMDA receptor activation results in high Ca2+ levels and activation of CaMK-IV, which in turn enhances transcription of more amyloidogenic APP751 and APP770 isoforms. (7) The high Ca2+ levels also increase vesicle binding to the plasma membrane and subsequent transport of APP via endocytosis to late-endosomes, where it can be processed by BACE1. Thus, increased NMDA receptor activation enhances amyloidogenic processing of APP. The α- and β-CTFs generated from nonamyloidogenic and amyloidogenic pathways are further processed by the γ-secretase complex, leading to the formation of (8) AICD fragment, and the P3 or (9) amyloid β peptides, respectively. Activation of mGluRs 2 and 5 is also thought to increase generation of amyloid β peptide.
Short treatments with NMDA receptor and mGluR 1 agonists have been shown to increase soluble APPα release and intracellular accumulation of the α-CTF fragment and to reduce amyloid β formation. This is apparent in cultured neurons and in astrocytes, which express mostly Kunitz protease inhibitor domain–containing APP.87,88 It is likely that NMDA receptor and mGluR 1 activation increases nonamyloidogenic APP processing by increased trafficking and activation of α-secretase at the cell membrane.87–89 There is evidence that the NMDA receptor, by interacting with synapse associated protein 97, can increase ADAM10 trafficking to synaptic membranes,89 whereas mGluR 1 activation can increase α-secretase activity.88 The increased α-secreatse activity appears to be mediated by downstream activation of PKC because phorbol esters, well-characterized PKC activators, and mGluR agonists have both been shown to increase nonamyloidogenic APP processing90 and because a PKC inhibitor, GF 109203X, can abolish mGluR 1 agonist–induced soluble APPα release, whereas mGluR 1 antagonists are unable to prevent phorbol ester–induced APP processing.88 As most APP is found at the cell membrane, NMDA receptor–induced trafficking and mGluR 1 activation of ADAM10 at the membrane could facilitate soluble APPα release and reduce amyloidogenic APP processing.87,88
Conversely, prolonged activation of NMDA receptors, a situation analogous to Alzheimer disease pathology, increases amyloidogenic APP processing. When neurons are activated by high glutamate concentrations for longer time periods (i.e., more than 24 h) there is a shift from APP695 expression to APP751 and APP770.91,92 This shift is mediated by activation of extrasynaptic NMDA receptors through Ca2+/calmodulin-dependent protein kinase (CaMK) IV.91,92 The longer APP isoforms have been shown to be more amyloidogenic, and so this shift in expression increases amyloid β levels.91 Excessive glutamate release from the presynaptic terminals has also been suggested as a mechanism for increased amyloid β production via NMDA receptor–mediated Ca2+ influx.92 Increased Ca2+ levels trigger fusion of synaptic vesicles to the plasma membrane, which enhances the rate of endocytosis to recycle these vesicles. Increased endocytosis subsequently removes APP from the plasma membrane, the site of nonamyloidogenic α-secretase cleavage, into the endosomes for amyloidogenic processing via β-secretase.93 More recent data have also shown that mGluRs are involved with increased amyloidogenic processing of APP; specifically mGluR 5 increases amyloid β1–40 production, whereas mGluR 2 increases amyloid β1–42 release.94,95
Glutamate-mediated synaptic plasticity and amyloid β peptide
Glutamate is essential in establishing new neural networks that form memories and assist learning through a process called long-term potentiation. This process is generated by high-frequency stimulation of the presynaptic plasma membrane, resulting in increased release of glutamate and activation of its receptors at the postsynaptic membrane. The AMPA and mGluRs are activated by the initial glutamate release, whereas NMDA receptors only become fully active after continuous synchronized glutamate stimulation following activation of the AMPA and mGluRs. The NMDA receptor activation allows Ca2+ to enter the postsynaptic cell, which subsequently triggers different kinase pathways and increases protein transcription. This process strengthens synapses and increases synaptic density.96,97 Long-term depression occurs when there is little stimulation at an established synapse or by asynchronous stimulation of iGluRs. Long-term depression has the opposite effect of long-term potentiation (i.e., reducing synaptic strength and preventing memory formation). Long-term depression can be caused by a reduction in the levels of NMDA and AMPA receptors at the postsynaptic membrane or by repeated weak stimulation of AMPA receptors that does not lead to the depolarization of the postsynaptic membrane and therefore does not fully activate NMDA receptors. This leads to a reduction in the number of neuronal spines on neurons and reduced synaptic activity. A decrease in long-term potentiation and an increase in long-term depression have both been observed in Alzheimer disease pathology.96,98
Many different paradigms have been used to show that amyloid β disrupts long-term potentiation and reduces synaptic plasticity.98,99 Cullen and colleagues99 reported that intracere-broventricular injections of amyloid β1–40 and amyloid β1–42 reduced the duration of high-frequency stimulation–induced long-term potentiation field excitatory postsynaptic potentials in the CA1 region of rat brains. This decrement in long-term potentiation was confirmed in studies using triple transgenic mice expressing mutant human APP, PS and tau, where intraneuronal amyloid β peptide was shown to accumulate with age and correlate with cognitive deficits observed in these mice. The cognitive deficits were observed to occur before amyloid β deposition, thus indicating that soluble amyloid β possibly disrupts normal neuronal function well before plaque formation.100 When another mouse model, Tg2576 mice, which also expressed mutant human APP and had an increased amyloid β burden, were fed a γ-secretase inhibitor to reduce amyloid β levels, there was a profound restoration of impaired long-term potentiation, confirming that amyloid β does affect synaptic plasticity.101
To understand the mechanisms whereby amyloid β can influence synaptic plasticity, many groups have examined the effects of amyloid β peptide derived from different sources in organotypic hippocampal slice preparations. These studies have shown that naturally occurring amyloid β peptides obtained from spinal fluid102 or directly from brains of individuals with Alzheimer disease103 and naturally secreted amyloid β oligos from cultured cells can affect long-term potentiation and synaptic density.104,105 On the basis of these results, it has been suggested that amyloid β dimers and larger aggregates are required for amyloid β–induced inhibition of long-term potentiation and that monomeric amyloid β peptide is not inhibitory.102,105 These effects could be reversed by removing amyloid β with monoclonal antibodies directed against the peptide.102 There is also evidence that specific concentrations of soluble amyloid β oligomers can inhibit long-term potentiation while increasing long-term depression.98,104
Organotypic hippocampal slices from wild-type mice and mice overexpressing mutant APP have also been used extensively to look at the effects of amyloid β on neuron morphology, spine density, long-term potentiation and long-term depression.98,105,106 Knafo and colleagues107 assessed changes in the morphology and density of dendritic spines from the dentate gyrus in mice expressing PS1 and Swedish APP (APPsw), a form of APP with a mutation at the β-cleavage site that increases amyloid β production. These results showed that the proximity of amyloid β plaques to the dendrites affected spine density and shape compared with litter-mate control mice. Dendrites passing through the plaque had a much lower spine density than dendrites that abutted the plaque but did not pass through it, whereas dendrites that were further removed from the plaque had a similar number of spines as those found in nontransgenic mice. These investigators also assessed the morphology of the spines, and whereas the number of spines was similar between the APPsw transgenic mice and nontransgenic controls in areas where plaques were not present, the number of large spines that are usually associated with memory was much higher in the nontransgenic mice. Thus, APPsw transgenic mice were unable to form synapses as readily as non-transgenic mice, an observation that correlates well with the cognitive deficit found in mice expressing APP/PS1.107 As the loss of large spines is found throughout the dentate gyrus of the mutant APP transgenic mice, it is likely that soluble forms of amyloid β peptides, rather than amyloid β–containing neuritic plaques, are responsible for this morphological deficit.
These studies have shown that high levels of soluble amyloid β can reduce the number and alter the morphology of dendritic spines in mutant APP transgenic mice and that exogenous amyloid β produces similar results in hippocampal slices and primary neurons. Using an elegant hippocampal slice culture system, Wei and colleagues106 further elucidated the effects of amyloid β proximity on synaptic plasticity. Neurons in the CA3 region of the hippocampus have long axons that form synapses with the neurons in the CA1 region. When neurons from the CA3 region were transfected with APP, CA1 neurons that were very close to the transfected cells showed reduced spine density, and the remaining spines demonstrated an altered morphology. Transfecting CA1 neurons also caused a reduction in spine density in non-transfected neurons that were close to the transfected cells. Spine density and morphological changes were reversed when cultures were treated with a γ-secretase inhibitor, indicating that amyloid β was responsible for reduced synaptic density.
The role of glutamate in amyloid β–dependent spine loss, long-term potentiation inhibition and long-term depression induction has also received considerable attention. The NMDA receptor antagonist AP-5 prevents amyloid β–induced spine loss, thus suggesting that glutamate is essential for amyloid β–induced changes in spine density and morphology.106 More recently, amyloid β–induced activation of extrasynaptic NR2B has been suggested to play a critical role in this process. When wild-type mouse hippocampal slice cultures were treated with either soluble amyloid β oligomers or the glutamate uptake inhibitor D,L-threo-β-benzyloxyaspartate there was an increase in extrasynaptic NR2B-mediated inhibition of long-term potentiation that was attenuated by the specific NR2B inhibitors ifenprodil and Ro 25–6981.104 Furthermore, it was shown that when glutamate levels were reduced by the glutamate scavenger system, glutamic pyruvic transaminase and pyruvate, amyloid β could no longer induce inhibition of long-term potentiation. Taken together, these data suggest that extrasynaptic NR2B activation can possibly mediate amyloid β–induced inhibition of long-term potentiation and that this depends to some extent on the presence of glutamate.
