Abstract
Leprosy (also known as Hansen’s disease) is an infectious peripheral neurological disorder caused by Mycobacterium leprae that even today leaves millions of individuals worldwide with life-long disabilities. The specific mechanisms by which this bacterium induces nerve injury remain largely unknown, mainly owing to ethical and practical limitations in obtaining affected human nerve samples. In addition to humans, nine-banded armadillos (Dasypus novemcinctus) are the only other natural host of M. leprae, and they develop a systemically disseminated disease with extensive neurological involvement. M. leprae is an obligate intracellular parasite that cannot be cultivated in vitro. Because of the heavy burdens of bacilli they harbor, nine-banded armadillos have become the organism of choice for propagating large quantities of M. leprae, and they are now advancing as models of leprosy pathogenesis and nerve damage. Although armadillos are exotic laboratory animals, the recently completed whole genome sequence for this animal is enabling researchers to undertake more sophisticated molecular studies and to develop armadillo-specific reagents. These advances will facilitate the use of armadillos in piloting new therapies and diagnostic regimens, and will provide new insights into the oldest known infectious neurodegenerative disorder.
Introduction
Nerve damage is the hallmark of leprosy. Diagnosis of the disease is based on clinical findings of anesthetic skin lesions or evidence of nerve damage in conjunction with the presence of Mycobacterium leprae. Peripheral nerves are affected early in the course of the disease. The unique ability of M. leprae to invade human peripheral nerves is the root cause of disfigurement and deformity in leprosy, and underlies the extreme social stigma and reduced quality of life that is generally associated with the disease (Scollard et al., 2006).
Antibiotic therapy can effectively cure leprosy. Multi-drug therapy (MDT) using a combination of rifampin, clofazimine and dapsone has sharply reduced the global burden of disease in the past 50 years, and only 228,474 new cases were reported during 2010 (http://www.who.int/lep/situation/en/). Unfortunately, however, MDT alone does not treat nerve damage, which is largely irreversible. Some 2- to 3-million people worldwide continue to suffer residual neurological affects of the disease, requiring lifelong management. The problem is more pronounced in highly endemic locales in Angola, Brazil, Central African Republic, Congo, India, Madagascar, Mozambique, Nepal and Tanzania (WHO, 2010).
Although M. leprae has been recognized as the causative agent of leprosy for more than a century, little is still known about the pathophysiology of the underlying nerve damage. It probably involves a complicated interplay of both host-inflammatory and bacterial-mediated events (Scollard, 2008; Wilder-Smith and Van Brakel, 2008). A major impediment to our understanding is obtaining suitable tissues for study. Leprotic lesions are highly focal and are usually distributed asymmetrically over the body (Nations and Barohn, 2002; Rodrigues and Lockwood, 2011), and ethical and practical limitations make it almost impossible to biopsy affected human nerves. When fresh samples can be obtained, they rarely contain a lesion, and those derived from amputated limbs are generally not suitable for detailed molecular analysis (Antunes et al., 2006). An effective animal model would greatly facilitate progress in this area, but most common laboratory animals (rat, rabbit, guinea pig, etc.) are naturally resistant to M. leprae. Although M. leprae does replicate when inoculated into the foot pads of mice, the infection remains localized to the foot (Shepard, 1960) and manifests without nerve involvement. Only the nine-banded armadillo reliably exhibits extensive neurological involvement following infection with M. leprae.
Armadillos have been used in medical research since the mid-1800s. Studies involving this animal have contributed to our knowledge of embryology (Storrs and Williams, 1968; Peppler and Stone, 1980), reproductive biology (D’Addamio et al., 1977; Peppler and Stone, 1980), immunology (Harboe et al., 1978; Guerra-Infante et al., 1996) and various infectious diseases, including syphilis and Chagas disease (Chaussinand and Besse, 1952; Wicher et al., 1983). However, the nine-banded armadillo has been most exploited as a model for leprosy (Peña et al., 2008; Scollard, 2008).
M. leprae manifests in armadillos as a systemically disseminated infection with similar structural and pathological changes as observed in tissues and nerves of humans with leprosy. Marked inflammation, with bacilli attached to the progressively demyelinating Schwann cells, can be observed on histopathological inspection of infected armadillo nerves, and a functional deficit can be demonstrated in leprotic nerves using electrophysiology (Scollard, 2008). As described below, infected armadillos closely recapitulate human leprosy, and they are therefore useful models for dissecting the underlying mechanisms of nerve damage and testing new therapeutic interventions.
