Abstract
Background/Aims
Chronic hepatitis B infection is a common cause of secondary membranous nephropathy (MN) in endemic areas. Lamivudine treatment improves renal outcome in patients with hepatitis B virus-associated MN (HBV-MN), but prolonged use leads to the emergence of lamivudine-resistant variants. We describe our experience treating lamivudine-resistant and other strains of HBV-MN with new antiviral drugs.
Methods
Of the 89 patients biopsied and diagnosed with MN from 1996 to 2011, 10 positive for hepatitis B surface antigen were recruited for this study. We investigated the clinical courses, therapeutic responses, and prognoses of patients with HBV-MN.
Results
The incidence of HBV-MN among the original 89 patients was 11.2%. Of these patients, four were treated with supportive care and six with antiviral drugs. One of the four patients treated with supportive care had a spontaneous remission. Four of the six patients treated with antiviral drugs were given lamivudine, and the other two were given entecavir. Two of the four patients treated with lamivudine achieved complete remission with seroconversion (i.e., development of anti-hepatitis B e antigen antibodies), whereas the other two had lamivudine-resistant strains, which were detected at 22 and 23 months after lamivudine treatment, respectively. We added adefovir to the treatment regimen for one of these patients, and for the other patient we substituted clevudine for lamivudine. Both of these patients experienced complete remission, as did the two patients initially treated with entecavir, neither of whom showed resistance to the drug.
Conclusions
New nucleoside analogues, such as entecavir, adefovir, and clevudine, can be effective for treatment of HBV-MN, including lamivudine-resistant strains.
Keywords: Hepatitis B, Membranous glomerulonephritis
INTRODUCTION
Membranous nephropathy (MN) is classified into idiopathic and secondary categories, with secondary causes identified in 20% of adult cases [1]. Frequent causes of secondary MN include autoimmune disease (e.g., systemic lupus erythematosus), infectious disease (e.g., hepatitis B virus [HBV] infection), tumors, and drugs or toxins. HBV infection is a common cause of MN in endemic areas [2,3].
The natural history of HBV-associated MN (HBV-MN) is not completely understood. Unlike affected children, adults with HBV-MN typically develop progressive disease [4]. Various management strategies have been tried, but there is no optimal drug choice for the treatment of HBV-MN. Although steroids and cytotoxic agents are the cornerstone treatments for idiopathic MN, their efficacy has not been established for HBV-MN. Furthermore, such drugs can cause deleterious hepatic flare-ups or even fatal decompensation by enhancing viral replication when the drugs are withdrawn [5]. Interferon-α therapy has shown mixed results in adults with HBV-MN, and it has side effects, such as flu-like symptoms [4,6].
Recently, lamivudine treatment for HBV-MN was shown to improve renal outcome without side effects [7]. However, a potential limitation of prolonged treatment with lamivudine is the emergence of drug-resistant strains following the induction and selection of HBV variants with mutations at the tyrosine-methionine-aspartate-aspartate (YMDD) motif of the DNA polymerase [8]. New drugs, such as entecavir, adefovir, and clevudine, have been introduced to treat chronic hepatitis B, but there are no clinical data regarding their efficacy for treating HBV-MN. In this study, we report our treatment experience of HBV-MN, including lamivudine-resistant strains, with several antiviral drugs, including entecavir, adefovir, and clevudine.
METHODS
Patient selection
Eighty-nine patients who were biopsied and diagnosed with MN between 1996 and 2011 formed the initial subject pool for this study, from which 10 patients were enrolled. The criteria for patient selection were: age of 15 years or more at the first clinical presentation; hepatitis B surface antigen (HBsAg) positivity and hepatitis B e antigen (HBeAg) positivity, or detection of circulating HBV DNA. Patients with MN secondary to another cause, such as neoplasm, infections other than hepatitis B, connective tissue disorder, or putative drugs and toxins, were excluded. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital.
