Abstract
Diffuse large B cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma in all countries and all age groups. DLBCL is potentially curable, and the outcome of patients with DLBCL has completely changed with the introduction of therapy involving the monoclonal antibody rituximab in combination with chemotherapy. Nonetheless, relapse is detected after treatment with rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone in approximately 30% of patients. It has recently become clear that DLBCL represents a heterogeneous admixture of quite different entities. Gene expression profiling has uncovered DLBCL subtypes that have distinct clinical behaviors and prognoses; however, incorporation of this information into treatment algorithms awaits further investigation. Future approaches to DLBCL treatment will use this new genetic information to identify potential biomarkers for prognosis and targets for treatment.
Keywords: Diffuse large B cell lymphoma, Therapeutics, Rituximab, Stem cell transplantation
INTRODUCTION
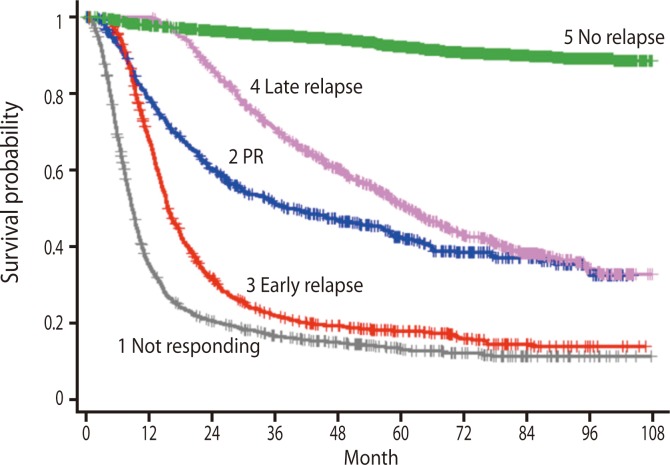
Diffuse large B cell lymphoma (DLBCL) is the most common lymphoid malignancy in adults and the most frequent subtype of non-Hodgkin lymphoma (NHL) in all countries around the world and every age group [1,2]. DLBCL is found most commonly in people who are middle-aged or elderly, with the median age at diagnosis of DLBCL in the sixth decade, and men are slightly more likely to develop DLBCL than women. Despite being classified as a single disease entity by the World Health Organization [2], DLBCL is a remarkably heterogeneous disease with considerable variation in clinical behavior, response to therapy, and long-term outcome (Fig. 1). Recent studies involving gene expression microarray analysis of DLBCL have revealed significant heterogeneity within this diagnosis [3]. The International Prognostic Index (IPI), which includes five parameters (age, performance status, stage, lactate dehydrogenase level, and extranodal site involvement), is the most commonly used means of risk stratification in DLBCL and has been further validated in the rituximab era [4]. The IPI score is used routinely to identify patients with high-risk disease. However, within the low or low-intermediate IPI group, there currently is no reliable way of prospectively identifying the subset of patients destined to do poorly, in terms of either primary refractory disease or an early relapse. Patients with identical IPI scores may exhibit striking variability in outcome, suggesting the presence of significant heterogeneity within each IPI category.
Figure 1.
Outcome according to response to first treatment in patients with diffuse large B cell lymphoma (based on GELA database; Courtesy of Bertrand Coiffier, Hospices Civils de Lyon, Lyon, France).
To predict the prognosis of patients with DLBCL at diagnosis, recent studies have examined the molecular origin of DLBCL to identify markers that stratify DLBCL cases based on the cell of origin [3,5-9]. Recent attempts using gene expression profiling (GEP) or immunohistochemical (IHC) markers to identify the cell of origin in DLBCL or the activation/suppression of signaling pathways are likely to be more successful in increasing our understanding of the biology of DLBCL and predicting the response to therapy and prognosis than the standard morphologic and clinical criteria used to date [10]. GEP has revealed that DLBCL consists of at least three major subtypes derived from B cells at different states of differentiation and with unique molecular pathogenesis: germinal center B cells (GCBs), activated B cells (ABCs), and primary mediastinal B cells.