Exploitation of the armadillo model requires careful description of the disease they manifest and the development of armadillo-specific reagents for modern molecular-based studies. The recently derived whole genome sequence (http://www.ncbi.nlm.nih.gov/genome/235) has provided an essential resource in this regard. The expression profile of genes associated with neuronal structure and function during peripheral nerve injury can now be discerned using M. leprae-infected armadillo nerves. Knowledge gained about the pathogenesis and prevention of nerve injury in leprosy might also be translatable to the study of other neurodegenerative diseases. In this Review, we briefly summarize some of the unique aspects of the armadillo that are relevant for modeling leprosy, especially with respect to neurodegeneration.
The armadillo: natural history and husbandry
This exotic-looking house-cat-sized animal is found only in the Americas and ranges from northern Argentina to the central United States. Armadillos can adapt to many diverse habitats, but are most abundant in low-lying and coastal areas. Their population has undergone extensive expansion in recent years (Taulman and Robbins, 1996). However, they do not hibernate and a poor tolerance of cold temperatures is the main factor limiting their range.
Their long lifespan (12 years) and cool body temperature (32–35°C – optimal for M. leprae) are the main physiological traits that first attracted the attention of leprosy researchers. However, armadillos also have other unusual characteristics, such as gestational diapause (whereby embryos do not implant for 4–5 months after fertilization) and polyembryony (always giving birth to monozygotic quadruplicate offspring). Although the animals do not breed reliably in captivity, gravid females taken from the wild will litter in captivity; the genetically identical offspring can be ideal models to study the role of host genetics and genomic factors in disease susceptibility (Misch et al., 2010).
Armadillos can be maintained by any laboratory that is capable of housing rabbits or other medium-sized mammals. We use modified rabbit cages with soft plastic flooring inserts and gang individual units together with a tunnel to separate the sleeping and feeding area from the litter pan side. A small plastic trash can with shredded paper functions as a sham burrow and enriches the environment.
Leprosy in armadillos
Susceptibility and natural infection
Leprosy lesions manifest mainly in cooler body regions, including the skin and mucous membranes of the upper respiratory tract (Yassin et al., 1975; Sato et al., 2007). For nearly a century, investigators attempted to grow M. leprae in various different animals with cool body temperatures (Couret, 1911). Following the discovery that the foot pads of mice (∼32°C) would support limited replication of M. leprae (Shepard, 1960), Kirchheimer and Storrs began experimentally infecting nine-banded armadillos (Kirchheimer and Storrs, 1971). The heavy burdens of bacilli that manifested in armadillos was a boon to leprosy research and made these animals the hosts of choice for propagating M. leprae. Concentrations of 109 to 1011M. leprae/gram of liver, spleen or lymph node are not uncommon among armadillos (Job, 2000; Truman et al., 2008).
Following the discovery that they were susceptible to M. leprae, a naturally occurring mycobacteriosis was found in free-ranging armadillos (Walsh et al., 1975). Subsequent studies confirmed that the causative agent was M. leprae, and showed that wild armadillos had harbored the infection for many decades before they were used in leprosy research (Truman et al., 1986). Exactly when armadillos became infected with M. leprae is still unclear. However, leprosy was introduced to the new world through colonization, and the animals must have acquired the infection from humans sometime in the last few hundred years. Notably, recent studies indicate that M. leprae can be transmitted zoonotically between humans and wild armadillos in the southern United States (Truman et al., 2011), and biomarkers of M. leprae infection have been reported among wild armadillos in Brazil, Colombia and Argentina (Truman, 2005; Truman and Fine, 2010).
Clinical manifestation
Armadillos show few overt signs of leprosy. Although susceptibility to lepromatous-type leprosy (affecting the skin) is a unique trait shared only by humans and armadillos, a large portion of the armadillo body is covered with armor, and skin lesions are not easily seen (Truman, 2008). Abrasions around the eyes, nose and feet are the most common signs but are also somewhat non-specific. In the laboratory, plantar ulceration is common in the later stages of infection (Fig. 1). Although M. leprae has no toxins and is not life-threatening in man, infected armadillos usually show profound anemia and compromised liver and renal functions, and eventually succumb to secondary complications of persistent bacteremia if not humanely sacrificed (Truman and Sanchez, 1993).
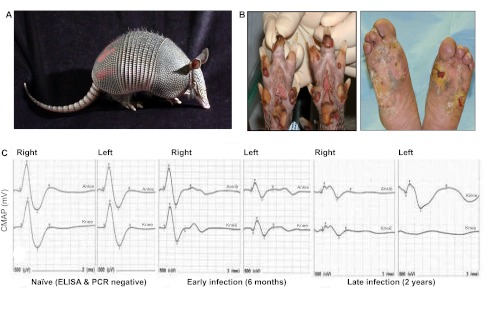
Fig. 1.
