SUMMARY
Mutations in SPG4, encoding the microtubule-severing protein spastin, are responsible for the most frequent form of hereditary spastic paraplegia (HSP), a heterogeneous group of genetic diseases characterized by degeneration of the corticospinal tracts. We previously reported that mice harboring a deletion in Spg4, generating a premature stop codon, develop progressive axonal degeneration characterized by focal axonal swellings associated with impaired axonal transport. To further characterize the molecular and cellular mechanisms underlying this mutant phenotype, we have assessed microtubule dynamics and axonal transport in primary cultures of cortical neurons from spastin-mutant mice. We show an early and marked impairment of microtubule dynamics all along the axons of spastin-deficient cortical neurons, which is likely to be responsible for the occurrence of axonal swellings and cargo stalling. Our analysis also reveals that a modulation of microtubule dynamics by microtubule-targeting drugs rescues the mutant phenotype of cortical neurons. Together, these results contribute to a better understanding of the pathogenesis of SPG4-linked HSP and ascertain the influence of microtubule-targeted drugs on the early axonal phenotype in a mouse model of the disease.
INTRODUCTION
Hereditary spastic paraplegia (HSP) is a heterogeneous group of inherited neurological disorders, mainly characterized by a bilateral and slowly progressive spasticity of the lower limbs caused by the degeneration of the corticospinal tracts (Reid, 2003; Fink, 2006). HSP shows a considerable genetic heterogeneity, with 45 loci mapped so far (Salinas et al., 2008). Mutations in the SPG4 gene encoding spastin, a member of the AAA (ATPase associated with diverse cellular activities) superfamily, are responsible for the most frequent form of autosomal dominant HSP (Hazan et al., 1999). More than 200 mutations have been described within the SPG4 coding sequence, including nonsense, frameshift, splice site and missense mutations as well as large-scale deletions (Hazan et al., 1999; Fonknechten et al., 2000; Depienne et al., 2007). Although some missense mutations clearly show a dominant-negative effect (Errico et al., 2002; Du et al., 2010; Solowska et al., 2010), the vast majority of SPG4 mutations, which affect the ATPase domain, are thought to cause this form of HSP by haploinsufficiency (Fonknechten et al., 2000; Charvin et al., 2003; Roll-Mecak and Vale, 2008; Riano et al., 2009).
The SPG4 gene directs the synthesis of four spastin isoforms through the use of alternative translation initiation sites, which generate a full-length protein of 68 kDa and a shorter isoform of 60 kDa, in addition to the alternative splicing of exon 4, which leads to two additional isoforms of 64 and 56 kDa, respectively (Claudiani et al., 2005). Like p60-katanin, spastin is involved in microtubule severing (Errico et al., 2002; Evans et al., 2005; Salinas et al., 2005; Roll-Mecak and Vale, 2005), a process by which long microtubules are cut into shorter and highly motile fragments (Baas et al., 2005). Spastin forms a ring-shape hexamer containing a prominent central pore into which the C-terminal tail of tubulin is attached and pulled. It has been proposed that the mechanical forces exerted by spastin on the C-terminal tail of tubulin destabilize tubulin-tubulin interactions in the microtubule lattice (White et al., 2007; Roll-Mecak and Vale, 2008). In the cytoplasm, spastin localizes to vesicular structures, tubular endoplasmic reticulum (ER) and predominantly to cellular regions characterized by extensive remodeling of the cytoskeleton, such as the centrosomes, the spindle poles and the mitotic cell midbody, in addition to neuron growth cones and axon branch points (Errico et al., 2004; Yu et al., 2008; Connell et al., 2009; Park et al., 2010). In neurons, spastin and katanin microtubule-severing activities provide an important source of non-centrosomal microtubules and have been shown to be essential for axon outgrowth and branching in vitro (Karabay et al., 2004; Riano et al., 2009; Yu et al., 2008). Functional studies in zebrafish and Drosophila revealed that spastin regulation of microtubule dynamics is crucial for motor neuron development and function (Wood et al., 2006; Trotta et al., 2004; Sherwood et al., 2004; Orso et al., 2005). We and others have reported that mice harboring an SPG4-truncated mutation develop a progressive axonal degeneration characterized by axonal swellings associated with impaired axonal transport (Tarrade et al., 2006; Kasher et al., 2009). Using primary cultures of cortical neurons from spastin-mutant mice, we have shown that these axonal swellings occur in a specialized region of the axon characterized by an abrupt transition between stable and dynamic microtubules, which suggests that alterations in microtubule dynamics primarily give rise to this mutant phenotype (Tarrade et al., 2006). However, no experimental evidence supporting this hypothesis has been brought forward so far, whereas other pathogenic mechanisms, including defective membrane trafficking or impaired endoplasmic reticulum (ER) morphogenesis, have been suggested to cause axonopathy in cell and animal models of SPG4-linked HSP (for reviews, see Blackstone et al., 2011; Lumb et al., 2012).
TRANSLATIONAL IMPACT.
Clinical issue
Hereditary spastic paraplegia (HSP) describes a heterogeneous group of neurodegenerative disorders that are mainly characterized by progressive and bilateral spasticity of the lower limbs due to the retrograde degeneration of the corticospinal tracts. Among the numerous proteins that can be affected in HSP, spastin, encoded by SPG4 (the most common HSP-associated gene), is altered in 40% of all autosomal-dominant HSP families. This paper characterizes in further detail the pathogenic processes that occur in a spastin-mutant mouse model of the disease that develops a progressive axonal degeneration of cortical neurons.
Results
Using primary cultures of cortical neurons from spastin-mutant mice, the authors explored the molecular and cellular mechanisms underlying the axon swelling phenotype that precedes the degeneration of cortical axons. This analysis reveals a marked and specific impairment of microtubule dynamics in spastin-mutant cortical axons. The impairment in microtubule dynamics is associated with a major disorganization of the microtubule network within the swellings that significantly alters axonal transport. Very low concentrations of microtubule-targeting drugs substantially rescue the axon phenotype associated with loss of spastin function in mammalian cortical neurons, thereby indicating that alterations in microtubule dynamics are a primary cause of the mouse mutant phenotype.
Implications and future directions
Although the molecular bases underlying the rescue of mouse mutant neurons by microtubule-targeting drugs need to be further dissected, these results provide new insights into potential suppressors of the early axonal phenotype in a mammalian model of HSP, which could guide future development of therapeutic strategies.
In the present study, we further analyzed primary cultures of cortical neurons from spastin-mutant mice to determine the molecular and cellular mechanisms underlying the axon swelling phenotype. Our analysis reveals an early, marked and specific impairment of microtubule dynamics in spastin-depleted cortical axons, which is associated with a major disorganization of the microtubule network within the swellings that alters retrograde axonal transport by increasing the frequency of cargo stalling. Furthermore, we show that nanomolar concentrations of microtubule-targeting drugs substantially rescue the axon phenotype associated with a loss of spastin function in mammalian cortical neurons.
RESULTS
Loss of spastin function is the pathogenic model underlying the axonopathy of SpΔ/Δ mouse mutant
We previously generated mice that carry a heterozygous (SpΔ/+) or homozygous (SpΔ/Δ) deletion of Spg4 exons 5 to 7, which leads to a premature stop codon and mimics 15% of the truncated mutations found in HSP patients (Tarrade et al., 2006). We initially reported that our heterozygous mice SpΔ/+ show a 50% decrease in wild-type spastin compared with controls and develop a milder phenotype than their homozygous siblings SpΔ/Δ, which suggests a causative mechanism of haploinsufficiency (Tarrade et al., 2006). However, because haploinsufficiency was recently questioned for SPG4-linked HSP (Solowska et al., 2008), we tried to further characterize the molecular mechanism associated with the Spg4 frameshift mutation of our mouse mutant by exploring whether SpΔ/Δ mice express a truncated spastin lacking the AAA domain. We therefore carried out an immunoblot analysis of brain lysates from 4-month-old Sp+/+, SpΔ/+ and SpΔ/Δ mice using the S51 antibody, directed against the N-terminal region of spastin (from amino acids 87 to 354) (Errico et al., 2004), which could thereby bind to the truncated protein. We also performed a control western blot with protein extracts from Cos-7 cells transfected with GFP-fused full-length or AAA-domain-depleted spastin (GFP-Sp+ and GFP-SpΔ) (Fig. 1A). As expected, our analysis confirmed that the S51 antibody recognizes the truncated spastin in transfected cells (Fig. 1A). However, SpΔ/+ and SpΔ/Δ mice did not show detectable levels of truncated spastin in vivo, although they exhibited a reduction (∼40%) or a lack of wild-type spastin, respectively (Fig. 1B,C). Furthermore, identical results were obtained with larger amounts of protein extracts (100 μg; data not shown), which thereby suggests that loss of spastin function might be responsible for the SpΔ/Δ mutant phenotype.
Fig. 1.
