SUMMARY
Neural stimulation can reduce the frequency of seizures in persons with epilepsy, but rates of seizure-free outcome are low. Vagus nerve stimulation prevents seizures by continuously activating noradrenergic projections from the brainstem to the cortex. Cortical norepinephrine then increases GABAergic transmission and increases seizure threshold. Another approach, responsive nervous stimulation, prevents seizures by reactively shocking the seizure onset zone in precise synchrony with seizure onset. The electrical shocks abort seizures before they can spread and manifest clinically. The goal of this study was to determine whether a hybrid platform in which brainstem activation triggered in response to impending seizure activity could prevent seizures. We chose the zebrafish as a model organism for this study because of its ability to recapitulate human disease, in conjunction with its innate capacity for tightly controlled high-throughput experimentation. We first set out to determine whether electrical stimulation of the zebrafish hindbrain could have an anticonvulsant effect. We found that pulse train electrical stimulation of the hindbrain significantly increased the latency to onset of pentylenetetrazole-induced seizures, and that this apparent anticonvulsant effect was blocked by noradrenergic antagonists, as is also the case with rodents and humans. We also found that the anticonvulsant effect of hindbrain stimulation could be potentiated by reactive triggering of single pulse electrical stimulations in response to impending seizure activity. Finally, we found that the rate of stimulation triggering was directly proportional to pentylenetetrazole concentration and that the stimulation rate was reduced by the anticonvulsant valproic acid and by larger stimulation currents. Taken as a whole, these results show that that the anticonvulsant effect of brainstem activation can be efficiently utilized by reactive triggering, which suggests that alternative stimulation paradigms for vagus nerve stimulation might be useful. Moreover, our results show that the zebrafish epilepsy model can be used to advance our understanding of neural stimulation in the treatment of epilepsy.
INTRODUCTION
A seizure is a sustained and synchronous elevation in brain electrical activity that can result in loss of consciousness and injury. Epilepsy is the condition of having recurrent and unprovoked seizures. Upwards of 0.5% of the general population suffers from epilepsy, or an estimated 60 million people worldwide (Hauser et al., 1991). Up to 50% of the people with epilepsy will continue to have seizures despite being given adequate trials of appropriately selected antiepileptic drugs (Shorvon, 2007). Medication refractory epilepsy (MRE) results in higher than expected rates of mortality (Zielinski, 1974), cognitive impairment (Aldenkamp, 2006) and psychosocial dysfunction (Pershad and Siddiqui, 1992) at a cost to the US economy of more than $12 billion annually (Begley et al., 1994). Therefore, the development of new treatment modalities for MRE is prudent.
Neural stimulation therapy is a viable adjunct to the treatment of MRE (Cascino, 2008). Currently, two modes of neural stimulation for treatment of epilepsy are in widespread use: vagus nerve stimulation (VNS) and responsive nervous stimulation (RNS). VNS was inspired by reports in the early 1900s that carotid sinus massage could in some cases terminate ongoing seizure activity, presumably by inducing retrograde vagus nerve activation (Salanova and Worth, 2007; Ghaemi et al., 2010). Stimulation of the left vagus nerve in the neck as therapy for epilepsy first came to the US market in 1997 (Cyberonics, Houston, TX). VNS appears to have an anticonvulsant effect by retrograde activation of the locus coeruleus (LC) in the brainstem (Groves et al., 2005), which results in elevated levels of norepinephrine in the cortex (Hasselmo, 1995; Krahl et al., 1998; Mamedov, 1993; Lim et al., 2005; Roosevelt et al., 2006; Follesa et al., 2007). Cortical norepinephrine ultimately facilitates γ-aminobutyric acid-producing (GABAergic) transmission, which increases seizure threshold (Barry et al., 1989; Nai et al., 2009). The VNS device is typically preconfigured to deliver electrical stimulations at a scheduled rate independently of perceived seizure activity, although patients and bystanders are also able to manually activate the device in response to seizures by swiping a magnet over the chest implant site.
The RNS device has been in clinical trials for several years. The company (Neuropace, Mountain View, CA) sought FDA approval in 2010 for use in patients with MRE. Although the mechanism of RNS is not precisely known, RNS is thought to work by delivering an electrical impulse directly to the seizure onset zone, which results in termination of seizure propagation (Osorio et al., 2001; Motamedi et al., 2002; Anderson et al., 2008). Traditionally, the RNS delivers electrical stimulation in response to newly initiated seizure activity and not on a scheduled basis, although scheduled stimulation paradigms have also proven effective.
TRANSLATIONAL IMPACT.
Clinical issue
Medication-refractory epilepsy affects at least 0.25% of the general population. The prevalence of medication-refractory epilepsy remains unchanged despite a recent surge in antiepileptic drug development. Neural stimulation is a viable treatment option for some people with medication refractory epilepsy, but it does not increase rates of seizure freedom. Currently, two modalities of neural stimulation are in widespread use for treatment of epilepsy: vagus nerve stimulation and responsive neural stimulation. Vagus nerve stimulation exerts an anticonvulsant effect by retrograde activation of brainstem relays that increase cortical norepinephrine levels and attenuate seizures. Responsive neural stimulation prevents seizures by delivering electrical shocks directly to the seizure onset zone in precise synchrony with seizure onset. The goal of this study was to test a hybrid system in which brain stem activation is triggered reactively in response to impending seizure activity.
Results
The authors report that non-triggered stimulation of the zebrafish hindbrain significantly delayed the onset of pentylenetetrazole (PTZ)-induced seizures by a mechanism that was dependent on α-noradrenergic mechanisms. They also found that statistical properties of the zebrafish cerebral field potential could be used to anticipate the occurrence of a seizure and trigger stimulations reactively. Finally, they found that reactive triggering of hindbrain stimulation could stave off (PTZ)-induced seizures indefinitely. Stimulation rates varied predictably with PTZ concentration and stimulator current magnitude.
Implications and future directions
These results indicate that combining the hindbrain-activating properties of vagus nerve stimulation with the reactive triggering of responsive neural stimulation results in a ‘demand-triggered’ stimulation paradigm that might have greater utility than either modality alone. They also support a potential role for reactive vagus nerve stimulation in the treatment of epilepsy. Moreover, these results indicate that zebrafish cerebral field potential is of sufficient complexity as to be amenable to analysis by seizure anticipation algorithms intended for use in humans. Together with the capacity to undertake tightly controlled and high-throughput experimentation in zebrafish, this study should promote development in the field of seizure anticipation. Finally, these results suggest that the hindbrain-forebrain noradrenergic circuitry is of sufficient density in the adult zebrafish to serve an anticonvulsant effect, which means that the zebrafish model might be useful for studying other aspects in which noradrenergic circuitry is involved, such as learning, memory and behavior.
