Abstract
Background
Most individuals with lung cancer have symptoms for several months before presenting to their GP. Earlier consulting may improve survival.
Aim
To evaluate whether a theory-based primary care intervention increased timely consulting of individuals with symptoms of lung cancer.
Design and setting
Open randomised controlled trial comparing intervention with usual care in two general practices in north-east Scotland.
Method
Smokers and ex-smokers aged ≥55 years were randomised to receive a behavioural intervention or usual care. The intervention comprised a single nurse consultation at participants’ general practice and a self-help manual. The main outcomes were consultations within target times for individuals with new chest symptoms (≤3 days haemoptysis, ≤3 weeks other symptoms) in the year after the intervention commenced, and intentions about consulting with chest symptoms at 1 and 6 months.
Results
Two hundred and twelve participants were randomised and 206 completed the trial. The consultation rate for new chest symptoms in the intervention group was 1.19 (95% confidence interval [CI] = 0.92 to 1.53; P = 0.18) times higher than in the usual-care group and the proportion of consultations within the target time was 1.11 (95% CI = 0.41 to 3.03; P = 0.83) times higher. One month after the intervention commenced, the intervention group reported intending to consult with chest symptoms 31 days (95% CI = 7 to 54; P = 0.012) earlier than the usual care group, and at 6 months this was 25 days (95% CI = 1.5 to 48; P = 0.037) earlier.
Conclusion
Behavioural intervention in primary care shortened the time individuals at high risk of lung disease intended to take before consulting with new chest symptoms (the secondary outcome of the study), but increases in consultation rates and the proportions of consultations within target times were not statistically significant.
Keywords: early detection of cancer, general practice, illness behaviour, lung neoplasms, randomised controlled trial
INTRODUCTION
Lung cancer is common and survival rates poor, particularly in the UK, where late stage at diagnosis has been highlighted as an important factor.4–4 Five-year survival rates are closely linked to disease stage at diagnosis, so there is potential to improve lung cancer survival by diagnosing it earlier. Recently, benefits have been reported for lung cancer screening with low-dose computed tomography, but with high false-positive rates and costs. Population screening is unlikely to be implemented in the near future and further trials (including UK Lung Screen) are being initiated.5–7
The alternative is to attempt to diagnose cancer early through rapid identification and investigation of those with symptoms. Lung cancer has been targeted for this by the National Awareness and Early Diagnosis Initiative in England and the Detect Cancer Early Programme in Scotland.8,9 Achieving this will be challenging, but there are some grounds for optimism.10 Most individuals with lung cancer have unrecognised symptoms for several months before seeking medical help and there are indications that consulting behaviour is modifiable; for example, the wait before consulting is shorter for those with particular experiences, including previous chest infections.11 There have been encouraging findings from a recent public awareness campaign in Doncaster, including increases in symptom awareness and referrals for chest X-ray.12 Few interventions, however, have been evaluated in randomised trials. A systematic review found only five randomised trials of interventions to promote cancer awareness and early presentation.13 None of the interventions targeted lung cancer and only one included lung cancer symptoms.14 These trials reported modest effects on cancer awareness and attitudes, but none reported effects on consulting behaviour.
Interventions that have been developed using research evidence and theory to understand fully the underlying problem and how it may be targeted, and use behaviour change techniques found effective in other situations, are more likely to be effective.15 A previous article described the development of a theory-based primary care intervention that seeks to reduce the time taken by individuals at high risk of lung cancer to consult.10 This study evaluates the effect of this intervention on actual and intended consulting behaviour.
How this fits in
Most individuals with lung cancer have symptoms for several months before seeking medical help. This study developed a general practice-based intervention to be delivered to individuals at high risk of lung cancer. The intervention reduced the time individuals reported they would wait before consulting with various chest symptoms. Although this is encouraging, increased rates of consulting within target times were not statistically significant, so larger trials are needed.
METHOD
A parallel-group randomised controlled trial was carried out in two general practices in north-east Scotland.
Participants
Participants were long-term smokers (at least 20 pack-years) aged ≥55 years, including ex-smokers if their cessation date was within 10 years. Smokers and ex-smokers were identified from practice computerised records and a sample selected (every nth name from an alphabetical list, where n = [number eligible]/[number required]); these individuals were sent a letter from their general practice, inviting them to take part in the study. Two waves of recruitment were conducted, the first to establish recruitment rates and the second to achieve the target sample size.
