SUMMARY
Local progression is the predominant failure pattern for pediatric intrinsic pontine gliomas. Overall outcome in this tumor is poor with reported median survivals of 8–12 months with localized radiation. In this phase II study, Motexafin-Gadolinium (MGd), a radiosensitizing agent that localizes in tumors with greater affinity than in normal tissues, was administered prior to daily radiation in attempt to improve the outcome in this disease.
Purpose
To evaluate the effect on one-year event-free survival (EFS) and overall survival (OS) of combining Motexafin-Gadolinium (MGd), a potent radiosensitizer, with daily fractionated radiotherapy in children with newly diagnosed intrinsic pontine gliomas.
Patients and Methods
Patients with newly diagnosed intrinsic pontine glioma were treated with MGd daily for 5 consecutive days each week, for a total of 30 doses. Patients received a 5–10 minute intravenous bolus of MGd, 4.4 mg/kg/day, given 2–5 hours prior to standard dose irradiation. Radiation therapy was administered at a daily dose of 1.8 Gy for 30 treatments over 6 weeks. The total dose was 54 Gy.
Results
Sixty eligible children received MGd daily, concurrent with 6 weeks of radiation therapy. The estimated one-year EFS was 18% ± 5% and the estimated one-year OS was 53% ± 6.5%. The most common grade 3–4 toxicities were lymphopenia, transient elevation of liver transaminases, and hypertension.
Conclusion
Compared to historical controls, the addition of Motexafin-Gadolinium to a standard 6-week course of radiation did not improve the survival of pediatric patients with newly diagnosed intrinsic pontine gliomas.
Keywords: Pediatric Pontine Glioma, Motexafin-Gadolinium, Radiation Therapy
INTRODUCTION
Ten to 20% of children with central nervous system tumors will be diagnosed with a brainstem glioma, and 70–80% of these will be diffuse intrinsic pontine gliomas. The prognosis of patients with intrinsic pontine gliomas is extremely poor. Standard therapy with daily radiation to a dose of 54 to 60 Gy results in median survival of 8–12 months with most studies reporting 2-year overall survival rates of 5–15%. (1–3)
Local in-field failures dominate, and prior approaches to therapy have investigated ways to intensify therapy. Strategies have included dose escalation using twice daily, hyperfractionated radiation therapy, and the addition of cytotoxic chemotherapy agents and radiosensitizing agents. (4–12) One such radiosensitizing agent is Motexafin-Gadolinium (MGd), an expanded metalloporphyrin that localizes in tumors with greater affinity than in normal tissues. It targets and inhibits oxidative stress-related proteins such as thioredoxin reductase, which in turn, is thought to lead to a reduced ability to repair oxidative damage induced by radiation. (13–15) The precise mechanism of action, however, is not fully elucidated. In pre-clinical models, MGd has a radiation sensitizer enhancement ratio of approximately 2. (16,17)
A Children’s Oncology Group phase I study of MGd in conjunction with involved field radiation therapy for pediatric intrinsic pontine gliomas found 4.4 mg/kg/day to be the maximum tolerated dose with primary dose-limiting toxicities of hypertension and transient elevations in serum transaminases. (18) In this Children’s Oncology Group phase II study, we sought to evaluate the effect on one-year event-free survival (EFS) and overall survival (OS) of combining MGd with daily, fractionated radiotherapy in children with newly diagnosed intrinsic pontine gliomas.
PATIENTS AND METHODS
Patient Eligibility
Patients age 21 years or younger with unifocal diffuse intrinsic pontine gliomas were eligible for this study. Brain MRI with gadolinium administration within 2 weeks of study entry was required; however, histologic verification was not required. Patients with tumors that diffusely involved the pons (greater than 50% intra-axial), the pons and medulla, the pons and midbrain or the entire brainstem were eligible. Tumors that contiguously involved the thalamus or upper cervical cord were permitted on study. Other eligibility criteria included: a Karnofsky or Lansky performance status of >/= 60; adequate bone marrow, renal and liver function; and a life expectancy of 8 weeks or greater. Patients who had prior cranial radiotherapy, any prior definitive therapy for this tumor, G6PD deficiency, biliary obstruction, or who were pregnant or breastfeeding were ineligible. The use of clinically necessary corticosteroids or anticonvulsants was permitted. Institutional review board approved consent was obtained from patients and/or their parents or legal guardians prior to study enrollment.