Patch clamp studies on the field excitatory postsynaptic currents after low-frequency stimulation of neurons in the CA1 region of hippocampal slices have shown that soluble amyloid β oligomer-induced long-term depression also depends on glutamate.108–110 When the glutamate scavenger, glutamic pyruvic transaminase, was used in combination with NMDA receptor and mGluR inhibitors, they were able to restore amyloid β–induced long-term depression to normal levels, showing that it was mediated by excessive glutamate stimulation of both NMDA receptor and mGluRs. The glutamate uptake inhibitor D,L-threo-β-benzyloxyaspartate as well as inhibitors of calcineurin and glycogen synthase kinase-3 (GSK-3) were then used to show that amyloid β–induced long-term depression depended on excessive glutamate levels and activation of these enzymes.108,110 There is also evidence that amyloid β peptide can alter the levels/efficiency of glutamate transporters, which are known to be decreased in APP transgenic mice and Alzheimer disease brains,59,111,112 thus suggesting a role for amyloid β in long-term depression by regulating glutamate clearance.
Amyloid β–mediated neuronal function and glutamate
The significance of amyloid β in synaptic plasticity is well established, but its role in regulating synaptic function is still poorly understood. Low concentrations (i.e., picomolar or nanomolar) of amyloid β1–40 have been shown to increase viability of cultured neurons,15,85 and there is evidence that amyloid β may have a role in normal synaptic function (Box 1).127 This is supported by recent data that showed depletion of endogenous amyloid β peptide by using an amyloid β antibody or small interfering RNA against murine APP can reduce long-term potentiation and contextual fear and reference memories in wild-type mice.128 There is also evidence that synaptic activity increases amyloid β production both in vitro and in vivo,95,105,129 whereas increased concentrations of soluble amyloid β in cells overexpressing APP depressed synaptic activity;130 thus it is likely that amyloid β may act as regulator of synaptic activity through a negative feedback loop. This is supported by electrophysiological studies that showed that amplitudes of NMDA and AMPA receptor–mediated excitatory postsynaptic currents were significantly enhanced in APP knockout hippocampal cultured neurons. A similar increase in synaptic transmission was detected when wild-type cultured neurons were treated with the γ-secretase inhibitor DAPT, which decreases levels of amyloid β peptides.131 Conversely, cultured slices overex-pressing APP showed a reduced NMDA receptor–dependent synaptic transmission.127 It is thus possible that dysfunction of the negative feedback loop mediated by amyloid β could lead to increased silencing of neurons by reducing synaptic transmission in affected areas of the brain in individuals with Alzheimer disease.
Box 1. The roles of amyloid β peptide in the function and control of the glutamate cycle and glutamatergic neurons.
Decrease synaptic density and alter the morphology of dendritic spines98,106,107,115
Disrupt the postsynaptic density and prevent NMDA and AMPA receptors reaching the cell surface120,121
AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA = N-methyl-d-aspartate.
One possible mechanism by which excess amyloid β could reduce synaptic transmission is by inducing endocytosis of NMDA and AMPA receptors from the postsynaptic membranes.108,113,132,133 This is especially important with respect to AMPA receptors, as trafficking of these receptors to and from the cell surface is very dynamic.119 Hsieh and colleagues108 showed that amyloid β–induced synaptic depression and dendritic spine loss are dependent on AMPA receptor removal from the postsynaptic membrane. They also suggested that endocytosis of GluR2 drives the removal of AMPA receptors from the cell surface, as cells transfected with a mutant form of GluR2, which is endocytosed more readily than wild-type GluR2, showed a reduction in AMPA as well as NMDA receptor–mediated transmission. Amyloid β has also been reported to regulate NMDA receptor trafficking in cortical neurons derived from mice overexpressing APPsw.133 These mice had the same total number of NMDA receptors, but fewer cell surface NMDA receptors than nontransgenic mice. Exogenous application of amyloid β1–42 to nontransgenic cortical neurons promoted NMDA receptor endocyto-sis, indicating that the reduced receptor levels at the cell surface of APPsw mice was due to increased removal rather than disrupted trafficking to the membrane.133
A role for striatal-enriched tyrosine phosphatase (STEP61) has recently been linked to amyloid β–induced synaptic function since it increases the endocytosis of NMDA and AMPA receptors from the postsynaptic membrane.133–136 The STEP61 is enriched in synapses, suggesting that it has a specialized role in cell signalling. The levels of STEP61 were reported to be increased in 2 mouse models of Alzheimer disease133 and in humans135 with Alzheimer disease. There is evidence that amyloid β peptides, delivered using the enriched conditioned media of cells overexpressing APP, can increase synaptic depression by enhancing STEP61 activity via multiple mechanisms.133,135,136 First, amyloid β is known to impair the ubiquitin proteasome system,137 which can increase STEP61 protein levels in APP transgenic mice and in neuronal cell cultures.135 Second, amyloid β can activate α7 nicotinic receptors or NMDA receptors and increase Ca2+ entry into the cell. This triggers protein phosphatase 2B, which activates STEP61 by dephosphorylation,138 leading to increased internalization of the NR1/NR2B receptor complex.133 Third, mGluR 5 activation has also been shown to increase STEP61 translation in rat hippocampal slices and synaptosomes, which leads to extracellular signal-related kinase1/2 (ERK1/2)-dependent de-phosphorylation of the GluR2 isoform and subsequent endocytosis of the AMPA receptor.136 Taken together these findings indicate that amyloid β can activate and increase protein levels of STEP61, resulting in enhanced dephosporylation and endocytosis of glutamate receptors and reduced synaptic function.
It has recently been reported that soluble amyloid β1–40 can disrupt the postsynaptic density (PSD), an electron dense thickening of the membrane comprising a network of proteins, including glutamate receptors, adhesion molecules, scaffolding proteins, cytoskeletal proteins and associated signalling molecules. The PSD, which is involved in maintaining synaptic homeostasis, is regulated by cytoskeletal protein PSD-95 and the master organizing proteins Shank and Homer.120,121 There is evidence that PSD-95 and Shank are especially important for ensuring that AMPA and NMDA receptors get to the cell surface and stay at the plasma membrane,120,139 and Homer is important in mGluR 1 localization.140 Amyloid β colocalizes with PSD-95115 and causes a breakdown of the PSD-95, Shank and Homer clusters and a reduction in levels of NMDA and AMPA receptors at the plasma membrane.121 These observations are supported by the analysis of amyloid β and PSD-95 levels in intact nerve terminals taken from Alzheimer disease and control brains, which showed a 19% decrease in PSD-95 levels and a 132% increase in the amyloid β levels in Alzheimer disease compared with control brains.141 One of the most important signalling proteins that is highly abundant in the PSD is Ca2+/CaMKII, which plays a role in the delivery of AMPA receptors to the synapses.142,143 There is evidence that mice overexpressing APP not only show reduced synaptic CaMKII and cell surface GluR1 levels, but also exhibit decreased AMPA receptor transmission as observed in amyloid β1–42 treated neurons.144,145 Thus, synaptic function is a closely controlled process requiring the appropriate activation of different types of proteins, including structural proteins controlling the architecture of pre- and postsynapses, kinase and phosphatases that mediate the effects of glutamate and other neurotransmitters. The complexity of amyloid β–induced synaptic dysfunction is evidenced by its ability to affect numerous elements of the synaptic machinery, which can directly influence pathological abnormalities in the brains of individuals with Alzheimer disease.
Glutamate release/uptake and amyloid β peptide
It is now well established that amyloid β peptides are produced constitutively by brain cells, including neurons, and are found in the nanomolar–picomolar range in the cere-brospinal fluid of healthy individuals,15,84 thus raising the possibility that these peptides under normal conditions may have a role in the regulation of brain neurotransmitter/modulator release. We and others have previously reported that amyloid β1–40/1–42 peptides can inhibit the release of acetylcholine, particularly from hippocampal and cortical regions of the rat brain.37,146,147 Additionally, there is evidence that amyloid β–related peptides can modulate the release of noradrenaline,148,149 but not of γ-aminobutyric acid,150 from rat brain slice preparations. As for glutamate release, it has been reported earlier that pretreatment of hippocampal slices with micromolar concentrations of amyloid β25–35 can potentiate glutamate release.150 More recently, using an in vitro slice preparation, we have shown that nanomolar concentrations of amyloid β1–40/1–42 peptides without pretreatment can potentiate potassium-evoked glutamate release from the hippocampus and cortex — the areas affected in Alzheimer disease. The effect is specific and appears to be mediated by direct interaction with the glutamatergic terminals.118 This idea is reinforced by the evidence that preincubation of nerve endings with amyloid β1–42 can potentiate [14C]glutamic acid release, at least in part, by interacting with an N-type voltage-operated calcium channel.148 These findings, together with the observation that glutamatergic terminals containing VGLUT1 are localized in close apposition to the APP-positive neurons in the hippocampus, provide an anatomical basis to suggest a role of endogenously derived amyloid β peptides in the presynaptic regulation of glutamate release from selected brain regions. At present, the underlying mechanisms by which amyloid β can potentiate glutamate release remains unclear, but it is of interest to note that nanomolar concentrations of amyloid β1–40 did not significantly alter glutamate release from the striatum, an area that is relatively spared in Alzheimer disease.118 Whether micromolar concentrations of amyloid β peptide can enhance glutamate release from striatal slices remains to be established. However, these results raise the possibility that preferential vulnerability of hippocampal and cortical neurons could relate, at least in part, to their sensitivity to amyloid β peptides.