Armadillos are the only natural host of leprosy, aside from humans. (A) A nine-banded armadillo. (B) Similarly wounded feet of an armadillo in the late stage of leprosy (left) and a human leprosy patient (right). (C) Representative wave forms illustrating progressive nerve conduction deficit in compound motor action potential (CMAP; mV; y-axis) leading to complete conduction block in late infection. The upper lines show responses to stimulation at the ankle, and the lower ones to the knee.
Immunology and histopathology
In both humans and armadillos, leprosy exhibits a wide immunological and histopathological spectrum, ranging from the polar extremes of tuberculoid (TT) and lepromatous (LL), with three indistinct borderline forms in between (Ridley and Jopling, 1966). The TT form is characterized by few individual skin lesions with well-organized granulomas containing few bacilli, whereas the LL form manifests as numerous poorly organized, diffuse skin lesions with high numbers of bacilli (Walker and Lockwood, 2006). Nerve damage occurs with all forms of the disease (Grimaud and Vallat, 2003; Scollard, 2008). The response of each individual seems to be innate and can be predicted by intra-dermal injection of M. leprae antigens (lepromin) in a Mitsuda reaction (Mitsuda, 1919; Krotoski et al., 1993). Like humans, armadillos can exhibit the full spectrum of histopathological responses to M. leprae, but ∼70% of infected armadillos exhibit the LL form (Job and Truman, 1999). Because animals with the LL form are more likely to develop heavy burdens of bacilli, they are the ones commonly selected for in vivo propagation of M. leprae and have been studied most.
Leprosy neuropathy in armadillos
Neuropathy in leprosy is caused by the alteration of host signaling pathways by M. leprae that are localized to the peripheral nerves, as well as inflammatory and immunological responses to the infection. These effects lead to the demyelination of Schwann cells, resulting in abnormal neural conduction and axon degeneration (Rambukkana, 2010). In addition to clinical observations of focal anesthesia, impaired sensation, nerve thickening and motor dysfunction, various methods have been developed to study impaired neural function and structural damage in leprosy neuropathy.
Electrophysiology
Electrophysiology is a convenient, non-invasive means to assess the functional characteristics of peripheral nerves. Demyelination of axons results in decreased nerve conduction velocity (NCV), and loss of axons causes decreased compound motor action potential (CMAP) (Franssen, 2008). Although their hard carapace and thick skin limit the number of nerves that can be examined in armadillos, techniques have been adapted to permit assessment of conduction in both hind limbs along the armadillo posterior tibial (PT) nerve, which lies beneath the skin surface between the ankle and knee and innervates the small lumbrical and flexor muscles of each foot. Peripheral conduction deficit among M. leprae-infected armadillos begins early in the course of their disease and progresses over time (Fig. 1). Decreased CMAP amplitude (<0.9 mV) is most common, but abnormal NCV (<40 m/second) can also be observed in infected armadillos (Fig. 1). Approximately 75% of experimentally infected armadillos develop a demonstrable conduction deficit in these PT nerves after infection; the onset of the deficit seems to coincide with the evolution of a detectable immune response to M. leprae [generally detectable IgM antibodies to phenolic glycolipid-1 (PGL1), considered a specific antigen for M. leprae]. In observations of more than 175 different armadillos, an increased anti-PGL1 antibody level was significantly correlated with decreased CMAP among the infected animals (r=−0.3058, P<0.003) (R.L. and R.W.T., unpublished data). Nearly all of the animals that developed a conduction deficit also eventually exhibited signs of clinical neuropathy in their foot pads. Increased anti-PGL1 antibody levels and decreased CMAP were also highly correlated with the clinical appearance of wounds under heavy calluses, hypertrophic nails (P<0.03) and nail avulsion (P<0.008, r=0.2–0.26). In addition, the flexor and lumbrical muscles of infected armadillos were atrophied, as evidenced by a 20% average decrease in physiological cross-sectional areas (P<0.02) compared with naïve animals (R.W.T., unpublished data). These data indicate that the lower extremity of armadillos becomes heavily involved with M. leprae and exhibits many of the same abnormalities seen in humans with leprosy. Sensory nerve conduction tests and F-wave analysis need further development.