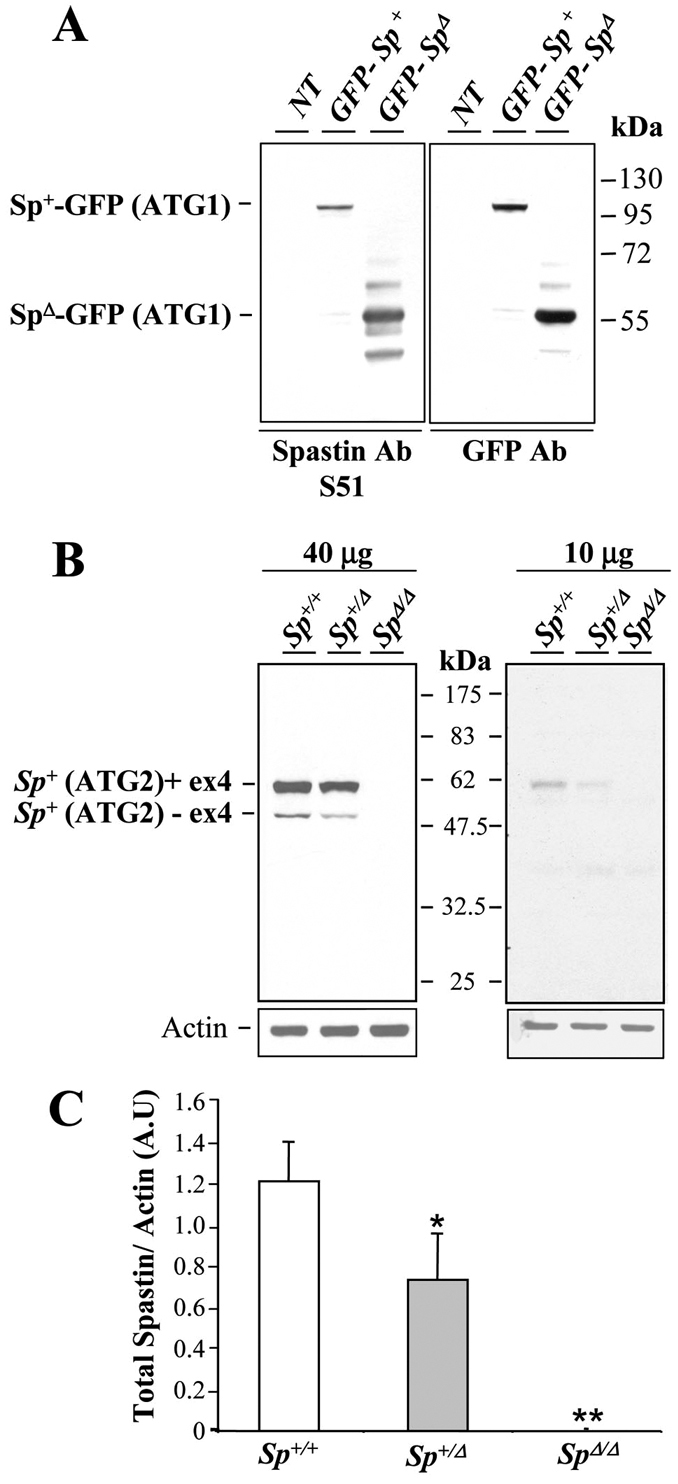
Absence of truncated spastin in SpΔ/Δ mutant mice. (A) Western blot analysis of protein extracted from non-transfected Cos-7 cells (NT) or transfected with constructs expressing GFP-Sp+ or GFP-SpΔ using S51 spastin polyclonal antibody (left panel) or GFP antibody (right panel). S51 antibody specifically recognizes the truncated spastin lacking its AAA domain (i.e. GFP-SpΔ), which is equally detected by the anti-GFP antibody. (B) Immunoblot analysis of spastin from 40 μg (left panel) or 10 μg (right panel) of 4-month-old Sp+/+, SpΔ/+ and SpΔ/Δ brain lysates using S51 spastin antibody. SpΔ/+ and SpΔ/Δ brain lysates do not show any traces of truncated spastin. Note the reduction or complete absence of wild-type spastin isoforms in SpΔ/+ and SpΔ/Δ mice, respectively. Actin was used as a loading control. (C) Quantification of spastin band density (including all spastin isoforms) normalized to actin values from three independent experiments. Spastin expression is reduced by 40% in SpΔ/+ mice compared with Sp+/+ mice, whereas no traces of wild-type spastin was detectable in SpΔ/Δ mutants. Vertical bars show s.e.m. *P<0.05, **P<0.001. A.U, arbitrary units.
Spastin mutation triggers axonal swellings in mouse cortical but not hippocampal neurons
Primary cultures of SpΔ/Δ cortical neurons revealed the presence of swellings in the distal part of the axons at the proximity of the growth cone (Tarrade et al., 2006). To determine whether this phenotype is restricted to cortical neurons or could be observed in other neuronal populations, we analyzed primary cultures of hippocampal neurons, in which spastin is normally highly expressed (Ma et al., 2006), from Sp+/+ and SpΔ/Δ mouse embryos. Hippocampal and cortical neurons were cultured in the same experimental conditions and processed at 6 days in vitro (DIV6) for acetylated α-tubulin immunolabeling. We first checked whether neurite outgrowth and branching were affected in SpΔ/Δ hippocampal neurons, which was not the case because they behaved similarly to Sp+/+ control neurons in that respect (Fig. 2A,B and data not shown). However, unlike SpΔ/Δ cortical neurons, hippocampal neurons from SpΔ/Δ mutant mice (n=4) did not show any axonal swellings in two independent experiments (Fig. 2B,G,H). To assess whether this observation was only due to a delayed occurrence of axonal swellings in these neurons, we analyzed the same experiments at DIV12 and confirmed the absence of swelling in cultured SpΔ/Δ hippocampal neurons (data not shown). These data suggest that a lack of spastin specifically affects cortical axon integrity in vitro. We then tested whether the sensitivity of cortical neurons to spg4 mutation could be explained by a cell-type specific expression of different spastin isoforms. To address this issue, we performed western blot analyses on 20 μg of total protein extracts from DIV6 cortical and hippocampal neurons using the S51 antibody (Errico et al., 2004) that recognizes four spastin isoforms including the long (translated from the first ATG) and short (translated from the second ATG) isoforms with or without exon 4. A similar expression pattern was observed between these two neuronal cell types, with a predominant expression of the short exon 4-containing isoform and a seeming absence of the full-length isoforms (Fig. 2I). Therefore, the cell specificity of axonal swelling cannot be assigned to a differential expression of spastin isoform(s). However, our findings show a selective sensitivity of mouse cortical neurons in response to the lack of spastin, which is consistent with the restricted phenotype observed in HSP patients.
Fig. 2.
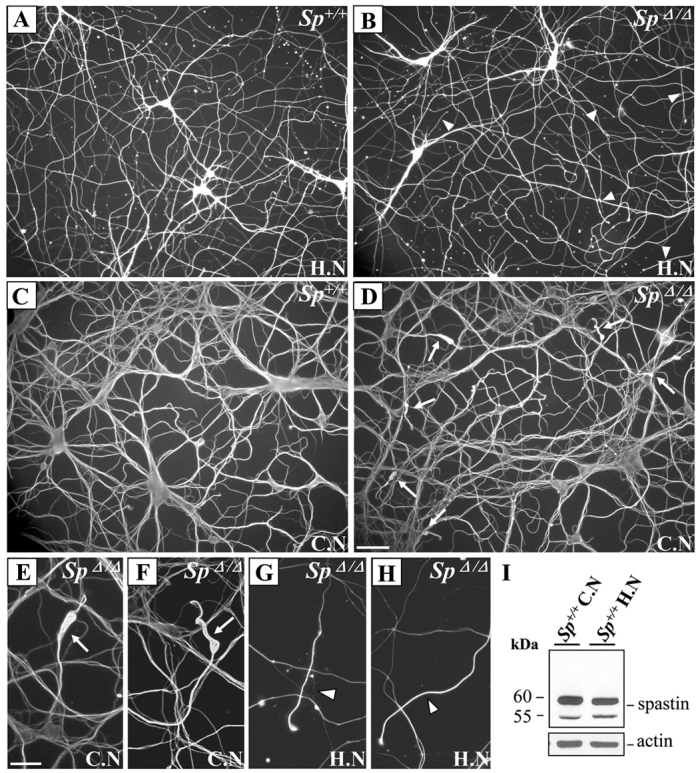
Spastin deletion specifically affects the integrity of cortical axons. (A–H) Immunodetection of acetylated α-tubulin in primary cultures of DIV6 hippocampal (A,B,G,H) and cortical neurons (C–F) from Sp+/+ (A,C) and SpΔ/Δ (B,D–H) embryos. Unlike SpΔ/Δ cortical neurons that show obvious axonal swellings (D–F; arrows), SpΔ/Δ hippocampal neurons do not display any neurite swellings in the distal part of their axons (arrowheads) or in other regions of the axon (B,G,H). Neurite density was not affected in SpΔ/Δ hippocampal neurons (B) compared with Sp+/+ hippocampal neurons (A). Images E–H represent higher magnification of SpΔ/Δ cortical (E,F) and hippocampal (G,H) axon distal regions. Scale bars: 50 μm (A–D); 20 μm (E–H). (I) Western blot analysis of spastin isoforms in protein lysates (20 μg) from primary cultures of Sp+/+ cortical and hippocampal neurons using the S51 spastin antiserum. Actin was used as a loading control. H.N, hippocampal neurons; C.N, cortical neurons.
