SUMMARY
Animal models mimicking human diseases have been used extensively to study the pathogenesis of autoimmune diseases and the efficacy of potential therapeutics. They are, however, limited with regard to their similarity to the human disease and cannot be used if the antagonist and its cognate receptor require high similarity in structure or binding. Here, we examine the induction of oxazolone-mediated features of atopic dermatitis (AD) in NOD-scid IL2Rγnull mice engrafted with human peripheral blood mononuclear cells (PBMC). The mice developed the same symptoms as immunocompetent BALB/c mice. Histological alterations induced by oxazolone were characterized by keratosis, epithelial hyperplasia and influx of inflammatory cells into the dermis and epidermis. The cellular infiltrate was identified as human leukocytes, with T cells being the major constituent. In addition, oxazolone increased human serum IgE levels. The response, however, required the engraftment of PBMC derived from patients suffering from AD, which suggests that this model reflects the immunological status of the donor. Taken together, the model described here has the potential to evaluate the efficacy of therapeutics targeting human lymphocytes in vivo and, in addition, might be developed further to elucidate molecular mechanisms inducing and sustaining flares of the disease.
INTRODUCTION
A large number of new drug candidates fail in clinical trials for a variety of reasons, including insufficient activity or unforeseen toxicity. This most probably reflects the predictive quality of the preclinical animal models and the difficulty in translating positive data in animal models to the patient. The pathophysiological mechanisms in the genetically heterogeneous and often aged patient population differ markedly from those observed in animals. This is particularly relevant for the 12-week-old inbred specific pathogen-free (SPF)-bred mouse, which is used widely for the development of disease models and efficacy profiling of new drugs to include potential treatment of chronic immuno-inflammatory disorders.
Atopic dermatitis (AD) is a relapsing chronic inflammatory disease of the skin characterized by rash, pruritus, eczema, xerosis and lichenification (Elias and Steinhoff, 2008; Elias et al., 2010). AD is a T-helper cell type 2 (TH2)-driven inflammation, with interleukin-4 (IL-4) and interleukin-13 (IL-13) playing key roles in the early edematous phase (Wollenberg et al., 2000; Kaminishi et al., 2002; Obara et al., 2002). Both interleukins activate the type II IL-4 receptor complex, which consists of IL-4 receptor α and IL-13 receptor α1, resulting in the generation of phenotypic symptoms such as IgM-IgE switch, fibrosis, epithelial hyperplasia and barrier dysfunction (Grewe et al., 1994; Mueller et al., 2002; Elias et al., 2003).
One animal model that closely reflects these features is the oxazolone-induced AD in hairless mice (Man et al., 2008). When applied to the skin of hairless mice in low doses for a period of 3 weeks, mice develop symptoms characteristic for AD including barrier dysfunction, secretion of IgE, epithelial cell hyperplasia, fibrosis and infiltration of inflammatory cells into the dermis and epidermis and secretion of TH2 cytokines. Although this model is useful in many respects, it does not reflect the variability observed in patients and cannot be used when murine protein structures and binding mechanism significantly differ from their human counterparts.
NOD-scid IL2Rγnull mice engrafted with human peripheral blood mononuclear cells (PBMC) are an attractive model for the study of human diseases (Shultz et al., 2007b; King et al., 2008). So far, humanized mice have been used to study autoimmune type 1 diabetes (Shultz et al., 2007a), thyroiditis (D’Eufemia et al., 1992) and rheumatoid arthritis (Tighe et al., 1990; Davis et al., 2002). Here, we report the development of oxazolone-induced AD-like features in NOD-scid IL2Rγnull mice engrafted with PBMC derived from patients suffering from AD. Mice developed the same features as those previously observed in immunocompetent mice. Challenge with oxazolone resulted in epithelial hyperplasia, keratosis and infiltration of inflammatory cells (consisting mainly of human T cells) into the dermis and epidermis. Engraftment alone without further treatment was not sufficient to induce AD. However, in contrast to the results obtained in immunocompetent mice, ethanol alone induced AD-like symptoms albeit milder than observed with oxazolone. Mice engrafted with PBMC derived from healthy volunteers and non-engrafted mice did not respond to oxazolone challenge, which suggests that in this specific model development of AD is dependent on the presence of lymphocytes from patients with a history of AD.