In contrast to glutamate release, amyloid β effects on glutamate uptake are somewhat controversial. Some studies have suggested that micromolar concentrations of amyloid β25–35 can enhance glutamate uptake by astrocytes, which is mediated by increased expression of the glutamate transporter GLAST and enhanced by ERK inhibition, but unaffected by antioxidants.151,152 However, nanomolar concentrations of the amyloid β25–35 peptide were found to increase extracellular glutamate levels by inhibiting its uptake in cultured neurons and astrocytes.116,153 Harris and colleagues153 proposed that oxidative stress induced by the amyloid β peptide damages glutamate transporters, preventing glutamate uptake, and this proposal is supported by the increased levels of conjugation observed between the reactive oxygen species 4-hydroxy-2-nonenal and GLT-1 in synaptosomal preparations treated with amyloid β peptide and in the Alzheimer disease brain.154 More recent data have confirmed that amyloid β1–40 reduces glutamate uptake, and this also suggests that oxidative damage, as well as decreased GLT-1 and GLAST levels, could mediate reduced glutamate clearance from the synapse.73 Interestingly, prolonged exposure of cultured microglia to a 5 μM concentration of amyloid β25–35 can increase the extracellular levels via reverse glutamate transporters.155 Thus amyloid β peptides can influence extracellular glutamate levels by regulating uptake and/or release of glutamate, which may be relevant in normal physiological and pathological conditions.155
Glutamate and amyloid β–mediated toxicity
Chronic exposure to amyloid β peptides can induce toxicity in a variety of cell lines and in primary rat and human cultured neurons. The toxicity of the peptide is related to its ability to form insoluble aggregates.84,156–158 However, recent evidence suggests that the most detrimental forms of amyloid β peptides are the soluble oligomers and that the insoluble amorphous or fibrillar deposits represent a less harmful inactivated form of the peptide.15 The mechanisms associated with amyloid β toxicity are not clearly defined, but appear to involve alterations in intracellular calcium, production of free radicals, phosphorylation of tau protein and/or activation of caspase and noncaspase pathways that culminate in programmed cell death.25,84,159 Studies on a variety of cell lines and primary cultured neurons suggest that amyloid β toxicity might be mediated either by interaction with a hydroxy-steroid dehydrogenase enzyme or by plasma membrane receptors for advanced glycation end products, class A scavenger receptor, p75 neurotrophin receptor, amylin or α7 nicotinic receptors.117,160–164 However, a number of studies have clearly indicated that amyloid β toxicity is mediated, at least in part, by glutamate-mediated excitotoxicity, which involves activation of the NMDA receptors, leading to elevated intra-cellular Ca2+ and consequent stimulation of a cascade of enzymes resulting in cell death.28,76,77,158 Amyloid β–induced excitotoxic cell death was first reported in the early 1990s, with initial reports indicating that prolonged exposure of amyloid β peptides, including amyloid β25–35 and amyloid β1–38 with glutamate induced greater cell death than exposure to amyloid β or glutamate alone in mouse cortical neuronal cultures.122 The role of glutamate receptors in this process has subsequently been shown through the use of glutamate receptor agonists and antagonists.123 When Mattson and colleagues123 exposed human primary neuronal cultures to amyloid β1–38/25–35 and either NMDA or KA, they showed that glutamate receptor activation increased cell death, possibly through a dysregulation of Ca2+ homeostasis, and that the NMDA receptor antagonist APV and the KA receptor antagonist DGG reduced cell death. This is supported by more recent data showing that inactivation of the NMDA receptor by antagonists, such as MK-801, AP5 or memantine, can protect neurons from amyloid β1–40/1–42 toxicity;124–126 that amyloid β1–40/1–42–induced neurodegeneration in the adult rat brain is mediated, in part, by activation of the NMDA receptor;165,166 and that transgenic mice exhibiting high levels of amyloid β peptide show increased vulnerability to excitotoxicity.167,168 Taken together, these studies suggest that glutamate receptor activation may be essential for amyloid β–induced cell death.
At present, it is unclear how amyloid β peptides can regulate activation of glutamate receptors, leading to the death of neurons. There is evidence that amyloid β peptide can bind directly to glutamate receptors, increasing their activity and leading to influx of Ca2+ in organotypic slice cultures. Antagonists of NMDA and AMPA receptors can prevent influx of Ca2+ and cell death.27 Alternatively, it is possible that amyloid β–related peptides can increase extracellular glutamate levels by potentiating release117,118,155 and/or inhibiting the uptake of the neurotransmitter,116,153 which can subsequently trigger death of neurons by excitotoxicity. It is therefore likely that a combination of reduced glutamate clearance from the synaptic cleft, increased release of glutamate from neurons and glia, and the subsequent activation of the glutamate receptors contribute to toxicity mediated by amyloid β–related peptides.
Glutamate and amyloid β–mediated tau phosphorylation
A number of earlier studies have suggested a critical role for tau in the genesis of amyloid β toxicity, but the cellular mechanisms by which tau mediates degeneration of neurons remain unclear. Using cultured neurons/cell lines, it has been shown that amyloid β1–40/1–42 can induce site-specific phosphorylation of tau protein by activating multiple kinases, including ERK1/2, PKC, cyclin-dependent kinases (CDK), Fyn kinase and GSK-3β. Given the evidence that tau phosphorylation occurs before the loss of neurons and that inhibition of tau phosphorylation can prevent amyloid β–induced neurodegeneration,169–172 it is likely that increased levels of phosphorylated tau can contribute to the death of neurons by triggering loss of microtubule binding, impaired axonal transport and neuritic dystrophy. A critical role of the phosphorylated tau protein in amyloid β–induced toxicity has been established by the evidence that cells undergoing amyloid β toxicity exhibit increased levels of tau phosphorylation,169,170 that inhibition of tau phosphorylation by blocking tau kinases can prevent cell death,171,173 and that neurons cultured from tau protein knockout mice are resistant to amyloid β toxicity.174
Given the importance of glutamate in amyloid β–mediated toxicity, a functional interrelationship between glutamate receptors and tau phosphorylation has long been investigated in a variety of experimental paradigms. We and others have shown that glutamate receptor antagonists can protect neurons against amyloid β1–40/1–42–mediated toxicity.124–126 This effect appears to be mediated, at least in part, by reversing or decreasing activation of tau kinases, such as ERK1/2 and GSK-3β, that are involved in the phosphorylation of tau protein rather than altering extracellular glutamate levels or regulation of amyloid β conformation or internalization.125 Apart from influencing tau phosphorylation directly, there is evidence that Fyn kinase can regulate glutamatergic NMDA receptor activation following amyloid β treatment by triggering phosphorylation and subsequent interaction of the NR2B subunit of the receptor with PSD-95. The NR2B/PSD-95/Fyn complex that is formed when NMDA receptors are activated perpetuates Ca2+ influx, which can activate 2 key tau kinases, GSK-3β175 and CDK5.176 It is therefore possible that NMDA receptor activation stabilizes the NR2B/PSD-95/Fyn complex, resulting in a persistent activation of the NMDA receptor channel and increasing tau phosphorylation either by direct phosphorylation of tau via Fyn kinase or by activating other tau kinases, such as GSK-3β or CDK5.177 It is also of interest to note that tau is required for the transportation of Fyn kinase to the postsynapse, where it forms a complex with PSD-95 and the NR2B subunit of the NMDA receptor. Disrupting the localization of Fyn kinase or the formation of the NR2B/PSD-95/Fyn complex was found to protect neurons against amyloid β–induced toxicity.178 Collectively, these results suggest not only increased tau phosphorylation, but also that tau-mediated transportation of Fyn kinase to postsynaptic sites is possibly required for the degeneration of neurons induced by amyloid β–related peptides.
Increased tau phosphorylation may also be a consequence of reduced dephosporylation of the protein. The serine/threonine phosphatases, such as protein phosphatases 1, 2A, 2B (i.e., calcineurin) and 5, have all been shown to dephosphorylate tau, and the activity of protein phosphatases 1, 2A and 5 is known to be reduced by 20%–30% in brains of individuals with Alzheimer disease.179–181 With protein phosphatases 1 and 2A accounting for 90% of serine/threonine phosphatase activity in mammalian cells182,183 and tau dephosporylation predominantly being mediated by protein phosphatase 2A,179 there is convincing evidence that down-regulation of this phosphatase can lead to increased tau hyperphosphorylation in the brains of individuals with Alzheimer disease. Inhibiting protein phosphatase 2A has been shown to increase tau hyperphosphorylation by reducing its dephosphorylation directly184,185 and by increasing the activity of tau kinases, such as CaMKII,184 GSK-3β186 and ERK1/2.187 In addition, protein phosphatase 2A has been shown to dephosphorylate tau in an NMDA and KA receptor–dependent manner.188–190 When rat cortical neurons were treated for 1 hour with 100 μM of glutamate or NMDA there was an initial reduction in phosphorylated tau, which was prevented when the cells were cotreated with okadaic acid, a protein phosphatase 1 and 2A inhibitor. This effect was only measured for the first 4 hours after glutamate or NMDA administration, and so the duration of this effect was not determined.188 This is supported by in vivo studies showing that mice injected with KA exhibit a transient reduction in tau phosphorylation and increase in protein phosphatase 2A activity190 followed by an increased tau hyperphosphorylation and reduced protein phosphatase 2A activity. Thus, it is likely that glutamate receptor activation may transiently reduce tau phosphorylation, but eventually leads to hyper-phosphorylation of the protein, at least in part by reducing protein phosphatase 2A activity. Collectively, these results raise the possibility that reducing tau dephosphorylation may also partly contribute to the increased phosphorylation of tau protein and subsequent degeneration of neurons and/or formation of tangles observed in Alzheimer disease.