Axonal transport is delayed in the distal region of SpΔ/Δ cortical axons
We previously reported that mitochondria and peroxysomes were abnormally accumulated in the proximal part of neurite swellings, which suggests a focal impairment of retrograde axonal transport in SpΔ/Δ cortical neurons (Tarrade et al., 2006). Here, we describe further exploration of the subcellular distribution of various organelles, such as endosomes, lysosomes and synaptic vesicles and show that they are all accumulated within axonal swellings (supplementary material Fig. S1A–C″), which indicates that the axonal transport defect of SpΔ/Δ cortical neurons is generalized to several, if not all, types of organelles. We next assessed retrograde axonal transport efficiency in primary cultures of SpΔ/Δ cortical neurons using the cholera toxin b subunit (CTb). CTb is internalized at the growth cone and retrogradely transported to the soma where it accumulates in the endoplasmic reticulum and Golgi (Lencer et al., 1999). Control Sp+/+ and mutant SpΔ/Δ cortical neurons were incubated with Alexa-Fluor-488-conjugated CTb at DIV6 for 15 minutes and washed in CTb-free medium for 30 minutes or 3 hours. After fixation, we evaluated the percentage of CTb-treated neurons showing an accumulation of the fluorescent toxin in their soma. Surprisingly, no significant difference was detected between the number of CTb-positive Sp+/+ and SpΔ/Δ neuron soma after 30 minutes or 3 hours of CTb washout (data not shown), suggesting that spastin depletion does not abolish retrograde axonal transport in cortical neurons. However, we observed an accumulation of CTb-positive vesicles in the proximal part of axonal swellings (supplementary material Fig. S1D–D″), as previously reported for other organelles, which confirms that retrograde axonal transport is locally perturbed in SpΔ/Δ cortical neurons.
To further characterize the deleterious effect of spastin depletion on axonal transport, we assessed mutant vesicular transport using phase-contrast video microscopy in DIV6 SpΔ/Δ cortical neurons, with special emphasis on axonal swellings (supplementary material Movie 1). The vesicles located within axonal swellings displayed apparent Brownian motion, whereas vesicles in non-swollen axons exhibited rapid linear movements towards the cell soma. This Brownian motion, which is reminiscent of the microtubule-unloaded cargo movement, was non-linear and led to non-productive translocation of the vesicles, resulting in their progressive accumulation in the proximal part of the swellings (supplementary material Movie 1). Moreover, we confirmed that retrograde axonal transport is not totally blocked in this specific region of the axon because we clearly observed vesicles leaving the swelling and being retrogradely transported towards the soma in a rapid and linear way, which resembles the movement of microtubule-based transport. Our results thus demonstrate that spastin depletion does not abolish retrograde axonal transport but increases cargo stalling in axon swellings, possibly by locally disturbing cargo loading on microtubules.
Major disorganization of the microtubule network within axonal swellings of spastin-deficient cortical neurons
In SpΔ/Δ cortical neurons, retrograde axonal transport is mainly impaired within and around the swellings. Because axonal swellings occur in a specific region of mutant axons, characterized by an abrupt transition between stable and dynamic microtubules (Tarrade et al., 2006), we examined whether this defect in axonal transport results from local perturbations of the microtubule network. To address this issue, we performed an ultrastructural study of axonal swellings with a special focus on microtubule architecture. Ultrathin sections of DIV6 primary cultures of Sp+/+ and SpΔ/Δ cortical neurons were processed for electron microscopy. This analysis revealed a major disorganization of the microtubule network within SpΔ/Δ cortical axonal swellings, which displayed unbundled, misoriented and curly microtubules (Fig. 3A–F, arrows) compared with Sp+/+ control axons where microtubules were organized in parallel arrays (Fig. 3G,H, arrows). Interestingly, we observed that some organelles are locally accumulated within the swellings but never entirely fill them, suggesting that swellings might primarily result from alterations of the microtubule network and not from an excessive accumulation of organelles (Fig. 3B,D). Our data thus indicate that spastin depletion affects microtubule organization in the distal part of cortical axons, which would lead to axonal transport defects within axon swellings.
Fig. 3.
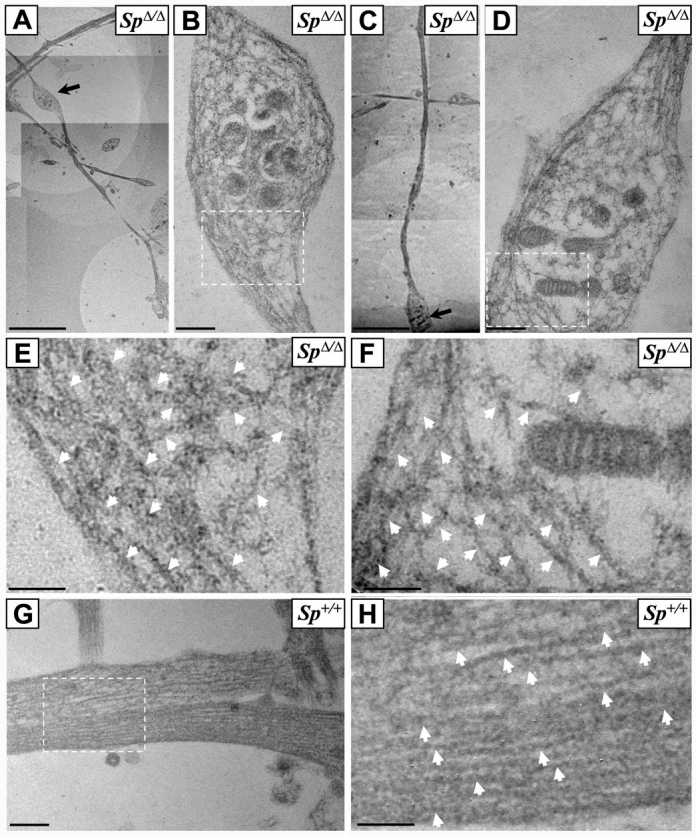
Ultrastructural analysis of SpΔ/Δ cortical neurons reveals a massive disorganization of the microtubule network within axonal swellings. (A–H) Ultrathin sections of DIV6 primary cultures of cortical neurons from SpΔ/Δ (A–F) and Sp+/+ mouse embryos (G–H). Images B and D are higher magnifications of axonal swellings indicated by arrows in images A and C, respectively. Images E, F and H are higher magnification of boxed regions in images B, D and G, respectively. Note the obvious disorganization and the tangled and bent aspect of microtubules within axonal swellings of SpΔ/Δ cortical neurons (B,D–F). This abnormal appearance of microtubules was never observed in Sp+/+ axons, in which microtubules are always organized in parallel arrays (G–H). White arrows indicate microtubules (E,F,H). Scale bars: 5 μm (A,C); 0.5 μm (B,D,G); 0.25 μm (E,F,H).
Early and marked impairment of microtubule disassembly in spastin-deficient cortical neurons
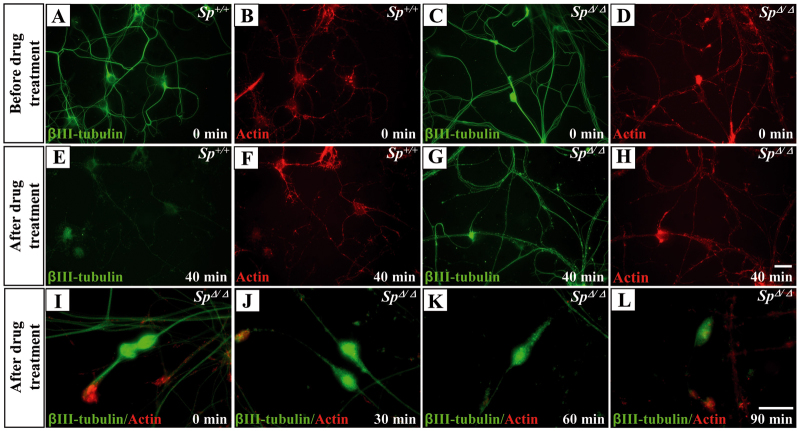
We previously reported that a marker of stable microtubules (detyrosinated-tubulin) was accumulated within axonal swellings, which suggested an excessive stabilization of the microtubules in this specific region (Tarrade et al., 2006). To confirm this aberrant stabilization, we first established that Tau, a microtubule-stabilizing protein, and δ2-tubulin, a marker of very long-lived microtubules (Paturle-Lafanechère et al., 1994), are similarly accumulated in the axonal swellings of SpΔ/Δ cortical neurons (supplementary material Fig. S2). To further demonstrate that spastin depletion leads to abnormal stabilization of the microtubule network, we compared the resistance of microtubules to the depolymerizing drug nocodazole in SpΔ/Δ and Sp+/+ cultured cortical neurons at DIV4 (i.e. before the occurrence of neurite swellings). When DIV4 Sp+/+ or SpΔ/Δ neurons were exposed to 30 μM nocodazole for 40 minutes, only residual tubulin staining was detectable in Sp+/+ cortical axons whereas a persistent and strong signal could be observed in SpΔ/Δ axons (Fig. 4A–H).
Fig. 4.
Impaired microtubule disassembly in SpΔ/Δ cultured cortical neurons. (A–L) Sp+/+ (A,B,E,F) and SpΔ/Δ (C,D,G–L) neurons at 4 days (A–H) or 6 days (I–L) post-plating. Neurons were incubated in the presence of 30 μM nocodazole for 40 minutes (E–H) or 40 μM nocodazole for 0 (I), 30 (J), 60 (K) or 90 minutes (L), then permeabilized in saponin-based buffer to extract free tubulin molecules and double-labeled for F-actin (red) and βIII-tubulin (green). After 40 minutes of exposure to 30 μM nocodazole, only a faint βIII-tubulin staining was still detectable in Sp+/+ cortical axons (E) whereas a persistent microtubule signal was observed in SpΔ/Δ axons (G). Note that the remaining microtubule signal was still present in SpΔ/Δ axonal swellings after 90-minute exposure to 40 μM nocodazole, whereas only a residual labeling was observed in the other regions of the axon shaft (L). Scale bars: 20 μm.