Tests for serum HBsAg, anti-HBs antibodies, HBeAg, and anti-HBe antibodies were carried out by radioimmunoassay (Abbott Laboratories, Chicago, IL, USA). Serum HBV DNA levels were measured by either Quantiplex branched DNA assay (Bayer Diagnostics, Berkeley, CA, USA; sensitivity > 7 × 105 copies/mL), VERSANT 3.0 branched DNA assay (Bayer HealthCare LLC, Tarrytown, NY, USA; sensitivity > 2 × 103 copies/mL), or a real-time polymerase chain reaction (RT-PCR) method (Abbott Real Time HBV Quantification kit, Abbott Molecular Inc., Abbott Park, IL, USA; sensitivity > 28 copies/mL). YMDD mutation was evaluated using a nested PCR and restriction fragment length polymorphism assay [9].
Renal tissue samples were routinely processed and stained with hematoxylin and eosin, periodic acid-Schiff, Masson's trichrome, Jones's silver, and Congo red stains. For direct immunofluorescence, a frozen section of the fresh tissue sample was stained with antisera against human C3, C4, C1q, IgA, IgM, IgG, and fibrinogen. Complete remission from proteinuria was defined by a proteinuria value ≤ 0.3 g/day in a patient with previous nephrotic range proteinuria. Partial remission was defined as a 50% reduction in urine protein and to a proteinuria level of < 3.5 g/day [10].
All data are presented as means ± standard deviations unless otherwise specified. Continuous variables at the start of treatment were compared by the Mann-Whitney test, categorical groups were compared with Fisher's exact test, and a p < 0.05 was considered to indicate statistical significance. Statistical analysis was performed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
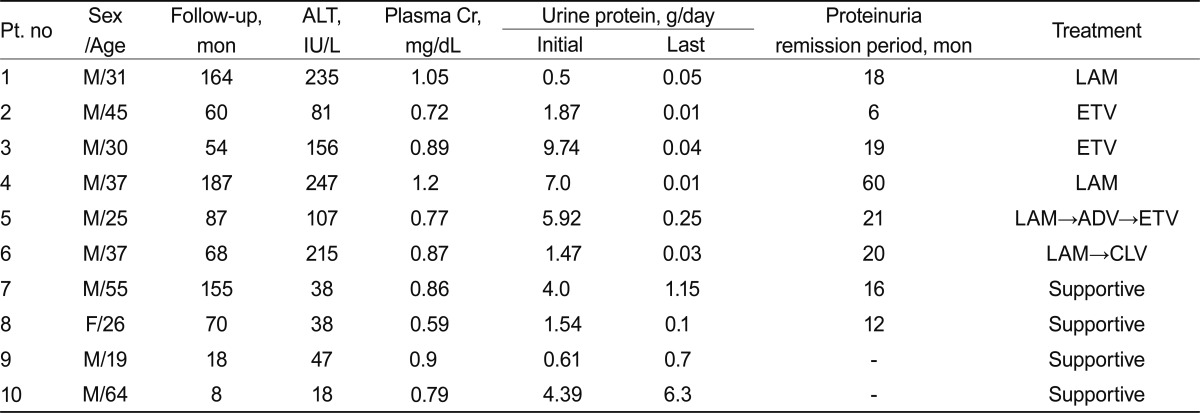
Of the 89 patients with MN, 65 (73%) had idiopathic MN and 24 (27%) had secondary MN. Of patients with secondary MN, 10 (37%) had HBV-MN. The clinical and laboratory findings of patients with HBV-MN are presented in Table 1. The patients included nine males and one female, with a mean age of 37 years (range, 19 to 64). Of these patients, five had nephrotic syndrome, and the other five had subnephrotic range proteinuria. The mean urinary protein excretion was 3.70 g/day (range, 0.50 to 9.74). The median serum creatinine concentration was 0.86 mg/dL (range, 0.59 to 1.2). All patients had HBsAg and circulating HBV DNA. Six patients had increased plasma alanine aminotransferase concentrations (≥ 1.5 times the upper limit of the normal range). The mean follow-up duration was 87 months (range, 8 to 187).
Table 1.
Clinical and laboratory findings of patients with hepatitis B virus-associated membranous nephropathy
Pt, patient, ALT, alanine aminotransferase; Cr, creatinine; LAM, lamivudine; ETV, entecavir; ADV, adefovir; CLV, clevudine.