Morphometry
The myelin sheath is crucial for maintaining nerve function, and M. leprae is known to damage this protective layer (Scollard, 2008). Similar to human LL cases, infected armadillo nerves demonstrate a heavy infiltration of M. leprae-loaded nucleated cells (mostly macrophages) in many fascicles of nerve trunks. In addition, by electron microscopy, M. leprae can be seen invading progressively demyelinating Schwann cells (Scollard, 2008) (Fig. 2). For quantitative studies of demyelination in infected armadillo nerves, myelinated fiber morphometry can be used to score the number, distribution, myelin area, axon area (de Blaquiere et al., 1994) and other physical parameters of myelinated fibers in 1-μm cross-sections of nerve trunks (Romero et al., 2000). Interestingly, studies of distal segments of PT nerves from infected armadillos in which electrophysiological abnormalities had been demonstrated showed only slight variations from normal, with no definite or statistically significant patterns of nerve fiber abnormality (D.M.S., unpublished data). These results vary from those reported for amputated fibers from humans with leprosy. However, it seems likely that the armadillo nerves studied were from animals that were at an earlier stage of pathogenesis of nerve injury than can be observed in and/or obtained from human leprosy patients.
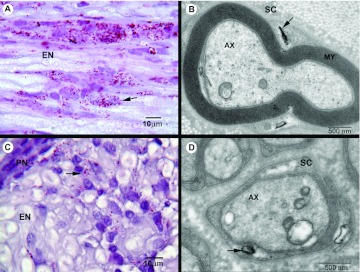
Fig. 2.
Inflammation,M. leprae infiltration and demyelination in infected armadillo PT nerve. (A) Longitudinal section showing a large number of acid-fast bacilli (AFB; arrow) in endoneurium (EN) of nerve. (B) Electron micrograph of a myelinated Schwann cell (SC) infected with M. leprae (arrow). AX, axon; MY, myelin sheath. (C) Cross-section of an infected nerve showing infiltration of M. leprae (arrow) in EN and perinurium (PN), as well as an infiltrate of mononuclear cells at the site of infection. (D) M. leprae (arrow) infecting non-myelinated Schwann cells in infected armadillo nerve.
Applying armadillos for the development of early diagnostics, therapeutics and vaccines
Although therapy is available, earlier diagnosis of leprosy could improve the quality of life after treatment. In addition, an effective vaccine is urgently needed to prevent leprosy transmission (Duthie et al., 2011). Since the 1960s, potential leprosy vaccine candidates have been tested in conventional laboratory mice (Shepard, 1966). However, as noted above, mice are naturally resistant to M. leprae infection, so results from mouse studies are of limited translational value (Shepard, 1965). The armadillo is the only animal model in which protection against dissemination of leprosy bacilli or progress of nerve damage can be evaluated. Bacterins of heat-killed M. leprae or viable BCG have been shown to protect armadillos against M. leprae challenge or enhance their immunity to the organism (Kirchheimer et al., 1978). Armadillos can also be applied for testing new diagnostic candidates because they are the only host in which the true status of infection can be determined, and the long incubation period of leprosy in humans can cause confounding results.
Armadillos also hold potential for the testing of leprosy therapies. For example, it will soon be possible to prospect for new leprosy drugs or interventions using end-points such as sustained viability of the inoculated bacilli or altered gene expression profile of M. leprae-infected armadillo nerves. Among the most interesting drug candidates are anti-neuropathic agents that are currently under development for the treatment of neural complications related to diabetes, HIV or cancer. Understanding the effectiveness of new therapies in a disease model alternative to human patients and the mouse footpad will also provide information on the pathogenic mechanisms involved in other neuropathic diseases, and might benefit further development of therapies for those conditions.
To test vaccines, diagnostics or drugs, armadillos can be infected with M. leprae by various routes. Although intravenous challenge is used most commonly for in vivo propagation of M. leprae, this route takes nearly 2 years to establish the infection and for clinical manifestation of leprosy. However, respiratory instillation, intradermal inoculation and intra-neural injection of bacilli are also effective means to establish infection in the armadillo. Depending on the compartment or disease phenotype of interest, armadillos can be infected by different routes to more rapidly manifest focal infections. Leprotic granulomas can be established within only a few weeks via direct injection of bacilli into armadillo skin or nerves; such short-term infections can be particularly useful for rapid screening of potential new drugs or therapies before committing to longer-term trials.