To compare microtubule stability between the swellings and other regions of SpΔ/Δ cortical axons, we performed another set of experiments in which DIV6 neurons were exposed to higher doses of nocodazole (40 μM) for longer periods of time (30, 60 or 90 minutes; Fig. 4I–L and data not shown). We have preliminarily assessed that this longer treatment does not affect the survival of cortical neurons (data not shown). Interestingly, after a 90-minute exposure, the labeling of microtubules was barely detectable along the axon shaft whereas a strong signal persisted in SpΔ/Δ axonal swelling (Fig. 4I–L). Together, our data indicate a marked and early impairment of microtubule disassembly all along the axon shaft of SpΔ/Δ cortical neurons, with an enhanced effect of microtubule stabilization within axonal swellings.
Spastin depletion decreases the number of microtubule plus ends in cortical axons
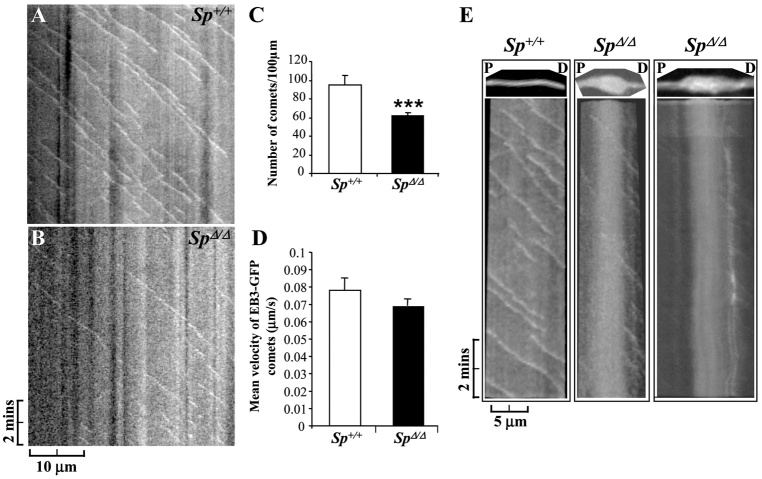
To assess whether the lack of microtubule severing reduces the number of dynamic plus ends within mutant cortical axons, we analyzed microtubule dynamics in Sp+/+ and SpΔ/Δ axons using the EB3-GFP fusion protein, which allowed us to visualize the plus ends of polymerizing microtubules (Stepanova et al., 2003). EB3 belongs to the microtubule plus-end tracking protein family (+TIPs), which binds to rapidly growing microtubule plus ends and gradually dissociates from microtubules when they convert from a polymerizing to a depolymerizing state. We electroporated Sp+/+ and SpΔ/Δ cortical neurons with an EB3-GFP construct and monitored the behavior of this fusion protein, which appears as moving comets within axons (Stepanova et al., 2003), by time-lapse videomicroscopy at DIV6 (Fig. 5 and supplementary material Movies 2–5). A total of 20 Sp+/+ and 29 SpΔ/Δ transfected axons were imaged and analyzed. Kymograph analysis of time-lapse recording revealed that the average number of EB3-GFP comets was significantly reduced in SpΔ/Δ axons (61.47±4.16) compared with Sp+/+ axons (95.26±9.35; P<0.001; Fig. 5A–C). Furthermore, the vast majority of EB3-GFP comets were moving in the anterograde direction in both Sp+/+ (98.97%) and SpΔ/Δ axons (96.24%), whereas the number of retrogradely moving comets per 100 μm of axonal length was not significantly different between Sp+/+ (1.31±0.80) and SpΔ/Δ axons (1.77±0.88; P=0,071; data not shown). Surprisingly, the mean velocity of EB3-GFP comets was not significantly decreased in SpΔ/Δ axons (0.069±0.004 μm/second) compared with Sp+/+ control axons (0.078±0.007 μm/second; P=0.236; Fig. 5D and supplementary material Movies 2–5), which suggests that spastin depletion does not affect microtubule polymerization rate. We also investigated the behavior of EB3-GFP comets within SpΔ/Δ axonal swellings, although the low frequency of swellings (∼5 axon swellings per 100 nuclei) associated with the small percentage of transfected neurons expressing the appropriate level of the fusion protein prevented a statistical analysis of EB3-GFP comet dynamics in the swellings compared with control axons. However, kymograph analysis of 10-minute time-lapse recordings in two SpΔ/Δ axonal swellings revealed that only very few EB3-GFP comets were moving within the swellings, as shown for non-swollen SpΔ/Δ axons, and that the vast majority of these moving comets are found in the distal end of the swellings (Fig. 5E and boxed regions of supplementary material Movies 2, 4 and 5).
Fig. 5.
Spastin loss of function decreases the number of dynamic microtubule plus ends in cortical axon shaft. (A–E) Time-lapse recording of EB3-GFP comets in DIV6 Sp+/+ (n=20) and SpΔ/Δ (n=29) cortical axons. (A,B) Kymographs of a Sp+/+ (A) and SpΔ/Δ (B) axon from a 10-minute time-lapse recording of EB3-GFP comets. These kymographs revealed a striking decrease in EB3-GFP moving comets in SpΔ/Δ axon shaft, which is associated with a weak movement of diffuse EB3-GFP protein compared with Sp+/+ axon. (C) Quantification of the average number of EB3-GFP comets per 100 μm of axonal length. (D) Quantification of the mean EB3-GFP comet velocity. The average number of EB3-GFP comets is significantly reduced in SpΔ/Δ axons compared with Sp+/+ axons (***P<0.001), whereas the mean comet speed is not affected by spastin depletion. Vertical bars show s.e.m. (E) Kymograph analysis of microtubule plus ends (i.e. EB3-GFP comets) in a selected Sp+/+ axonal region (left panel; see supplementary material Movie 2) and two distinct SpΔ/Δ swollen axons (middle and right panels; supplementary material Movies 4 and 5). The analyzed regions are boxed in supplementary material Movies 2, 4 and 5 and are presented on the top of each kymograph. Kymographs showed that only very few EB3-GFP comets are moving within swellings and that the vast majority of these moving comets are located in the most distal end of the swelling. P, proximal; D, distal. (A,B,E) Anterograde comets are represented by diagonal lines leading to the bottom right, whereas stationary comets (i.e. Fig. 5E, right panel) are shown by vertical white lines.
Together, these results demonstrate that the loss of spastin microtubule-severing activity significantly impairs the number of dynamic microtubule plus ends within cortical axon shafts, without affecting the polymerization rate of the remaining plus ends.
Substoichiometric concentrations of microtubule-targeting drugs alleviate the pathological phenotype of spastin-deficient cortical neurons
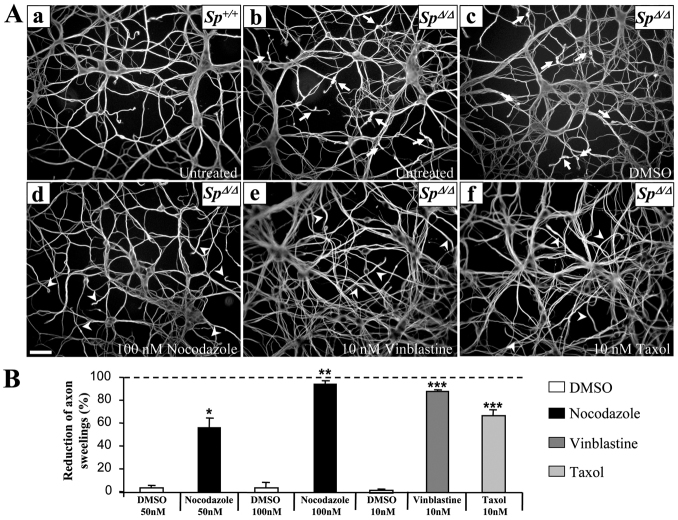
Finally, we checked whether a modulation of microtubule dynamics could modify the pathological phenotype of SpΔ/Δ cortical neurons. To preserve the overall architecture of neuron microtubule network, we used tubulin- and microtubule-targeting drugs at nanomolar concentrations, which are known to only modify microtubule dynamic instability (Jordan and Wilson, 1990; Jordan et al., 1991; Toso et al., 1993; Jordan et al., 1993; Tanaka et al., 1995; Vasquez et al., 1997; Witte et al., 2008). SpΔ/Δ neurons were treated before (DIV2) or as soon as the first swellings start to appear (DIV5) (Tarrade et al., 2006) with 50 nM nocodazole (DIV2), 100 nM nocodazole (DIV5), 10 nM vinblastine (DIV5), 10 nM taxol (DIV5) or with DMSO (control vehicle), fixed at DIV6 and immunolabeled with an acetylated α-tubulin antibody. These nanomolar doses of microtubule-targeting drugs do not affect the viability of cultured SpΔ/Δ cortical neurons at either DIV2 or DIV5, as determined by the mean percentage of apoptotic neurons with or without treatment in three independent experiments (n>1000 neurons in each condition, data not shown). Moreover, the number of neurite swellings per 100 nuclei was assessed in more than 1000 neurons per embryo (n=4) and for each experimental condition. Interestingly, we observed a significant and reproducible effect of the three microtubule-targeting agents on the number of axon swellings in SpΔ/Δ cortical neurons. Treatment of mutant neurons at DIV5 with 100 nM nocodazole successfully rescued the pathological phenotype of these neurons, reducing the number of neurite swellings by 95.1±2.6%, in comparison with SpΔ/Δ neurons treated with DMSO (reduction of 4.15±4%, P<0.001; Fig. 6Ab–Ad,B). Indeed, means of 6.8±0.6, 6.4±0.3 and 0.5±0.2 axonal swellings per 100 nuclei were respectively obtained for untreated, DMSO-treated and 100 nM nocodazole-treated SpΔ/Δ neurons. Moreover, the treatment of SpΔ/Δ DIV5 neurons with 10 nM vinblastine or 10 nM taxol decreased the swelling number by 88.5±1.14% and 67±5.08%, respectively, compared with the DMSO-treated control neurons (1±0,2%, P=0.001; Fig. 6Ac,Ae,Af,B). Means of 6.6±0.4, 6.5±0.7, 0.8±0.2 and 2.2±0.3 axonal swellings per 100 nuclei were obtained for untreated, DMSO-, 10 nM vinblastin- and 10 nM taxol-treated SpΔ/Δ neurons, respectively. Each set of experiments was reproduced three times independently. Because of the well-established role of microtubule dynamics in axon outgrowth, we assessed the effect of each drug treatment on neurite density. Importantly, neurite outgrowth was not affected when DIV5 SpΔ/Δ cortical neurons were treated with 100 nM nocodazole, 10 nM vinblastine or 10 nM taxol compared with untreated or DMSO-treated neurons (data not shown). To explore whether an earlier treatment could similarly prevent the formation of axonal swellings, we treated mutant cortical neurons with 50 nM nocodazole at DIV2. Interestingly, the treatment at DIV2 equally abolished the formation of neurite swellings but significantly affected neurite outgrowth (supplementary material Fig. S3).