More recently, a number of studies have indicated that caspase- and calpain-mediated proteolytic cleavage of tau protein, in addition to enhanced phosphorylation, may have a role in the amyloid β–induced degeneration of neurons. There is evidence that 43–50 kDa truncated tau generated by caspase-3 tends to assemble more rapidly into filaments than full-length tau and has been detected before the formation of tangles and cell death.191–194 Similarly, 17 kDa tau generated by calpain following treatment with amyloid β–related peptides was also detected before enhanced tau phosphorylation or neurite degeneration in cultured neurons. This proteolytic cleavage of tau may lead to neurodegeneration either directly, as reported in various cell lines and neurons, or indirectly by reducing the pool of full-length tau available for binding to microtubules.195,196 There is evidence that NMDA receptor–mediated activation of calpain may be involved in triggering the generation of 17 kDa tau fragments, which can lead to the degeneration of neurons, as both NMDA receptor antagonists and calpain inhibitors are found to protect neurons from cell death.197 Thus, NMDA receptor–regulated tau cleavage and hyperphosphorylation of the protein may represent 2 different mechanisms by which tau can cause cell death.
Glutamate antagonists in the treatment of Alzheimer disease
Owing to the critical role of glutamate in both amyloid β–and tau-mediated pathology, glutamate receptor antagonists have been viewed as good therapeutic targets for many years. Originally the noncompetitive NMDA receptor antagonists MK-801 and phencyclidine were tried as therapies for Alzheimer disease.198,199 These compounds bind to sites within the NMDA receptor channel complex and prevent further Ca2+ entry by blocking the pore. Unfortunately, these drugs had a slow onset of action and bound irreversibly to the channel, preventing normal physiologic entry of Ca2+ into the cell. This led to severe psychotomimetic side effects, such as hallucinations, ataxia and memory loss, thus precluding their use as therapeutics for Alzheimer disease and also other conditions where NMDA receptors were therapeutic targets.198,200 Subsequently, other NMDA receptor antagonists, including amantadine and dextromethorphan, have shown promising results in the treatment of other forms of dementia, but have failed to serve as good therapeutics for Alzheimer disease.200,201 Interestingly, memantine, which was first synthesized by Eli Lilly in 1968 as a derivative of amantadine, was found to be a noncompetitive, low to moderate–affinity NMDA receptor antagonist that can prevent pathological activation of the receptor without affecting its physiologic functions.201–204 This antagonist exhibits a lower binding affinity than MK-801 and phencyclidine, but has a higher affinity than the endogenous NMDA receptor antagonist, the magnesium ion, which normally blocks the voltage-dependent activation of the NMDA receptor. Further studies indicate that memantine preferentially blocks NMDA receptor activity when the channel is excessively open. Since the NMDA receptor channels are open during normal synaptic activity for only milliseconds, memantine is unable to act or accumulate in the channel, thus selectively sparing normal synaptic activity. However, during prolonged receptor activation, as occurs under prolonged depolarization or excitotoxic conditions, memantine becomes an effective blocker of NMDA receptor activity.202,203,205 The pharmacokinetics of memantine and its ability to protect neurons in a variety of in vitro and in vivo animal models suggest a favourable clinical safety profile of the antagonist in the treatment of neurodegenerative disorders. These results, together with some positive data from human clinical trials, convinced the European Union in 2002 and the U.S. Federal Drug Administration in in 2003 to approve memantine for the treatment of moderate-to-severe Alzheimer disease.203,205
Accumulated evidence suggests that memantine can provide protection against a variety of insults, including quinolinic acid–induced toxicity, brain trauma, ischemia and amyloid β1–40/1–42–mediated neurotoxicity under in vitro and in vivo conditions.124,126,166,206 We have recently reported that memantine can significantly protect rat primary cortical cultured neurons against amyloid β1–42–induced toxicity by attenuating activation of tau kinases (i.e., GSK-3β and ERK-1/2) and phosphorylation of tau protein.125 The drug also reduces amyloid β–induced cleavage of caspase-3 and dephosphorylation of cyclic AMP response element-binding protein, both of which are critical for cell survival. However, memantine did not alter either the conformation or internalization of amyloid β peptide, and it was unable to attenuate amyloid β–induced potentiation of extracellular glutamate levels.125 In addition to its effect on toxicity, memantine is able to reverse amyloid β–induced long-term potentiation deficits in mouse hippocampal slice cultures207 and in the CA1 region of the adult rat hippocampus under in vivo conditions.114 Consistent with these results, several studies have demonstrated beneficial effects of memantine on cognitive deficits and Alzheimer disease–related pathology in transgenic mice overexpressing amyloid β peptides.208–210 There is evidence that memantine can reduce amyloid β plaque burden and increase synaptic density in the hippocampus of Tg2576 transgenic mice.211 Triple transgenic mice treated daily with 30 mg/kg of memantine for 3 months also showed improvement in cognitive performance at 3 different age groups, which was accompanied by reduced levels of hyperphosphorylated tau and increased levels of inactive GSK-3β.208 The triple transgenic mice also showed a reduction in insoluble amyloid β, but an increase in soluble amyloid β levels without any alterations in APP, α-CTF or β-CTF after memantine treatment. Thus, it is likely that the antagonist does not alter APP levels/processing, but changes amyloid β aggregation or clearance in animal models of Alzheimer disease.208 This is somewhat contrary to previous studies that have shown lower APP levels and altered APP processing resulting in a reduction in amyloid β1–40/1–42 production and/or secretion after memantine treatment under in vivo and in vitro conditions.91,212,213 Whether the discrepancy could be due to long-term (30 mg/kg/d memantine for 3 mo)208 versus short-term (i.e., 20 mg/kg/d for only 8 d)91 treatments with memantine or other factors remains to be established. However, these results, taken together, indicate that memantine is able to attenuate both tau- and amyloid β–mediated pathology along with an effect on the cognitive performance of the animal, validating its therapeutic potential in the treatment of Alzheimer disease pathology.
The clinical effects of memantine have been widely studied, and a number of trials and meta-analyses have shown beneficial effects of the drug on the global status, cognition, behaviour and function of individuals with moderate to severe Alzheimer disease without any major adverse effects.214–216 It has also been shown to improve behavioural disturbances, such as agitation and aggression. Its mechanism of action as an NMDA receptor antagonist differs from the cholinesterase inhibitors approved for the treatment of Alzheimer disease (i.e., donepezil, galantamine and revastigmine), suggesting a theoretical potential to be used in combination with these drugs. Some clinical trials and other lines of experimental data have suggested that the combination of memantine and cholinesterase inhibitors is safe and might provide additive benefits. The side effect profile of memantine is very different from that of the cholinesterase inhibitors, thus allowing its use in individuals with Alzheimer disease who cannot tolerate cholinesterase inhibitors because of their gastrointestinal or cardiac adverse effects. The data on the efficacy of memantine for individuals with mild to moderate Alzheimer disease is somewhat less compelling, with some studies demonstrating no benefit beyond placebo either as monotherapy or in conjunction with cholinesterase inhibitors.204,217,218 Unfortunately, to date, clinical studies have only examined the symptomatic benefits of mematine with a limited dose of the drug. Given its purported mechanism of action as an NMDA receptor antagonist, it is of interest to determine whether higher doses of memantine with acceptable tolerability might have disease-modifying effects, such as protection of neurons against excitotoxicity by attenuating tau phosphorylation, which may provide a rationale for the use of the drug in individuals with mild Alzheimer disease or even those at earlier stages.204 Some recent studies have suggested that second-generation analogues of memantine, nitromemantines, may be more neuroprotective than memantine at a lower dosage in both in vitro and in vivo animal models.219 These modified drugs not only share memantine’s ability to block NMDA receptors, but can also nitrosylate 1 of 5 cysteines in NMDA receptors. Since S-nitrosylation of the NR2A subunit has been shown to modulate NMDA receptor activity,203 it is likely that nitromemantines may provide a means to specifically target NMDA receptors without causing dangerous side effects, such as lowering blood pressure, that are associated with other nitrosylating drugs like nitroglycerin. However, more research is needed to establish the efficacy of nitromemantines in protecting neurons in animal models as well as clinical safety for their use in humans with Alzheimer disease.
Acknowledgements
The authors are grateful to CIHR and the Canada Research Chairs program for their support to the research programs.
Footnotes
Competing interests: None declared for T.J. Revett. G.B. Baker declares educational grant support from Pfizer Canada and travel and speaker fees from the Turkish Association for Psychopharmacology. J.H. Jhamandas and S. Kar are supported in part by a CIHR operating grant paid to their institution.