Of the 10 patients with HBV-MN, six received antiviral drugs and four were given supportive care, including angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Two of the four patients who received supportive care had nephrotic syndrome, and the other two patients had subnephrotic range proteinuria. One of the patients with nephrotic syndrome achieved partial remission, and the other had persistent proteinuria until being lost to follow-up at 8 months. One of the two patients with subnephrotic range proteinuria experienced spontaneous remission at the 12-month follow-up, but the other patient's subnephrotic proteinuria persisted.
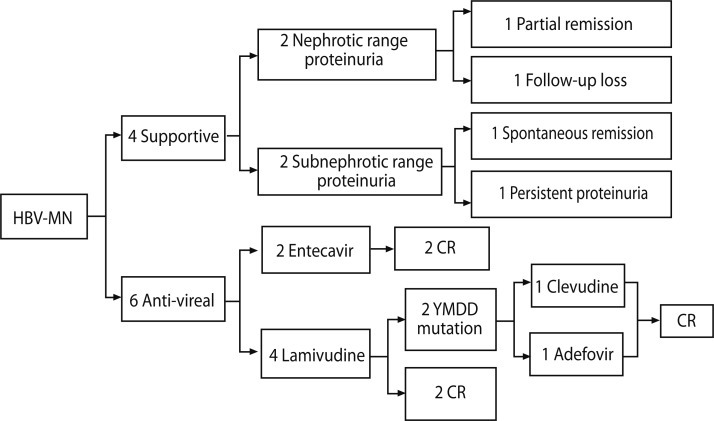
Of the six patients treated with antiviral drugs, three had nephrotic range proteinuria and three had subnephrotic range proteinuria. Four patients were treated with lamivudine and the other two with entecavir. Of four patients receiving lamivudine treatment, two achieved complete remission with seroconversion (i.e., development of anti-HBe antibodies), whereas the other two had lamivudine-resistant strains with mutations at the YMDD motif of the DNA polymerase, which was detected at 22 and 23 months following lamivudine treatment, respectively. After detection of lamivudine resistance, adefovir was added to one patient's drug regimen, and lamivudine was switched to clevudine for the other patient. Although both patients with lamivudine-resistant strains reached remission from proteinuria and virologic responses during lamivudine treatment, they achieved complete remission after their respective changes in drug therapy. Following the addition of adefovir dipivoxil, the first patient's HBV DNA copies dropped from 24,194,771 to 78,047 at the 12-month follow-up, and the patient who had clevudine treatment saw virologic response and urinary protein excretion below 0.3 g/day within 5 months; however, myopathy occurred 6 months after clevudine treatment, so entecavir was substituted for clevudine at that point (Fig. 1).
Figure 1.
Flow chart illustrating the clinical course and treatment of 10 patients in the study. Numbers of boxes represent number of patients. HBV-MN, hepatitis B virus-associated membranous nephropathy; CR, complete remission; YMDD, tyrosine-methionine-aspartate-aspartate.
As mentioned above, two of the six patients treated with antiviral agents received entecavir from the beginning. One of these had nephrotic syndrome and the other had subnephrotic range proteinuria. They both achieved seroconversion, with development of anti-HBe antibodies, and urinary protein excretion below 0.3 g/day after entecavir treatment, one within 6 months and the other at 19 months. No side effects or resistant strains were observed.
DISCUSSION
In this study, we report our experience treating HBV-MN, including lamivudine-resistant strains, with several antiviral drugs. The results show that in addition to lamivudine, the new antiviral drugs entecavir, adefovir, and clevudine were effective for treating HBV-MN. For lamivudine-resistant strains, switching to clevudine was effective for one patient in this study and adding adefovir was effective for another. Our data also indicate that entecavir, which is now the first-line agent for treatment of chronic B hepatitis [11], was an effective treatment for HBV-MN.
Various management strategies have been tried for HBV-MN, but the ideal drug has yet to be found. Steroid and cytotoxic agents may do more harm than good by enhancing viral replication [5], and interferon-a has produced mixed results in adults with HBV-MN, in many cases inducing significant side effects [4,6]. On the other hand, current lamivudine treatment has improved renal outcome in HBV-MN with no side effects [7]. Similar effects have been reported for other types of HBV-associated glomerulonephritis [12], and lamivudine was also an effective treatment for HBV-MN in our study; two patients treated with lamivudine in our study achieved urinary protein excretions below 0.3 g/day with seroconversion (i.e., development of anti-HBe antibodies).