Intervention
A consultation with a trained nurse guided each participant through a self-help manual, which the participant took home, and developed ‘if–then’ action plans (full details are published separately).10 The manual provided information, used behaviour-change techniques and sought to: engage high-risk individuals, using logos, celebrity endorsement by Liz Dawn (from Coronation Street), and ‘special attention’ messages; increase the salience and personal relevance of symptoms; reinforce the benefits of early intervention in lung cancer and other chest diseases using patient stories and frequent positive messages; sanction early consultation using messages from doctors, other patients, and Liz Dawn; provide prompts to self-monitoring; provide simple symptoms checklists linked to ‘if–then’ action plans; and tackle barriers to consultation using ‘if–then’ coping plans.16,17 Participants allocated to the intervention group who did not attend the nurse consultation were sent the self-help manual by post. The intervention was appraised at, and refined after, focus groups and interviews with patients from the target group, GPs, and individuals with lung cancer. The control group received usual care at their general practice, which included patient-initiated consultations, opportunistic smoking-cessation advice and, if applicable, annual reviews for chronic obstructive pulmonary disease (COPD).
Outcomes
The primary outcome was the number of general practice consultations, within target times, for individuals with new chest symptoms (≤3 days for haemoptysis and ≤3 weeks for other symptoms) in the year after the start of intervention. However, data on symptom duration were missing for most consultations, so the primary outcome was analysed in two parts: first, number of general practice consultations with new chest symptoms and, secondly, the proportion of those who consulted (and for whom duration data were available) that were within the target time. The secondary outcome was intention to consult with symptoms by a given time. This was assessed using four items: intervention, self-efficacy, knowledge, and mood; each requiring forced-choice direct estimation of the time taken before making an appointment to see a doctor for a given chest symptom scenario. Each item had 11 options ranging from <1 day to >6 months. Internal consistency (Cronbach’s alpha) was 0.78. These were converted into ‘number of days’ to give the analysis more meaning (for example, 3 weeks to 21 days), with <1 day converted to 1 day and >6 months to 180 days. The a priori decision was for the main outcome to be a single measure of intention combining all four items, but with the four items also presented separately for clinical relevance.
Additional measures of process were:
self-efficacy for consulting without delay; 10 items each with a 10-point scale (from ‘not at all confident’ to ‘totally confident’) summed to give scores ranging from 10 to 100, Cronbach’s alpha was 0.85;
knowledge of symptoms of lung disease; 21 symptoms with ‘yes’, ‘no’, ‘not sure’ answers, expressed as a percentage of answers correct;
risk perception; ‘how would you rate your chance of getting lung disease?’, with a five-point scale ranging from ‘very low’ to ‘very high’;
Adverse effects were assessed by:
the Hospital Anxiety and Depression Scale (HADS), a validated scale measuring anxiety (7 items, range 0–21) and depression (7 items, range 0–21), with minimal confounding by somatic symptoms;18
the Cancer Worry Scale, a six-item scale (range 6–24) with internal consistency (Cronbach’s alpha) of 0.88 in this study; 19,20 and
general practice consultation rates with anxiety or depression.
Wider health service effects were measured by total general practice consultations, and chest radiograph and respiratory medicine referrals.
Respiratory fitness for treatment was assessed by spirometry, which was measured for participants attending nurse consultations and categorised using Global initiative for chronic Obstructive Lung Disease (GOLD) criteria for COPD.21
Self-report data were collected by questionnaires at recruitment (pre-intervention) and 1 and 6 months after the nurse consultation. For the control group, who did not have a nurse consultation, matching dates were allocated by pairing each control participant at randomisation to an intervention participant and using the latter’s date of nurse consultation. For intervention-group participants who did not attend the nurse consultation, the date of posting their manual was used. Data on consultations and referrals during the years before and after the nurse consultation were collected by review of general practice case notes. Data on duration of symptoms were collected from both records of consultations in case notes and short questionnaires that participants were asked to complete if they consulted.
Sample size
It was estimated that, without intervention, 35% of participants would consult for respiratory symptoms,22 of whom 20% (7% of the total) would consult within target times.11 It was judged that an increase to 25% would be clinically worthwhile; a sample of 200 (100 in each group) would provide 90% power at 5% two-sided significance to detect this difference.
Randomisation
Invitations to participate and randomisation in equal numbers to intervention and control groups were both conducted at the start of the trial. Responses from consenting participants were stratified by general practice and given a unique identification number as they were received. When blocks of 24 had accumulated, random numbers were generated by the project senior statistician and assigned to the previously ordered identification numbers.
Blinding
Data entry from questionnaires was blind to group allocation. Detailed protocols and rules were used for abstraction of data from case notes, but blinding was not possible because indicators of nurse consultations were present in the case notes.