Post-genomic advances
Armadillos are the most abundant xenarthran, and Dasypus novemcinctus was one of 24 species selected for the Mammalian Genome Project (http://www.broadinstitute.org/scientific-community/science/projects/mammals-models/29-mammals-project) at Baylor College of Medicine (BCM) and the Broad Institute. All sequence reads are deposited in the National Center for Biotechnology Information (NCBI) trace archive, and assemblies, scaffolds and BLAST access will be hosted by the BCM Human Genome Sequencing Center; these will also be available in relevant public databases, such as NCBI and Ensemble. A 2X assembly has now been extended to a 6X level for use in cross-species comparison (Lindblad-Toh et al., 2011). Although annotation of the sequence is only at a preliminary stage, with only nine known genes and 8116 gene-scaffolds identified (https://www.hgsc.bcm.edu/content/armadillo-genome-project), the available sequence has significantly benefited efforts to advance the armadillo model.
Genomic diversity and disease susceptibility
The newly available genome sequence data has been used to study the genetic diversity of nine-banded armadillos in Mexico (Arteaga et al., 2012) and the United States, and to examine the influence of climatic niches on the gene flow (Arteaga et al., 2011). Genomic polymorphisms have been employed to estimate paternity and maternity in armadillos (Prodöhl et al., 1998), and examination of expressed sequence tags will probably yield further insight into pregnancy control and the physiological basis of polyembryony and diapause (Lynch et al., 2011).
Several single nucleotide polymorphisms (SNPs) have been associated with human susceptibility to M. leprae infection (Misch et al., 2010). Armadillos also show variable responses to M. leprae infection, and the availability of genome sequence data has enabled the identification of corresponding SNPs and analyses of their association with disease progression. For example, a survey of Toll-like receptor (TLR) polymorphism in armadillos found that a nonsynonymous polymorphism [A1879G (R627G) in TLR1] was significantly associated with resistance to leprosy (R.S., unpublished data). Although many other polymorphisms in TLR1, TLR2 and TLR4 have been found to be associated with leprosy susceptibility and manifestation in humans (Hawn et al., 2007; Misch et al., 2008) and are present at similar positions in armadillos, their association with susceptibility to infection was not statistically significant. A relatively small percentage of armadillos are resistant to M. leprae, and large sample sizes might be needed for conclusive genetic analysis. Genome-wide SNP linkage analysis and melting-curve-based assays have been used effectively to identify new SNPs in human studies (Misch et al., 2008; Yang et al., 2012), and the application of similar approaches might be possible with armadillos once the genome is fully annotated.
Gene expression
Microarrays have been widely used for genome-wide expression studies, but microarrays specific for armadillos have yet to be developed. However, cross-species hybridization using human gene chips has identified a total of 1546 differentially regulated genes in the nerves of infected armadillos. Upregulation of genes associated with inflammation, degeneration and regeneration activities in infected armadillo nerves (R.S., unpublished data) occurs on spinal cord (Bareyre and Schwab, 2003) and peripheral nerve (Gillen et al., 1995) injury. Further study of the molecular mechanisms of nerve damage using other methods in armadillos has been limited because of the lack of armadillo-specific antibodies, but it should be possible to overcome this limitation soon.
The recently derived whole genome sequence of the armadillo also enables the design of armadillo-specific quantitative PCR assays. Using these assays, genes associated with inflammation [e.g. those encoding cytokines such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α)] and constitutively expressed neuro-proteins (such as PGP9.5, β-tubulin and neurofilament) were found to be upregulated in affected armadillo nerves. In contrast, growth factors required for regeneration of the peripheral nervous system (such as DLK-1 and NGF-β) were found to be downregulated in affected nerves; such alterations might prevent the regeneration of peripheral nerve tissue in leprosy. Following the alterations in gene expression patterns could be useful for monitoring the effectiveness of neuro-regenerative or protective agents for treating nerve injury.
Development of armadillo-specific reagents
Antibodies for armadillo molecular markers are required for in vivo and in vitro studies of ultrastructural changes in the affected nerves using immunohistochemistry; the development of such reagents was limited until recently owing to the unavailability of the genomic sequence. Now that the draft sequence is available, a large number of armadillo-specific antibodies for cytokines, immunological markers and neurological markers are being developed (Adams et al., 2005; Peña et al., 2008). Once the armadillo genome is fully annotated and available in a browsable format (similar to those for human, mouse and other common animal models), new antibodies and other armadillo-specific regents will rapidly become available.
Conclusions and future prospects
As reviewed here, M. leprae infection of the armadillo closely recapitulates features of human leprosy and is the best available model of early nerve injury in this disease. Although immunological and molecular reagents (such as recombinant antigens, antibodies or pre-designed gene expression arrays) are not yet readily available, the armadillo genome sequence (6X coverage) has enabled molecular studies and is facilitating efforts to develop armadillo-specific research reagents (Peña et al., 2008). Armadillos were established as the host of choice for in vivo propagation of leprosy bacilli; more recently, they have enabled steps forward in our understanding of leprosy pathogenesis. As the only animal host that develops neurological involvement with M. leprae, they are uniquely suited for studying the underlying mechanism of nerve injury and piloting new interventions. The use of armadillos in leprosy research will continue to expand our understanding of this disease, as well as that of other neurological disorders.