A potential limitation of prolonged treatment with lamivudine is the emergence of drug-resistant virus strains, the frequency of which has been shown to increase with time: 24% at 1 year, 38% at 2 years, 50% at 3 years, and 67% at 4 years [8,13]. In our study, two of four patients (50%) treated with lamivudine experienced lamivudine resistance within 2 years. American Association for the Study of Liver Disease (AASLD) guidelines recommend adding adefovir to lamivudine, or substituting entecavir for lamivudine, to treat patients with lamivudine-resistant HBV [14]. In our case, the addition of adefovir (as per AASLD guidelines) helped one patient with lamivudine resistance, as indicated by the reduction in HBV DNA copies at 12 months, but further follow-up data were not available. After switching the other lamivudine-resistant patient to clevudine, virologic response and urinary protein excretion below 0.3 g/day occurred within 5 months. Clevudine has potent antiviral activity, but its efficacy against YMDD-mutant HBV has not yet been established [14,15]. Fortunately, the renal and liver outcomes of our clevudine patient were good; however, the patient experienced myopathy 6 months after starting clevudine treatment, necessitating a change to entecavir. Clevudine-associated myopathy has been seen in previous reports [16].
Medical personnel started using entecavir to treat chronic hepatitis B in 2005, and now it is the first-line agent for treatment of naïve chronic hepatitis B patients due to its strong HBV suppressive effect and low resistance rate [11,17,18] Recently, Ikee et al. [19] reported the clinical effects of entecavir in HBV-related MN, showing HBeAg seroconversion and HBV clearance. In our study, two patients were treated with entecavir as an initial therapy, and they went into complete remission and seroconversion with development of anti-HBe antibodies within 13 and 19 months, respectively. Entecavir resistance was not observed, and there were no side effects. Therefore, entecavir could also be a first-line agent for treatment of HBV-MN.
Spontaneous complete remission of nephrotic syndrome with seroconversion to anti-HBe is uncommon among adult patients with HBV-MN [4]. In our study, four patients were treated with supportive care only (i.e., without antiviral therapy), two with nephrotic syndrome and two with subnephrotic range proteinuria. One of the patients with nephrotic syndrome experienced partial remission after 132 months, and the other was lost to follow-up at 8 months. Of the two patients with subnephrotic range proteinuria, one had a spontaneous remission and the other had persistent proteinuria. Thus, as in previous research, we did not observe spontaneous resolution of HBV-MN in patients with nephrotic syndrome. On the other hand, one of our patients with subnephrotic range proteinuria in HBV-MN seemed to have had a spontaneous remission following supportive care.
In most reports, the diagnosis of HBV-MN was based on the persistence of circulating HBV or HBV DNA, the absence of other causative agents, and/or the presence of HBV-specific antigen or viral genomes in the glomerulus [20]. We did not perform immunohistochemical staining with antibodies against HBsAg, HBeAg, or hepatitis B core antigen in our study. It is not practical to perform indirect fluorescent antibody assays in all centers. Moreover, the association between MN and HBs antigenemia is well-established in endemic regions [21]. Therefore, even though we were unable to perform such staining, we believe this study has clinical value.
To date, there have been no reports of treatment for HBV-MN with lamivudine-resistant strains, even though the frequency of lamivudine-resistant HBV mutant strains is increasing. In our study, the addition of adefovir to lamivudine and the substitution of clevudine for lamivudine were both effective against lamivudine-resistant strains of HBV-MN. In addition, entecavir was effective for treating HBV-MN as an initial therapy. Therefore, new nucleoside analogues, such as entecavir, adefovir, and clevudine, can be effective initial treatments for HBV-MN and/or as rescue therapies in cases of lamivudine-resistance.
Acknowledgments
This study was supported by a grant (A102065) from the Korea Healthcare Technology R&D project, Ministry of Health and Welfare, Republic of Korea.