Drug Administration
MGd was administered at a dose of 4.4 mg/kg/day by intravenous (IV) push over 5–10 minutes, 2 to 5 hours prior to scheduled radiation. MGd was administered for a total of 30 doses, and was not administered on days in which radiation was not delivered. Toxicity assessment was based on National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. MGd was discontinued permanently for grade 4 hematologic or non-hematologic toxicities. For grade 3 hematologic toxicity, MGd was continued with supportive care. For grade 3 non-hematologic toxicity, MGd was held until the toxicity was grade 1 or lower, and then restarted at a 25% dose reduction. If grade 3 toxicity recurred at the reduced dose, then the drug was permanently discontinued and radiation was continued alone.
Radiation Therapy
Radiation therapy was administered at a daily dose of 1.8 Gy for 30 fractions over 6 weeks, for a total dose of 54 Gy. No radiation dose reductions were permitted. The treatment fields encompassed the entire tumor plus margin. 3D planning was encouraged but not mandated, and intensity-modulated radiation therapy (IMRT) was allowed. Proton therapy was not permitted. Gross tumor volume (GTV) was defined as the enhancing and non-enhancing tumor volume as visualized on the treatment planning scan; the use of T2-weighted or FLAIR MR sequences was encouraged to assist in outlining the GTV. The clinical tumor volume (CTV) was the GTV plus a margin of 1.5 cm in all directions, not extending outside of brain and with care taken when extending it inferiorly to minimize the amount of normal cervical cord included. The planning tumor volume (PTV) was the CTV plus a margin (not to exceed 5 mm) in all directions to account for daily setup variation and patient movement. For both 2D and 3D treatment planning, the prescription point was at or near the isocenter, with the goal of the treatment plan to completely encompass the PTV within the 95% isodose surface. Normal tissue dose constraints were employed for the cervical spinal cord, optic chiasm, optic nerves, cochlea, supratentorial brain, hypothalamus, and the pituitary.
Response Assessment
Response assessment with gadolinium-enhanced brain MRI was performed 6 weeks, 3 months and 6 months after completing therapy. Thereafter, an MRI was obtained every 6 months until disease relapse. Tumor response criteria were determined by changes in size using the maximal 2-dimensional cross-sectional tumor measurements, using either T1 or T2 weighted MRI images (whichever gave the best estimate of tumor size). In addition, tumor length was measured and recorded to provide a volumetric response determination; however, the 3D tumor volume change was not used to determine response status. Response was characterized as complete response (disappearance of all abnormal signal within the brainstem and return to normal size), partial response (≥50% decrease in the sum of the products of the two perpendicular diameters of the target lesion), stable disease (neither sufficient decrease/increase in the products of the two perpendicular diameters of the target lesion to qualify for partial response/progressive disease), or progressive disease (≥25% increase in the cross-sectional area of the largest two diameters of the target lesion, appearance of new lesions, or worsening neurologic status not explained by causes unrelated to tumor progression in the setting of any increase in tumor size). Patients continued on protocol therapy unless there was evidence for progressive disease, toxicity meeting criteria for cessation of MGd, refusal of protocol therapy by patient/parent, or the treating physician determined it to be in the patient’s best interest to terminate study therapy.
Statistical Analysis
The primary efficacy endpoint evaluated whether study therapy resulted in a one-year EFS rate higher than the historical baseline of 21.9%, which is the maximum likelihood estimates derived from an exponential model during the first year from study Children’s Cancer Group (CCG)-9941. (19) Event-free survival (EFS) was defined as the time from study enrollment to the first occurrence of disease progression, disease relapse, a second malignant neoplasm (SMN), or death from any cause. Both ACNS0222 and CCG-9941 were assumed to have exponential distribution. The test of comparison is based on a one-sided, one-sample test of proportions,
where p̂ is the estimated proportion of patients who are event-free at 1 year, p=0.219, and V̂(p̂) is the estimated asymptotic variance of p̂. Since censoring in this disease in the first year of follow-up is minimal, the test statistic is based on the simple estimate of the proportion of patients who are event-free at one year. The estimated variance in this case is p̂(1−p̂)/n, where n is the total sample size.