In this study, we combine the brainstem noradrenergic pathway utilized by VNS with the reactive triggering paradigm utilized by RNS using the zebrafish (Danio rerio) epilepsy model. First, we examined whether or not stimulation of the hindbrain in the vicinity of the LC could attenuate seizures in the zebrafish, as it does in rodent and human. Next, we examined whether or not the anticonvulsant effect of hindbrain stimulation could be invoked and sustained by reactive triggering based on statistical properties of the cerebral field potential, which is the zebrafish equivalent to the human and rodent electroencephalogram (EEG). Finally, we determined whether the rate of hindbrain stimulation could be affected by varying the concentration of proconvulsants, by the addition of anticonvulsants and by changing the stimulator current amplitude and duration. The net result of this study is a fully functioning neural stimulation paradigm for the zebrafish, and indirect evidence that the reactive triggering paradigm of RNS might be efficacious at preventing seizures when combined with the brainstem activating properties of VNS.
RESULTS
Anticonvulsant effect of hindbrain stimulation
In rodents, activation of noradrenergic efferent neurons in the LC results in α-noradrenergic facilitation of cortical GABA-B receptors, which results in neuronal inhibition and a resistance to seizures. Although the zebrafish brain lacks a neocortex and formal hippocampus, the equivalent forebrain structures (posterior and lateral pallium) receive noradrenergic afferents from the zebrafish LC (Ma, 1994a; Ma, 1994b; Ma, 1997). Pentylenetetrazole (PTZ) is a proconvulsant that inhibits GABA-A receptors, which results in uncompensated neuronal excitation and seizures (Jim et al., 1989; Bloms-Funke et al., 1996; Huang et al., 2001). Intuitively then, stimulation of the zebrafish hindbrain in the vicinity of the LC could attenuate PTZ-induced seizures.
To test this hypothesis, we compared the latency to onset of behavioral seizure activity in fish that received electrical hindbrain stimulation to the latency in fish that did not. Specifically, fish in the experimental group were anesthetized with eugenol and immobilized using previously described methods (Pineda et al., 2011). Next, a stimulator wire was inserted into the hindbrain and electrical stimulation was applied. The fish were then released and allowed to recover from anesthesia and resume full swimming activity. Next, the fish were placed in a bath containing 15 mM PTZ and the time to onset of seizure activity was measured visually to the nearest second (supplementary material Movie 1). PTZ delivery to the CNS is by way of movement across the gills and into the bloodstream. Drug delivery by immersion is very efficient in fish and is the most common route of medication delivery in fisheries (Hunn and Allen, 1974; Neiffer and Stamper, 2009; Stewart et al., 2012). Fish in the control group underwent exactly the same protocol except that electrical stimulation was not applied after stimulator wire insertion.
The onset latency to behavioral seizure activity was significantly elevated in fish that received hindbrain stimulation compared with fish that received electrode insertion surgery but no stimulation (Fig. 1). Also, the anticonvulsant effect of hindbrain stimulation was partially blocked by pretreatment with the nonselective α-noradrenergic antagonist phenoxybenzamine (POB) (Fig. 1). Cumulatively, these results suggest that hindbrain stimulation in the vicinity of the LC promotes resistance to seizures by noradrenergic mechanisms in zebrafish, just as it does in rodents and human.
Fig. 1.
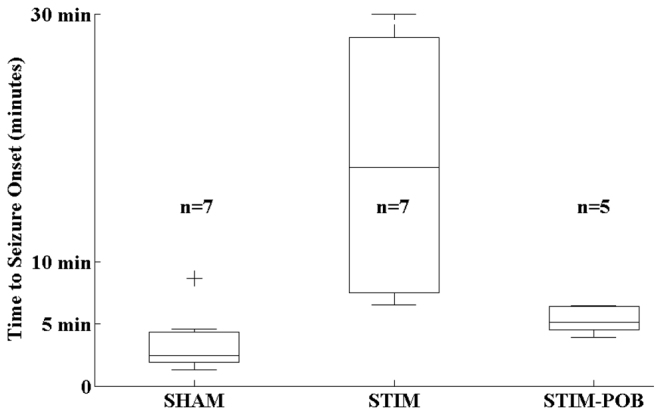
Latency to onset of Stage III behavioral seizure activity. Onset of Stage III behavioral seizure activity was measured in response to 15 mM PTZ in fish that had stimulator wire insertion and no stimulation (SHAM), stimulation (STIM) or stimulation in the presence of 25 μM POB. Hindbrain stimulation increases seizure onset latency. The effect was partially blocked by the α-noradrenergic antagonist POB. Bars indicate the data median. Boxes and whiskers indicate the 25th to 75th and 5th to 95th percentiles, respectively. Crosses indicate outliers.
Entropy changes in the cerebral field potential at seizure onset
To develop our hybrid neural stimulator, we needed to find a way of triggering hindbrain stimulation in close temporal proximity to seizure onset. Arguably, the most reliable indicator of impending seizure onset in humans and rodents is a reduction in the statistical dispersion of the electroencephalogram (EEG) (Abasolo et al., 2008; Escudaro et al., 2009; Raiesdana et al., 2009; Zandi et al., 2009). We have developed a method for recording the time-varying electric field over the zebrafish cerebrum (the cerebral field potential), which we believe to be the equivalent of the human and rodent EEG (Pineda et al., 2011). To determine whether the dispersion of the cerebral field potential might be of use in triggering our stimulator, we investigated changes in the scalar entropy of the field potential in response to PTZ application.
Scalar entropy is a quantitative measure of a signal’s statistical dispersion (Shannon, 1951) and is estimated according to:
where S1. is the scalar entropy and p(n) is the probability of observing occurrence n out of N total occurrences. Conceptually, a signal with a wide distribution is expected to have a larger entropy estimate, whereas signals with more narrow distributions have smaller entropy estimates. From the equation, it is clear that if a signal containing N different points is maximally dispersed (all points being different) then the distribution is a flat horizontal line with a maximal entropy of log2N. This is represented by the blue horizontal dashed line in Fig. 2A. Likewise, if a signal is minimally dispersed (all points being the same) then the distribution is a vertical line with entropy of 0. This is represented by the red vertical dashed line in Fig. 2A.
Fig. 2.