Footnotes
No potential conflict of interest relevant to this article is reported.
References
- 1.Jefferson JA, Couser WG. Therapy of membranous nephropathy associated with malignancy and secondary causes. Semin Nephrol. 2003;23:400–405. doi: 10.1016/s0270-9295(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 2.Zeng CH, Chen HM, Wang RS, et al. Etiology and clinical characteristics of membranous nephropathy in Chinese patients. Am J Kidney Dis. 2008;52:691–698. doi: 10.1053/j.ajkd.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Lee EJ, Lee SH, Won JJ, et al. Etiology and clinical course of secondary membranous nephropathy. Korean J Med. 2005;68:407–416. [Google Scholar]
- 4.Lai KN, Li PK, Lui SF, et al. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324:1457–1463. doi: 10.1056/NEJM199105233242103. [DOI] [PubMed] [Google Scholar]
- 5.Lai KN, Tam JS, Lin HJ, Lai FM. The therapeutic dilemma of the usage of corticosteroid in patients with membranous nephropathy and persistent hepatitis B virus surface antigenaemia. Nephron. 1990;54:12–17. doi: 10.1159/000185802. [DOI] [PubMed] [Google Scholar]
- 6.Conjeevaram HS, Hoofnagle JH, Austin HA, Park Y, Fried MW, Di Bisceglie AM. Long-term outcome of hepatitis B virus-related glomerulonephritis after therapy with interferon alfa. Gastroenterology. 1995;109:540–546. doi: 10.1016/0016-5085(95)90343-7. [DOI] [PubMed] [Google Scholar]
- 7.Tang S, Lai FM, Lui YH, et al. Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int. 2005;68:1750–1758. doi: 10.1111/j.1523-1755.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 8.Liaw YF, Leung NW, Chang TT, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B:Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172–180. doi: 10.1053/gast.2000.8559. [DOI] [PubMed] [Google Scholar]
- 9.Allen MI, Gauthier J, DesLauriers M, et al. Two sensitive PCR-based methods for detection of hepatitis B virus variants associated with reduced susceptibility to lamivudine. J Clin Microbiol. 1999;37:3338–3347. doi: 10.1128/jcm.37.10.3338-3347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattran DC, Appel GB, Hebert LA, et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 11.Yuen MF, Lai CL. Treatment of chronic hepatitis B: evolution over two decades. J Gastroenterol Hepatol. 2011;26(Suppl 1):138–143. doi: 10.1111/j.1440-1746.2010.06545.x. [DOI] [PubMed] [Google Scholar]
- 12.Yi Z, Jie YW, Nan Z. The efficacy of anti-viral therapy on hepatitis B virus-associated glomerulonephritis: a systematic review and meta-analysis. Ann Hepatol. 2011;10:165–173. [PubMed] [Google Scholar]
- 13.Lau DT, Khokhar MF, Doo E, et al. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828–834. doi: 10.1053/jhep.2000.17912. [DOI] [PubMed] [Google Scholar]
- 14.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 15.Fu L, Liu SH, Cheng YC. Sensitivity of L-(-)2,3-dideoxythiacytidine resistant hepatitis B virus to other antiviral nucleoside analogues. Biochem Pharmacol. 1999;57:1351–1359. doi: 10.1016/s0006-2952(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 16.Tak WY, Park SY, Cho CM, et al. Clinical, biochemical, and pathological characteristics of clevudine-associated myopathy. J Hepatol. 2010;53:261–266. doi: 10.1016/j.jhep.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 18.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 19.Ikee R, Ishioka K, Oka M, et al. Hepatitis B virus-related membranous nephropathy treated with entecavir. Nephrology (Carlton) 2010;15:266. doi: 10.1111/j.1440-1797.2009.01165.x. [DOI] [PubMed] [Google Scholar]
- 20.Lai AS, Lai KN. Viral nephropathy. Nat Clin Pract Nephrol. 2006;2:254–262. doi: 10.1038/ncpneph0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson RJ, Couser WG. Hepatitis B infection and renal disease: clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 1990;37:663–676. doi: 10.1038/ki.1990.32. [DOI] [PubMed] [Google Scholar]