The test was performed after the last enrolled patient was followed for a minimum of 1 year. The criteria for significance was the upper 90th percentile of the standard normal distribution, so that the test was performed at a nominal 10% type I error level.
The secondary efficacy endpoint was overall survival (OS), defined as the time to death from any cause. Nonparametric EFS and overall survival curves were computed using the product-limit (Kaplan-Meier) estimates, with standard errors via the Greenwood formula. Standard descriptive statistical methods were used to summarize the rates of individual toxicities. The analyses were based on the data set as of December 31, 2010.
RESULTS
A total of 64 patients, median age 6.4 years (range 1.7 to 17.3 years), were enrolled between June 2007 and November 2008. Of the 64 enrolled patients, 30 (47%) were male and 42 (66%) were Caucasian. The observed accrual rate of approximately 3.4 patients/month exceeded the expected rate. Two patients were declared ineligible, one due to a major deviation in informed consent and one did not meet eligibility criteria for stage and extent of disease. In addition, two patients did not receive any treatment and went off the study soon after enrollment, one due to progressive neurologic dysfunction before the start of treatment with removal from the protocol by the treating physician, and one due to withdrawal of consent by the parent.
Primary Endpoint
Survival
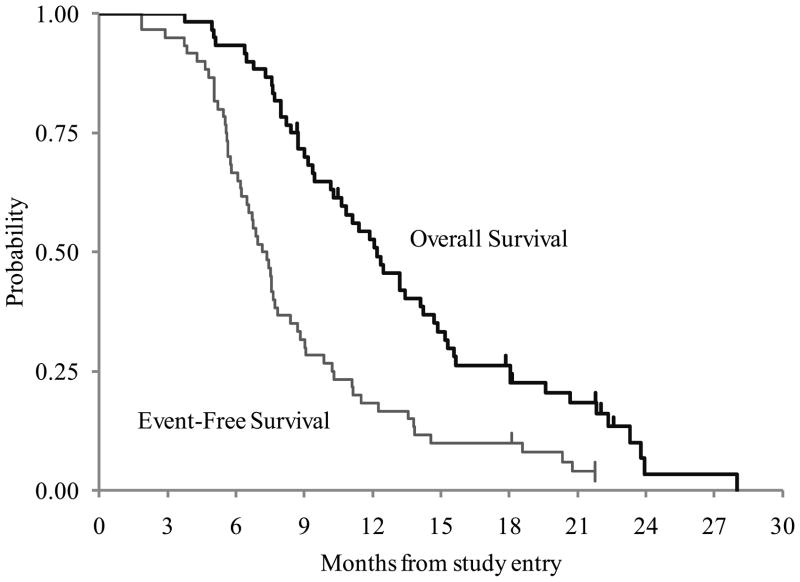
The Kaplan Meier estimate of one-year EFS was 18 ± 5% and the one-year OS was 53 ± 6.5% (Figure 1). Of the 60 patients, 55 patients progressed/relapsed with a median time to relapse of 7.2 months (range of 1.9 to 21.7 months). There were 53 deaths, including 50 patients who progressed and 3 patients died due to disease without documented radiological progression. The median time to death was 11.4 months (range of 3.7 to 28 months); 28 of the deaths occurred within the first year. Only two of the 60 patients were alive at last contact with no progression. The time on study for these two patients was 18.1 and 21.7 months.
FIGURE 1.