Statistical methods
Analysis was by intention to treat, with participants analysed according to the trial arm to which they were randomised. For counts of consultations by participants, Poisson regression was used to produce an estimate of the ratio of consultation rates between the two treatment groups. The proportion of consultations within the target time was analysed using a generalised linear model with a binomial distribution and logit link function. For outcome measures from questionnaires, linear mixed effects models were used to estimate the treatment differences at 1 month and 6 months, taking into account the correlation between the repeated outcomes. Main analyses were of all observed data adjusted for baseline count or score, age (in years), sex, and practice. This assumes that missing data were at random, therefore sensitivity analyses were conducted with all participants using multiple methods of missing value imputation, including the median value from completers, median values within a scale, hot-deck of completers (random selected value from completers), and last value carried forward, to check for important differences.23
RESULTS
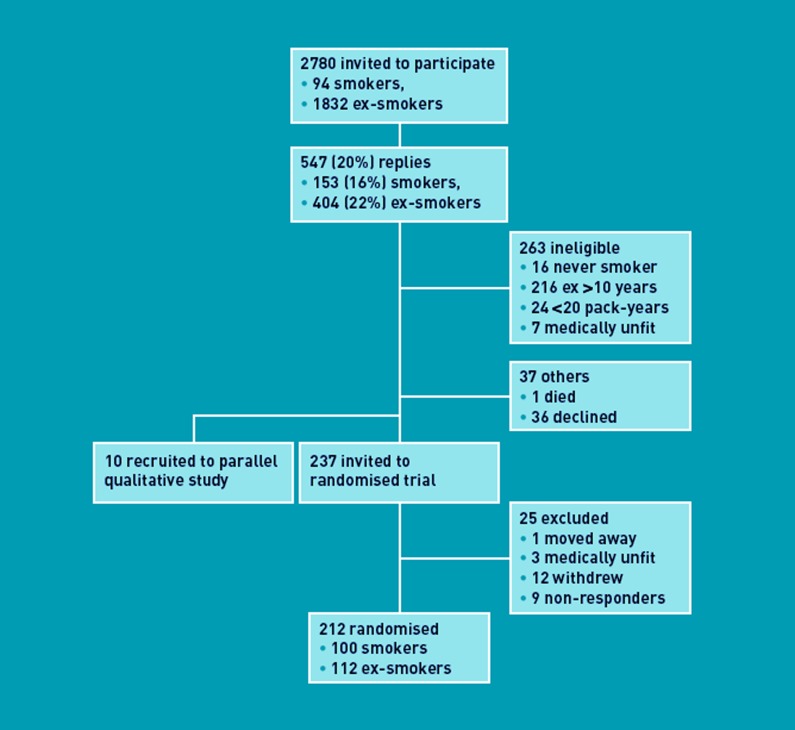
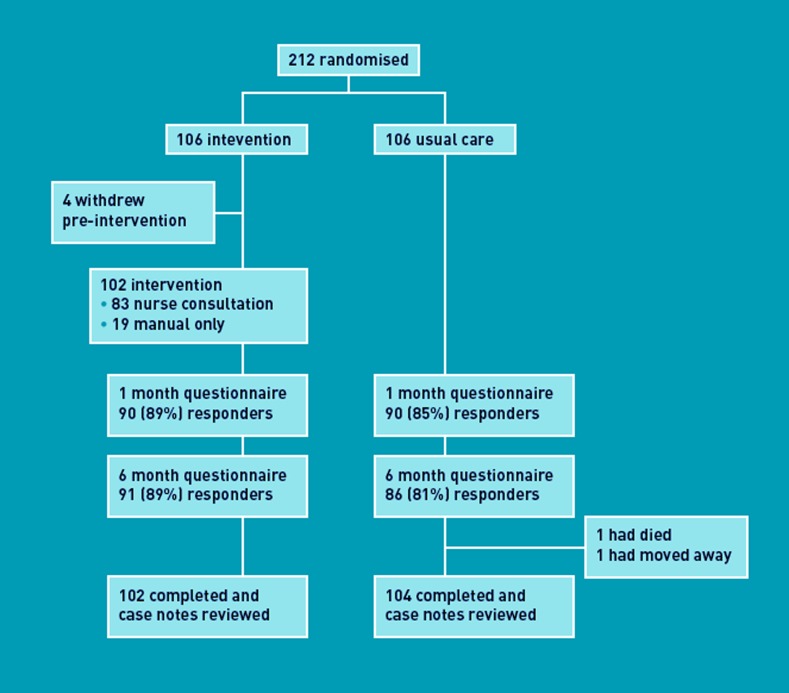
Recruitment took place between 26 May 2008 and 28 February 2009. Of 2780 smokers and ex-smokers initially approached, 212 were eligible, consented, and randomised into two equal groups (Figure 1). There were no important differences between groups (Table 1). Of 82 intervention group participants, spirometry was normal (forced expiratory volume in one second/forced vital capacity [FEV1/FVC]≥0.7) for 40 (49%). The remainder had spirometric criteria for COPD: five (6%) mild (FEV1≥80% of predicted); 24 (29%) moderate (FEV1<80% and ≥50% of predicted); eight (10%) severe (FEV1<50% and ≥30% of predicted); and five (6%) very severe (FEV1<30% of predicted). Follow-up took place between 29 October 2008 and 10 June 2010, with 206 (102 intervention, 104 control) participants completing the trial (Figure 2).