DLBCL is typically treated with combination chemoimmunotherapy that includes the antibody rituximab and anthracyclines [11-15]. Various regimens cure approximately 60% to 70% of patients with DLBCL [4]; however, some patients either fail to respond to rituximab-based therapy or relapse early, or experience a poor outcome with second-line or salvage therapies.
FRONT-LINE TREATMENT
Common treatment algorithms for the management of DLBCL are divided into strategies for localized disease (Ann Arbor stages I and II) and advanced-stage disease (stages III and IV). Until recently, cyclophosphamide, hydroxydaunorubicin, vincristine, prednisolone (CHOP) chemotherapy administered every 21 days (CHOP21) remained the standard therapy for DLBCL, with a long-term overall survival (OS) rate of approximately 40% [16].
In the 1980s, several studies were performed using aggressive combination-chemotherapy regimens such as MACOP-B and ProMACE-CytaBOM in an attempt to improve the results of DLBCL treatment but these programs were costly, difficult to administer, and appeared to be more toxic than CHOP [17-19]. The Southwest Oncology Group (SWOG) and the Eastern Cooperative Oncology Group (ECOG) initiated a prospective, randomized, phase III trial that compared CHOP with three aggressive multi-agent regimens [16]. When compared with these intensive chemotherapy regimens, the standard CHOP regimen produced similar survival outcomes in patients with advanced NHL. However, fatal toxic reactions were less common in patients treated with CHOP, thus establishing CHOP as the standard of care for patients with DLBCL. This finding has been confirmed by other trials comparing more aggressive chemotherapy regimens with standard CHOP therapy [20,21]. Several trials have subsequently explored modifications of the CHOP regimen based on dose-intensity and dose-density in an attempt to improve its efficacy [22-24].
In 1997, rituximab became the first monoclonal antibody approved for use by the Food and Drug Administration for follicular lymphoma, and this immunotherapy was soon applied to DLBCL and other B-cell NHLs [25]. Rituximab is an antibody directed against the CD20 protein, which is primarily found on the surface of B cells and is present on many lymphoma cells. Although the mechanism is not completely understood, rituximab is thought to induce lysis of lymphoma cells through complement-mediated cytolysis, antibody-dependent cell cytotoxicity, and direct induction of apoptosis. In addition, rituximab acts synergistically with chemotherapy [26].
Rituximab significantly improves treatment outcome in DLBCL. A large randomized phase III study demonstrated improved OS in patients with DLBCL who were treated with rituximab with CHOP (R-CHOP) therapy [27]. Other studies showed that combined rituximab and chemotherapy clearly prolonged event-free survival (EFS) and OS in elderly patients [12,15]. The MabThera International Trial Group study compared a CHOP-like chemotherapy regimen and the same regimen with the addition of rituximab in patients under 60 years of age with good prognosis and demonstrated prolonged EFS and OS in the rituximab-treated group [14]. Based on the results of these clinical trials, R-CHOP therapy is now considered to be the 'standard therapy' in DLBCL, especially for young low-risk and elderly DLBCL patients. However, it has not yet provided a satisfactory outcome in patients in the high-risk group according to IPI classification.
DIFFERENT TREATMENT STRATEGIES FOR DIFFERENT STAGES OF DISEASE
The benefit of 3 cycles of CHOP followed by involved field radiotherapy (IFRT) (5-year-OS of 95%) in patients with limited-stage DLBCL (60 years or younger with no adverse risk factors) was confirmed in a series from the British Columbia Cancer Agency [28]. The ECOG 1484 study showed that the addition of IFRT to CHOP (eight cycles) prolonged disease free survival (DFS) in patients with limited-stage DLBCL who had achieved complete remission (CR) compared with CHOP alone (6-year-DFS 73% for IFRT plus CHOP vs. 56% for CHOP alone) [29]. However, in the GELA LNH 93-4 study, the addition of radiotherapy to four cycles of CHOP did not provide any advantage over four cycles of CHOP alone for the treatment of elderly patients with low-risk localized aggressive lymphoma.
In the SWOG 0014 study, the addition of rituximab to CHOP (three cycles) and IFRT showed efficacy in patients with limited-stage DLBCL. In historical comparison, these results were better than those for patients treated without rituximab. R-CHOP (three cycles) with IFRT or R-CHOP (six cycles) with or without IFRT was recommended for patients with non-bulky (less than 10 cm) DLBCL [30].