ACNS0222 Event-Free and Overall Survival
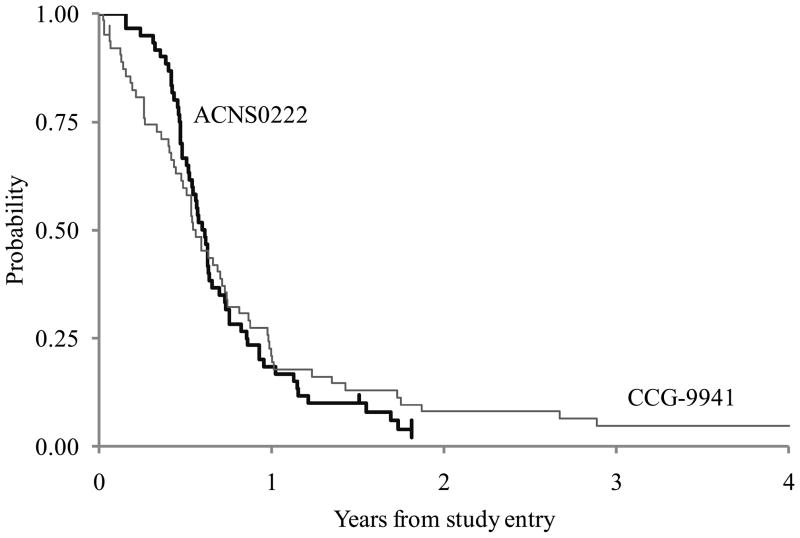
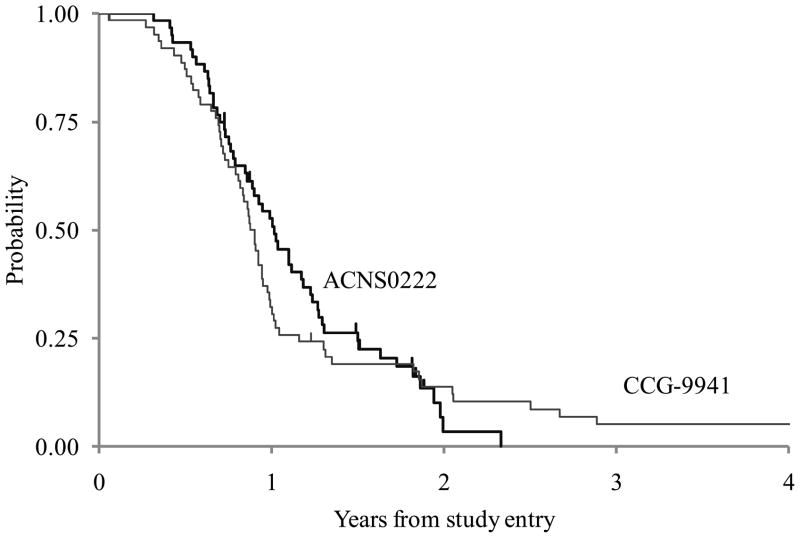
Figure 2 gives a comparison of the EFS of ACNS0222 and CCG-9941. For ACNS0222, 46 patients progressed and 3 patients died without documented progression within 1 year. There was no censoring before year 1. The estimated proportion of patients who were event-free at 1-year on ACNS0222 was 0.183 (11/60). The one-sided p-value of the test of comparison based on a one-sided, one-sample test of proportions was 0.76. We failed to reject the null hypothesis that the one-year EFS rate on the current treatment is the same or lower than the historical baseline of 21.9% at the 0.1 significance level. The one-year OS for ACNS0222 was 53 ± 7% compared to 32 ± 6% for CCG-9941, and is demonstrated in Figure 3.
FIGURE 2.
Comparison of Event-Free Survival between ACNS0222 and CCG-9941
FIGURE 3.
Comparison of Overall Survival between ACNS0222 and CCG9941
Secondary Endpoints
Toxicity
The reporting period form for radiation therapy with concurrent MGd was submitted for all 60 eligible patients who received treatment. No deaths on treatment or within one month of the end of treatment were reported. The most common grade 3–4 toxicity was lymphopenia (16.7%), which is likely at least partially related to concomitant steroid treatment. The second and third most common grade 3–4 toxicities were transient elevation of liver transaminases (11.7%) and hypertension (8.3%), both known side effects of MGd.