Figure 1.
Recruitment to the randomised trial and parallel qualitative study.
Table 1.
Characteristics of participants randomised
| Characteristic | Intervention (n = 106) | Control (n = 106), n (%) |
|---|---|---|
| Practice A, n (%) | 51 (48) | 51 (48) |
| Practice B, n (%) | 55 (52) | 55 (52) |
| Male, n (%) | 61 (58) | 64 (61) |
| Mean age, years (SD) | 64.2 (6.0) | 64.6 (7.0) |
| Current smoker, n (%) | 45 (42) | 55 (52) |
| Chronic obstructive pulmonary disease, n (%) | 30/102 (29) | 23/104 (22) |
| MRC dyspnoea grade, n (%) | ||
| 0: no shortness of breath | 9 (8) | 7 (7) |
| 1: only on strenuous exercise | 21 (20) | 29 (27) |
| 2: on hurrying or walking up slight hill | 42 (40) | 32 (30) |
| 3: makes walking slower or with stops | 16 (15) | 8 (8) |
| 4: stops after 100 yards on level | 16 (15) | 28 (26) |
| 5: housebound with breathlessness | 2 (2) | 2 (2) |
| Employment, n (%) | ||
| Full time | 17 (17) | 16 (15) |
| Part-time work | 9 (9) | 10 (10) |
| Unemployed | 4 (4) | 4 (4) |
| Retired | 58 (58) | 56 (54) |
| Other, such as invalid/disabled/carer | 12 (12) | 18 (17) |
| Missing | 6 | 2 |
| Highest educational qualification, n (%) | ||
| School leaving certificate to A level | 28 (29) | 33 (31) |
| Vocational/technical qualifications | 21 (21)) | 20 (19) |
| University degree | 5 (5) | 4 (4) |
| Other | 9 (9) | 10 (10) |
| None of these | 35 (34) | 38 (36) |
| Missing | 8 | 1 |
| Scottish Index of Multiple Deprivation, n (%) | ||
| 1: most deprived | 23 (22) | 20 (19) |
| 2 | 27 (25) | 38 (36) |
| 3 | 23 (22) | 23 (22) |
| 4 | 16 (15) | 10 (9) |
| 5: least deprived | 17 (16) | 15 (14) |
| Home ownership, n (%) | ||
| Own home | 66 (65) | 59 (59) |
| Rent home | 31 (31) | 41 (41) |
| Other | 4 (4) | 6 (6) |
| Missing | 5 (5) | – |
| Living arrangements, n (%) | ||
| Alone | 30 (29) | 37 (35) |
| With spouse/partner | 62 (60) | 67 (63) |
| With other family | 7 (7) | 2 (2) |
| Other | 4 (4) | 0 (0) |
| Missing | 3 (3) | – |
MRC = Medical Research Council. SD = standard deviation.
Figure 2.
Trial profile.
The adjusted ratio of consultation rates with new chest symptoms in the intervention versus control group was 1.19 (95% confidence interval [CI] = 0.92 to 1.53) (Table 2). Data on symptom duration were available for 105 of 250 (42%) consultations in the year after intervention commenced. In the intervention group, 52 of 65 (80%) consultations were within the target time, compared with 31 of 40 (78%) for the control group. Adjusted for age, practice, sex, and number within target time in the pre-intervention period, the rate ratio in the intervention versus control group was 1.11 (95% CI = 0.41 to 3.03; P = 0.83).
Table 2.