Fig. 6.
Microtubule-targeting drugs rescue the pathological phenotype of SpΔ/Δ cortical neurons. (A) Immunolabeling of acetylated α-tubulin on DIV6 primary cultures of Sp+/+ (Aa) and SpΔ/Δ (Ab–Af) cortical neurons untreated (Aa,Ab) or treated 5 days post-plating with 100 nM of nocodazole (Ad), 10 nM of vinblastine (Ae), 10 nM of taxol (Af) or the equivalent volume of DMSO (vehicule; Ac). Note the absence of axonal swellings in the distal region of SpΔ/Δ cortical neurons treated with microtubule-targeting drugs (Ad–Af; arrowheads) compared with untreated or DMSO-treated SpΔ/Δ neurons (Ab,Ac; arrows). The neurite morphology of mutant neurons treated with microtubule-targeting drugs appears similar to that of Sp+/+ neurons (Aa). Scale bar: 50 μm. (B) The percentage of axonal swellings in SpΔ/Δ cortical neuron cultures was evaluated at DIV6. Note that the nanomolar concentration of microtubule targeting-drugs significantly decreases the proportion of neurite swellings in primary cultures of SpΔ/Δ neurons compared with DMSO-treated cultures. Asterisks indicate statistically different percentages between DMSO-treated neurons and 100 nM nocodazole-, 10 nM vinblastine- or 10 nM taxol-treated cells: *P<0.05, **P<0.001, ***P<0.0001. Vertical bars indicate s.e.m. More than 1000 neurons were analyzed per experimental condition.
Together, our data show that axonal swellings of spastin-deficient neurons result from a defect in the regulation of microtubule dynamics in cortical axons. Importantly, substoichiometric concentrations of microtubule-targeting drugs are able to rescue the main pathological phenotype of SpΔ/Δ mutant cortical neurons at DIV5, without affecting neurite density or neuron survival.
DISCUSSION
The goal of this study was to investigate the molecular mechanisms responsible for the phenotype of spastin knockout mutant mice using primary cultures of cortical neurons. We here provide compelling evidence that the lack of mouse spastin leads to abnormal stabilization and organization of the microtubule network along cortical axons associated with the occurrence of swellings. This abnormal stabilization of the microtubule network in SpΔ/Δ cortical neurons is consistent with previous studies in Drosophila showing that the knockdown of D-spastin equally causes an increased stabilization of the microtubule network at the neuromuscular junction associated with synaptic growth and neurotransmission defects (Trotta et al., 2004; Orso et al., 2005). Because alterations of microtubule dynamics are also observed in SpΔ/Δ non-swollen axons, and microtubule-targeting drugs prevent the occurrence of axonal swellings in mutant cortical neurons, our data strongly suggest that misregulation of microtubule dynamics is a primary event in the axonopathy of our mouse mutant and, thereby, in the pathogenesis of SPG4-linked HSP.
On the basis of the well-characterized role of spastin in microtubule severing (Evans et al., 2005; White et al., 2007), we propose that the abnormal dynamics of microtubules results from the loss of spastin microtubule-severing activity. Recent studies have shown that spastin preferentially severs microtubules harboring specific post-translational modifications of their C-terminal tail (Roll-Mecak and Vale, 2008; Labroid et al., 2010). For example, the use of an antibody against the exposed glutamate residues on the tubulin C-terminal tail totally inhibits spastin-mediated severing, whereas such inhibition never occurs with an antibody directed against tyrosinated residues located in the same region (Roll-Mecak and Vale, 2008). Furthermore, spastin overexpression in neurons preferentially disrupts detyrosinated (Glu-tubulin) and acetylated (i.e. stable microtubules) rather than tyrosinated microtubules (i.e. dynamic microtubules) (Riano et al., 2009), demonstrating that spastin selectively severs specific subpopulations of microtubules. Accordingly, we previously showed that the lack of spastin microtubule-severing activity in knockout mice induces an accumulation of detyrosinated and δ2-tubulin (two markers of stable microtubules) within axonal swellings of SpΔ/Δ cortical neurons (Tarrade et al., 2006). Moreover, we have here established that spastin loss of function strikingly decreases the number of dynamic microtubule plus ends within the axon shaft of cortical neurons. Importantly, spastin knockdown was similarly shown to reduce the number of microtubules plus ends in zebrafish developing neurons (Butler et al., 2010) whereas its overexpression was reported to increase the number of EB3 comets in rat hippocampal neurons (Qiang et al., 2010). Together, these results suggest that spastin loss of function leads to the formation of longer and ‘long-lived’ microtubules that are potentially submitted to various post-translational modifications (such as detyrosination and acetylation), which can influence the recruitment of microtubule-associated proteins (MAP) and therefore change the dynamic properties of these polymers. For example, it has been shown that long-lived detyrosinated microtubules become less susceptible to the depolymerizing activity of the molecular motor kinesin 13 (Peris et al., 2009), which additionally increases microtubule stability. Long-lived microtubules could also be recognized by microtubule-stabilizing proteins (like Tau and MAP1B), further enhancing their stability. This paradigm is supported by the local accumulation of Tau in axonal swellings of spastin-deficient cortical neurons as shown in this study (see also Kasher et al., 2009).
Furthermore, several studies have shown that the increased density of MAP along microtubules significantly impairs plus-end-directed microtubule transport by reducing the frequency of interaction between kinesin and microtubules (for review, see Baas and Qiang, 2005). The increased stability of the microtubule network caused by spastin depletion might thus be primarily responsible for the impaired anterograde axonal transport of mitochondria and organelles described in non-swollen SpastΔE7/ΔE7 cortical axons from another mutant mouse (Kasher et al., 2009). Interestingly, whereas anterograde transport is affected all along SpastΔE7/ΔE7 axons (Kasher et al., 2009), we and others have described a specific alteration in retrograde axonal transport in the distal part of both SpastΔE7/ΔE7 and SpΔ/Δ cortical axons (Kasher et al., 2009). Intriguingly, although the axonal swellings of SpastΔE7/ΔE7 mice are morphologically similar to those observed in the spinal cord of our SpΔ/Δ mice, SpastΔE7/ΔE7 axonal swellings are stochastically distributed along the axons of cultured neurons (Kasher et al., 2009), whereas axonal swellings only occur in the distal part of SpΔ/Δ axons in our cultures (Tarrade et al., 2006). The discrepancy between these two mouse models might be due either to different genetic background or to diverse experimental conditions used for primary cultures of neurons.