Five patients went off protocol therapy for toxicity: (1) one developed a grade 4 pain syndrome, felt to be possibly related to study drug; (2) one experienced a grade 4 allergic reaction after receiving MGd, a rare but known possible reaction to the drug; (3) one was given MGd for only 6 days due to a grade 3 ALT elevation which remained a grade 3 during radiotherapy; (4) one had grade 4 leukopenia and grade 4 neutropenia; and (5) one had grade 3 hypertension and grade 3 headache before being removed from therapy due to elevation of liver enzymes.
Response Assessment
Although response assessment was not a study objective, the disease evaluation by the institutions at the end of week 12 is summarized in Table 1. Of those who completed therapy, seven patients did not have their disease status evaluated at week 12. Seventeen of the 18 patients with partial response subsequently progressed and 16 died; 21 of the 24 patients with stable disease later progressed and 21 died. Eight patients did not complete the scheduled therapy, including 5 patients who went off protocol therapy due to toxicity before being evaluated and 3 who were withdrawn by the parent or physician.
TABLE 1.
Institutional Disease Response Evaluation at Week 12 on ACNS0222
| Evaluation at Week 12 | # of Patients | # With Subsequent Relapse or PD | # Who Subsequently Died |
|---|---|---|---|
| Partial Response | 18 | 17 | 16 |
| Stable Disease | 24 | 21 | 21 |
| Progressive Disease | 3 | 3 | 3 |
| Not Evaluated | 7 | 6 | 6 |
| Off Protocol Due to Toxicity | 5 | 5 | 4 |
| Withdrawal by Parent/Physician | 3 | 3 | 3 |
| Total | 60 | 55 | 53 |
DISCUSSION
Pediatric diffuse intrinsic pontine gliomas have a dismal prognosis, with local progression the cause for death in nearly all patients. Efforts to improve outcome have focused on ways to improve local control. Preclinical studies have demonstrated that Motexafin-Gadolinium, like naturally occurring porphyrins, selectively accumulates in tumors. (13) This agent enhances radiation-induced apoptosis, possibly via induction of futile redox cycling and depletion of intracellular antioxidants such as glutathione, ascorbate, lipoate and others. (13–15) A prior phase I study of MGd and radiation for intrinsic pontine gliomas showed good tolerability at a dose of 4.4 mg/kg/d, which was the dose used in this phase II study. (18)
In this study, the primary objective was to determine whether treatment with radiation therapy and concurrent Motexafin-Gadolinium would result in a one-year EFS rate higher than the historical baseline of 21.9%, which was derived from an exponential model from study CCG-9941. CCG-9941 was the most recent randomized group-wide study for diffuse pontine glioma, in which patients received one of two pre-radiation chemotherapy regimens followed by hyperfractionated radiation therapy to 72 Gy in twice-daily fractions of 1 Gy. (19) The estimated one-year EFS in ACNS0222 was 18% ± 5%; thus, the results of this study demonstrate no apparent survival advantage with the addition of MGd to standard radiation therapy for pediatric pontine glioma. The comparison of EFS between ACNS0222 and CCG-9941 is shown in Figure 2.
This study, like prior studies, failed to improve upon the results achieved by standard radiation therapy alone for intrinsic pontine gliomas. Several studies conducted by POG (Pediatric Oncology Group), CCG (Children’s Cancer Group), and COG (Children’s Oncology Group) between 1984 and 2005 investigated different approaches of intensifying therapy in an attempt to improve outcomes. (4–7, 18–22) These studies evaluated hyperfractionated radiation therapy and the addition of cytotoxic chemotherapy agents and radiosensitizing agents to radiation therapy. Patients enrolled on earlier trials (POG8495 (4,6) and CCG9882 (7, 20)) had higher estimated 2-year EFS (11 ± 2%) and 2-year OS (18 ± 2%) rates compared to patients enrolled on more recent trials after 1990 (2-yr EFS, 4 ± 1% and 2-yr OS, 9 ± 2%). This difference likely is due to inadvertent inclusion of patients with focal low-grade gliomas in the early studies in which CT rather than MRI may have been used for inclusion. The more recent studies (POG9836 (21), CCG9941 (19), A09712 (18), POG9239 (5) and ACNS0126 (22)) represent a more homogeneous population, based on imaging entry criteria. All 5 of these studies reported 2-year EFS rates below 10% with estimated 1-year EFS and OS rates of 14%±1.9% and 32%±2.6, respectively (estimated EFS and OS for the aforementioned studies determined by COG statistical office, personal communication).