R-CHOP21 chemotherapy has been the standard treatment for patients with advanced stage DLBCL based on results of the GELA study demonstrating that the addition of rituximab to CHOP21 improved progression-free survival (PFS) and OS in elderly patients with advanced DLBCL [11,16,31]. These findings have been confirmed in additional randomized trials [12,32].
Prior to the introduction of rituximab, six cycles of dose dense CHOP (CHOP14) as first-line therapy was found to be superior to six cycles of CHOP21 in patients older than 60 years [23]. In the RICOVER 60 trial, the addition of rituximab to six or eight cycles of CHOP14 (R-CHOP14) improved clinical outcomes in elderly patients compared with CHOP14 alone [13,33]. Over a median observation time of 82 months, both EFS and OS were significantly improved after R-CHOP14 [33]. Ongoing randomized studies are evaluating the role of R-CHOP14 versus R-CHOP21 [34,35]. Although randomized trials have found the French ACVBP-R regimen to be superior to R-CHOP in younger patients, toxicity precludes its use in older patients [36,37].
Dose-adjusted (DA) R-EPOCH has shown significant activity in untreated patients with DLBCL [34,38], and an ongoing phase III randomized trial is evaluating DA R-EPOCH versus R-CHOP in untreated patients with DLBCL.
DIFFERENT TREATMENT STRATEGIES FOR GCB AND ABC DLBCL SUBTYPES
To segregate DLBCL into biologically meaningful subgroups that might allow identification of rational therapeutic targets, the Leukemia and Lymphoma Molecular Profiling Project began gene expression analysis of DLBCL biopsy samples using DNA microarrays and identified biologically distinct and prognostically meaningful molecular subgroups of DLBCL [8]. Recent gene expression microarray analyses of DLBCL have revealed significant heterogeneity within this diagnosis [3]. For example, GCB and ABC DLBCL subtypes are derived from B cells at different stages of differentiation. GCB DLBCL appears to arise from GCBs, whereas ABC DLBCL likely arises from post-GCBs that are blocked during plasmacytic differentiation [39].
Analysis of molecular subtype and outcome following upfront CHOP treatment shows a statistically significant difference in 5-year-OS of the DLBCL subtypes: 59% for GCB DLBCL and 31% for ABC DLBCL, independent of IPI risk group [3,40]. Because this analysis was performed on biopsies obtained in the pre-rituximab era, a second analysis was performed on 233 biopsies obtained from patients treated with R-CHOP [39]. Similarly, patients with GCB DLBCL had a more favorable survival than those with ABC DLBCL, with 3-year-OS rates of 84% and 56%, respectively (p < 0.001), and, as expected, the OS of both GCB and ABC DLBCL was better than in the pre-rituximab era.
GEP is not yet popular for routine clinical use. In its place, investigators have developed IHC models, and immunostaining algorithms have been used to differentiate between these two subtypes using combinations of CD10, BCL6, IRF4/MUM1, GCET1, and FOXP1 [41,42]. Despite variable reproducibility, these approaches have successfully distinguished GCB from non-GCB DLBCL in a number of clinical trials [5,6,43].
Although the technology of IHC is more established than that of GEP it is not without standardization and reproducibility issues, highlighting the fact that even apparently simplified approaches have limitations and are not always reliable [44,45]. Recent comparative analysis revealed that the rate of misclassification using IHC is high, especially for GCB subtypes, ranging from 30% to 60% [46]. This study also showed that GEP successfully predicted PFS and OS based upon GCB versus ABC subtypes, whereas none of the five IHC algorithms was able to do so. Attempts to adopt IHC surrogates for GEP findings have met with only modest and controversial success.
It is not necessary to perform any kind of genetic testing to render a diagnosis of DLBCL. However, it is clear that genetic factors and/or the cell of origin are key determinants of specific subtypes and prognosis [45]. So far, none of the fascinating data emanating from GEP and other technologies have translated into routine clinical practice.