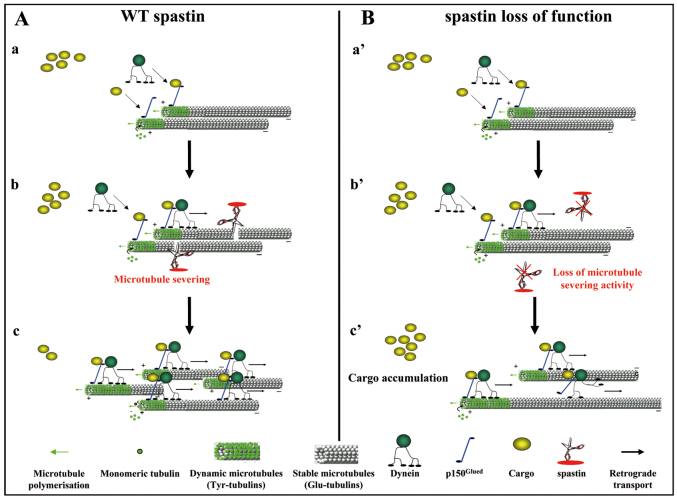
In our model, the specific localization of the swellings in the distal region of the axon suggests a higher sensitivity of this area to the lack of spastin microtubule-severing activity and therefore provides a potential explanation for the dying-back axonopathy in SPG4-linked HSP. This increased sensitivity might be explained by spastin enrichment in the distal portion of the axons in normal conditions (Errico et al., 2004; Yu et al., 2008) but can also be generated by the lack of a highly specialized function of spastin in this particular region. Because loss of spastin causes alterations in both microtubule dynamics and retrograde axonal transport in this region, we propose that spastin could participate in the local coupling of microtubule severing and axonal transport. Using live imaging, we show that the vesicles located within axonal swellings display Brownian motion that is reminiscent of that described for microtubule-uncharged cargoes, and that the local accumulation of organelles at the swelling proximal region results from delayed and impaired transport rather than from a complete obstruction of retrograde axonal transport. Our results therefore suggest that spastin depletion might locally alter cargo loading onto microtubules. Interestingly, tubulin post-translational modifications were shown to influence the recruitment of protein complexes such as MAPs (i.e. Tau, MAP1B) or plus-end tracking proteins (p150Glued, EB1, CLIP170) onto microtubules (Verhey and Gaertig, 2007). In primary cultures of mouse fibroblasts and neurons from tubulin tyrosine ligase (TTL) knockout mice, the accumulation of detyrosinated tubulin leads to the abnormal localization of the CAP-Gly plus-end-tracking proteins (+TIPs) such as p150Glued, a member of the dynein complex, and CLIP170 (Peris et al., 2006). Because p150Glued has a crucial role in cargo loading and microtubule retrograde transport (Vaughan et al., 2002) and CLIP in linking organelle membranes to microtubules (Pierre et al., 1992; Rickard and Kreis, 1996), we propose that the accumulation of detyrosinated tubulin within SpΔ/Δ axonal swellings might affect the recruitment of these +TIPs to microtubule plus ends and thereby the efficiency of retrograde axonal transport. This hypothesis leads us to propose a model in which spastin microtubule-severing activity creates novel microtubule plus ends, in the distal part of the axons, that are used as local nucleation points for the synthesis of tyrosinated dynamic microtubules as well as for p150Glued and CLIP cargo loading onto microtubules (Fig. 7A). Alterations of spastin function might thus decrease dynamic microtubule plus ends (Butler et al., 2010), which would dampen microtubule dynamics as well as cargo loading onto microtubules and thereby axonal transport (Fig. 7B).
Fig. 7.
Hypothetical model of spastin function. (A,B) Model of spastin function in the distal part of cortical axons in physiological (A) and pathological (B) conditions. Cargoes that should be retrogradely transported are recruited on microtubule plus ends via p150Glued, a member of the dynactin complex. Then, the recruitment of the molecular motor dynein via p150Glued allows retrograde transport of cargoes to the soma along microtubules (Aa; Ba′). In the distal part of the axon, spastin severs microtubules to generate new microtubules plus ends (Ab) that might serve as local nucleation points for the synthesis of dynamic microtubules (Ac) and also improve retrograde axonal transport efficiency by increasing the number of microtubules plus ends, and thereby the capacity of cargo loading onto microtubules via p150Glued (Ac). In this region of the axon, loss of spastin microtubule-severing activity (Bb′) might reduce the number of microtubule plus ends, which will subsequently affect both microtubule dynamics and cargo loading efficiency (Bc′).
Interestingly, neuropathological studies of patients with SPG4-linked HSP reveal the presence of axonal swellings in the corticospinal tracts and posterior columns (Kasher et al., 2009). This observation indicates that the pathogenic mechanisms responsible for the axonopathy in the spastin knockout mice (Tarrade et al., 2006; Kasher et al., 2009) seem to be conserved in the human disease and that the two existing mouse models are thus particularly relevant to study the physiopathology of SPG4-linked HSP.
Finally, we show that nanomolar concentrations of microtubule-targeting-drugs, such as nocodazole, vinblastine or taxol, alleviate the main pathological feature of spastin-depleted cortical neurons by dramatically reducing the number of axon swellings. Our findings corroborate a previous analysis in Drosophila, which showed that the treatment of spastin-depleted larvae with low doses of vinblastine (i.e. 5 nM) significantly attenuates the pathological phenotype associated with either D-spastin knockdown or overexpression of a mutated (K467R) D-spastin (Orso et al., 2005). Our analysis thus reveals that the pathogenic processes generated by spastin loss-of-function, which could be similarly rescued in Drosophila and mouse models, are highly conserved between invertebrates and vertebrates. Although high concentrations of nocodazole and vinblastine destabilize microtubules and taxol stabilizes microtubules at high doses, low and substoichiometric concentrations of each of these three compounds, as used in the present study, have the same effect and dampen microtubule dynamic instability without a significant impact on microtubule mass (for review, see Jordan and Wilson, 2004; Jordan and Wilson, 1990; Jordan et al., 1991; Toso et al., 1993; Jordan et al., 1993; Tanaka et al., 1995; Vasquez et al., 1997). This paradigm thus implies that under our experimental conditions, these low doses of taxol would not further enhance the whole microtubular mass stability, which would have worsened the phenotype of mutant cortical axons, but might instead prevent the formation of axonal swellings by locally restraining microtubule dynamic instability at the end of axons, with minimal effect on microtubule structural integrity. Importantly, treatments of Aplysia bag cell neurons with similar doses of taxol and vinblastine to those used here resulted in the same effect on growing axons in vitro. Indeed, at these nanomolar concentrations, both drugs suppress microtubule extension during growth cone steering and turning by dampening microtubule dynamics (Suter et al., 2004). Using videomicroscopy, Suter and colleagues demonstrated that taxol or vinblastine application causes microtubules to undergo long pauses in their assembly, which subsequently block microtubule advance within the growth cone (Suter et al., 2004). Similarly, low doses of nocodazole, taxol or vinblastine might prevent the formation of novel axonal swellings in SpΔ/Δ cortical neurons by blocking microtubule assembly in the distal region of the axon.
In conclusion, our analysis shows that a modulation of microtubule dynamics using different microtubule-targeting drugs can efficiently reduce the occurrence of axonal swellings in mammalian spastin-deficient neurons. Although the molecular bases underlying the rescue of SpΔ/Δ neurons by these drugs need to be further dissected, our results provide new insights into potential suppressors of the early axonal phenotype in mammalian models of HSP, which might orientate future development of therapeutic strategies.
METHODS
Antibodies
The following primary antibodies were used: acetylated α-tubulin (clone 6-11B-1, Sigma), actin (clone AC-40, Sigma), EEA1 (clone 14, BD Transduction Laboratories), Lamp-1 (clone 1D4B, PharMingen), snap23a (G-16, Santa Cruz Biotechnology), β-III tubulin (TUJ-1, Covance), Tau-1 (clone PC1C6, Millipore), δ2-tubulin (kindly provided by Annie Andrieux, Grenoble Institute of Neuroscience, Grenoble, France), phosphorylated NF 200 (SMI 31, Chemicon), Caspase 3 (R&D Systems), GFP (Invitrogen) and the S51 rabbit polyclonal antiserum against spastin [kindly provided by Elena Rugarli, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany)] (Errico et al., 2004).
Western blot analysis
Immunoblots were performed using 10–40 μg of total brain protein extracts from 4-month-old Sp+/+, SpΔ/+ and SpΔ/Δ mice (Tarrade et al., 2006) or 5 μg of total protein lysate from Cos-7 mock cells or cells transfected with GFP-Sp+ or GFP-SpΔ (Tarrade et al., 2006). Protein extracts were prepared using an SDS-lysis buffer (25 mM sodium sulfate pH 7.2, 5 mM EDTA, 1% SDS) supplemented with 1 mM PMSF and a cocktail of protease inhibitors (Roche), and dosed with a BCA assay. The extracts were subjected to electrophoresis on 10% SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were incubated overnight at 4°C with spastin polyclonal antiserum S51 (1:1000), anti-GFP polyclonal antibody (1:6000) or anti-actin monoclonal antibody (1:10,000). After several washes in PBS-T buffer (PBS, 0.05% Tween 20), membranes were incubated for one hour at room temperature with horseradish peroxidase-labeled goat anti-rabbit or anti-mouse antibodies (Santa Cruz Biotechnology). Immunostained proteins were visualized using an enhanced chemiluminescence detection system (Santa Cruz Biotechnology). Spastin levels from 4-month-old Sp+/+, SpΔ/+ and SpΔ/Δ mouse brain extracts were estimated by quantifying the immunoblot band density normalized to actin values (ImageJ software, NIH, Bethesda, MD) in three independent experiments. Values were statistically compared using an unpaired t-test (Statview software).
Primary cultures of cortical and hippocampal neurons
Primary cultures of cortical neurons were prepared from Sp+/+ and SpΔ/Δ embryos at 14.5 days post coitum (d.p.c.) as described previously (Tarrade et al., 2006). Primary cultures of hippocampal neurons were prepared from 17.5 d.p.c. Sp+/+ and SpΔ/Δ embryos. Hippocampi were dissected in Mg2+, Ca2+-free HBSS, and incubated with trypsin (0.25% trypsin in Mg2+, Ca2+-free HBSS) for 20 minutes at 37°C. Hippocampi were then washed three times in Mg2+, Ca2+-free HBSS and mechanically dissociated by repeated triturations in Neurobasal Medium (Invitrogen) supplemented with 2% B27 (Invitrogen). Hippocampal neurons were plated as previously described for cortical neurons (Tarrade et al., 2006). Cells were maintained for 6 or 12 days at 37°C in 5% CO2. The assessment of neurite outgrowth was performed using a stereological method as previously reported (Ronn et al., 2000). All animal procedures were performed in accordance with institutional guidelines (agreement B91-228-2 and 3429).