Consultation rates during the year before and after the nurse consultation or matched control date
| Intervention (n = 102) | Control (n = 104) | Adjusted analysisa | |||||
|---|---|---|---|---|---|---|---|
| General practice consultations | Consultations, n | Consulters, n (%) | Consultations, n | Consulters, n (%) | Unadjusted RR ratio (95% CI) | RR (95% CI) | P-value |
| New chest symptom | |||||||
| Year before | 98 | 45 (44) | 118 | 55 (53) | |||
| Year after | 138 | 56 (55) | 112 | 53 (51) | 1.26 (0.98 to 1.61) | 1.19 (0.92 to 1.53) | 0.18 |
| Any chest symptom | |||||||
| Year before | 137 | 45 (44) | 145 | 55 (53) | |||
| Year after | 188 | 56 (55) | 139 | 54 (52) | 1.38 (1.11 to 1.72) | 1.20 (0.96 to 1.51) | 0.11 |
| Anxiety or depression | |||||||
| Year before | 22 | 8 (8) | 27 | 15 (14) | |||
| Year after | 22 | 13 (13) | 33 | 14 (13) | 0.68 (0.40 to 1.17) | 0.51 (0.27 to 0.93) | 0.029 |
| Median (IQR) | Median (IQR) | ||||||
| Any reason | |||||||
| Year before | 745 | 6 (4–10) | 851 | 7 (5–10.5) | |||
| Year after | 833 | 8 (4–11) | 787 | 7 (4–10) | 1.08 (0.98 to 1.19) | 1.15 (1.04 to 1.27) | 0.005 |
IQR = interquartile range. RR = rate ratio
Adjusted analysis included age, sex, and practice.
The intervention group reported their intention to consult as statistically significantly sooner: 31 days (95% CI = 7 to 54) earlier at 1 month, and 25 days (95% CI = 1.5 to 48) earlier at 6 months. Of individual scenarios, the biggest difference was that concerning weight loss (Table 3). Re-analyses using multiple methods of imputation of missing data did not make meaningful changes to the findings (data not shown).
Table 3.
Intention, self-efficacy, knowledge, and mood before intervention and 1 and 6 months afterwards
| Intention to consult (days before consulting) | Intervention | Control | Unadjusted model | Adjusted modela | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | DD | (95% CI) | DD | (95% CI) | P-value | |
| All four scenarios combined | |||||||||
| Baseline | 102 | 124 (154) | 101 | 118 (113) | |||||
| 1 month | 86 | 66 (88) | 89 | 102 (116) | –31 | (–62.6 to 0.65) | –30.6 | (–54.4 to –6.82) | 0.012 |
| 6 months | 89 | 70 (108) | 83 | 94 (110) | –26.8 | (–59.2 to 5.53) | –24.9 | (48.3 to –1.47) | 0.037 |
| New persistent dry cough | |||||||||
| Baseline | 105 | 33 (52) | 104 | 33 (47) | |||||
| 1 month | 88 | 18 (37) | 89 | 24 (40) | –3.73 | (–15.1 to 7.60) | –3.27 | (–12.8 to 6.26) | 0.50 |
| 6 months | 89 | 21 (39) | 86 | 26 (41) | –4.80 | (–16.5 to 6.95) | –6.72 | (–16.7 to 3.27) | 0.19 |
| Newly short of breath in day-to-day activities | |||||||||
| Baseline | 104 | 35 (54) | 104 | 27 (42) | |||||
| 1 month | 87 | 16 (29) | 89 | 23 (38) | –4.85 | (–14.9 to 5.18) | –6.61 | (–14.0 to 0.74) | 0.078 |
| 6 months | 89 | 17 (33) | 84 | 21 (35) | –5.05 | (–15.2 to 5.08) | –7.38 | (–15.3 to 0.57) | 0.069 |
| Coughing up phlegm with signs of blood | |||||||||
| Baseline | 104 | 7.4 (20) | 103 | 5.5 (10) | |||||
| 1 month | 89 | 3.6 (5.2) | 89 | 4.4 (5.4) | –6.61 | (–14.0 to 0.74) | 0.16 | (–2.22 to 2.55) | 0.89 |
| 6 months | 90 | 5.5 (19) | 84 | 4.0 (4.6) | –7.38 | (–15.3 to 0.57) | 1.23 | (–1.00 to 3.46) | 0.28 |
| Losing weight | |||||||||
| Baseline | 105 | 48 (56) | 103 | 56 (61) | |||||
| 1 month | 88 | 30 (42) | 89 | 51 (59) | –21.1 | (–36.2 to –6.00) | –16.9 | (–30.3 to3.53) | 0.014 |
| 6 months | 90 | 25 (36) | 83 | 42 (50) | –16.9 | (–29.8 to –4.02) | –11.5 | (–22.6 to –0.50) | 0.041 |
| Self-efficacy (score from 10 to 100) | |||||||||
| Baseline | 95 | 69.7 (23.7) | 99 | 73.5 (19.0) | |||||
| 1 month | 83 | 74.4 (22.0) | 82 | 74.5 (16.8) | –0.27 | (–6.20 to 5.66 | 2.81 | (–2.42 to 8.04) | 0.29 |