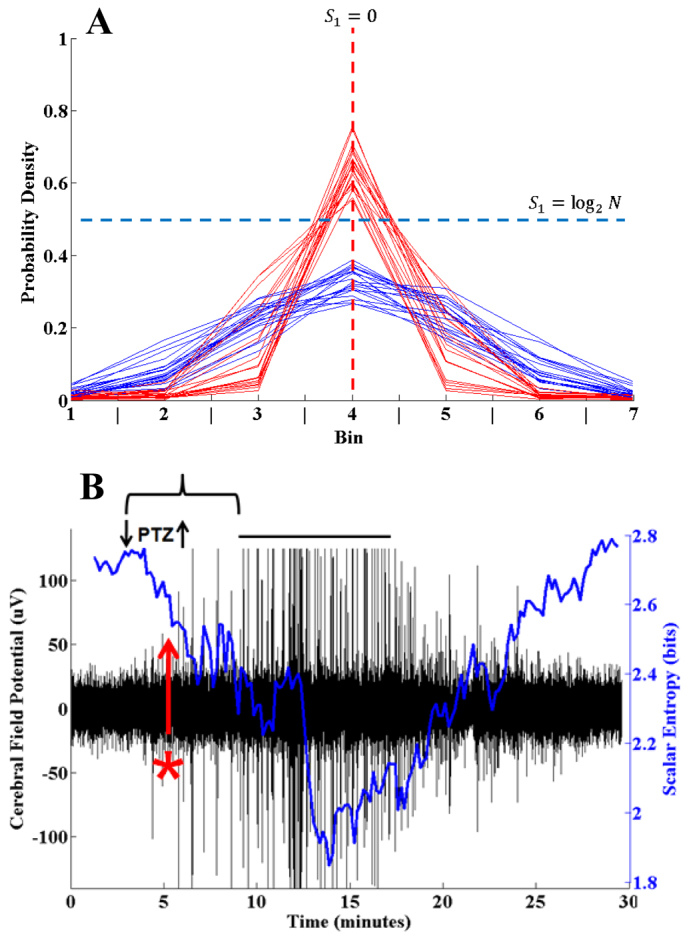
Changes in zebrafish cerebral field potential in response to PTZ. (A) Probability densities of 20 consecutive 10-second forebrain field potential recordings from before (red) and after (blue) PTZ exposure. The field potential was sampled at 64 Hz. Samples are normalized on a scale of 0 to 1 and then individual points sorted into one of seven equidistant probability bins. Blue traces are obtained in the baseline state (prior to PTZ application). Red traces are obtained in the pre-seizure state (after PTZ application, but before electrographic seizure activity). The horizontal blue dashed line represents a theoretical probability density function in which all points in the sample are different and the entropy is maximal and equal to log2N where N is the number of points in the sample. The vertical red dashed line represents a probability density function in which all points are the same and the entropy is minimal and equal to zero. The blue traces more closely approximate the blue horizontal dashed line, indicating a wider dispersion and higher entropy in the baseline state. The red traces more closely approximate the red vertical dashed line, indicating a narrower dispersion and lower entropy in the pre-seizure state. (B) Simultaneous forebrain field potential and scalar entropy for a PTZ-induced seizure. PTZ was applied at the down arrow and then removed at the up arrow. Following a latency (bracket), an electrographic seizure occurs (over-score), as evidenced by the marked increase in the amplitude of the cerebral field potential (black). The scalar entropy (blue) estimated on the field potential data decreases following PTZ exposure and prior to seizure onset. Thus, a 3 s.d. reduction in scalar entropy (red asterisk) from the mean of a rolling baseline average can be used as a trigger for reactive hindbrain stimulation.
To qualitatively determine whether the scalar entropy of the zebrafish cerebral field potential changes in response to PTZ application, we plotted the distributions of 20 consecutive 10-second traces of field potential recording before (blue) and after (red) PTZ exposure (Fig. 2A). As shown, the pre-exposure traces (blue) have a wider distribution and would be expected to have a larger scalar entropy estimate. Likewise, the post-exposure traces (red) assume a more narrow distribution and would be expected to have a smaller entropy estimate.
The entropy-reducing effect of PTZ exposure on the zebrafish cerebral field potential is further illustrated in supplementary material Movie 2. The video begins approximately 2 minutes after PTZ application and about 1 minute prior to seizure onset. At time 0:10 there is a ‘dropout’ in the background frequencies of the field potential that corresponds to the reduction in entropy prior to seizure onset (at 0:50). Based on Fig. 2A and supplementary material Movie 2, it seems likely that PTZ application produces a reduction in the scalar entropy of the cerebral field potential well before seizure onset that could be potentially useful as a trigger for the stimulator.
To determine a triggering threshold, we recorded sequential seizures and simultaneously performed rolling estimates of scalar entropy (Fig. 2B). After 3 minutes, 0.06 ml of 1 M PTZ stock solution was added to the 4 ml recording chamber to produce a PTZ exposure concentration of 15 mM. Following 3 minutes of PTZ exposure, the recording chamber was evacuated and replenished with tank water. After a brief latency (bracket), a seizure was recorded (over-score) as evidenced by an increase in the amplitude of the field potential (black). The criterion for determining seizure onset is based on a sustained increase in signal power over baseline and is described elsewhere (Pineda et al., 2011).
Following PTZ exposure but prior to seizure onset, there was a reduction in the scalar entropy (blue) of the signal greater than three standard deviations (3 s.d.) below the baseline mean (Fig. 2B, red asterisk). We estimated scalar entropy on nine such seizures. The baseline entropy was 2.6±0.3 bits (mean ± s.e.m.). The minimum entropy was 1.4±0.6 bits. The time between a 3 s.d. reduction in scalar entropy and seizure onset was 190±22 seconds. Based on these results, we concluded that we could use an entropy reduction of 3 s.d. below the baseline mean as the threshold for triggering the hindbrain stimulator.
Reactive hindbrain stimulation
The reactive hindbrain stimulator is shown in Fig. 3A. The forebrain field potential was recorded via a high gain amplifier (upper left), the amplifier output was fed to a central processing unit (CPU) by way of an analog to digital converter (ADC), and the CPU estimated the scalar entropy in real time (Fig. 3B). After establishing a 3-minute rolling baseline average, the hindbrain stimulator (upper right of Fig. 3A) was armed. When the scalar entropy fell below the baseline mean by 3 s.d., hindbrain stimulation was triggered by a transistor-transistor logic (TTL) signal from the digital to analog converter (DAC). A single stimulation was triggered once every 10 seconds so long as the entropy of the forebrain field potential was 3 s.d. below the baseline mean. Data obtained during stimulation and for 5 milliseconds afterwards was not buffered into the rolling entropy estimates so as to not taint the entropy estimates with stimulator artifact.
Fig. 3.