In reporting the negative results of the diffuse intrinsic pontine glioma patients enrolled on ACNS0126, which investigated concurrent and adjuvant temozolomide with conventional radiation, Cohen and colleagues hypothesized that one explanation for the lack of efficacy of temozolomide in this disease may be that it fails to adequately penetrate the target tissue. (22) This hypothesis raises the possibility that despite an agent having good central nervous system penetrance, the ability to reach the pons/tumor may be impacted by local factors or by regional variation in the blood-brain barrier. In the prior phase I study of MGd and radiation for pontine gliomas, an MR analysis was performed and demonstrated that only 21% of scans demonstrated any enhancement at baseline. By day 30 of treatment, MGd uptake was seen in 52% of cases. (18) The increase in tumoral uptake of MGd suggests at least some penetration of target tissue; however, this did not translate into an improvement in outcome in this phase II study of MGd and radiation.
Since previous efforts to intensify or modulate the effects of local irradiation with investigational agents have not resulted in an improvement in outcome for children with intrinsic pontine gliomas, recent efforts focus on the therapeutic incorporation of molecularly targeted agents. (23–25) The ongoing study within the Children’s Oncology Group is evaluating vorinostat (suberoylanilide hydroxamic acid, SAHA), an oral histone deacetylase (HDAC) inhibitor that has been shown to inhibit growth of malignant gliomas and enhance radiation sensitivity of these tumors in preclinical studies. (26–29) SAHA is given concurrent to, and in maintenance therapy following, standard local irradiation for this disease. To aid in the selection of appropriate molecularly targeted agents, more knowledge of the biology of diffuse intrinsic pontine gliomas is needed. (30)
In summary, the use of concurrent Motexafin-Gadolinium with standard, once-daily radiation therapy did not result in an improvement in the event-free survival of pediatric patients with newly diagnosed diffuse pontine gliomas.
Acknowledgments
Research Supported by: COG Grant U10 CA98543-06.
FUNDING ACKNOWLEDGMENT
This research was supported by COG Grant CA 98543 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 2.Smith MA, Freidlin B, Ries LA, et al. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90:1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- 3.Laigle-Donadey F, Doz F, Delattre JY. Brainstem gliomas in children and adults. Current opinion in oncology. 2008;20:662–667. doi: 10.1097/CCO.0b013e32831186e0. [DOI] [PubMed] [Google Scholar]
- 4.Freeman CR, Krischer JP, Sanford RA, et al. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. International journal of radiation oncology, biology, physics. 1993;27:197–206. doi: 10.1016/0360-3016(93)90228-n. [DOI] [PubMed] [Google Scholar]
- 5.Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. International journal of radiation oncology, biology, physics. 1999;43:959–964. doi: 10.1016/s0360-3016(98)00501-x. [DOI] [PubMed] [Google Scholar]
- 6.Freeman CR, Krischer J, Sanford RA, et al. Hyperfractionated radiotherapy in brain stem tumors: results of a Pediatric Oncology Group study. International journal of radiation oncology, biology, physics. 1988;15:311–318. doi: 10.1016/s0360-3016(98)90010-4. [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group Phase I/II Trial. Cancer. 1993;72:1414–1421. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Walter AW, Gajjar A, Ochs JS, et al. Carboplatin and etoposide with hyperfractionated radiotherapy in children with newly diagnosed diffuse pontine gliomas: a phase I/II study. Med Pediatr Oncol. 1998;30:28–33. doi: 10.1002/(sici)1096-911x(199801)30:1<28::aid-mpo9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Michalski A, Bouffet E, Taylor RE, et al. The addition of high-dose tamoxifen to standard radiotherapy does not improve the survival of patients with diffuse intrinsic pontine glioma. J Neurooncol. 2010;100:81–88. doi: 10.1007/s11060-010-0141-9. [DOI] [PubMed] [Google Scholar]