The contemporary relevance of the role of GEP and/or the use of IHC surrogates in the rituximab era is somewhat controversial [45]. It has been proposed that BCL2-positive DLBCL benefits from the addition of rituximab to CHOP chemotherapy, whereas BCL2-negative cases do not [47]. Other studies suggest that the addition of rituximab to CHOP benefits BCL6-negative DLBCL, but not BCL6-positive cases [48]. It therefore appears that patients with ABC DLBCL benefit from the addition of rituximab, but those with GCB DBLCL do not. Some reports suggest that the use of rituximab eliminates the prognostic differences between these two groups [48-50] although other studies do not support this notion.
Even though GCB DLBCL has a better prognosis than ABC DLBCL, more than of 30% of patients are not cured. BCL6 is an important modulator of B cell development in the germinal center, and mutations/translocations in BCL6 enhance its inhibitory effects on apoptotic stress responses and promote proliferation, both of which are associated with treatment failure [51-55]. An interesting and potentially important observation is the effect of topoisomerase II inhibition on BCL6 expression through ubiquitin-mediated protein degradation and possibly transcriptional inhibition [56]. The German high-grade lymphoma study group showed that addition of the topoisomerase II inhibitor etoposide to CHOP (CHOEP) significantly improved the EFS of younger, but not older, patients with untreated DLBCL [23,24]. The higher frequency of GCB DLBCL in younger patients may explain why the benefit of etoposide was only found in patients under 60 years and not in older patients.
Interestingly, the positive effect of including etoposide in CHOEP was lost when rituximab was also added (R-CHOEP) [23]. This may reflect the overall salutary effect of rituximab on the outcome of both GCB and ABC DLBCL, rather than a specific effect on BCL6. In this regard, the DA-EPOCH-R regimen, which was designed to inhibit topoisomerase II, might be particularly effective in GCB DLBCL, in part due to its effective inhibition of topoisomerase II and BCL6.
ABC DLBCL is characterized by the expression of genes associated with survival and proliferation and has an inferior clinical outcome. Based on in vitro evidence that the proteasome inhibitor bortezomib blocked degradation of phosphorylated inhibitor of kappa B and consequently inhibited nuclear factor-kappa B activity in ABC DLBCL cell lines, bortezomib was combined with DA-EPOCH in patients with relapsed/refractory DLBCL [57-59]. Bortezomib alone had no activity against DLBCL, but when combined with chemotherapy demonstrated a significantly higher response and median OS in ABC DLBCL than in GCB DLBCL (response, 83% vs. 13%; p = 0.0004 and OS, 10.38 months vs. 3.4 months; p = 0.0026). These results suggest that bortezomib enhances the effectiveness of chemotherapy in ABC, but not GCB DLBCL, and provide a rational therapeutic approach based on genetically distinct DLBCL subtypes [60]. When bortezomib was combined with R-CHOP in patients with previously untreated DLBCL to assess its toxicity and efficacy [60], there was no difference between patients with GCB and ABC DLBCL, suggesting that bortezomib overcame the adverse prognostic effect of the ABC DLBCL subtype. Based on these studies, a randomized study of R-CHOP + bortezomib in untreated patients with ABC DLBCL is ongoing (Pyramid study).
A recent study suggesting that lenalidomide may be preferentially effective in ABC DLBCL [61] also warrants further investigation.
SALVAGE TREATMENT FOR PATIENTS WITH RELAPSED DLBCL
Although the adoption of R-CHOP as the new standard of care has improved outcomes for DLBCL, many patients still relapse. In such cases, the standard approach for fit patients with DLBCL has been to proceed toward salvage therapy and consolidation with autologous stem cell transplantation (ASCT).
The 1995 PARMA trial is the only randomized trial comparing ASCT versus salvage chemotherapy [62]. This study reported a significantly superior OS and EFS in patients who underwent salvage ASCT. Based on this study, ASCT has become the standard of care in patients less than 60 years old with chemosensitive relapsed or primary refractory aggressive NHL.