Contributors: T.J. Revett and S. Kar designed the study. T.J. Revett acquired the data. T.J. Revett, G.B. Baker, J.H. Jhamandas and S. Kar analyzed the data and wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerreiro RJ, Gustafson DR, Hardy J. The genetic architecture of Alzheimer’s disease: beyond APP, PSENs and APOE. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St George-Hyslop PH, Petit A. Molecular biology and genetics of Alzheimer’s disease. C R Biol. 2005;328:119–30. doi: 10.1016/j.crvi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–9. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 6.Seshadri S, Fitzpatrick AL, Ikram MA, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–40. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollingworth P, Harold D, Jones L, et al. Alzheimer’s disease genetics: current knowledge and future challenges. Int J Geriatr Psychiatry. 2011;26:793–802. doi: 10.1002/gps.2628. [DOI] [PubMed] [Google Scholar]
- 9.Lambert JC, Amouyel P. Genetics of Alzheimer’s disease: New evidences for an old hypothesis? Curr Opin Genet Dev. 2011;21:295–301. doi: 10.1016/j.gde.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–26. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 11.de la Torre JC. How do heart disease and stroke become risk factors for Alzheimer’s disease? Neurol Res. 2006;28:637–44. doi: 10.1179/016164106X130362. [DOI] [PubMed] [Google Scholar]
- 12.Hölscher C. Diabetes as a risk factor for Alzheimer’s disease: insulin signalling impairment in the brain as an alternative model of Alzheimer’s disease. Biochem Soc Trans. 2011;39:891–7. doi: 10.1042/BST0390891. [DOI] [PubMed] [Google Scholar]
- 13.Chen J-H, Lin K-P, Chen Y-C. Risk factors for dementia. J Formos Med Assoc. 2009;108:754–64. doi: 10.1016/S0929-6646(09)60402-2. [DOI] [PubMed] [Google Scholar]
- 14.Butterfield DA, Pocernich CB. The glutamatergic system and Alzheimer’s disease: therapeutic implications. CNS Drugs. 2003;17:641–52. doi: 10.2165/00023210-200317090-00004. [DOI] [PubMed] [Google Scholar]
- 15.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–84. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 16.Combs B, Voss K, Gamblin TC. Pseudohyperphosphorylation has differential effects on polymerization and function of tau isoforms. Biochemistry. 2011;50:9446–56. doi: 10.1021/bi2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mi K, Johnson GV. The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2006;3:449–63. doi: 10.2174/156720506779025279. [DOI] [PubMed] [Google Scholar]
- 18.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl. 1998;53:127–40. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- 20.Chow VW, Mattson MP, Wong PC, et al. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeBlanc AC, Chen HY, Autilio-Gambetti L, et al. Differential APP gene expression in rat cerebral cortex, meninges, and primary astroglial, microglial and neuronal cultures. FEBS Lett. 1991;292:171–8. doi: 10.1016/0014-5793(91)80861-v. [DOI] [PubMed] [Google Scholar]
- 22.Mills J, Reiner PD. Regulation of amyloid precursor protein cleavage. J Neurochem. 1999;72:443–60. doi: 10.1046/j.1471-4159.1999.0720443.x. [DOI] [PubMed] [Google Scholar]
- 23.Murphy MP, Levine H. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–23. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Näslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–7. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 25.Blurton-Jones M, Laferla FM. Pathways by which Aβ facilitates tau pathology. Curr Alzheimer Res. 2006;3:437–48. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- 26.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberdi E, Sanchez-Gomez MV, Cavaliere F, et al. Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–72. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium. 2010;47:183–9. doi: 10.1016/j.ceca.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45:583–95. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Dasilva KA, Shaw JE, McLaurin J. Amyloid-β fibrillogenesis: structural insight and therapeutic intervention. Exp Neurol. 2010;223:311–21. doi: 10.1016/j.expneurol.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Morgan D. Immunotherapy for Alzheimer’s disease. J Intern Med. 2011;269:54–63. doi: 10.1111/j.1365-2796.2010.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pahnke J, Walker LC, Scheffler K, et al. Alzheimer’s disease and blood-brain barrier function — Why have anti-β-amyloid therapies failed to prevent dementia progression? Neurosci Biobehav Rev. 2009;33:1099–108. doi: 10.1016/j.neubiorev.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samadi H, Sultzer D. Solanezumab for Alzheimer’s disease. Expert Opin Biol Ther. 2011;11:787–98. doi: 10.1517/14712598.2011.578573. [DOI] [PubMed] [Google Scholar]
- 34.DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5:417–21. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- 35.Francis PT. Glutamatergic systems in Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18:S15–21. doi: 10.1002/gps.934. [DOI] [PubMed] [Google Scholar]
- 36.Bell KF, Bennett DA, Cuello AC. Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J Neurosci. 2007;27:10810–7. doi: 10.1523/JNEUROSCI.3269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kar S, Slowikowski SP, Westaway D, et al. Interactions between β-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci. 2004;29:427–41. [PMC free article] [PubMed] [Google Scholar]
- 38.Morris JC. Challenging assumptions about Alzheimer’s disease: mild cognitive impairment and the cholinergic hypothesis. Ann Neurol. 2002;51:143–4. doi: 10.1002/ana.10135. [DOI] [PubMed] [Google Scholar]
- 39.Bell KFS, Cuello AC. Altered synaptic function in Alzheimer’s disease. Eur J Pharmacol. 2006;545:11–21. doi: 10.1016/j.ejphar.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 40.Dijk SN, Francis PT, Stratmann GC, et al. Cholinomimetics increase glutamate outflow via an action on the corticostriatal pathway: implications for Alzheimer’s disease. J Neurochem. 1995;65:2165–9. doi: 10.1046/j.1471-4159.1995.65052165.x. [DOI] [PubMed] [Google Scholar]
- 41.Tsang SWY, Pomakian J, Marshall GA, et al. Disrupted muscarinic M1 receptor signalling correlates with loss of protein kinase C activity and glutamatergic deficit in Alzheimer’s disease. Neurobiol Aging. 2007;28:1381–7. doi: 10.1016/j.neurobiolaging.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Kowall NW, Beal MF. Glutamate-, glutaminase-, and taurine-immunoreactive neurons develop neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1991;29:162–7. doi: 10.1002/ana.410290208. [DOI] [PubMed] [Google Scholar]
- 43.Bussière T, Giannakopoulos P, Bouras C, et al. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol. 2003;463:281–302. doi: 10.1002/cne.10760. [DOI] [PubMed] [Google Scholar]
- 44.Fremeau RT, Jr, Troyer MD, Pahner I, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–60. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 45.Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- 46.Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 47.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 48.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 49.Gerber U, Gee CE, Benquet P. Metabotropic glutamate receptors: intracellular signalling pathways. Curr Opin Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Schools GP, Kimelberg HK. mGluR3 and mGluR5 are the predominant metabotropic glutatmate receptor mRNAs expressed in hippocampal astrocytes acutely isolated from young rats. J Neurosci Res. 1999;58:533–43. doi: 10.1002/(sici)1097-4547(19991115)58:4<533::aid-jnr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 51.Vogt KE, Nicoll RA. Glutamate and γ-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci U S A. 1999;96:1118–22. doi: 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 53.Storck T, Schulte S, Hofmann K, et al. Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89:10955–9. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pines G, Danbolt NC, Bjoras M, et al. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–7. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 55.Shashidharan P, Huntley GW, Meyer T, et al. Neuron-specific human glutamate transporter: molecular cloning, characterization and expression in human brain. Brain Res. 1994;662:245–50. doi: 10.1016/0006-8993(94)90819-2. [DOI] [PubMed] [Google Scholar]
- 56.Kashani A, Lepicard E, Poirel O, et al. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol Aging. 2008;29:1619–30. doi: 10.1016/j.neurobiolaging.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Kirvell SL, Esiri M, Francis PT. Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer’s disease. J Neurochem. 2006;98:939–50. doi: 10.1111/j.1471-4159.2006.03935.x. [DOI] [PubMed] [Google Scholar]
- 58.Sokolow S, Luu SH, Nandy K, et al. Preferential accumulation of amyloid β in presynaptic glutamatergic terminals (VGLUT1 and VGLUT2) in Alzheimer’s disease cortex. Neurobiol Dis. 2012;45:381–7. doi: 10.1016/j.nbd.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacob CP, Koutsilieri E, Bartl J, et al. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alzheimers Dis. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- 60.Scott HA, Gebhardt FM, Mitrovic AD, et al. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol Aging. 2011;32:553.e1–11. doi: 10.1016/j.neurobiolaging.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Robinson SR. Changes in the cellular distribution of glutamine synthetase in Alzheimer’s disease. J Neurosci Res. 2001;66:972–80. doi: 10.1002/jnr.10057. [DOI] [PubMed] [Google Scholar]
- 62.Hynd MR, Scott HL, Dodd PR. Selective loss of NMDA receptor NR1 subunit isoforms in Alzheimer’s disease. J Neurochem. 2004;89:240–7. doi: 10.1111/j.1471-4159.2003.02330.x. [DOI] [PubMed] [Google Scholar]
- 63.Hynd MR, Scott HL, Dodd PR. Differential expression of N-methyl D-aspartate receptor NR2 isoforms in Alzheimer’s disease. J Neurochem. 2004;90:913–9. doi: 10.1111/j.1471-4159.2004.02548.x. [DOI] [PubMed] [Google Scholar]