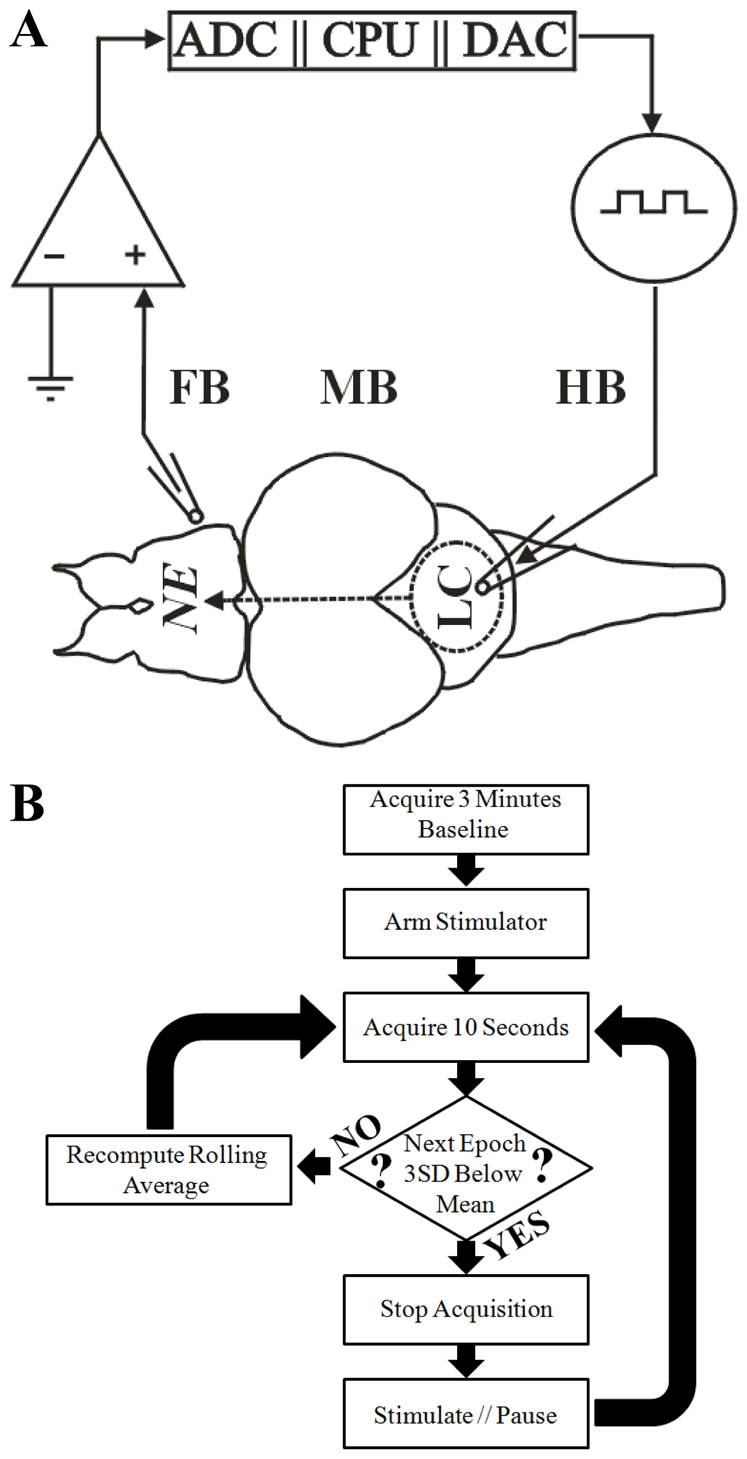
Reactive hindbrain stimulator. (A) Scheme of the reactive neural stimulation circuit. Forebrain (FB) field potential is recorded via high-gain amplifier (upper left) and fed into the control element consisting of an analog-digital converter (ADC) and a central processing unit (CPU). When the CPU detects seizure activity, it triggers the hindbrain (HB) stimulator (upper right; 0.1 mA/0.1 milliseconds) in the locus coeruleus (LC) via a digital to analog converter (DAC) resulting in norepinephrine (NE) delivery to the FB and an anticonvulsant effect as discussed (MB, midbrain). (B) Flow diagram for control and triggering of reactive hindbrain stimulation. Following the acquisition of a 3-minute baseline, the hindbrain stimulator was armed. Scalar entropy was estimated on consecutive 10-second epochs of cerebral field potential data. Stimulation was triggered when the entropy was greater than 3 s.d. below the baseline mean. Data acquisition was paused for 5 milliseconds to allow stimulator artifact to dissipate so that entropy estimates would not be contaminated and skewed by stimulator artifact.
An example of reactive hindbrain stimulation for PTZ-induced seizures is shown in real time (Fig. 4A). The black tracing shows the cerebral field potential, the blue tracing shows the scalar entropy and reactively triggered pulse electrical stimulations are marked with red asterisks (Fig. 4A). After establishing a 3-minute baseline, PTZ was applied to the recording chamber as before, but this time the recording chamber was not evacuated and PTZ exposure was continuous. At 6 minutes, there was a reduction in the scalar entropy (blue) of the field potential that triggered a volley of hindbrain stimulations as noted by the red asterisks. A total of 21 separate stimulations (in three volleys) were triggered over a 30-minute time period. No seizure occurred in this fish despite 30 minutes of continuous PTZ exposure. In stark contrast to Fig. 1A, where 7/7 un-stimulated fish (SHAM) had seizures within 10 minutes of continuous PTZ exposure and 7/7 fish that received a single round of 30 stimulations (STIM) seized within 30 minutes of continuous PTZ exposure, the fish that received reactive hindbrain stimulations received significantly less than 30 stimulations and did not have seizures at all. These results show conclusively that reactive hindbrain stimulation can be triggered (based on statistical properties of the cerebral field potential) and produce a significant anticonvulsant affect beyond that imparted by non-triggered stimulations.
Fig. 4.
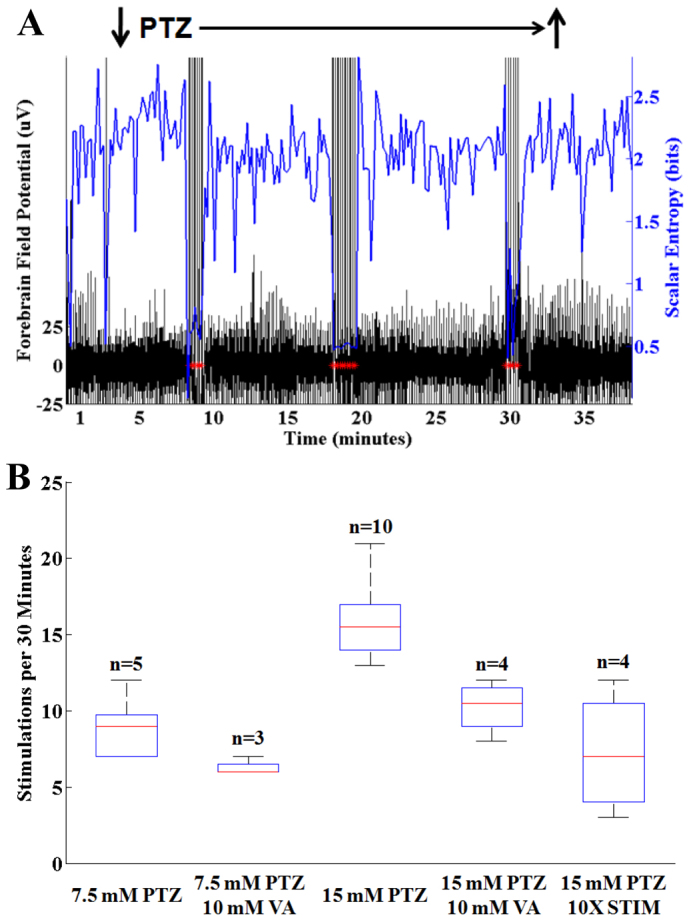
Reactive hindbrain stimulation in the presence of PTZ. (A) Simultaneous recording of forebrain field potential (black) and scalar entropy (blue). PTZ was applied continuously (down arrow). Following a latency, there was a decrease in scalar entropy that results in a volley of hindbrain stimulations (red asterisks). A total of three stimulation volleys with 21 total hindbrain stimulations occurred over a 30-minute interval. Despite more than 30 minutes of exposure to PTZ, no seizure occurred. The amplifier registered a stimulation artifact in the EEG tracing with each stimulation. (B) Histogram showing number of stimulations per 30-minute PTZ exposure time. The stimulation rate was directly dependent on PTZ concentration and was reduced by the addition of valproic acid (VA). Use of a stimulator pulse that delivers 10× the charge per stimulation (10XSTIM) resulted in significantly fewer hindbrain stimulations over a 30-minute period in the presence of 15 mM PTZ. Bars indicate the data median. Boxes and whiskers indicate the 25th to 75th and 5th to 95th percentiles, respectively.