Several regimens exist for salvage lymphoma therapy including ifosphamide, carboplatin, etoposide (ICE), etoposide, methy prednisolone, high-dose cytarabine, cisplatin (ESHAP), dexamethasone, cisplatin, cytarabine (DHAP), and dexamethasone, cisplatin, gemcitabine. These regimens have various response rates. Although the standard of salvage therapy is still being debated, the addition of rituximab to the salvage regimen appears to benefit relapsed patients, especially those previously unexposed to rituximab. The Hemato-Oncologie voor Volwassenen Nederland group randomized relapsed patients to DHAP with or without rituximab. After two cycles, 75% of the patients in the rituximab with DHAP (R-DHAP) arm had responsive disease compared with 54% in the DHAP arm (p = 0.01). With a median follow-up of 24 months, there was a significant difference in PFS (52% vs. 31%; p < 0.002) and OS in favor of the R-DHAP arm [63]. Moreover, rituximab does not appear to impair stem cell engraftment or adversely affect transplantation toxicity and is associated with improved PFS when administered before ASCT for DLBCL [64].
One study conducted a retrospective review of patients treated with rituximab with ICE (R-ICE) and compared them with historical controls treated with ICE alone. R-ICE given for three cycles produced a CR in 53% of patients, and none of the patients had treatment-related toxicity that precluded ASCT [65]. It is important to note that patients in both studies had received prior induction chemotherapy without rituximab.
Another study showed that among patients with relapsed or refractory DLBCL who received rituximab with ESHAP as salvage therapy with curative intent, those previously exposed to rituximab had very low CR and overall response rates [66]. The most effective regimen for salvage chemotherapy after R-CHOP failure was addressed by a prospective multicenter phase III study Collaborative Trial in Relapsed Aggressive Lymphoma [67,68], in which DLBCL patients were randomized to receive salvage R-ICE or R-DHAP. After three courses, responders underwent high-dose therapy, and ASCT. There was no statistical difference between R-ICE and R-DHAP in overall response rate (63.5% vs. 62.8%), 3-year-PFS (31% vs. 42%), and 3-year-OS (47% vs. 51%), suggesting that either regimen can be used for salvage therapy. Because nearly all patients with DLBCL currently receive front-line R-CHOP, these data call into question our current strategies for salvage therapy, particularly for patients who relapse within 1 year of initial therapy.
STEM CELL TRANSPLANTATION
The PARMA study did not enroll patients over 60 years old, and there are no comparative data for ASCT versus non-transplantation as salvage therapy in this age group. Two non-randomized studies have been published since 2000 comparing the outcomes of autologous versus myeloablative allogeneic stem cell transplantation (SCT) as treatment specifically for DLBCL patients. One study reported that allogeneic SCT patients had significantly worse 1-year-rates of OS, PFS, and treatment-related mortality (TRM) than ASCT patients, but the differences were not significant at 3 or 5 years [69]. Another study reported a significantly worse 3-year-TRM for allogeneic compared with autologous SCT patients, but no difference in survival outcomes [70]. Neither study reported a significant difference in the risk of relapse or disease progression between the two treatment groups at any time interval. One randomized study compared autologous peripheral blood SCT (PBSCT) versus bone marrow transplantation (BMT) as treatment for patients with aggressive NHL (61% with DLBCL) [71]. Patients who underwent autologous PBSCT had a significantly longer OS, but not EFS, than those who underwent autologous BMT.
A study that investigated the impact of three courses of intensified CHOP versus no CHOP prior to two cycles of induction followed by first-line ASCT for patients with de novo aggressive NHL found that patients who received intensified CHOP had significantly better OS and EFS than those who did not [72].
CONCLUSIONS
DLBCL is a clinically and biologically diverse disease that cannot easily be subdivided into distinct disease entities because of overlapping morphology and pathogenetic features. Currently there are no reliable markers to prospectively identify patients in each subgroup. R-CHOP is the standard therapy in elderly and low-risk young patients. As we better understand the biological explanation for this heterogeneity, we hope to develop more specific and more effective therapies for high-risk patients. The ultimate aim is to improve the outcome of patients with DLBCL through the selection of individualized treatment regimens.
Acknowledgments
This work was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0620220-1).
Footnotes
No potential conflict of interest relevant to this article is reported.