| 6 months | 86 | 71.5 (19.8) | 76 | 76.8 (15.5) | –4.00 | (–9.43 to 1.44) | –1.54 | (–6.45 to 3.37) | 0.54 |
| Knowledge (% correct) | |||||||||
| Baseline | 106 | 68.4 (22.2) | 106 | 68.9 (24.0) | |||||
| 1 month | 90 | 74.7 (17.4) | 90 | 64.4 (20.4) | 10.5 | (4.90 to 16.0) | 10.4 | (5.32 to 15.6) | <0.001 |
| 6 months | 91 | 69.1 (18.0) | 85 | 67.0 (23.2) | 2.00 | (–4.111 to 8.11) | 2.03 | (–3.86 to 7.92) | 0.50 |
| Perceived risk (score out of 5) | |||||||||
| Baseline | 105 | 3.27 (1.01) | 103 | 3.39 (0.89) | |||||
| 1 month | 89 | 3.22 (1.04) | 87 | 3.33 (0.95) | –0.13 | (–0.43 to 0.16) | –0.05 | (–0.28 to 0.18) | 0.68 |
| 6 months | 90 | 3.23 (0.98) | 84 | 3.25 (0.86) | 0.03 | (–0.25 to 0.30) | 0.07 | (–0.15 to 0.30) | 0.53 |
| Cancer Worry Scale (score from 6 to 24) | |||||||||
| Baseline | 106 | 8.38 (2.87) | 104 | 8.84 (2.85) | |||||
| 1 month | 90 | 9.56 (3.69) | 88 | 9.93 (3.73) | –0.39 | (–1.47 to 0.68) | –0.014 | (–0.81 to 0.78) | 0.97 |
| 6 months | 89 | 9.52 (3.25) | 84 | 9.14 (3.12) | 0.38 | (–0.55 to 1.31) | 0.79 | (0.16 to 1.41) | 0.014 |
| Anxiety (HADS score from 0 to 21) | |||||||||
| Baseline | 104 | 4.63 (3.52) | 104 | 5.69 (4.30) | |||||
| 1 month | 89 | 5.11 (3.61) | 90 | 5.96 (4.08) | –0.68 | (–1.81 to 0.44) | 0.04 | (–0.70 to 0.78) | 0.92 |
| 6 months | 89 | 5.29 (3.63) | 85 | 5.72 (4.12) | –0.44 | (–1.57 to 0.69) | 0.32 | (–0.45 to 1.09) | 0.41 |
| Depression (HADS score from 0 to 21) | |||||||||
| Baseline | 105 | 5.01 (3.44) | 106 | 5.86 (4.06) | |||||
| 1 month | 89 | 5.55 (3.61) | 89 | 6.21 (3.81) | –0.52 | (–1.59 to 0.56) | 0.06 | (–0.67 to 0.80) | 0.86 |
| 6 months | 90 | 5.44 (3.45) | 85 | 6.28 (3.89) | 0.86 | (–1.93 to 0.20) | –0.19 | (–0.89 to 0.51) | 0.59 |
Adjusted analysis included baseline score, age group, sex, and practice. DD = days difference. HADS = Hospital Anxiety and Depression Scale.
There was strong evidence that the total consultation rate was higher in the intervention group (Table 2): the median number of consultations for any reason increased from six in the year before intervention to eight in the year after, while remaining unchanged at seven in the control group. The adjusted consultation rate ratio was 1.15 (95% CI = 1.04 to 1.27).
There was no evidence of a difference between groups in self-efficacy or perceived risk at 1 month or 6 months. Knowledge scores were significantly higher for the intervention group compared to controls at 1 month, but not after 6 months (Table 3). Cancer Worry Scores were not statistically different at 1 month, but were found to be higher in the intervention group at 6 months (0.79, 95% CI = 0.16 to 1.41) (Table 3). HADS scores were not affected by the intervention, nor were there differences in GP consultation rates for anxiety or depression (Table 2).
The numbers of chest radiograph referrals from the intervention group increased from eight (for eight participants) in the pre-intervention year to 17 (for 15 participants) in the year after the intervention commenced. From the control group, there were 11 referrals (10 participants) pre-intervention and 13 (12 participants) afterwards. Numbers of participants with respiratory medicine referrals increased from one in the pre-intervention year to 11 in the year after the intervention commenced. Respective figures for the control group were one and four. The numbers were too small to permit formal statistical testing.
DISCUSSION
Summary
For individuals at risk of lung cancer, a theory-based intervention in primary care shortened the intended time to consultation with new chest symptoms but, while consultation rates increased, this was not statistically significant. The intervention caused a small increase in cancer worry, but this did not translate into anxiety or depression.