- 10.Allen J, Siffert J, Donahue B, et al. A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer. 1999;86:1064–1069. doi: 10.1002/(sici)1097-0142(19990915)86:6<1064::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Marcus KJ, Dutton SC, Barnes P, et al. A phase I trial of etanidazole and hyperfractionated radiotherapy in children with diffuse brainstem glioma. International journal of radiation oncology, biology, physics. 2003;55:1182–1185. doi: 10.1016/s0360-3016(02)04391-2. [DOI] [PubMed] [Google Scholar]
- 12.Bernier-Chastagner V, Grill J, Doz F, et al. Topotecan as a radiosensitizer in the treatment of children with malignant diffuse brainstem gliomas: results of a French Society of Paediatric Oncology Phase II Study. Cancer. 2005;104:2792–2797. doi: 10.1002/cncr.21534. [DOI] [PubMed] [Google Scholar]
- 13.Hashemy SI, Ungerstedt JS, Zahedi Avval F, et al. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. The Journal of biological chemistry. 2006;281:10691–10697. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- 14.Lecane PS, Karaman MW, Sirisawad M, et al. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer research. 2005;65:11676–11688. doi: 10.1158/0008-5472.CAN-05-2754. [DOI] [PubMed] [Google Scholar]
- 15.Magda D, Lecane P, Miller RA, et al. Motexafin gadolinium disrupts zinc metabolism in human cancer cell lines. Cancer research. 2005;65:3837–3845. doi: 10.1158/0008-5472.CAN-04-4099. [DOI] [PubMed] [Google Scholar]
- 16.Miller RA, Woodburn K, Fan Q, et al. In vivo animal studies with gadolinium (III) texaphyrin as a radiation enhancer. International journal of radiation oncology, biology, physics. 1999;45:981–989. doi: 10.1016/s0360-3016(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, Zakian K, Thaler H, et al. Effects of Motexafin gadolinium on tumor metabolism and radiation sensitivity. International journal of radiation oncology, biology, physics. 2001;49:1381–1390. doi: 10.1016/s0360-3016(00)01566-2. [DOI] [PubMed] [Google Scholar]
- 18.Bradley KA, Pollack IF, Reid JM, et al. Motexafin gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: a Children’s Oncology Group phase I study. Neuro-oncology. 2008;10:752–758. doi: 10.1215/15228517-2008-043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennings MT, Sposto R, Boyett JM, et al. Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children’s Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:3431–3437. doi: 10.1200/JCO.2002.04.109. [DOI] [PubMed] [Google Scholar]
- 20.Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial. Cancer. 1994;74:1827–1834. doi: 10.1002/1097-0142(19940915)74:6<1827::aid-cncr2820740628>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatric blood & cancer. 2008;50:227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 22.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro-oncology. 2011;13:410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas-Kogan DA, Banerjee A, Kocak M, et al. Phase I trial of tipifarnib in children with newly diagnosed intrinsic diffuse brainstem glioma. Neuro-oncology. 2008;10:341–347. doi: 10.1215/15228517-2008-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broniscer A, Baker JN, Tagen M, et al. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol. 2010;28:4762–4768. doi: 10.1200/JCO.2010.30.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro-oncology. 2007;9:145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ugur HC, Ramakrishna N, Bello L, et al. Continuous intracranial administration of suberoylanilide hydroxamic acid (SAHA) inhibits tumor growth in an orthotopic glioma model. J Neurooncol. 2007;83:267–275. doi: 10.1007/s11060-007-9337-z. [DOI] [PubMed] [Google Scholar]
- 27.Chinnaiyan P, Vallabhaneni G, Armstrong E, et al. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005;62:223–229. doi: 10.1016/j.ijrobp.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 28.Yin D, Ong JM, Hu J, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13:1045–1052. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 29.Eyupoglu IY, Hahnen E, Buslei R, et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93:992–999. doi: 10.1111/j.1471-4159.2005.03098.x. [DOI] [PubMed] [Google Scholar]
- 30.Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28:1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]