- 64.Williams C, Mehrian Shai R, Wu Y, et al. Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer’s disease. PLoS ONE. 2009;4:e4936. doi: 10.1371/journal.pone.0004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pellegrini-Giampietro DE, Bennett MV, Zukin RS. AMPA/kainate receptor gene expression in normal and Alzheimer’s disease hippocampus. Neuroscience. 1994;61:41–9. doi: 10.1016/0306-4522(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 66.Yasuda RP, Ikonomovic MD, Sheffield R, et al. Reduction of AMPA-selective glutamate receptor subunits in the entorhinal cortex of patients with Alzheimer’s disease pathology: a biochemical study. Brain Res. 1995;678:161–7. doi: 10.1016/0006-8993(95)00178-s. [DOI] [PubMed] [Google Scholar]
- 67.Dewar D, Chalmers DT, Graham DI, et al. Glutamate metabotropic and AMPA binding sites are reduced in Alzheimer’s disease: an autoradiographic study of the hippocampus. Brain Res. 1991;553:58–64. doi: 10.1016/0006-8993(91)90230-s. [DOI] [PubMed] [Google Scholar]
- 68.Aronica E, Dickson DW, Kress Y, et al. Non-plaque dystrophic dendrites in Alzheimer hippocampus: a new pathological structure revealed by glutamate receptor immunocytochemistry. Neuroscience. 1998;82:979–91. doi: 10.1016/s0306-4522(97)00260-1. [DOI] [PubMed] [Google Scholar]
- 69.Albasanz JL, Dalfo E, Ferrer I, et al. Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer’s disease and dementia with Lewy bodies correlates with stage of Alzheimer’s-disease-related changes. Neurobiol Dis. 2005;20:685–93. doi: 10.1016/j.nbd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Lee HG, Ogawa O, Zhu X, et al. Aberrant expression of meta-botropic glutamate receptor 2 in the vulnerable neurons of Alzheimer’s disease. Acta Neuropathol. 2004;107:365–71. doi: 10.1007/s00401-004-0820-8. [DOI] [PubMed] [Google Scholar]
- 71.Masliah E, Alford M, DeTeresa R, et al. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol. 1996;40:759–66. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 72.Li S, Mallory M, Alford M, et al. Glutamate transporter alterations in Alzheimer’s disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56:901–11. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Matos M, Augusto E, Oliveira CR, et al. Amyloid-β peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156:898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Parihar MS, Brewer GJ. Mitoenergetic failure in Alzheimer disease. Am J Physiol Cell Physiol. 2007;292:C8–23. doi: 10.1152/ajpcell.00232.2006. [DOI] [PubMed] [Google Scholar]
- 75.Greenamyre JT, Maragos WF, Albin RL, et al. Glutamate transmission and toxicity in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:421–30. doi: 10.1016/0278-5846(88)90102-9. [DOI] [PubMed] [Google Scholar]
- 76.Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30:379–87. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ulas J, Weihmuller FB, Brunner LC, et al. Selective increase of NMDA-sensitive glutamate binding in the striatum of Parkinson’s disease, Alzheimer’s disease, and mixed Parkinson’s disease/Alzheimer’s disease patients: an autoradiographic study. J Neurosci. 1994;14:6317–24. doi: 10.1523/JNEUROSCI.14-11-06317.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikonomovic MD, Mizukami K, Warde D, et al. Distribution of glutamate receptor subunit NMDAR1 in the hippocampus of normal elderly and patients with Alzheimer’s disease. Exp Neurol. 1999;160:194–204. doi: 10.1006/exnr.1999.7196. [DOI] [PubMed] [Google Scholar]
- 80.Panegyres PK, Zafiris-Toufexis K, Kakulas BA. The mRNA of the NR1 subtype of glutamate receptor in Alzheimer’s disease. J Neural Transm. 2002;109:77–89. doi: 10.1007/s702-002-8238-9. [DOI] [PubMed] [Google Scholar]
- 81.Sze C, Bi H, Kleinschmidt-DeMasters BK, et al. N-methyl-D-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer’s disease. J Neurol Sci. 2001;182:151–9. doi: 10.1016/s0022-510x(00)00467-6. [DOI] [PubMed] [Google Scholar]
- 82.Allinson TM, Parkin ET, Turner AJ, et al. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–52. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 83.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 84.Clippingdale AB, Wade JD, Barrow CJ. The amyloid-β peptide and its role in Alzheimer’s disease. J Pept Sci. 2001;7:227–49. doi: 10.1002/psc.324. [DOI] [PubMed] [Google Scholar]
- 85.Plant LD, Boyle JP, Smith IF, et al. The production of amyloid β peptide is a critical requirement for the viability of central neurons. J Neurosci. 2003;23:5531–5. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roberson MR, Harrell LE. Cholinergic activity and amyloid precursor protein metabolism. Brain Res Brain Res Rev. 1997;25:50–69. doi: 10.1016/s0165-0173(97)00016-7. [DOI] [PubMed] [Google Scholar]
- 87.Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates α-secretase amyloid precursor protein processing and inhibits amyloid-β production. J Neurosci. 2009;29:4442–60. doi: 10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee RK, Jimenez J, Cox AJ, et al. Metabotropic glutamate receptors regulate APP processing in hippocampal neurons and cortical astrocytes derived from fetal rats. Ann N Y Acad Sci. 1996;777:338–43. doi: 10.1111/j.1749-6632.1996.tb34443.x. [DOI] [PubMed] [Google Scholar]
- 89.Marcello E, Gardoni F, Mauceri D, et al. Synapse-associated protein-97 mediates alpha-secretase ADAM10 trafficking and promotes its activity. J Neurosci. 2007;27:1682–91. doi: 10.1523/JNEUROSCI.3439-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.da Cruz e Silva OA, Rebelo S, Vieira SI, et al. Enhanced generation of Alzheimer’s amyloid-β following chronic exposure to phorbol ester correlates with differential effects on α and ɛ isozymes of protein kinase C. J Neurochem. 2009;108:319–30. doi: 10.1111/j.1471-4159.2008.05770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bordji K, Becerril-Ortega J, Nicole O, et al. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-β production. J Neurosci. 2010;30:15927–42. doi: 10.1523/JNEUROSCI.3021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lesné S, Ali C, Gabriel C, et al. NMDA receptor activation inhibits α-secretase and promotes neuronal amyloid-β production. J Neurosci. 2005;25:9367–77. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cirrito JR, Kang JE, Lee J, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim SH, Fraser PE, Westaway D, et al. Group II metabotropic glutamate receptor stimulation triggers production and release of Alzheimer’s amyloidβ42 from isolated intact nerve terminals. J Neurosci. 2010;30:3870–5. doi: 10.1523/JNEUROSCI.4717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang BL. Neuronal protein trafficking associated with Alzheimer disease: from APP and BACE1 to glutamate receptors. Cell Adh Migr. 2009;3:118–28. doi: 10.4161/cam.3.1.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–99. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- 97.Morris RG. Long-term potentiation and memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:643–7. doi: 10.1098/rstb.2002.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shankar GM, Li S, Mehta TH, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cullen WK, Suh YH, Anwyl R, et al. Block of LTP in rat hippocampus in vivo by β-amyloid precursor protein fragments. Neuroreport. 1997;8:3213–7. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- 100.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 101.Townsend M, Qu Y, Gray A, et al. Oral treatment with a γ-secretase inhibitor improves long-term potentiation in a mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2010;333:110–9. doi: 10.1124/jpet.109.163691. [DOI] [PubMed] [Google Scholar]
- 102.Klyubin I, Betts V, Welzel AT, et al. Amyloid β protein dimer containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–7. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shankar GM, Bloodgood BL, Townsend M, et al. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–75. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li S, Jin M, Koeglsperger T, et al. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–38. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Selkoe DJ. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–13. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wei W, Nguyen LN, Kessels HW, et al. Amyloid β from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–6. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Knafo S, Alonso-Nanclares L, Gonzalez-Soriano J, et al. Widespread changes in dendritic spines in a model of Alzheimer’s disease. Cereb Cortex. 2009;19:586–92. doi: 10.1093/cercor/bhn111. [DOI] [PubMed] [Google Scholar]
- 108.Hsieh H, Boehm J, Sato C, et al. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–43. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim JH, Anwyl R, Suh YH, et al. Use-dependent effects of amyloidogenic fragments of β-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001;21:1327–33. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li S, Hong S, Shepardson NE, et al. Soluble oligomers of amyloidβ protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masliah E, Alford M, Mallory M, et al. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp Neurol. 2000;163:381–7. doi: 10.1006/exnr.2000.7386. [DOI] [PubMed] [Google Scholar]