The effect of PTZ concentration, valproic acid and stimulator current on stimulation rate
If hindbrain stimulation triggering is truly reactive and in proportion to the demand imposed by PTZ, then the stimulation rate should be directly proportional to PTZ concentration and should be reduced in the presence of valproic acid, an anticonvulsant. To test this, we subjected fish to reactive hindbrain stimulation in the presence of varying PTZ concentrations with and without valproic acid. As shown in Fig. 4B, we found that higher PTZ concentrations resulted in higher stimulation rates and that the addition of valproic acid to the recording chamber resulted in reduced stimulation rates. Valproic acid attenuates PTZ seizures in part by inhibiting GABA transaminase (Lee et al., 2010). These results show that valproic acid also partially attenuates entropy reductions in the forebrain field potential mediated by PTZ.
We selected the magnitude of our stimulation current empirically on the basis of published results of other studies involving electrical stimulation of fish brain (Demski et al., 1975). Because the degree of forebrain norepinephrine release should be proportional to the stimulating current, we expect that larger stimulation currents will result in lower stimulation rates. To test this directly, we performed a series of experiments in which we used 0.1 μC of charge per stimulation (0.5 mA/0.2 milliseconds) instead of delivering 0.01 μC of charge per stimulation (0.1 mA/0.1 milliseconds). We observed significantly fewer stimulations per 30-minute time period with the larger charge delivery (Fig. 4B, 10XSTIM).
To be sure that our triggered stimulations were not random occurrences, we obtained 30-minute recordings in four fish with the stimulator armed but without PTZ. We recorded one stimulation in one fish (not shown). Collectively, these results corroborate the following: that stimulation was triggered reactively according to the ‘demand’ of the recording environment imposed by PTZ, that we did not overdrive the system with our preselected stimulation parameters and that stimulations were not random spontaneous occurrences. In these experiments, the mean entropy was 2.3±0.3 bits. The entropy at which stimulation initially triggered was 1.5±0.3 bits. The trough entropy during a stimulation volley was 0.9±0.2 bits.
Anatomical correlation
To verify the site of the stimulating electrode, we examined formalin-fixed cross-sections for a tract corresponding to the electrode insertion site. We were able to confidently recognize four distinct anatomical regions on the basis of differences in cross-sectional anatomy using 100-μm sections through the hindbrain. We identified the distal-most aspect of the necropsy tract left by the stimulating electrode and charted these in Fig. 5A. Despite not having used a stereotactic device, we were generally within 1 mm of the approximate region of the LC.
Fig. 5.
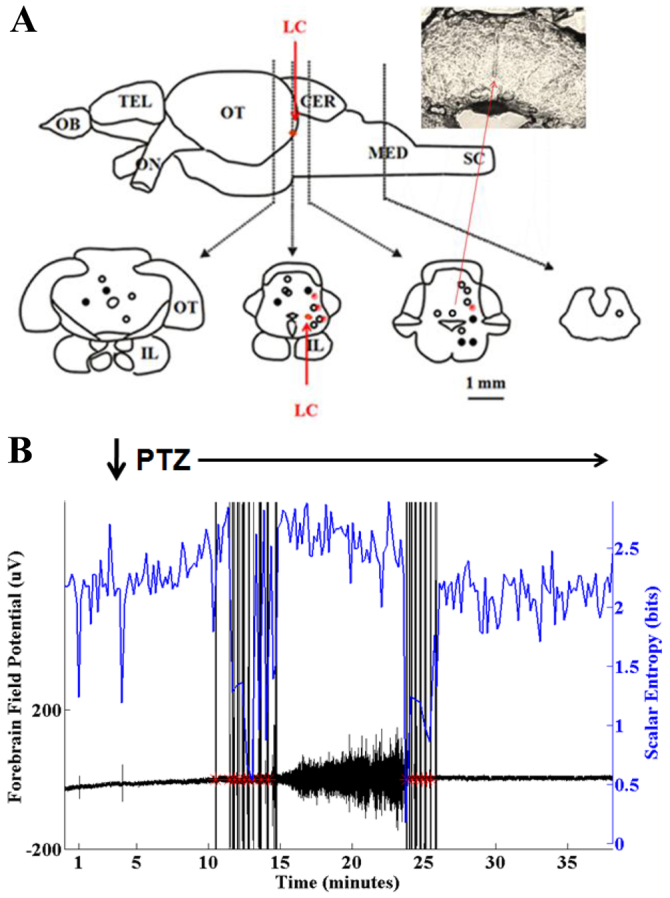
Anatomical correlation. (A) Histologic locations of distal-most aspect of stimulating electrode as shown on necropsy with a representative section shown in the insert. In each instance, the stimulating electrode was located within 1 mm of the area deemed to be most consistent with the LC. Symbols on the left side of the templates represent experiments done in the presence of 7.5 mM PTZ and symbols on the right side of the templates represent experiments done in the presence of 15 mM PTZ. Open circles represent experiments done in the presence of PTZ alone. Filled circles represent experiments done in the presence of PTZ and 10 mM valproic acid. Red dots represent experiments done in the presence of 15 mM PTZ using 0.1 μC of charge (0.5 mM/0.2 milliseconds) per stimulation. All other experiments were performed using 0.01 μC of charge (0.1 mM/0.1 milliseconds) per stimulation. OB, olfactory bulb; TEL, telencephalon; CER, cerebellum; MED, medulla; SC, spinal cord; IL, inferior lobe; LC, locus coeruleus. (B) Reactive hindbrain stimulation using a far caudal stimulation site. Following a latency after PTZ application (down arrow) the scalar entropy (blue) fell and stimulation was triggered (red asterisks). Despite stimulation, an electrographic seizure occurred as evidenced by the increased amplitude of the cerebral field potential (black). A second round of stimulation terminated this seizure. These results imply that more caudal stimulation sites are less effective at attenuating seizures.
The small size of the zebrafish cerebrum and hindbrain necessitated the use of monopolar stimulation, with the active electrode being inserted directly into the brain parenchyma and the reference electrode being placed subcutaneously at the same rostro-caudal location. The net result is a high volume monopolar stimulator configuration, which we expect to result in reduced anatomic specificity. Indeed, there was no significant difference in stimulation rate versus distance from the LC when comparing stimulation sites 1 mm caudal and upwards of 2 mm rostral to sites closest LC (not shown). However, as shown in Fig. 5B, a more caudal reactive stimulation site did not block PTZ seizure occurrence. In this case, PTZ was applied to the recording chamber and there was a reduction in entropy that triggered a stimulation volley, but a seizure occurred anyway. The seizure eventually terminated and a second stimulation volley was triggered as a result of this. The reduced efficiency of the far caudal stimulation site was probably due to the lack of cross-activation of ascending projections.
DISCUSSION
Our results show that combining the hindbrain-activating properties of VNS with the reactive triggering of RNS results in a ‘demand-triggered’ stimulation paradigm that might ultimately prove to have greater utility than either modality alone. Specifically, we showed that it was possible to use the changing statistical properties of the zebrafish cerebral field potential to trigger hindbrain stimulation in reaction to proconvulsant exposure in order to attenuate PTZ-induced seizures. These results implicate a potential role for reactive vagus nerve stimulation in the treatment of epilepsy. Given the large numbers of patients with implanted vagus nerve stimulators who continue to have seizures, further investigation into the possibility of a hybrid VNS-RNS stimulation platform is warranted.