TRANSLATIONAL IMPACT.
Clinical issue
Atopic dermatitis (AD) is a relapsing, chronic inflammatory skin disease characterized by rash, pruritus, eczema, xerosis and lichenification. Many animal models have been developed to study the disease in vivo; one involves exposing the skin of hairless mice to oxazolone, a haptenizing agent that induces many features of human AD. However, this model does not recapitulate the variability observed in human AD patients, and it cannot be used to study mechanisms or test drugs that involve human-specific molecules or mechanisms. Thus, similar to many other disease models, better models of AD are required to increase the potential of preclinical trials to test new drug candidates for activity and toxicity.
Results
This study describes a new model of AD involving oxazolone-induced pathology in immune-compromised mice engrafted with human peripheral mononuclear cells (PBMCs). On treatment with oxazolone, mice engrafted with PBMCs from AD patients – but not with PBMCs from healthy donors – developed the same pathological signs as observed in immunocompetent oxazolone-exposed mice, including epithelial hyperplasia, IgE secretion and infiltration of inflammatory cells into the dermis and epidermis. Importantly, however, the cellular infiltrate was of human origin, predominantly T cells, as was the IgE. Unlike the model involving immunocompetent mice, symptoms were also observed in response to ethanol (used as a control) in the new model, suggesting that primed lymphocytes from AD patients respond to ethanol as a contact allergic.
Implications and future directions
This report introduces a new in vivo model of AD involving cells from patients, opening up opportunities to study human-specific molecules and mechanisms in this disease. The data suggest that applying this model in AD research might improve the predictability of future phase II clinical trials, as it more closely represents the heterogeneity and complexity of AD patients than previously established models.
RESULTS
Selection of donors
In light of the previous observation that atopic milieus support immunological responses (Reichle et al., 2011), we decided to use PBMC isolated from patients suffering from AD for engraftment. Three patients were selected who exhibited a SCORAD (severity scoring of atopic dermatitis) index between 16 and 48. The cells were first analyzed in vitro with regard to their capacity for IL-4-mediated IgE secretion, which was a requirement for their use in the mouse study. PBMC were isolated and incubated as previously described (Kobayashi et al., 2009). Some 4×106 cells were incubated for 14 days in the presence (50 ng/ml) or absence of IL-4. The induction of human IgE (hIgE) and human IgG (hIgG) synthesis was measured in the supernatant by immunoassay and turbidimetric measurement, respectively. PBMC from all three patients responded to the exposure of IL-4 with increased synthesis of hIgE, whereas hIgG levels remained unaffected. IgE levels increased by a factor of 166 from 2.13±1.8 ng/ml in the control sample to 332±87.2 in the treated sample. A group of healthy volunteers without any known history of atopic diseases served as control. We also observed the responsiveness of PBMC to IL-4 in this group. Expression levels increased from 0.92±1 ng/ml to 104±85 ng/ml. For the animal studies, we selected one donor with no responsiveness to IL-4 and one donor who responded to IL-4 with similar elevated hIgE levels as the AD patients.
Engraftment of NOD-scid IL2Rγnull mice with PBMC and induction of immunological responses
In all experiments NOD-scid IL2Rγnull mice were divided into two groups: those engrafted with PBMC derived from patients suffering from AD and those engrafted with PBMC from healthy volunteers without any history of atopic diseases (non-AD). On day one, NOD-scid IL2Rγnull mice were engrafted with 4×106 PBMC from the selected donors as described in Methods.