Acknowledgments
We thank Kyle Andrews, Anitria Carter, Thomas P. Gillis, Dave Giurintano, Kim Gusman, Michael T. Kearney, James L. Krahenbuhl, Greg McCormick, Roena Stevenson and Heidi Zhang. We thank Disease Models & Mechanisms for Traveling Fellowships to visit the Center for Regenerative Medicine, University of Edinburgh, Edinburgh, Scotland, UK.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any financial or competing interests.
FUNDING
This work is supported by the Department of Health and Humans Services, Health Resources and Services Administration’s, National Hansen’s Disease Program, and the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID IAA-2646).
REFERENCES
- Adams J. E., Peña M. T., Gillis T. P., Williams D. L., Adams L. B., Truman R. W. (2005). Expression of nine-banded armadillo (Dasypus novemcinctus) interleukin-2 in E. coli. Cytokine 32, 219–225 [DOI] [PubMed] [Google Scholar]
- Antunes S. L. G., Chimelli L. M., Rabello E. T., Valentim V. C., Corte-Real S., Sarno E. N., Jardim M. R. (2006). An immunohistochemical, clinical and electroneuromyographic correlative study of the neural markers in the neuritic form of leprosy. Braz. J. Med. Biol. Res. 39, 1071–1081 [DOI] [PubMed] [Google Scholar]
- Arteaga M. C., McCormack J. E., Eguiarte L. E., Medellín R. A. (2011). Genetic admixture in multidimensional environmental space: asymmetrical niche similarity promotes gene flow in armadillos (Dasypus novemcinctus). Evolution 65, 2470–2480 [DOI] [PubMed] [Google Scholar]
- Arteaga M. C., Piñero D., Eguiarte L. E., Gasca J., Medellín R. A. (2012). Genetic structure and diversity of the nine-banded armadillo in Mexico. J. Mammal. 93, 547–559 [Google Scholar]
- Bareyre F. M., Schwab M. E. (2003). Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci. 26, 555–563 [DOI] [PubMed] [Google Scholar]
- Chaussinand R., Besse P. (1952). Inoculation of Hansen and Stefansky bacilli in the rainbow perch Eupomotis gibbosus. Preliminary note. (Abstract). Int. J. Lepr. Other Mycobact. Dis. 20, 420–421 [Google Scholar]
- Couret M. (1911). The behavior of Bacillus leprae in cold-blooded animals. J. Exp. Med. 13, 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addamio G. H., Roussel J. D., Storrs E. E. (1977). Response of the nine-banded armadillo (Dasypus novemcinctus) to gonadotropins and steroids. Lab. Anim. Sci. 27, 482–489 [PubMed] [Google Scholar]
- de Blaquiere G. E., Curtis J., Pereira J. H., Turk J. L. (1994). Denatured muscle grafts for nerve repair in an experimental model of nerve damage in leprosy. 1. A functional and morphometric study. Int. J. Lepr. Other Mycobact. Dis. 62, 55–63 [PubMed] [Google Scholar]
- Duthie M. S., Gillis T. P., Reed S. G. (2011). Advances and hurdles on the way toward a leprosy vaccine. Hum. Vaccin. 7, 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H. (2008). Electrophysiology in demyelinating polyneuropathies. Expert Rev. Neurother. 8, 417–431 [DOI] [PubMed] [Google Scholar]
- Gillen C., Gleichmann M., Spreyer P., Müller H. W. (1995). Differentially expressed genes after peripheral nerve injury. J. Neurosci. Res. 42, 159–171 [DOI] [PubMed] [Google Scholar]
- Grimaud J., Vallat J. M. (2003). [Neurological manifestations of leprosy]. Rev. Neurol. (Paris) 159, 979–995 [PubMed] [Google Scholar]
- Guerra-Infante F., Quesada-Pascual F., Estrada-Parra S., Santos-Argumedo L. (1996). Evolution of lymphocyte populations in armadillos (Dasypus novemcinctus) inoculated with M. leprae. Int. J. Lepr. Other Mycobact. Dis. 64, 152–158 [PubMed] [Google Scholar]
- Harboe M., Closs O., Rees R. J., Walsh G. P. (1978). Formation of antibody against Mycobacterium leprae antigen 7 in armadillos. J. Med. Microbiol. 11, 525–535 [DOI] [PubMed] [Google Scholar]
- Hawn T. R., Misch E. A., Dunstan S. J., Thwaites G. E., Lan N. T., Quy H. T., Chau T. T., Rodrigues S., Nachman A., Janer M., et al. (2007). A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur. J. Immunol. 37, 2280–2289 [DOI] [PubMed] [Google Scholar]