References
- 1.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009:523–531. doi: 10.1182/asheducation-2009.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 3.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 4.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 5.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15:5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 7.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 8.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 9.Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 10.Lee NR, Song EK, Jang KY, et al. Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma. 2008;49:247–256. doi: 10.1080/10428190701824536. [DOI] [PubMed] [Google Scholar]
- 11.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 12.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 13.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 14.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 15.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 17.Klimo P, Connors JM. MACOP-B chemotherapy for the treatment of diffuse large-cell lymphoma. Ann Intern Med. 1985;102:596–602. doi: 10.7326/0003-4819-102-5-596. [DOI] [PubMed] [Google Scholar]
- 18.Shipp MA, Harrington DP, Klatt MM, et al. Identification of major prognostic subgroups of patients with large-cell lymphoma treated with m-BACOD or M-BACOD. Ann Intern Med. 1986;104:757–765. doi: 10.7326/0003-4819-104-6-757. [DOI] [PubMed] [Google Scholar]
- 19.Browne MJ, Hubbard SM, Longo DL, et al. Excess prevalence of Pneumocystis carinii pneumonia in patients treated for lymphoma with combination chemotherapy. Ann Intern Med. 1986;104:338–344. doi: 10.7326/0003-4819-104-3-338. [DOI] [PubMed] [Google Scholar]
- 20.Itoh K, Ohtsu T, Wakita H, et al. Dose-escalation study of CHOP with or without prophylactic G-CSF in aggressive non-Hodgkin's lymphoma. Ann Oncol. 2000;11:1241–1247. doi: 10.1023/a:1008361513544. [DOI] [PubMed] [Google Scholar]
- 21.Linch DC, Smith P, Hancock BW, et al. A randomized British National Lymphoma Investigation trial of CHOP vs. a weekly multi-agent regimen (PACEBOM) in patients with histologically aggressive non-Hodgkin's lymphoma. Ann Oncol. 2000;11(Suppl 1):87–90. [PubMed] [Google Scholar]
- 22.Tilly H, Lepage E, Coiffier B, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102:4284–4289. doi: 10.1182/blood-2003-02-0542. [DOI] [PubMed] [Google Scholar]
- 23.Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634–641. doi: 10.1182/blood-2003-06-2095. [DOI] [PubMed] [Google Scholar]
- 24.Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004;104:626–633. doi: 10.1182/blood-2003-06-2094. [DOI] [PubMed] [Google Scholar]
- 25.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 26.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 27.Coiffier B, Feugier P, Mounier N, et al. Long-term results of the GELA study comparing R-CHOP and CHOP chemotherapy in older patients with diffuse large B-cell lymphoma show good survival in poor-risk patients [abstract] J Clin Oncol. 2007;25(18 suppl):8009. [Google Scholar]
- 28.Shenkier TN, Voss N, Fairey R, et al. Brief chemotherapy and involved-region irradiation for limited-stage diffuse large-cell lymphoma: an 18-year experience from the British Columbia Cancer Agency. J Clin Oncol. 2002;20:197–204. doi: 10.1200/JCO.2002.20.1.197. [DOI] [PubMed] [Google Scholar]
- 29.Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin's lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032–3038. doi: 10.1200/JCO.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 30.Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28:4170–4176. doi: 10.1200/JCO.2009.27.3441. [DOI] [PubMed] [Google Scholar]
- 31.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 32.Canales MA, de la Serna J, Sabin P, et al. Up-front treatment of diffuse large-b cell lymphoma (DLBCL) in elderly patients with rituximab in combination with CHOP-like chemotherapy: a multicenter study on the current clinical management [abstract] Blood. 2005;106:4778. [Google Scholar]