Retrograde labeling experiments
Retrograde axonal transport was analyzed in Sp+/+ and SpΔ/Δ cortical neurons using the neuronal retrograde tracer cholera toxin B (CTb) conjugated with Alexa Fluor 488 (Molecular Probes). Six days after plating, Sp+/+ and SpΔ/Δ neurons were incubated with 1μg/ml of CTb Alexa Fluor 488 for 15 minutes at 37°C in Neurobasal Medium plus B27 and washed in CTb-free medium for 30 minutes or 3 hours. Cortical neurons were fixed with 2% paraformaldehyde in 1× PBS supplemented with 0.16 M sucrose for 10 minutes at 37°C. Cells were processed for immunolabeling of acetylated α-tubulin or actin and mounted with Vectashield and Dapi for observation. For each experimental condition, we analyzed more than 100 neurons and scored the percentage of soma showing an accumulation of CTb. Control and mutant scores were statistically compared using an unpaired t-test (Statview software).
Time-lapse video microscopy
For phase-contrast video microscopy, DIV6 SpΔ/Δ cortical neurons were placed in a chamber at 37°C under 5% of CO2. Individual axonal swelling was imaged using an Axiophot microscope (Zeiss). The movie is composed of 500-millisecond stills taken every 30 seconds over a period of 9 hours. To visualize microtubule plus-end dynamics, Sp+/+ and SpΔ/Δ cortical neurons were transfected before plating with 1 μg of DNA (EB3-GFP; kindly provided by Fatiha Nothias, UMR CNRS 7224/INSERM U952/UPMC, Paris, France), using the Nucleofector apparatus and the Basic Nucleofector Kit for primary mammalian neurons (Lonza, Basel, Switzerland). Time-lapse recording of EB3-GFP comets were performed at 37°C on DIV6 Sp+/+ and SpΔ/Δ transfected cortical neurons using a Leica DMI 6000B inverted spinning disk microscope with a 63× immersion objective. Images were acquired every 2 seconds over a period of 10 minutes. A minimal total of 20 axons were analyzed per genotype. Comet velocity and number were estimated using Kymograph analysis (yt, images; Fiji software), with lines drawn along the axon. Kymograph production and analysis were automated using a Fiji macro designed and written by P.M. Statistical analyses were performed using the Student’s t-test (Statview software). Kymographs of swollen axons were generated by analyzing the behavior of EB3-GFP comets in the whole volume of the swellings using the same method.
Electron microscopy
Primary cultures of DIV6 cortical neurons were fixed with 4% paraformaldehyde and 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and processed for electron microscopy as described previously (Tarrade et al., 2006; Frugier et al., 2000). Ultrathin sections were stained with uranyl acetate and lead citrate, and examined with a Tecnai F20 transmission electron microscope (Philips) at 200 kV.
Nocodazole susceptibility assay
Cultured Sp+/+ and SpΔ/Δ cortical neurons were treated with 30 μM nocodazole (Sigma) in DMSO or with DMSO alone for 40 minutes, permeabilized in PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, and 2 mM MgCl2, pH 6.9) with 0.02% saponin and 10 μM taxol, and fixed in PHEM buffer with 2% paraformaldehyde and 0.05% glutaraldehyde. The whole microtubule network was analyzed using a ßIII-tubulin antibody; cell morphology was visualized by actin filament immunostaining with phalloidin. Moreover, we carried out another set of experiments in which SpΔ/Δ cortical neurons were treated with 40 μM nocodazole for 0, 30, 60 or 90 minutes before fixation.
Pharmacological treatment of cortical neurons
Primary cultures of Sp+/+ and SpΔ/Δ cortical neurons were treated 2 or 5 days after plating with 50 nM nocodazole, 100 nM nocodazole, 10 nM vinblastine (Sigma), 10 nM taxol (Sigma) or DMSO (as a control) in Neurobasal Medium supplemented with 2% B27. Six days after plating, cortical neurons were fixed with 2% paraformaldehyde and 0.16 M sucrose in PBS for 10 minutes at 37°C, processed through immunolabeling of acetylated α-tubulin and mounted with Vectashield and Dapi. The number of neurite swellings per 100 nuclei was determined for each condition and scores were statistically compared using an unpaired t-test (Statview software). Each set of experiments was reproduced three times independently and more than 1000 neurons were analyzed per experiment.
Immunocytochemistry
After several washes in PBS, cells were fixed with 2% paraformaldehyde and 0.16 M sucrose for 10 minutes at 37°C, permeabilized with 0.1% Triton X-100 in PBS for 5 minutes and left in blocking reagent (3% BSA and 5% normal goat or donkey serum in PBS) for 1 hour at room temperature. Primary antibodies were diluted to the appropriate concentrations in the blocking reagent and incubated with cells overnight at 4°C. Cells were subsequently washed four times with PBS, incubated for 1 hour at room temperature with the appropriate secondary antibodies and washed again in PBS. Cells were mounted with Vectashield and Dapi and observed with an epifluorescence microscope (Zeiss Axiophot).
Supplementary Material
Acknowledgments
We are grateful to Elena Rugarli and Annie Andrieux for providing us with spastin and δ2-tubulin antibodies, respectively. We thank Alain Thorel and Mohamed Sennour (Centre des Matériaux, Ecole des Mines, Evry, France) for their help in electron microscopy, and Richard Schwartzmann from the Cell Imaging and Flow Cytometry facility of the IFR83 (Paris, France) for his precious help in time-lapse videomicroscopy of EB3-GFP comets. We also thank the SPATAX network for helpful discussions, Genopole (Evry) for constant support to P.A.C. and the Neuropôle de Recherche Francilien (Nerf) for the post-doctoral fellowship attributed to J.L.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
J.M. and C.F. conceived the project and designed the experiments. C.F., A.T. and L.P. performed all experiments and, together with J.M., analyzed the data. S.C., P.M., C.D., S.D. and N.R. provided technical assistance. P.C. and D.J. supervised A.T. and L.P. in their respective laboratories and had insightful comments on the project. C.F., J.H., L.P. and J.M. wrote the manuscript.
FUNDING
The IFR83 facility is supported by the Conseil regional d’Ile-de-France. This work was supported by INSERM, the Fédération pour la Recherche sur le Cerveau, GIS-ANR Maladies Rares, Université d’Evry and the Conseil Régional d’Ile de France to J.M.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.008946/-/DC1
REFERENCES
- Baas P. W., Qiang L. (2005). Neuronal microtubules: when the MAP is the roadblock. Trends Cell Biol. 15, 183–187 [DOI] [PubMed] [Google Scholar]
- Baas P. W., Karabay A., Qiang L. (2005). Microtubules cut and run. Trends Cell Biol. 15, 518–524 [DOI] [PubMed] [Google Scholar]
- Blackstone C., O’Kane C. J., Reid E. (2011). Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nat. Rev. Neurosci. 12, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R., Wood J. D., Landers J. A., Cunliffe V. T. (2010). Genetic and chemical modulation of spastin-dependent axon outgrowth in zebrafish embryos indicates a role for impaired microtubule dynamics in hereditary spastic paraplegia. Dis. Model. Mech. 3, 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvin D., Cifuentes-Diaz C., Fonknechten N., Joshi V., Hazan J., Melki J., Betuing S. (2003). Mutations of SPG4 are responsible for a loss of function of spastin, an abundant neuronal protein localized in the nucleus. Hum. Mol. Genet. 12, 71–78 [DOI] [PubMed] [Google Scholar]
- Claudiani P., Riano E., Errico A., Andolfi G., Rugarli E. I. (2005). Spastin subcellular localization is regulated through usage of different translation start sites and active export from the nucleus. Exp. Cell Res. 309, 358–369 [DOI] [PubMed] [Google Scholar]
- Connell J. W., Lindon C., Luzio J. P., Reid E. (2009). Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic 10, 42–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C., Fedirko E., Forlani S., Cazeneuve C., Ribaï P., Feki I., Tallaksen C., Nguyen K., Stankoff B., Ruberg M., et al. (2007). Exon deletions of SPG4 are a frequent cause of hereditary spastic paraplegia. J. Med. Genet. 44, 281–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., Ozdowski E. F., Kotowski I. K., Marchuk D. A., Sherwood N. T. (2010). Functional conservation of human Spastin in a Drosophila model of autosomal dominant-hereditary spastic paraplegia. Hum. Mol. Genet. 19, 1883–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico A., Ballabio A., Rugarli E. I. (2002). Spastin, the protein mutated in autosomal dominant hereditary spastic paraplegia, is involved in microtubule dynamics. Hum. Mol. Genet. 11, 153–163 [DOI] [PubMed] [Google Scholar]
- Errico A., Claudiani P., D’Addio M., Rugarli E. I. (2004). Spastin interacts with the centrosomal protein NA14, and is enriched in the spindle pole, the midbody and the distal axon. Hum. Mol. Genet. 13, 2121–2132 [DOI] [PubMed] [Google Scholar]
- Evans K. J., Gomes E. R., Reisenweber S. M., Gundersen G. G., Lauring B. P. (2005). Linking axonal degeneration to microtubule remodeling by Spastin-mediated microtubule severing. J. Cell Biol. 168, 599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. K. (2006). Hereditary spastic paraplegia. Curr. Neurol. Neurosci. Rep. 6, 65–76 [DOI] [PubMed] [Google Scholar]
- Fonknechten N., Mavel D., Byrne P., Davoine C. S., Cruaud C., Bönsch D., Samson D., Coutinho P., Hutchinson M., McMonagle P., et al. (2000). Spectrum of SPG4 mutations in autosomal dominant spastic paraplegia. Hum. Mol. Genet. 9, 637–644 [DOI] [PubMed] [Google Scholar]