Strengths and limitations
The primary outcome was analysed in two parts because data on symptom duration were missing for most consultations, despite the fact that data were collected from both case notes and questionnaires. This approach meant the findings on numbers of consultations for new chest symptoms were unaffected by missing data, but the data on proportions of consultations within target times should be viewed cautiously. Missing data on symptom duration may have been due to patients not remembering or GPs not recording the information. The former may be more likely for symptoms of long duration and this could explain the higher rates of symptoms within the target time that were found, compared to previous research.11 By splitting in two the effect size upon which the sample size was based, study power was lost and the study was, in the first place, only powered to detect a large difference. Thus while effects on consultations appeared encouraging, they were not statistically significant.
The secondary outcome, self-reported consulting intentions, is not equivalent to behaviour, but is the most proximal preceding factor and gives a strong indication of the likelihood that a behaviour will be performed.24 Translation of intentions into action is particularly likely when there are high levels of self-efficacy for early consultation, as has been found.25 By identifying and approaching potential participants from general practice, full data were obtained at all stages of recruitment. Initial recruitment rates were low, as is typical in this population; it is possible that participants are more interested in health, but their characteristics, weighted towards higher levels of deprivation, are typical of the wider high-risk group.
Comparison with existing literature
This trial adds weight to the limited existing research on interventions to reduce the time before consultation with symptoms of cancer. The recently reported public awareness campaign in Doncaster had encouraging effects but the authors called for randomised trials and the first of these are provided for lung cancer symptoms.12 Regarding other cancers, a systematic review of five randomised trials and three more recent randomised trials shows they have reported increased knowledge and awareness from various interventions on symptoms of colorectal, breast, prostate, and oral cancer, and melanoma.13,26–28 One randomised trial in the Netherlands included some lung cancer among other cancer symptoms and reported benefits to consulting intentions,14 but previous trials have not measured behaviour. Most interventions in these randomised trials have been leaflets and booklets but one trial on breast awareness showed that one-to-one interaction, as in the present intervention, is more effective than literature alone.28
Implications for research and practice
The findings of this study provide encouragement that intervention in primary care may lead to earlier consultation with symptoms of lung cancer, but fall short of proof. Evidence of an effect on actual consulting behaviour and its size is needed and will require a larger trial with more complete recording of symptom duration. The latter may require participants to be contacted after each consultation. Some additional observations from the present study are encouraging. The data collected on dyspnoea and spirometry show that severe lung disease is uncommon among the target group, so many would have been fit for aggressive treatment (surgery and radiotherapy). Rates of attendance at the intervention were high, suggesting that members of the target group are receptive. Furthermore, consultation rates with GPs and practice nurses averaged seven per year, so there are plenty of opportunities to engage patients.
By intervening in primary care, it is possible to shorten the time individuals at high risk of lung cancer intend to take before consulting with important symptoms. More research is needed to determine whether there is an effect on actual consulting behaviour and whether this is large enough to translate into improvements in prognosis.
Acknowledgments
Grateful thanks to the research nurses Marie Balment and Vivien Vaughan who delivered the intervention, and the interested patients who took part in the trial. Also to the staff at the general practices involved in the study, particularly Dr Peter Watson, Dr Iain Small, Susan Reynolds, and Michelle Bibby. Thank you also to Liz Dawn for providing words of support for the intervention and trial, and to Michael Head and Carol Sherriff who, with Phil Wilson (chair), oversaw the trial as the independent trial steering committee.
Funding
The study was funded by a project grant from Cancer Research UK, CRUK Grant Reference Number C542/A8695.
Ethical approval
Full ethical approval for the study was granted by the North of Scotland Research Ethics Committee (REC reference number: 08/S0801/13) on 15 February 2008.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Trial registration
UKCRN 3804; ISRCTN 22421875. The full protocol is available from the corresponding author.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.The Scottish Executive Health Department. Cancer scenarios: an aid to planning cancer services in Scotland in the next decade. Edinburgh: The Scottish Executive; 2001. [Google Scholar]