- 33.Pfreundschuh M, Ziepert M, Zeynalova S, et al. Six versus eight cycles of biweekly CHOP-14 with or without R in elderly patients (pts) with aggressive CD20+ B-cell lymphomas: Seven-year FU of the RICOVER-60 trial of the DSHNHL [abstract] J Clin Oncol. 2011;29(15 suppl):8029. [Google Scholar]
- 34.Purroy N, Lopez A, Vallespi T, Gironella M, Bergua J, Sancho JM. Dose-adjusted EPOCH plus rituximab (DA-EPOCH-R) in untreated patients with poor risk large B-cell lymphoma. A phase 2 study conducted by the Spanish PETHEMA group [abstract] Blood. 2009;114:2701. doi: 10.1111/bjh.13273. [DOI] [PubMed] [Google Scholar]
- 35.Delarue R, Tilly H, Salles G, et al. R-CHOP14 compared to R-CHOP21 in elderly patients with diffuse large B-cell lymphoma: results of the interim analysis of the LNH03-6B GELA study [abstract] Blood. 2009;114:406. [Google Scholar]
- 36.Recher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378:1858–1867. doi: 10.1016/S0140-6736(11)61040-4. [DOI] [PubMed] [Google Scholar]
- 37.Reyes F, Lepage E, Ganem G, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005;352:1197–1205. doi: 10.1056/NEJMoa042040. [DOI] [PubMed] [Google Scholar]
- 38.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717–2724. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiestner A, Staudt LM. Towards molecular diagnosis and targeted therapy of lymphoid malignancies. Semin Hematol. 2003;40:296–307. doi: 10.1016/s0037-1963(03)00194-x. [DOI] [PubMed] [Google Scholar]
- 41.Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–4594. doi: 10.1200/JCO.2007.15.9277. [DOI] [PubMed] [Google Scholar]
- 42.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29:200–207. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunleavy K, Janik J, Gea-Banacloche J, et al. Phase I/II study of bortezomib alone and bortezomib with dose-adjusted EPOCH chemotherapy in relapsed or refractory aggressive B-cell lymphoma [abstract] Blood. 2004;104:1385. [Google Scholar]
- 44.de Jong D, Xie W, Rosenwald A, et al. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications (a study from the Lunenburg Lymphoma Biomarker Consortium) J Clin Pathol. 2009;62:128–138. doi: 10.1136/jcp.2008.057257. [DOI] [PubMed] [Google Scholar]
- 45.Bagg A. B cells behaving badly: a better basis to behold belligerence in B-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2011;2011:330–335. doi: 10.1182/asheducation-2011.1.330. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117:4836–4843. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]
- 47.Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2: associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–4284. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 48.Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006;107:4207–4213. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nyman H, Adde M, Karjalainen-Lindsberg ML, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood. 2007;109:4930–4935. doi: 10.1182/blood-2006-09-047068. [DOI] [PubMed] [Google Scholar]
- 50.Saito B, Shiozawa E, Usui T, et al. Rituximab with chemotherapy improves survival of non-germinal center type untreated diffuse large B-cell lymphoma. Leukemia. 2007;21:2563–2566. doi: 10.1038/sj.leu.2404844. [DOI] [PubMed] [Google Scholar]
- 51.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 52.Paik JH, Jeon YK, Park SS, et al. Expression and prognostic implications of cell cycle regulatory molecules, p16, p21, p27, p14 and p53 in germinal centre and non-germinal centre B-like diffuse large B-cell lymphomas. Histopathology. 2005;47:281–291. doi: 10.1111/j.1365-2559.2005.02222.x. [DOI] [PubMed] [Google Scholar]
- 53.Wilson WH, Teruya-Feldstein J, Fest T, et al. Relationship of p53, bcl-2, and tumor proliferation to clinical drug resistance in non-Hodgkin's lymphomas. Blood. 1997;89:601–609. [PubMed] [Google Scholar]
- 54.Ranuncolo SM, Polo JM, Dierov J, et al. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat Immunol. 2007;8:705–714. doi: 10.1038/ni1478. [DOI] [PubMed] [Google Scholar]
- 55.Pasqualucci L, Migliazza A, Basso K, Houldsworth J, Chaganti RS, Dalla-Favera R. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood. 2003;101:2914–2923. doi: 10.1182/blood-2002-11-3387. [DOI] [PubMed] [Google Scholar]