Animals were treated with IL-4 (AD n=5; non-AD n=8), ethanol (AD n=16; non-AD n=9) or oxazolone (AD n=17; non-AD n=9) as described in Methods. For the challenge, engrafted animals were divided into two cohorts: animals treated with oxazolone and animals treated with ethanol (AD1, oxazolone n=8 or ethanol n=7; AD2, oxazolone n=6 or ethanol n=5; AD3, oxazolone n=3 or ethanol n=4; non-AD1, oxazolone n=4 or ethanol n=4; non-AD2, oxazolone n=5 or ethanol n=5). A cohort of non-engrafted NOD-scid IL2Rγnull mice challenged with oxazolone served as control (n=4). To compare the immunological responses to those of immunocompetent mice, a cohort of BALB/c mice challenged with oxazolone (n=9) and ethanol (n=9) according to the same regimen as the engrafted NOD-scid IL2Rγnull mice was included in this study.
Appearance of the skin, weight loss and behavioral changes and hIgG and hIgE were monitored throughout the experiment. In the oxazolone-treated cohort of BALB/c mice we observed pruritus, reddening of the skin and mild scaling, whereas the ethanol group was unaffected (data not shown). However, the pathomorphological changes seemed less pronounced than in hairless mice. In the group of engrafted NOD-scid IL2Rγnull mice we observed reddening of the skin and the appearance of scales and eczema upon treatment only in rare cases, suggesting that the inflammatory response was less pronounced than in immunocompetent mice.
Neither the BALB/c nor the engrafted NOD-scid IL2Rγnull mice developed epithelial barrier loss, as indicated by transepithelial water loss levels, which did not change throughout the experiment (data not shown).
None of the engrafted NOD-scid IL2Rγnull mice developed symptoms of AD spontaneously, nor did they show any symptoms of graft versus host disease (GVHD). The behavior of all mice was normal and they did not lose weight throughout the experiment; on the contrary, most of the animals gained weight. The median weight gain in the oxazolone-challenged group was +2.1% and +0.8% in the ethanol-challenged group.
Histological analysis of the skin
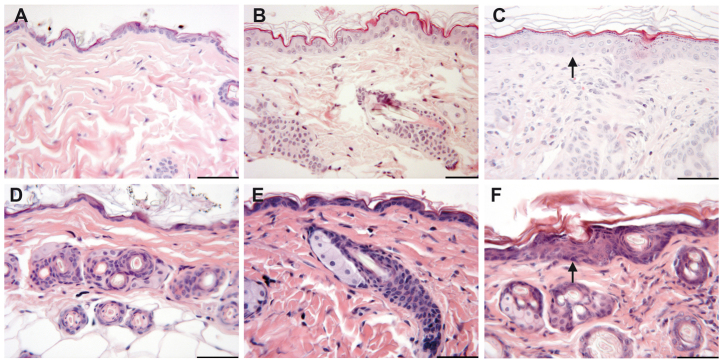
In order to clarify whether oxazolone had an effect on the architecture of the skin, skin sections from BALB/c mice and engrafted NOD-scid IL2Rγnull mice treated with oxazolone or ethanol were analyzed. Non-challenged BALB/c mice, BALB/c mice challenged with ethanol and non-engrafted SCID mice treated with oxazolone served as controls in this experiment. As shown in Fig. 1F, NOD-scid IL2Rγnull mice engrafted with PBMC derived from a patient suffering from AD displayed the same morphological changes as the immunocompetent counterpart (Fig. 1C). Epithelial hyperplasia was accompanied by influx of inflammatory cells and keratosis. By contrast, non-engrafted NOD-scid IL2Rγnull mice treated with oxazolone did not display any alterations (Fig. 1E) and looked similar to the skin of untreated and non-engrafted NOD-scid IL2Rγnull mice (Fig. 1D). BALB/c mice challenged with ethanol as carrier showed slightly increased keratosis.
Fig. 1.
Challenge with oxazolone results in epithelial hyperplasia and influx of inflammatory cells into the epidermis and dermis and keratosis as observed in immunocompetent mice. (A–C) HE-stained photomicrographs of skin sections from BALB/C mice: (A) no treatment, (B) challenged with ethanol and (C) challenged with oxazolone. (D–F) HE-stained skin sections from NOD-scid IL2Rγnull mice: (D) non-engrafted no treatment, (E) non-engrafted challenged with oxazolone and (F) engrafted challenged with oxazolone. Arrows indicate epithelial hyperplasia. Scale bars: 50 μm.