- 112.Scott HL, Pow DV, Tannenberg AE, et al. Aberrant expression of the glutamate transporter excitatory amino acid transporter 1 (EAAT1) in Alzheimer’s disease. J Neurosci. 2002;22:RC206. doi: 10.1523/JNEUROSCI.22-03-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao WQ, Santini F, Breese R, et al. Inhibition of calcineurin-mediated endocytosis and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloidβ oligomer-induced synaptic disruption. J Biol Chem. 2010;285:7619–32. doi: 10.1074/jbc.M109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klyubin I, Wang Q, Reed MN, et al. Protection against Aβ-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiol Aging. 2011;32:614–23. doi: 10.1016/j.neurobiolaging.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 115.Lacor PN, Buniel MC, Chang L, et al. Synaptic targeting by Alzheimer’s-related amyloid β oligomers. J Neurosci. 2004;24:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernández-Tomé P, Brera B, Arevalo MA, et al. β-amyloid25–35 inhibits glutamate uptake in cultured neurons and astrocytes: modulation of uptake as a survival mechanism. Neurobiol Dis. 2004;15:580–9. doi: 10.1016/j.nbd.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 117.Chin JH, Ma L, MacTavish D, et al. Amyloid β protein modulates glutamate-mediated neurotransmission in the rat basal forebrain: involvement of presynaptic neuronal nicotinic acetylcholine and metabotropic glutamate receptors. J Neurosci. 2007;27:9262–9. doi: 10.1523/JNEUROSCI.1843-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kabogo D, Rauw G, Amritraj A, et al. β-amyloid-related peptides potentiate K+-evoked glutamate release from adult rat hippocampal slices. Neurobiol Aging. 2010;31:1164–72. doi: 10.1016/j.neurobiolaging.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 119.Opazo P, Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci. 2011;46:1–8. doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 120.Gong Y, Lippa CF. Review: disruption of the postsynaptic density in Alzheimer’s disease and other neurodegenerative dementias. Am J Alzheimers Dis Other Demen. 2010;25:547–55. doi: 10.1177/1533317510382893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roselli F, Hutzler P, Wegerich Y, et al. Disassembly of shank and homer synaptic clusters is driven by soluble β-amyloid(1–40) through divergent NMDAR-dependent signalling pathways. PLoS ONE. 2009;4:e6011. doi: 10.1371/journal.pone.0006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koh JY, Yang LL, Cotman CW. β-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990;533:315–20. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- 123.Mattson MP, Cheng B, Davis D, et al. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–89. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Floden AMS, Li S, Combs CK. β-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor α and NMDA receptors. J Neurosci. 2005;25:2566–75. doi: 10.1523/JNEUROSCI.4998-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song MS, Rauw G, Baker GB, et al. Memantine protects rat cortical cultured neurons against β-amyloid-induced toxicity by attenuating tau phosphorylation. Eur J Neurosci. 2008;28:1989–2002. doi: 10.1111/j.1460-9568.2008.06498.x. [DOI] [PubMed] [Google Scholar]
- 126.Tremblay R, Chakravarthy B, Hewitt K, et al. Transient NMDA receptor inactivation provides long-term protection to cultured cortical neurons from a variety of death signals. J Neurosci. 2000;20:7183–92. doi: 10.1523/JNEUROSCI.20-19-07183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 128.Puzzo D, Privitera L, Fa M, et al. Endogenous amyloid β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 2011;69:819–30. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–22. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 130.Ting JT, Kelley BG, Lambert TJ, et al. Amyloid precursor protein overexpression depresses excitatory transmission through both presynaptic and postsynaptic mechanisms. Proc Natl Acad Sci U S A. 2007;104:353–8. doi: 10.1073/pnas.0608807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Priller C, Bauer T, Mitteregger G, et al. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–21. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kurup P, Zhang Y, Xu J, et al. Aβ-mediated NMDA receptor endo-cytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–57. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Snyder EM, Nong Y, Almeida CG, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–8. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 134.Braithwaite SP, Adkisson M, Leung J, et al. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP) Eur J Neurosci. 2006;23:2847–56. doi: 10.1111/j.1460-9568.2006.04837.x. [DOI] [PubMed] [Google Scholar]
- 135.Kurup P, Zhang Y, Venkitaramani DV, et al. The role of STEP in Alzheimer’s disease. Channels (Austin) 2010;4:347–50. doi: 10.4161/chan.4.5.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y, Venkitaramani DV, Gladding CM, et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–6. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oh S, Hong HS, Hwang E, et al. Amyloid peptide attenuates the proteasome activity in neuronal cells. Mech Ageing Dev. 2005;126:1292–9. doi: 10.1016/j.mad.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 138.Paul S, Nairn AC, Wang P, et al. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- 139.Ehrlich I, Klein M, Rumpel S, et al. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–81. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kammermeier PJ. Surface clustering of metabotropic glutamate receptor 1 induced by long Homer proteins. BMC Neurosci. 2006;7:1. doi: 10.1186/1471-2202-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gylys KH, Fein JA, Yang F, et al. Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165:1809–17. doi: 10.1016/s0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayashi Y, Shi SH, Esteban JA, et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 143.Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by α-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–11. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gu Z, Liu W, Yan Z. β-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem. 2009;284:10639–49. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao D, Watson JB, Xie CW. Amyloid β prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol. 2004;92:2853–8. doi: 10.1152/jn.00485.2004. [DOI] [PubMed] [Google Scholar]
- 146.Farr SA, Banks WA, Uezu K, et al. Antibody to β-amyloid protein increases acetylcholine in the hippocampus of 12 month SAMP8 male mice. Life Sci. 2003;73:555–62. doi: 10.1016/s0024-3205(03)00322-9. [DOI] [PubMed] [Google Scholar]
- 147.Kar S, Seto D, Gaudreau P, et al. β-amyloid-related peptides inhibit potassium-evoked acetylcholine release from rat hippocampal slices. J Neurosci. 1996;16:1034–40. doi: 10.1523/JNEUROSCI.16-03-01034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bobich JA, Zheng Q, Campbell A. Incubation of nerve endings with a physiological concentration of Aβ1–42 activates CaV2.2(N-Type)-voltage operated calcium channels and acutely increases glutamate and noradrenaline release. J Alzheimers Dis. 2004;6:243–55. doi: 10.3233/jad-2004-6305. [DOI] [PubMed] [Google Scholar]
- 149.Li M, Smith CP. β-amyloid1–40 inhibits electrically stimulated release of [3H]norepinephrine and enhances the internal calcium response to low potassium in rat cortex: prevention with a free radical scavenger. Brain Res Bull. 1996;39:299–303. doi: 10.1016/0361-9230(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 150.Arias C, Arrieta I, Tapia R. β-Amyloid peptide fragment 25–35 potentiates the calcium-dependent release of excitatory amino acids from depolarized hippocampal slices. J Neurosci Res. 1995;41:561–6. doi: 10.1002/jnr.490410416. [DOI] [PubMed] [Google Scholar]
- 151.Abe K, Misawa M. The extracellular signal-regulated kinase cascade suppresses amyloidβ protein-induced promotion of glutamate clearance in cultured rat cortical astrocytes. Brain Res. 2003;979:179–87. doi: 10.1016/s0006-8993(03)02899-3. [DOI] [PubMed] [Google Scholar]
- 152.Rodriguez-Kern A, Gegelashvili M, Schousboe A, et al. β-amyloid and brain-derived neurotrophic factor, BDNF, up-regulate the expression of glutamate transporter GLT-1/EAAT2 via different signaling pathways utilizing transcription factor NF-kappaB. Neurochem Int. 2003;43:363–70. doi: 10.1016/s0197-0186(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 153.Harris ME, Wang Y, Pedigo NW, Jr, et al. Amyloid β peptide (25–35) inhibits Na+-dependent glutamate uptake in rat hippocampal astrocyte cultures. J Neurochem. 1996;67:277–86. doi: 10.1046/j.1471-4159.1996.67010277.x. [DOI] [PubMed] [Google Scholar]
- 154.Lauderback CM, Hackett JM, Huang FF, et al. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Aβ1–42. J Neurochem. 2001;78:413–6. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 155.Noda M, Nakanishi H, Akaike N. Glutamate release from mi-croglia via glutamate transporter is enhanced by amyloid-β peptide. Neuroscience. 1999;92:1465–74. doi: 10.1016/s0306-4522(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 156.Pike CJ, Burdick D, Walencewicz AJ, et al. Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993;13:1676–87. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yankner BA. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron. 1996;16:921–32. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 158.Smith WW, Gorospe M, Kusiak JW. Signaling mechanisms underlying Aβ toxicity: potential therapeutic targets for Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2006;5:355–61. doi: 10.2174/187152706784111515. [DOI] [PubMed] [Google Scholar]
- 159.Roth KA. Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol. 2001;60:829–38. doi: 10.1093/jnen/60.9.829. [DOI] [PubMed] [Google Scholar]
- 160.El Khoury J, Hickman SE, Thomas CA, et al. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996;382:716–9. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 161.Kuner P, Schubenel R, Hertel C. β-amyloid binds to p57NTR and activates NFκB in human neuroblastoma cells. J Neurosci Res. 1998;54:798–804. doi: 10.1002/(SICI)1097-4547(19981215)54:6<798::AID-JNR7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 162.Wang HY, Lee DH, Davis CB, et al. Amyloid peptide Aβ(1–42) binds selectively and with pM affinity to α7 nicotinic acetylcholine receptors. J Neurochem. 2000;75:1155–61. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- 163.Yan SD, Chen X, Fu J, et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 164.Jhamandas JH, MacTavish D. Antagonist of the amylin receptor blocks β-amyloid toxicity in rat cholinergic basal forebrain neurons. J Neurosci. 2004;24:5579–84. doi: 10.1523/JNEUROSCI.1051-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Harkany T, Abraham I, Timmerman W, et al. β-amyloid neurotoxicity is mediated by a glutamate-triggered excitotoxic cascade in rat nucleus basalis. Eur J Neurosci. 2000;12:2735–45. doi: 10.1046/j.1460-9568.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- 166.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, et al. Neuroprotection by memantine against neurodegeneration induced by β-amyloid (1–40) Brain Res. 2002;958:210–21. doi: 10.1016/s0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- 167.Guo Q, Fu W, Sopher BL, et al. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–6. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 168.Moechars D, Lorent K, Van Leuven F. Premature death in transgenic mice that overexpress a mutant amyloid precursor protein is preceded by severe neurodegeneration and apoptosis. Neuroscience. 1999;91:819–30. doi: 10.1016/s0306-4522(98)00599-5. [DOI] [PubMed] [Google Scholar]