Our results also suggest that the zebrafish cerebral field potential is of sufficient complexity to make it amenable to analysis by algorithms commonly used in the quest for seizure anticipation in human. Specifically, we showed that decreases in the entropy of the forebrain field potential consistently precede PTZ-induced seizures in zebrafish, much in the same way that changes in the entropy of the EEG precede seizures in rodents and humans.
Seizure detection and anticipation are much-studied topics in the field of epilepsy (Lehnertz et al., 2001; Litt and Echauz, 2002; Mormann et al., 2006; Mormann et al., 2007). However, variations in recording conditions and the innate non-stationarity of the human EEG have thus far hampered development of seizure anticipation-prevention paradigms (Zoldi et al., 2000; Riecke et al., 2003). We found that spontaneous 3 s.d. entropy changes in the zebrafish cerebral field potential were sparse, as evidenced by the fact that we observed only one spontaneous stimulation in a single fish (out of four) despite 30 minutes of recording, which suggests stationarity in the signal. It might be that the zebrafish forebrain is not capable of generating the same degree of complex dynamics that human and rodent brains generate, which would make for a more stationary signal. However, it might also be that the stationarity we observed resulted from a degree of experimental control not afforded by mammalian models. At any rate, this stationarity affords a stable platform for the evaluation of prospective seizure anticipation algorithms.
Finally, our results suggest that the hindbrain-forebrain noradrenergic projections in the zebrafish are of sufficient density to be of functional utility. Specifically, we showed that hindbrain stimulation resulted in an increase in latency to onset of PTZ-induced seizures and that this anticonvulsant effect was dependent, at least in part, on noradrenergic mechanisms. These results suggest that the zebrafish might be useful in other areas where noradrenergic mechanisms are involved, namely learning, memory and behavior (Berridge and Waterhouse, 2003; Sara and Herve-Minvielle, 2008).
In summary, we have developed a functional neural stimulation paradigm for the zebrafish that combines different elements of existing neural stimulation paradigms for epilepsy that are used in humans. Our results imply a role for the zebrafish in the study of neural stimulation as therapy for epilepsy. Moreover, our results warrant further investigation into the possibility of hybrid VNS-RNS neural stimulation paradigms.
METHODS
Animal care and use
For the purposes of this study, female adult laboratory strain WIK zebrafish (9–14 months old) were used. All protocols and procedures were approved by The Ohio State University’s Institutional Animal Care and Use Committee (IACUC Protocol #2010A0141). Fish were maintained in aerated tank water and fed to satiety twice daily on weekdays, once daily on weekends. All experiments were performed 2–6 hours after a weekday morning feeding.
Anesthesia induction and maintenance
Anesthesia was induced and maintained prior to stimulator wire insertion in all described experiments. Fish were immersed in tank water baths containing eugenol (clove oil extract), as described elsewhere (Grush et al., 2004). Eugenol induces anesthesia by blocking fast voltage-gated sodium channels (Cho et al., 2008). Eugenol also has an antinociceptive effect on central and peripheral pain circuits by acting on the transient receptor potential, vanilloid subtype 1 (TRPV1) (Vriens et al., 2009). Anesthesia was induced by immersing the fish in 15 p.p.m. eugenol. Induction of anesthesia was deemed complete when the fish lost posture and rolled to the side. Anesthesia was maintained by continued immersion in 7.5 p.p.m. eugenol. Aversive maneuvers such as ‘tail flips’ and ‘swim attempts’ were taken to be signs of inadequate anesthesia and prompted higher eugenol exposure concentrations. In previous studies (Pineda et al., 2011), we found that a maintenance concentration of 7.5 p.p.m. eugenol was sufficient to maintain comfort, preserve spontaneous respiration and not attenuate PTZ-induced seizures.
Static and reactive hindbrain stimulation
A monopolar stimulating electrode was fashioned from a length of 0.005 inch tungsten (Hypertriton, Cap-Aux-Meules, QC, Canada) wire. The proximal portions of the tungsten were coated with a brush-on epoxy (LTB-400; Gardner Bender, Milwaukee, WI) for electrical insulation, with the distal 0.5 mm being left bare. We used a Grass Instruments (Quincy, MA) S88 stimulator in series with a Grass Instruments CCU1A constant current stimulus isolation unit to deliver stimulations. Stimulator wire placement was achieved using established anatomical landmarks (Rupp et al., 1996; Rink and Wullimann, 2004). The reference wire was placed under the skin at the same rostro-caudal location as the active electrode.
In experiments where hindbrain stimulation was static, each fish received two electrical stimulations, 1 minute apart. An electrical stimulation consisted of 15 square-wave pulses (0.1 mA/0.1 milliseconds) at a rate of one pulse per second. Because of the brief duration of stimulator wire insertion, tracts were not visible on necropsy in these fish. Unless otherwise specified, in experiments where hindbrain stimulation was reactive, single square wave pulses (0.1 mA/0.1 milliseconds) were delivered at a rate of not more than once per 10 seconds. Because of the prolonged duration of the reactive stimulation experiments, necropsy tracts were visible on histologic section, which allowed verification of the stimulator wire insertion site.
Determining the effect of static hindbrain stimulation on latency to onset of behavioral seizure activity
Free swimming zebrafish larvae show predictable stages of behavioral seizure activity in response to proconvulsants (Baraban et al., 2005). Stage I seizure activity consists of increased swimming. Stage II seizure activity consists of increased aversive or ‘whirlpool’ maneuvers. Stage III seizure activity consists of clonic convulsion with loss of posture and rolling to the side. Our previous works suggest that adult zebrafish also exhibit these same stages of behavioral seizure activity (Pineda et al., 2011). We have included supplementary material Movie 1 in support of this claim.
To determine the effects of hindbrain stimulation on the latency to onset of behavioral seizure activity, fish were anesthetized, immobilized and stimulated as described above. Next, the stimulator wires were removed and the fish were allowed to recover from anesthesia to the point that they resumed free swimming. They were then immersed in tank water containing 15 mM PTZ and the time to onset of Stage III seizure activity was measured. Each fish in these experiments recovered full swim activity and was later euthanized as described below.
Recording the cerebral forebrain field potential
The technique for recording the forebrain field potential and computing the power content of the signal was the same as described previously (Pineda et al., 2011). The forebrain field potential is the zebrafish equivalent to the human and rodent EEG. In short, we used a high gain amplifier (5000 V/V) and a six-pole bandpass filter with corner frequencies of 1.6–16 Hz. The raw cerebral field potential tracings shown in the figures were recorded using the Tracer Daq data acquisition program (Measurement Computing Corporation, Norton, MA). A truncated cerebral field potential recording of a PTZ-induced seizure is shown in supplementary material Movie 2. Supplementary material Figs S1–S6 show a typical PTZ seizure at varying time scales. In experiments where entropy estimates were made, the sampling rate was 64 Hz (a power of two). Otherwise, the sampling rate was 50 Hz (supplementary material Movie 2 and Figs S1–S6).