In order to identify the cellular infiltrate as human leukocytes, sections from the skin were stained with an antibody directed against human CD45 (Fig. 2A). Human leukocytes infiltrated the epidermis and dermis in engrafted NOD-scid IL2Rγnull mice challenged with oxazolone (Fig. 2Ad). As expected, leukocytes in skin sections of BALB/c challenged with ethanol or oxazolone (Fig. 2Aa,Ac) and of non-engrafted NOD-scid IL2Rγnull mice (Fig. 2Ab) were not stained with this antibody. The cellular infiltrate consisted mainly of T cells (Fig. 2B). An antibody directed against murine and human CD3 identified T cells in the epidermis and dermis of both, oxazolone treated BALB/c and engrafted NOD-scid IL2Rγnull mice (Fig. 2Bc,Bd). BALB/c mice treated with ethanol displayed a scarce infiltration of cells into the dermis and epidermis (Fig. 2Ba). No cells were stained in the skin of non-engrafted controls (Fig. 2Bb).
Fig. 2.
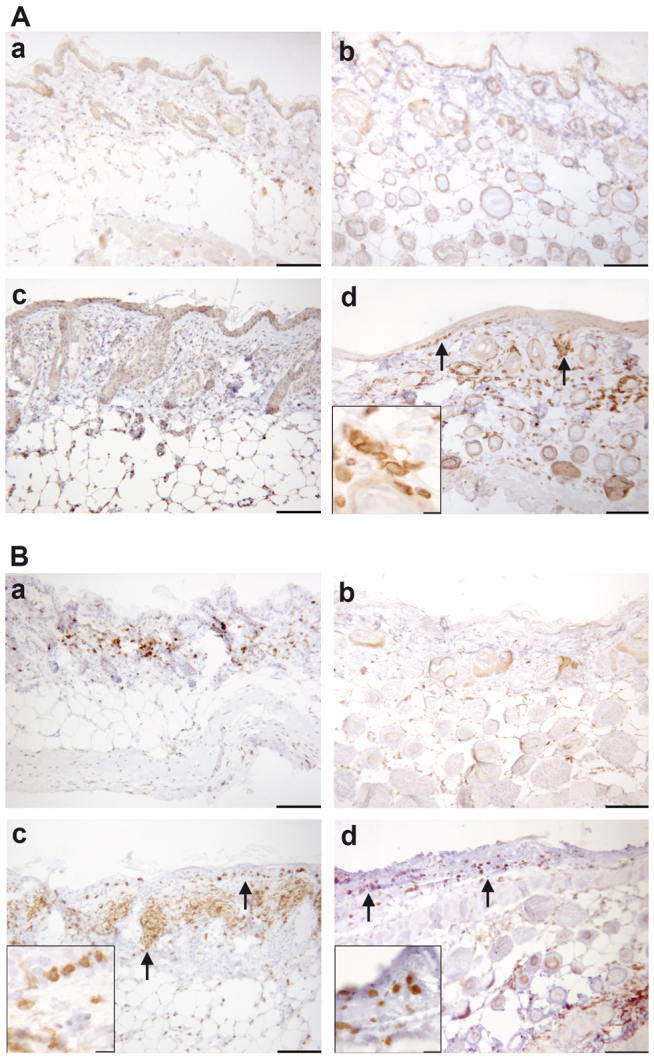
Upon challenge with oxazolone, human leukocytes (mainly consisting of T cells) infiltrate the dermis and epidermis. (A,B) Photomicrographs of immunohistochemically stained paraffin sections of the skin: (A) stained with anti-hCD45 antibody and (B) stained with anti-CD3 antibody. Skin samples for both A and B were taken from the following mice: BALB/c mouse challenged with ethanol (a); non-engrafted NOD-scid IL2Rγnull mouse (b); BALB/c mouse challenged with oxazolone (c); and engrafted NOD-scid IL2Rγnull mouse treated with oxazolone (d). Arrows indicate invaded human leukocytes. Scale bars: 100 μm; 10 μm (insets).