- 169.Alvarez G, Munoz-Montano JR, Satrustegui J, et al. Regulation of tau phosphorylation and protection against β-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord. 2002;4:153–65. doi: 10.1034/j.1399-5618.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 170.Brion JP, Anderton BH, Authelet M, et al. Neurofibrillary tangles and tau phosphorylation. Biochem Soc Symp. 2001;(67):81–8. doi: 10.1042/bss0670081. [DOI] [PubMed] [Google Scholar]
- 171.Ferrer I, Gomez-Isla T, Puig B, et al. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- 172.Zheng WH, Bastianetto S, Mennicken F, et al. Amyloid β peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 2002;115:201–11. doi: 10.1016/s0306-4522(02)00404-9. [DOI] [PubMed] [Google Scholar]
- 173.Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739:280–97. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 174.Rapoport M, Dawson HN, Binder LI, et al. Tau is essential to β-amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99:6364–9. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lesort M, Jope RS, Johnson GV. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3β and Fyn tyrosine kinase. J Neurochem. 1999;72:576–84. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- 176.Hernandez P, Lee G, Sjoberg M, et al. Tau phosphorylation by cdk5 and Fyn in response to amyloid peptide Aβ (25–35): involvement of lipid rafts. J Alzheimers Dis. 2009;16:149–56. doi: 10.3233/JAD-2009-0933. [DOI] [PubMed] [Google Scholar]
- 177.Lee G, Thangavel R, Sharma VM, et al. Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J Neurosci. 2004;24:2304–12. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–97. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 179.Liu F, Grundke-Iqbal I, Iqbal K, et al. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–50. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 180.Liu F, Iqbal K, Grundke-Iqbal I, et al. Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer’s disease. J Biol Chem. 2005;280:1790–6. doi: 10.1074/jbc.M410775200. [DOI] [PubMed] [Google Scholar]
- 181.Gong CX, Shaikh S, Wang JZ, et al. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–8. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 182.Sontag E, Luangpirom A, Hladik C, et al. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 183.Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, et al. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp Neurol. 2001;168:402–12. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 184.Bennecib M, Gong CX, Grundke-Iqbal I, et al. Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett. 2001;490:15–22. doi: 10.1016/s0014-5793(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 185.Gong CX, Lidsky T, Wegiel J, et al. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000;275:5535–44. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 186.Qian W, Shi J, Yin X, et al. PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3β. J Alzheimers Dis. 2010;19:1221–9. doi: 10.3233/JAD-2010-1317. [DOI] [PubMed] [Google Scholar]
- 187.Pei JJ, Gong CX, An WL, et al. Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer’s disease. Am J Pathol. 2003;163:845–58. doi: 10.1016/S0002-9440(10)63445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Adamec E, Mercken M, Beermann ML, et al. Acute rise in the concentration of free cytoplasmic calcium leads to dephosphorylation of the microtubule-associated protein tau. Brain Res. 1997;757:93–101. doi: 10.1016/s0006-8993(97)00166-2. [DOI] [PubMed] [Google Scholar]
- 189.Fleming LM, Johnson GV. Modulation of the phosphorylation state of tau in situ: the roles of calcium and cyclic AMP. Biochem J. 1995;309:41–7. doi: 10.1042/bj3090041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liang Z, Liu F, Iqbal K, et al. Dysregulation of tau phosphorylation in mouse brain during excitotoxic damage. J Alzheimers Dis. 2009;17:531–9. doi: 10.3233/JAD-2009-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cotman CW, Poon WW, Rissman RA, et al. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–12. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 192.Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2003;100:10032–7. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rissman RA, Poon WW, Blurton-Jones M, et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–30. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu MC, Kobeissy F, Zheng W, et al. Dual vulnerability of tau to calpains and caspase-3 proteolysis under neurotoxic and neurode-generative conditions. ASN Neuro. 2011;3:e00051. doi: 10.1042/AN20100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Park SY, Ferreira A. The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates β amyloid-induced neurodegeneration. J Neurosci. 2005;25:5365–75. doi: 10.1523/JNEUROSCI.1125-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis. A possible regulation of Alzheimer’s disease. FEBS J. 2006;273:3437–43. doi: 10.1111/j.1742-4658.2006.05352.x. [DOI] [PubMed] [Google Scholar]
- 197.Amadoro G, Ciotti MT, Costanzi M, et al. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103:2892–7. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kornhuber J, Weller M. Psychotogenicity and N-methyl-D-aspartate receptor antagonism: implications for neuroprotective pharmacotherapy. Biol Psychiatry. 1997;41:135–44. doi: 10.1016/S0006-3223(96)00047-9. [DOI] [PubMed] [Google Scholar]
- 199.Doraiswamy PM. The role of the N-methyl-D-aspartate receptor in Alzheimer’s disease: therapeutic potential. Curr Neurol Neurosci Rep. 2003;3:373–8. doi: 10.1007/s11910-003-0019-8. [DOI] [PubMed] [Google Scholar]
- 200.Farlow MR. NMDA receptor antagonists. A new therapeutic approach for Alzheimer’s disease. Geriatrics. 2004;59:22–7. [PubMed] [Google Scholar]
- 201.Sonkusare SK, Kaul CL, Ramarao P. Dementia of Alzheimer’s disease and other neurodegenerative disorders — memantine, a new hope. Pharmacol Res. 2005;51:1–17. doi: 10.1016/j.phrs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 202.Wenk GL, Parsons CG, Danysz W. Potential role of N-methyl-D- aspartate receptors as executors of neurodegeneration resulting from diverse insults: focus on memantine. Behav Pharmacol. 2006;17:411–24. doi: 10.1097/00008877-200609000-00007. [DOI] [PubMed] [Google Scholar]
- 203.Lipton SA, Chen HS. Paradigm shift in NMDA receptor drug development. Expert Opin Ther Targets. 2005;9:427–9. doi: 10.1517/14728222.9.3.427. [DOI] [PubMed] [Google Scholar]
- 204.Herrmann N, Li A, Lanctot K. Memantine in dementia: a review of the current evidence. Expert Opin Pharmacother. 2011;12:787–800. doi: 10.1517/14656566.2011.558006. [DOI] [PubMed] [Google Scholar]
- 205.Parsons CG, Gilling K. Memantine as an example of a fast, voltagedependent, open channel N-methyl-D-aspartate receptor blocker. Methods Mol Biol. 2007;403:15–36. doi: 10.1007/978-1-59745-529-9_2. [DOI] [PubMed] [Google Scholar]
- 206.Rao VL, Dogan A, Todd KG, et al. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- 207.Rammes G, Hasenjager A, Sroka-Saidi K, et al. Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 meta-botropic glutamate receptors in mediating the synaptotoxic effects of β-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology. 2011;60:982–90. doi: 10.1016/j.neuropharm.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 208.Martinez-Coria H, Green KN, Billings LM, et al. Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. Am J Pathol. 2010;176:870–80. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2004;311:677–82. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]
- 210.Van Dam D, De Deyn PP. Cognitive evaluation of disease-modifying efficacy of galantamine and memantine in the APP23 model. Eur Neuropsychopharmacol. 2006;16:59–69. doi: 10.1016/j.euroneuro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 211.Dong H, Yuede CM, Coughlan C, et al. Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2008;33:3226–36. doi: 10.1038/npp.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Alley GM, Bailey JA, Chen D, et al. Memantine lowers amyloid-β peptide levels in neuronal cultures and in APP/PS1 transgenic mice. J Neurosci Res. 2010;88:143–54. doi: 10.1002/jnr.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ray B, Banerjee PK, Greig NH, et al. Memantine treatment decreases levels of secreted Alzheimer’s amyloid precursor protein (APP) and Aβ peptide in the human neuroblastoma cells. Neurosci Lett. 2010;470:1–5. doi: 10.1016/j.neulet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-tosevere Alzheimer’s disease. N Engl J Med. 2003;348:1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 215.van Dyck CH, Tariot PN, Meyers B, et al. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:136–43. doi: 10.1097/WAD.0b013e318065c495. [DOI] [PubMed] [Google Scholar]
- 216.Winblad B, Jones RW, Wirth Y, et al. Memantine in moderate to severe Alzheimer’s disease: a meta-analysis of randomised clinical trials. Dement Geriatr Cogn Disord. 2007;24:20–7. doi: 10.1159/000102568. [DOI] [PubMed] [Google Scholar]
- 217.Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer’s disease: results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis. 2007;11:471–9. doi: 10.3233/jad-2007-11409. [DOI] [PubMed] [Google Scholar]
- 218.Schneider LS, Dagerman KS, Higgins JP, et al. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68:991–8. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- 219.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–8. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]