- 3.Cancer Research UK. Cancer survival statistics. http://info.cancerresearchuk.org/cancerstats/survival/?a=5441 (accessed 22 Nov 2012)
- 4.Campbell NC, Elliott AM, Sharp L, et al. Rural and urban differences in stage at diagnosis of colorectal and lung cancers. Br J Cancer. 2001;84(7):910–914. doi: 10.1054/bjoc.2000.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sox HC. Better evidence about screening for lung cancer. N Engl J Med. 2011;365(5):455–457. doi: 10.1056/NEJMe1103776. [DOI] [PubMed] [Google Scholar]
- 7.Field JK, Baldwin D, Brain K, et al. UKLS Team. CT screening for lung cancer in the UK: position statement by UKLS investigators following the NLST report. Thorax. 2011;66(8):736–737. doi: 10.1136/thoraxjnl-2011-200351. [DOI] [PubMed] [Google Scholar]
- 8.Richards MA. The size of the prize for earlier diagnosis of cancer in England. Br J Cancer. 2009;101(Suppl 2):S125–129. doi: 10.1038/sj.bjc.6605402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Scottish Government. Detect cancer early. http://www.scotland.gov.uk/Topics/Health/Services/Cancer/Detect-Cancer-Early (accessed 28 Nov 2012)
- 10.Smith SM, Murchie P, Devereux G, et al. Developing a complex intervention to reduce time to presentation with symptoms of lung cancer. Br J Gen Pract. 2012 doi: 10.3399/bjgp12X654579. DOI: 10.3399/bjgp12X654579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SM, Campbell NC, MacLeod U, et al. Factors contributing to the time taken to consult with symptoms of lung cancer: a cross-sectional study. Thorax. 2009;64(6):523–531. doi: 10.1136/thx.2008.096560. [DOI] [PubMed] [Google Scholar]
- 12.Athey VL, Suckling RJ, Tod AM, et al. Early diagnosis of lung cancer: evaluation of a community-based social marketing intervention. Thorax. 2011;67(5):412–417. doi: 10.1136/thoraxjnl-2011-200714. [DOI] [PubMed] [Google Scholar]
- 13.Austoker J, Bankhead C, Forbes LJL, et al. Interventions to promote cancer awareness and early presentation: systematic review. Br J Cancer. 2009;101(Suppl 2):S31–S39. doi: 10.1038/sj.bjc.6605388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Nooijer J, Lechner L, Candel M, de Vries H. Short- and long-term effects of tailored information versus general information on determinants and intentions related to early detection of cancer. Prev Med. 2004;38(6):694–703. doi: 10.1016/j.ypmed.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Campbell NC, Murray E, Darbyshire J, et al. Designing and evaluating complex interventions to improve health care. BMJ. 2007;334(7591):455–459. doi: 10.1136/bmj.39108.379965.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michie S, Johnston M, Francis J, et al. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol. 2008;57:660–680. [Google Scholar]
- 17.Michie S, Johnston M, Abraham C, et al. Strengthening evaluation and implementation by specifying components of behaviour change interventions. http://www.ucl.ac.uk/health-psychology/BCTtaxonomy/ (accessed 22 Nov 2012) [DOI] [PMC free article] [PubMed]
- 18.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Rees G, Fry A, Cull A, Sutton S. Illness perceptions and distress in women at increased risk of breast cancer. Psychol Health. 2004;19:749–765. [Google Scholar]
- 20.Lerman C, Trock B, Rimer B, et al. Psychological and behavioural implications of abnormal mammograms. Ann Intern Med. 1991;114(8):657–661. doi: 10.7326/0003-4819-114-8-657. [DOI] [PubMed] [Google Scholar]
- 21.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Spirometry for healthcare providers. http://www.gpcme.co.nz/pdf/GOLD%20SpirometryFull%5B1%5D.pdf (accessed 22 Nov 2012)
- 22.Simpson CR, Helms PJ, Taylor MW, Baxter-Jones ADG. Respiratory morbidity in primary care: a population based study, using practice from the Scottish Continuous Morbidity Recording Research Database. Health Bull (Edinb) 2000;58(6):489–496. [PubMed] [Google Scholar]
- 23.White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. doi: 10.1136/bmj.d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajzen I. The theory of planned behavior. Organ Behav Hum Dec. 1991;50:179–211. [Google Scholar]
- 25.Johnston DW, Johnston M, Pollard B, et al. Motivation is not enough: prediction of risk behavior following diagnosis of coronary heart disease from the theory of planned behavior. Health Psychol. 2004;23(5):533–538. doi: 10.1037/0278-6133.23.5.533. [DOI] [PubMed] [Google Scholar]
- 26.Morgan PD, Fogel J, Tyler ID, Jones JR. Culturally targeted educational intervention to increase colorectal health awareness among African Americans. J Health Care Poor Underserved. 2010;21(3 Suppl):S132–S147. doi: 10.1353/hpu.0.0357. [DOI] [PubMed] [Google Scholar]
- 27.Idriss NZ, Alikhan A, Baba K, Armstrong AW. Online, video-based patient education improves melanoma awareness: a randomized controlled trial. Telemed J E Health. 2009;15(10):992–997. doi: 10.1089/tmj.2009.0055. [DOI] [PubMed] [Google Scholar]
- 28.Linsell L, Forbes LJ, Kapari M, et al. A randomised controlled trial of an intervention to promote early presentation of breast cancer in older women: effect on breast cancer awareness. Br J Cancer. 2009;101(Suppl 2):S40–S48. doi: 10.1038/sj.bjc.6605389. [DOI] [PMC free article] [PubMed] [Google Scholar]