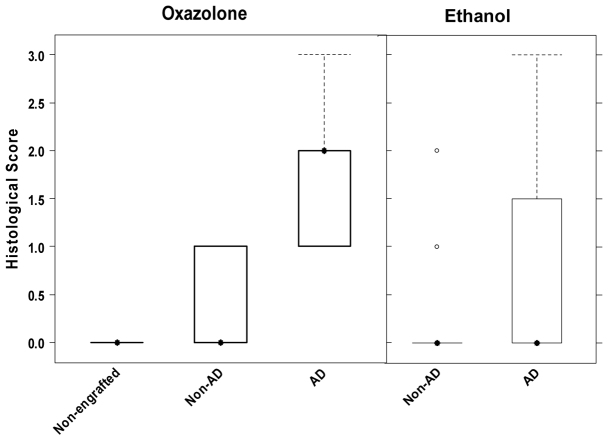
Stained sections of skin samples from all treated groups were classified according to a histological score as described in Methods. NOD-scid IL2Rγnull mice engrafted with PBMC derived from a donor with no atopic history served as control. This analysis revealed a major difference between immunocompetent mice and mice with a humanized immune system. The control group treated with carrier alone did not respond to the challenge. By contrast, in engrafted NOD-scid IL2Rγnull mice, we observed histological alterations in the ethanol-challenged group, albeit in most cases less severe than in the oxazolone-challenged group (Fig. 3). The median value of the assessed histological score in the oxazolone-treated group was 2 (n=12) as compared with 0 in the ethanol-challenged group (n=13). Surprisingly, in the cohort engrafted with PBMC with no atopic history almost no alterations were visible (oxazolone n=9, ethanol n=9). Statistical analysis (Kruskal-Wallis followed by multiple comparisons) revealed a statistically significant difference between the oxazolone-treated groups with and without atopic history (P<0.001 for AD versus non-AD mice treated with oxazolone). In the ethanol-challenged group, the difference was statistically insignificant (P=0.22 for AD versus non-AD mice treated with ethanol).
Fig. 3.
Challenge with oxazolone results in histological changes in NOD-scid IL2Rγnull mice engrafted with PMBCs derived from patients with AD. Histological changes were classified according to a histological score and depicted in a boxplot diagram. Sample sizes: non-engrafted n=4, non-AD n=9 and AD n=12. P<0.01 for AD vs non-AD treated with oxazolone. P=0.22 for AD versus non-AD treated with ethanol (Kruskal-Wallis test followed by multiple comparisons).
Secretion of human IgG and human IgE in response to challenge with ethanol and oxazolone
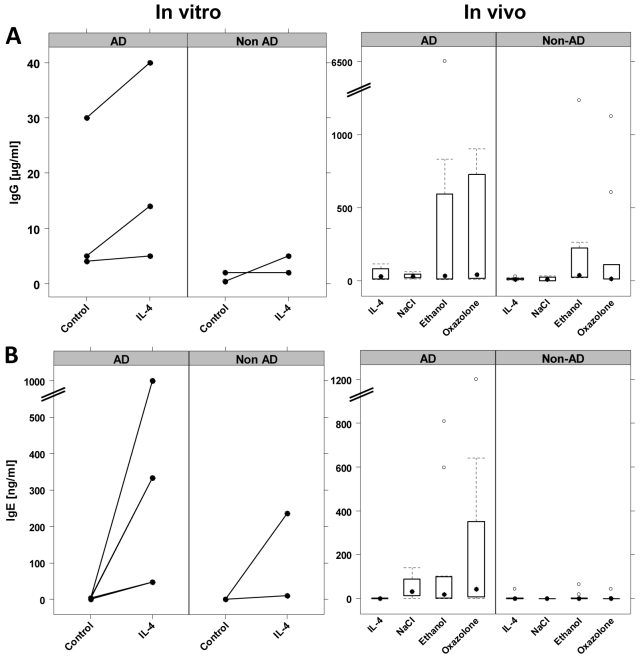
In order to analyze hIgG and hIgE levels, blood samples were taken from engrafted mice at day 23 and these immunoglobulins were measured by turbidimetric immunoassay in the serum of the mice. Human IgG levels were also taken as a proof of successful engraftment of the animals. Human IgG concentrations below detection levels (<0.2 μg/ml) were considered as indicative of non-engraftment and respective animals were excluded from the study (AD, oxazolone n=3, ethanol n=3; non-AD, n=0). All measurements were performed in two groups: mice engrafted with PBMC derived from patients suffering from AD and mice engrafted with PBMC derived from two healthy volunteers without any history of atopic diseases. All PBMC were analyzed simultaneously in vitro for their capacity to respond to IL-4. Fig. 4 depicts individual levels of hIgE of the respective donors in response to IL-4 under cell culture conditions. In order to compare the responsiveness of PBMC in vitro and in vivo, a cohort of animals was treated with IL-4 in addition to the cohorts treated with ethanol or oxazolone. As shown in Fig. 4A, engrafted animals treated with IL-4 in both groups (AD and non-AD) showed similar hIgG levels as observed previously (King et al., 2008). We observed a high variability, with levels reaching a mean value of 66.8±81 