- Job C. K. (2000). Developments in experimental leprosy. Indian J. Lepr. 72, 143–154 [PubMed] [Google Scholar]
- Job C. K., Truman R. W. (1999). Mitsuda-negative, resistant nine-banded armadillos and enhanced Mitsuda response to live M. leprae. Int. J. Lepr. Other Mycobact. Dis. 67, 475–477 [PubMed] [Google Scholar]
- Kirchheimer W. F., Storrs E. E. (1971). Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of lepromatoid leprosy in an experimentally infected armadillo. Int. J. Lepr. Other Mycobact. Dis. 39, 693–702 [PubMed] [Google Scholar]
- Kirchheimer W. F., Sanchez R. M., Shannon E. J. (1978). Effect of specific vaccine on cell-mediated immunity of armadillos against M. leprae. Int. J. Lepr. Other Mycobact. Dis. 46, 353–357 [PubMed] [Google Scholar]
- Krotoski W. A., Mroczkowski T. F., Rea T. H., Almodovar P. I., Clements B. C., Neimes R. E., Kahkonen M. K., Job C. K., Hastings R. C. (1993). Lepromin skin testing in the classification of Hansen’s disease in the United States. Am. J. Med. Sci. 305, 18–24 [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K., Garber M., Zuk O., Lin M. F., Parker B. J., Washietl S., Kheradpour P., Ernst J., Jordan G., Mauceli E., et al. (2011). A high-resolution map of human evolutionary constraint using 29 mammals. Nature 478, 476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch V. J., Leclerc R. D., May G., Wagner G. P. (2011). Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat. Genet. 43, 1154–1159 [DOI] [PubMed] [Google Scholar]
- Misch E. A., Macdonald M., Ranjit C., Sapkota B. R., Wells R. D., Siddiqui M. R., Kaplan G., Hawn T. R. (2008). Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl. Trop. Dis. 2, e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misch E. A., Berrington W. R., Vary J. C., Jr, Hawn T. R. (2010). Leprosy and the human genome. Microbiol. Mol. Biol. Rev. 74, 589–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda K. (1919). On the value of skin reaction with emulsion of leproma. Jpn. J. Dermatol. Urol. 19, 697–708 [Google Scholar]
- Nations S. P., Barohn R. J. (2002). Peripheral neuropathy due to leprosy. Curr. Treat. Options Neurol. 4, 189–196 [DOI] [PubMed] [Google Scholar]
- Peña M. T., Adams J. E., Adams L. B., Gillis T. P., Williams D. L., Spencer J. S., Krahenbuhl J. L., Truman R. W. (2008). Expression and characterization of recombinant interferon gamma (IFN-gamma) from the nine-banded armadillo (Dasypus novemcinctus) and its effect on Mycobacterium leprae-infected macrophages. Cytokine 43, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler R. D., Stone S. C. (1980). Plasma progesterone level during delayed implantation, gestation and postpartum period in the armadillo. Lab. Anim. Sci. 30, 188–191 [PubMed] [Google Scholar]
- Prodöhl P. A., Loughry W. J., McDonough C. M., Nelson W. S., Thompson E. A., Avise J. C. (1998). Genetic maternity and paternity in a local population of armadillos assessed by microsatellite DNA markers and field data. Am. Nat. 151, 7–19 [DOI] [PubMed] [Google Scholar]
- Rambukkana A. (2010). Usage of signaling in neurodegeneration and regeneration of peripheral nerves by leprosy bacteria. Prog. Neurobiol. 91, 102–107 [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. (1966). Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34, 255–273 [PubMed] [Google Scholar]
- Rodrigues L. C., Lockwood D. N. (2011). Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect. Dis. 11, 464–470 [DOI] [PubMed] [Google Scholar]
- Romero E., Cuisenaire O., Denef J. F., Delbeke J., Macq B., Veraart C. (2000). Automatic morphometry of nerve histological sections. J. Neurosci. Methods 97, 111–122 [DOI] [PubMed] [Google Scholar]
- Sato N., Fujimura T., Masuzawa M., Yogi Y., Matsuoka M., Kanoh M., Riley L. W., Katsuoka K. (2007). Recombinant Mycobacterium leprae protein associated with entry into mammalian cells of respiratory and skin components. J. Dermatol. Sci. 46, 101–110 [DOI] [PubMed] [Google Scholar]