- 56.Kurosu T, Fukuda T, Miki T, Miura O. BCL6 overexpression prevents increase in reactive oxygen species and inhibits apoptosis induced by chemotherapeutic reagents in B-cell lymphoma cells. Oncogene. 2003;22:4459–4468. doi: 10.1038/sj.onc.1206755. [DOI] [PubMed] [Google Scholar]
- 57.Allen C, Saigal K, Nottingham L, Arun P, Chen Z, Van Waes C. Bortezomib-induced apoptosis with limited clinical response is accompanied by inhibition of canonical but not alternative nuclear factor-{kappa}B subunits in head and neck cancer. Clin Cancer Res. 2008;14:4175–4185. doi: 10.1158/1078-0432.CCR-07-4470. [DOI] [PubMed] [Google Scholar]
- 58.Houldsworth J, Petlakh M, Olshen AB, Chaganti RS. Pathway activation in large B-cell non-Hodgkin lymphoma cell lines by doxorubicin reveals prognostic markers of in vivo response. Leuk Lymphoma. 2008;49:2170–2180. doi: 10.1080/10428190802428369. [DOI] [PubMed] [Google Scholar]
- 59.Strauss SJ, Higginbottom K, Juliger S, et al. The proteasome inhibitor bortezomib acts independently of p53 and induces cell death via apoptosis and mitotic catastrophe in B-cell lymphoma cell lines. Cancer Res. 2007;67:2783–2790. doi: 10.1158/0008-5472.CAN-06-3254. [DOI] [PubMed] [Google Scholar]
- 60.Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 61.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Response of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) with nongerminal center B-cell phenotype to lenalidomide (L) alone or in combination with rituximab (R) [abstract] J Clin Oncol. 2010;28(15 suppl):8038. [Google Scholar]
- 62.Olivieri A, Santini G, Patti C, et al. Upfront high-dose sequential therapy (HDS) versus VACOP-B with or without HDS in aggressive non-Hodgkin's lymphoma: long-term results by the NHLCSG. Ann Oncol. 2005;16:1941–1948. doi: 10.1093/annonc/mdi399. [DOI] [PubMed] [Google Scholar]
- 63.Vellenga E, van Putten WL, van 't Veer MB, et al. Rituximab improves the treatment results of DHAP-VIM-DHAP and ASCT in relapsed/progressive aggressive CD20+ NHL: a prospective randomized HOVON trial. Blood. 2008;111:537–543. doi: 10.1182/blood-2007-08-108415. [DOI] [PubMed] [Google Scholar]
- 64.Fenske TS, Hari PN, Carreras J, et al. Impact of pre-transplant rituximab on survival after autologous hematopoietic stem cell transplantation for diffuse large B cell lymphoma. Biol Blood Marrow Transplant. 2009;15:1455–1464. doi: 10.1016/j.bbmt.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- 66.Martin A, Conde E, Arnan M, et al. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93:1829–1836. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]
- 67.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gisselbrecht C, Glass B, Mounier N, et al. R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by autologous stem cell transplantation: CORAL study [abstract] J Clin Oncol. 2009;27(15 suppl):8509. [Google Scholar]
- 69.Lazarus HM, Zhang MJ, Carreras J, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol Blood Marrow Transplant. 2010;16:35–45. doi: 10.1016/j.bbmt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aksentijevich I, Jones RJ, Ambinder RF, Garrett-Mayer E, Flinn IW. Clinical outcome following autologous and allogeneic blood and marrow transplantation for relapsed diffuse large-cell non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:965–972. doi: 10.1016/j.bbmt.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 71.Vose JM, Sharp G, Chan WC, et al. Autologous transplantation for aggressive non-Hodgkin's lymphoma: results of a randomized trial evaluating graft source and minimal residual disease. J Clin Oncol. 2002;20:2344–2352. doi: 10.1200/JCO.2002.09.138. [DOI] [PubMed] [Google Scholar]
- 72.van Imhoff GW, van der Holt B, Mackenzie MA, et al. Impact of three courses of intensified CHOP prior to high-dose sequential therapy followed by autologous stem-cell transplantation as first-line treatment in poor-risk, aggressive non-hodgkin's lymphoma: comparative analysis of Dutch-Belgian Hemato-Oncology Cooperative Group Studies 27 and 40. J Clin Oncol. 2005;23:3793–3801. doi: 10.1200/JCO.2005.07.039. [DOI] [PubMed] [Google Scholar]