- Frugier T., Tiziano F. D., Cifuentes-Diaz C., Miniou P., Roblot N., Dierich A., Le Meur M., Melki J. (2000). Nuclear targeting defect of SMN lacking the C-terminus in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 9, 849–858 [DOI] [PubMed] [Google Scholar]
- Hazan J., Fonknechten N., Mavel D., Paternotte C., Samson D., Artiguenave F., Davoine C. S., Cruaud C., Dürr A., Wincker P., et al. (1999). Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat. Genet. 23, 296–303 [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Wilson L. (1990). Kinetic analysis of tubulin exchange at microtubule ends at low vinblastine concentrations. Biochemistry 29, 2730–2739 [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Wilson L. (2004). Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–265 [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Thrower D., Wilson L. (1991). Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 51, 2212–2222 [PubMed] [Google Scholar]
- Jordan M. A., Toso R. J., Thrower D., Wilson L. (1993). Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc. Natl. Acad. Sci. USA 90, 9552–9556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabay A., Yu W., Solowska J. M., Baird D. H., Baas P. W. (2004). Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. J. Neurosci. 24, 5778–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasher P. R., De Vos K. J., Wharton S. B., Manser C., Bennett E. J., Bingley M., Wood J. D., Milner R., McDermott C. J., Miller C. C., et al. (2009). Direct evidence for axonal transport defects in a novel mouse model of mutant spastin-induced hereditary spastic paraplegia (HSP) and human HSP patients. J. Neurochem. 110, 34–44 [DOI] [PubMed] [Google Scholar]
- Lacroix B., Van Dijk J., Gold N. D., Guizetti J., Aldrian-Herrada G., Rogowski K., Gerlich D. W., Janke C. (2010). Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J. Cell Biol. 189, 945–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer W. I., Hirst T. R., Holmes R. K. (1999). Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450, 177–190 [DOI] [PubMed] [Google Scholar]
- Lumb J. H., Connell J. W., Allison R., Reid E. (2012). The AAA ATPase spastin links microtubule severing to membrane modelling. Biochim. Biophys. Acta 1823, 192–197 [DOI] [PubMed] [Google Scholar]
- Ma D. L., Chia S. C., Tang Y. C., Chang M. L., Probst A., Burgunder J. M., Tang F. R. (2006). Spastin in the human and mouse central nervous system with special reference to its expression in the hippocampus of mouse pilocarpine model of status epilepticus and temporal lobe epilepsy. Neurochem. Int. 49, 651–664 [DOI] [PubMed] [Google Scholar]
- Orso G., Martinuzzi A., Rossetto M. G., Sartori E., Feany M., Daga A. (2005). Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J. Clin. Invest. 115, 3026–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. H., Zhu P. P., Parker R. L., Blackstone C. (2010). Hereditary spastic paraplegia proteins REEP1, spastin and atlastin-1 coordinate microtubule interactions with the tubular ER network. J. Clin. Invest. 120, 1097–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturle-Lafanechère L., Manier M., Trigault N., Pirollet F., Mazarguil H., Job D. (1994). Accumulation of delta 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J. Cell Sci. 107, 1529–1543 [DOI] [PubMed] [Google Scholar]
- Peris L., Thery M., Fauré J., Saoudi Y., Lafanechère L., Chilton J. K., Gordon-Weeks P., Galjart N., Bornens M., Wordeman L., et al. (2006). Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J. Cell Biol. 174, 839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris L., Wagenbach M., Lafanechère L., Brocard J., Moore A. T., Kozielski F., Job D., Wordeman L., Andrieux A. (2009). Motor-dependent microtubule disassembly driven by tubulin tyrosination. J. Cell Biol. 185, 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre P., Scheel J., Rickard J. E., Kreis T. E. (1992). CLIP-170 links endocytic vesicles to microtubules. Cell 70, 887–900 [DOI] [PubMed] [Google Scholar]
- Qiang L., Yu W., Liu M., Solowska J. M., Baas P. W. (2010). Basic fibroblast growth factor elicits formation of interstitial axonal branches via enhanced severing of microtubules. Mol. Biol. Cell 21, 334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid E. (2003). Science in motion: common molecular pathological themes emerge in the hereditary spastic paraplegias. J. Med. Genet. 40, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riano E., Martignoni M., Mancuso G., Cartelli D., Crippa F., Toldo I., Siciliano G., Di Bella D., Taroni F., Bassi M. T., et al. (2009). Pleiotropic effects of spastin on neurite growth depending on expression levels. J. Neurochem. 108, 1277–1288 [DOI] [PubMed] [Google Scholar]
- Rickard J. E., Kreis T. E. (1996). CLIPs for organelle-microtubule interactions. Trends Cell Biol. 6, 178–183 [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A., Vale R. D. (2005). The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr. Biol. 15, 650–655 [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A., Vale R. D. (2008). Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 451, 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronn L. C., Ralets I., Hartz B. P., Bech M., Berezin A., Berezin V., Moller A., Bock E. (2000). A simple procedure for quantification of neurite outgrowth based on stereological principles. J. Neurosci. Methods 100, 25–32 [DOI] [PubMed] [Google Scholar]
- Salinas S., Carazo-Salas R. E., Proukakis C., Cooper J. M., Weston A. E., Schiavo G., Warner T. T. (2005). Human spastin has multiple microtubule-related functions. J. Neurochem. 95, 1411–1420 [DOI] [PubMed] [Google Scholar]
- Salinas S., Proukakis C., Crosby A., Warner T. T. (2008). Hereditary spastic paraplegia: clinical features and pathogenic mechanisms. Lancet Neurol. 7, 1127–1138 [DOI] [PubMed] [Google Scholar]
- Sherwood N. T., Sun Q., Xue M., Zhang B., Zinn K. (2004). Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2, 2094–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowska J. M., Morfini G., Falnikar A., Himes B. T., Brady S. T., Huang D., Baas P. W. (2008). Quantitative and functional analyses of spastin in the nervous system: implications for hereditary spastic paraplegia. J. Neurosci. 28, 2147–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowska J. M., Garbern J. Y., Baas P. W. (2010). Evaluation of loss of function as an explanation for SPG4-based hereditary spastic paraplegia. Hum. Mol. Genet. 19, 2767–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T., Slemmer J., Hoogenraad C. C., Lansbergen G., Dortland B., De Zeeuw C. I., Grosveld F., van Cappellen G., Akhmanova A., Galjart N. J. (2003). Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J. Neurosci. 23, 2655–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter D. M., Schaefer A. W., Forscher P. (2004). Microtubule dynamics are necessary for Src family kinase-dependent growth cone steering. Curr. Biol. 14, 1194–1199 [DOI] [PubMed] [Google Scholar]
- Tanaka E., Ho T., Kirschner M. W. (1995). The role of microtubule dynamics in growth cone motility and axonal growth. J. Cell Biol. 128, 139–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrade A., Fassier C., Courageot S., Charvin D., Vitte J., Peris L., Thorel A., Mouisel E., Fonknechten N., Roblot N., et al. (2006). A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubule composition. Hum. Mol. Genet. 15, 3544–3558 [DOI] [PubMed] [Google Scholar]
- Toso R. J., Jordan M. A., Farrell K. W., Matsumoto B., Wilson L. (1993). Kinetic stabilization of microtubule dynamic instability in vitro by vinblastine. Biochemistry 32, 1285–1293 [DOI] [PubMed] [Google Scholar]
- Trotta N., Orso G., Rossetto M. G., Daga A., Broadie K. (2004). The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr. Biol. 14, 1135–1147 [DOI] [PubMed] [Google Scholar]
- Vasquez R. J., Howell B., Yvon A. M., Wadsworth P., Cassimeris L. (1997). Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 8, 973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. S., Miura P., Henderson M., Byrne B., Vaughan K. T. (2002). A role for regulated binding of p150 (Glued) to microtubule plus ends in organelle transport. J. Cell Biol. 158, 305–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey K. J., Gaertig J. (2007). The tubulin code. Cell Cycle 6, 2152–2160 [DOI] [PubMed] [Google Scholar]
- White S. R., Evans K. J., Lary J., Cole J. L., Lauring B. (2007). Recognition of C-terminal amino acids in tubulin by pore loops in spastin is important for microtubule severing. J. Cell Biol. 176, 995–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte H., Neukirchen D., Bradke F. (2008). Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180, 619–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. D., Landers J. A., Bingley M., McDermott C. J., Thomas-McArthur V., Gleadall L. J., Shaw P. J., Cunliffe V. T. (2006). The microtubule-severing protein spastin is essential for axon outgrowth in the zebrafish embryo. Hum. Mol. Genet. 15, 2763–2771 [DOI] [PubMed] [Google Scholar]
- Yu W., Qiang L., Solowska J. M., Karabay A., Korulu S., Baas P. W. (2008). The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell 19, 1485–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.