- Scollard D. M. (2008). The biology of nerve injury in leprosy. Lepr. Rev. 79, 242–253 [PubMed] [Google Scholar]
- Scollard D. M., Adams L. B., Gillis T. P., Krahenbuhl J. L., Truman R. W., Williams D. L. (2006). The continuing challenges of leprosy. Clin. Microbiol. Rev. 19, 338–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C. (1960). The experimental disease that follows the injection of human leprosy bacilli into foot-pads of mice. J. Exp. Med. 112, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C. (1965). Vaccination against experimental infection with Mycobacterium leprae. Am. J. Epidemiol. 81, 150–163 [DOI] [PubMed] [Google Scholar]
- Shepard C. C. (1966). Vaccination against human leprosy bacillus infections of mice: protection by BCG given during the incubation period. J. Immunol. 96, 279–283 [PubMed] [Google Scholar]
- Storrs E. E., Williams R. J. (1968). A study of monozygous quadruplet armadillos in relation to mammalian inheritance. Proc. Natl. Acad. Sci. USA 60, 910–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulman J. F., Robbins L. W. (1996). Recent range expansion and distributional limits of the nine-banded armadillo (Dasypus novemcinctus) in the United States. J. Biogeogr. 23, 635–648 [Google Scholar]
- Truman R. (2005). Leprosy in wild armadillos. Lepr. Rev. 76, 198–208 [PubMed] [Google Scholar]
- Truman R. W. (2008). Leprosy. In The Biology of the Xenarthra (ed. Vizcaíno S. F., Loughry W. J.), pp. 111–119 Gainesville, FL: University Press of Florida [Google Scholar]
- Truman R., Fine P. E. (2010). ‘Environmental’ sources of Mycobacterium leprae: issues and evidence. Lepr. Rev. 81, 89–95 [PubMed] [Google Scholar]
- Truman R. W., Sanchez R. M. (1993). Armadillos: models for leprosy. Lab. Anim. 22, 28–32 [Google Scholar]
- Truman R. W., Shannon E. J., Hagstad H. V., Hugh-Jones M. E., Wolff A., Hastings R. C. (1986). Evaluation of the origin of Mycobacterium leprae infections in the wild armadillo, Dasypus novemcinctus. Am. J. Trop. Med. Hyg. 35, 588–593 [DOI] [PubMed] [Google Scholar]
- Truman R. W., Andrews P. K., Robbins N. Y., Adams L. B., Krahenbuhl J. L., Gillis T. P. (2008). Enumeration of Mycobacterium leprae using real-time PCR. PLoS Negl. Trop. Dis. 2, e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman R. W., Singh P., Sharma R., Busso P., Rougemont J., Paniz-Mondolfi A., Kapopoulou A., Brisse S., Scollard D. M., Gillis T. P., et al. (2011). Probable zoonotic leprosy in the southern United States. N. Engl. J. Med. 364, 1626–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. L., Lockwood D. N. (2006). The clinical and immunological features of leprosy. Br. Med. Bull. 77–78, 103–121 [DOI] [PubMed] [Google Scholar]
- Walsh G. P., Storrs E. E., Burchfield H. P., Cotrell E. H., Vidrine M. F., Binford C. H. (1975). Leprosy-like disease occurring naturally in armadillos. J. Reticuloendothel. Soc. 18, 347–351 [PubMed] [Google Scholar]
- WHO (2010). Leprosy Today. Geneva, Switzerland: WHO [Google Scholar]
- Wicher K., Kalinka C., Walsh G. P. (1983). Attempt to infect the nine-banded armadillo with Treponema pallidum. Int. Arch. Allergy Appl. Immunol. 70, 285–287 [DOI] [PubMed] [Google Scholar]
- Wilder-Smith E. P., Van Brakel W. H. (2008). Nerve damage in leprosy and its management. Nat. Clin. Pract. Neurol. 4, 656–663 [DOI] [PubMed] [Google Scholar]
- Yang Q., Liu H., Low H. Q., Wang H., Yu Y., Fu X., Yu G., Chen M., Yan X., Chen S., et al. (2012). Chromosome 2p14 is linked to susceptibility to leprosy. PLoS ONE 7, e29747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin A., el Shennawy M., el Enany G., Wassef N. F., Shoeb S. (1975). Leprosy of the upper respiratory tract. A clinical bacteriological, histopathological and histochemical study of twenty cases. J. Laryngol. Otol. 89, 505–511 [DOI] [PubMed] [Google Scholar]