Reactive hindbrain stimulation
As described above, the high gain amplifier shown in Fig. 3A was realized using the apparatus described in Pineda et al. (Pineda et al., 2011). A Measurement Computing (MCC, Norton, MA) data acquisition (USB-1208HS-4AO) card served as both the ADC and DAC. The MCC card was interfaced to the CPU via Matlab’s (MathWorks, Natick, MA) Data Acquisition Toolbox. Scalar entropy was estimated in real time with Matlab Script. The techniques used optimize bin number and to correct for bias in entropy estimates are described elsewhere (Hall and Sarkar, 2011). As mentioned above, a Grass Instruments S88 stimulator in series with a Grass Instruments CCU1A constant current stimulus isolation unit was used to deliver electrical stimulations. For the purposes of this portion of the study, fish were exposed to either 7.5 or 15 mM PTZ, with or without 10 mM valproic acid for 30 minutes. Unless otherwise stated, stimulations consisted of single square wave pulses (0.1 mA/0.1 milliseconds) triggered at most once per 10 seconds based on a 3 s.d. reduction from baseline mean entropy.
Verification of stimulating electrode sites
Following each experiment, the fish were euthanized by immersion in tricaine (MS-222; Sigma, 160 μg/ml) until all respiratory movements had ceased for 3 minutes. Fish were then decapitated. The head was placed in a vial of 20% sucrose and 4% paraldehyde and refrigerated for 4 weeks. Next, 100 μm sections were cut from the fixed specimens using a cryostat. The sections were examined carefully for a tract corresponding to the electrode insertion site. The distal-most aspect of the necropsy tract was then charted. The techniques used for preparing these sections are described in further detail elsewhere (Hall and Behbehani, 1997).
Supplementary Material
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
C.W.H. prepared the manuscript and performed the data collection. R.P. and C.E.B. participated in the study design and data analysis. All authors edited the manuscript.
FUNDING
This study was partially funded by the National Institute of Neurological Disorders and Stroke (United States National Institutes of Health) grant number P30 NS045758.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.009423/-/DC1
REFERENCES
- Abásolo D., Escudero J., Hornero R., Gómez C., Espino P. (2008). Approximate entropy and auto mutual information analysis of the electroencephalogram in Alzheimer’s disease patients. Med. Biol. Eng. Comput. 46, 1019–1028 [DOI] [PubMed] [Google Scholar]
- Aldenkamp A. P. (2006). Cognitive impairment in epilepsy: State of affairs and clinical relevance. Seizure 15, 219–220 [Google Scholar]
- Anderson W., Kossoff E., Bergey G., Jallo G. (2008). Implantation of a responsive neurostimulator device in patients with refractory epilepsy. Neurosurg. Focus 25, E12. [DOI] [PubMed] [Google Scholar]
- Baraban S. C., Taylor M. R., Castro P. A., Baier H. (2005). Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131, 759–768 [DOI] [PubMed] [Google Scholar]
- Barry D. I., Wanscher B., Kragh J., Bolwig T. G., Kokaia M., Brundin P., Björklund A., Lindvall O. (1989). Grafts of fetal locus coeruleus neurons in rat amygdala-piriform cortex suppress seizure development in hippocampal kindling. Exp Neurol. 106, 125–132 [DOI] [PubMed] [Google Scholar]
- Begley C. E., Famulari M., Annegers J. F., Lairson D. R., Reynolds T. F., Coan S., Dubinsky S., Newmark M. E., Leibson C., So E. L., et al. (1994). The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 35, 1230–1243 [DOI] [PubMed] [Google Scholar]
- Berridge C. W., Waterhouse B. D. (2003). The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 42, 33–84 [DOI] [PubMed] [Google Scholar]
- Bloms-Funke P., Madeja M., Muhoff U., Speckmann E. J. (1996). Effects of pentylenetetrazole on GABA receptors expressed in oocytes of Xenopus laevis: extra- and intracellular sites of action. Neurosci. Lett. 205, 115–118 [DOI] [PubMed] [Google Scholar]
- Cascino G. D. (2008). When drugs and surgery don’t work. Epilepsia 49, 79–84 [DOI] [PubMed] [Google Scholar]
- Cho J. S., Kim T. H., Lim J. M., Song J. H. (2008). Effects of eugenol on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 1243, 53–62 [DOI] [PubMed] [Google Scholar]
- Demski L. S., Bauer D. H., Gerald J. W. (1975). Sperm release evoked by electrical stimulation of the fish brain: A functional-anatomical study. J. Exp. Zool. 191, 215–231 [DOI] [PubMed] [Google Scholar]
- Escudero J., Hornero R., Abásolo D. (2009). Interpretation of the auto-mutual information rate of decrease in the context of biomedical signal analysis. Application to electroencephalogram recordings. Physiol. Meas. 30, 187–199 [DOI] [PubMed] [Google Scholar]
- Follesa P., Biggio F., Gorini G., Caria S., Talani G., Dazzi L., Puligheddu M., Marrosu F., Biggio G. (2007). Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 117, 28–34 [DOI] [PubMed] [Google Scholar]
- Ghaemi K., Elsharkawy A. E., Schulz R., Hoppe M., Polster T., Pannek H., Ebner A. (2010). Vagus nerve stimulation: Outcome and predictors of seizure freedom in long-term follow-up. Seizure 19, 264–268 [DOI] [PubMed] [Google Scholar]
- Groves D. A., Bowman E. M., Brown V. J. (2005). Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci. Lett. 379, 174–179 [DOI] [PubMed] [Google Scholar]
- Grush J., Noakes D. L., Moccia R. D. (2004). The efficacy of clove oil as an anesthetic for the zebrafish, Danio rerio (Hamilton). Zebrafish 1, 46–53 [DOI] [PubMed] [Google Scholar]
- Hall C. W., Behbehani M. M. (1997). The medial preoptic nucleus of the hypothalamus modulates activity of nitric oxide sensitive neurons in the midbrain periaqueductal gray. Brain Res. 765, 208–217 [DOI] [PubMed] [Google Scholar]
- Hall C. W., Sarkar A. (2011). Mutual information in natural position order of electroencephalogram is significantly increased at seizure onset. Med. Biol. Eng. Comput. 49, 133–141 [DOI] [PubMed] [Google Scholar]
- Hasselmo M. E. (1995). Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav. Brain Res. 67, 1–27 [DOI] [PubMed] [Google Scholar]
- Hauser W. A., Annegers J. F., Kerland L. T. (1991). Prevalence of epilepsy in Rochester, Minnesota. Epilepsia 32, 429–445 [DOI] [PubMed] [Google Scholar]
- Huang R. Q., Bell-Horner C. L., Dibas M. I., Covey D. F., Drewe J. A., Dillon G. H. (2001). Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: mechanism and site of action. J. Pharmacol. Exp. Ther. 298, 986–995 [PubMed] [Google Scholar]
- Hunn J. B., Allen J. L. (1974). Movement of drugs across the gills of fishes. Ann. Rev. Pharmacol. 14, 47–54 [Google Scholar]
- Jim K. F., Lathers C. M., Farris V. L., Pratt L. F., Spivey W. H. (1989). Suppression of pentylenetetrazol-elicited seizure activity by intraosseous lorazepam in pigs. Epilepsia 30, 480–486 [DOI] [PubMed] [Google Scholar]
- Krahl S. E., Clark K. B., Smith D. C., Browning R. A. (1998). Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 39, 709–714 [DOI] [PubMed] [Google Scholar]
- Lee Y., Kim D., Kim Y. H., Lee H., Lee C. J. (2010). Improvement of pentylenetetrazol-induced learning deficits by valproic acid in the adult zebrafish. Eur. J. Pharmacol. 643, 225–231 [DOI] [PubMed] [Google Scholar]
- Lehnertz K., Andrzejak R. G., Arnhold J., Kreuz T., Mormann F., Rieke C., Widman G., Elger C. E. (2001). Nonlinear EEG analysis in epilepsy: its possible use for interictal focus localization, seizure anticipation, and prevention. J. Clin. Neurophysiol. 18, 209–222 [DOI] [PubMed] [Google Scholar]
- Lim E. P., Tan C.H, Jay T. M., Dawe G. S. (2005). Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J. Neurochem. 92, 368–374 [DOI] [PubMed] [Google Scholar]
- Litt B., Echauz J. (2002). Prediction of epileptic seizures. Lancet Neurol. 1, 22–30 [DOI] [PubMed] [Google Scholar]
- Ma P. M. (1994a). Catecholaminergic systems in the zebrafish. I. Number, morphology, and histochemical characteristics of neurons in the locus coeruleus. J. Comp. Neurol. 344, 242–255 [DOI] [PubMed] [Google Scholar]
- Ma P. M. (1994b). Catecholaminergic systems in the zebrafish. II. Projection pathways and pattern of termination of the locus coeruleus. J. Comp. Neurol. 344, 256–269 [DOI] [PubMed] [Google Scholar]
- Ma P. M. (1997). Catecholaminergic systems in the zebrafish. III. Organization and projection pattern of medullary dopaminergic and noradrenergic neurons. J. Comp. Neurol. 381, 411–427 [PubMed] [Google Scholar]
- Mamedov Z. G. (1993). Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. Neurophys. 25, 201–203 [DOI] [PubMed] [Google Scholar]
- Mormann F., Elger C. E., Lehnertz K. (2006). Seizure anticipation: from algorithms to clinical practice. Curr. Opin. Neurol. 19, 187–193 [DOI] [PubMed] [Google Scholar]
- Mormann F., Andrzejak R. G., Elger C. E., Lehnertz K. (2007). Seizure prediction: the long and winding road. Brain 130, 314–333 [DOI] [PubMed] [Google Scholar]
- Motamedi G. K., Lesser R. P., Miglioretti D. L. (2002). Optimizing parameters for terminating cortical afterdischarges with pulse stimulation. Epilepsia 43, 836–846 [DOI] [PubMed] [Google Scholar]
- Nai Q., Dong H. W., Hayar A., Linster C., Ennis M. (2009). Noradrenergic regulation of GABAergic inhibition of main olfactory bulb mitral cells varies as a function of concentration and receptor subtype. J. Neurophysiol. 101, 2472–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiffer D. L., Stamper M. A. (2009). Fish sedation, anesthesia, analgesia and euthanasia: Considerations, methods and types of drugs. Inst. Lab. Animal Res. 50, 343–360 [DOI] [PubMed] [Google Scholar]
- Osorio I., Frei M. G., Manly B. F., Sunderam S., Bhavaraju N. C., Wilkinson S. B. (2001). An introduction to contingent (closed-loop) brain electrical stimulation for seizure blockage, to ultra-short-term clinical trials, and to multidimensional statistical analysis of therapeutic efficacy. J. Clin. Neurophysiol. 18, 533–544 [DOI] [PubMed] [Google Scholar]
- Pershad D., Siddiqui R. S. (1992). Psychosocial dysfunction in adults with epilepsy. Int. J. Rehabil. Res. 15, 258–261 [DOI] [PubMed] [Google Scholar]
- Pineda R., Beattie C. E., Hall C. W. (2011). Recording the adult zebrafish cerebral field potential during pentylenetetrazole seizures. J. Neurosci. Methods 200, 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiesdana S., Golpayegani S. M., Firoozabadi S. M., Mehvari Habibabadi J. (2009). On the discrimination of pathophysiological states in epilepsy by means of dynamical measures. Comput. Biol. Med. 39, 1073–1082 [DOI] [PubMed] [Google Scholar]
- Rieke C., Mormann F., Andrzejak R. G., Kreuz T., David P., Elger C. E., Lehnertz K. (2003). Discerning nonstationarity from nonlinearity in seizure-free and preseizure EEG recordings from epilepsy patients. IEEE Trans. Biomed. Eng. 50, 634–639 [DOI] [PubMed] [Google Scholar]
- Rink E., Wullimann M. F. (2004). Connections of the ventral telencephalon (subpallium) in the zebrafish (Danio rerio). Brain Res. 1011, 206–220 [DOI] [PubMed] [Google Scholar]
- Roosevelt R. W., Smith D. C., Clough R. W., Jensen R. A., Browning R. A. (2006). Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 1119, 124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp B., Reichert H., Wullimann M. F. (1996). The zebrafish brain: a neuroanatomical comparison with the goldfish. Anat. Embryol. 194, 187–203 [DOI] [PubMed] [Google Scholar]
- Salanova V., Worth R. (2007). Neurostimulators in epilepsy. Curr. Neurol. Neurosci. 7, 315–319 [DOI] [PubMed] [Google Scholar]
- Sara S. J., Herve-Minvielle A. (2008). Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part i: Principles of functional organization. Curr. Neuropharmacol. 6, 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C. E. (1951). Prediction and entropy of printed English. The Bell System Technical Journal 30, 50–64 [Google Scholar]
- Shorvon S. D. (2007). The epidemiology and treatment of chronic and refractory epilepsy. Epilepsia 37, S1–S3 [DOI] [PubMed] [Google Scholar]
- Stewart A. M., Desmond D., Kyzar E., Gaikwad S., Roth A., Riehl R., Collins C., Monnig L., Green J., Kalueff A. V. (2012). Perspectives of zebrafish models of epilepsy: What, how and where next? Brain Res. Bull 87, 135–143 [DOI] [PubMed] [Google Scholar]
- Vriens J., Appendino G., Nilius B. (2009). Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 75, 1262–1279 [DOI] [PubMed] [Google Scholar]
- Zandi A. S., Dumont G. A., Javidan M., Tafreshi R. (2009). An entropy-based approach to predict seizures in temporal lobe epilepsy using scalp EEG. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1, 228–231 [DOI] [PubMed] [Google Scholar]
- Zielinski J. J. (1974). Epilepsy and mortality rate and cause of death. Epilepsia 15, 191–201 [DOI] [PubMed] [Google Scholar]
- Zoldi S., Krystal A., Greenside H. (2000). Stationarity and redundancy of multichannel EEG data recorded during generalized tonic-clonic seizures. Brain Topogr. 12, 187–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