μg/ml versus 63.0±86 μg/ml in the non-AD group. There was no difference between these values and those from engrafted animals without any treatment (data not shown). Administration of ethanol or oxazolone apparently had an impact on hIgG levels in the AD and non-AD groups, although the change in levels did not reach statistical significance. The variability was high; some animals did not show a rise in hIgG levels like the untreated group and some animals responded significantly. The ethanol-treated group reached values of 907±1834 μg/ml and the oxazolone-treated group values of 304±395 μg/ml. There was, however, no correlation between the observed histological score and the IgG levels, indicating that IgG levels were not related to pathomorphological changes. Pearson product moment analysis revealed a correlation coefficient of −0.0065 and a P value of 0.66.
Fig. 4.
Treatment-dependent human IgG and IgE secretion. (A,B) PBMC derived from three different patients suffering from AD and from two healthy volunteers (non-AD) were analyzed in vitro and in vivo with respect to their capacity to secrete human IgG (A) and IgE (B). Sample sizes: in vitro AD n=3; in vitro non-AD n=2; in vivo AD after treatment with IL-4 n=5, NaCl n=4, ethanol n=13 or oxazolone n=12; in vivo non-AD after treatment with IL-4 n=8, NaCl n=4, ethanol n=8 or oxazolone n=9. (A) hIgG secretion. IL-4 had moderate effects on IgG levels in vitro and in vivo, whereas ethanol and oxazolone induced the secretion of hIgG. (B) hIgE secretion. IL-4 induced IgE secretion in vitro in the AD group and in one donor of the non-AD group, whereas in vivo IL-4 failed to induce hIgE in both groups. Ethanol and oxazolone induced hIgE secretion in the AD group but not the non-AD group. IgE levels in the four different groups were significantly different. Kruskal-Wallis test followed by multiple comparisons revealed that the oxazolone- and ethanol-treated groups were significantly different to the IL-4-treated group (P=0.01 and P=0.01, respectively).
In contrast to exposure to IL-4 in vitro, where hIgE levels of PBMC derived from patients with AD increased by a factor of 166 from 2.13±1.8 ng/ml in the control sample to 332±87.2 ng/ml in the IL-4-treated sample, hIgE levels did not increase in engrafted animals treated with IL-4 (Fig. 4B). In the AD and non-AD groups, hIgE concentrations leveled at 1±1.6 ng/ml and 0.27±0.54 ng/ml, respectively. Values did not differ from values obtained in non-treated animals. The pattern, however, changed significantly when animals were challenged with oxazolone or ethanol. In the non-AD group, neither treatment had an impact on hIgE levels, which remained as low as in the IL-4-treated group. By contrast, oxazolone and ethanol induced the secretion of hIgE in the AD group. Although some animals remained irresponsive and the variability was high, IgE levels in the four different groups were significantly different. Kruskal-Wallis test followed by multiple comparisons revealed a significant difference between the oxazolone- and ethanol-treated groups and the IL-4-treated group (P=0.01 and P=0.01, respectively). In addition, there was a correlation between hIgE levels and the histological score. Pearson product moment analysis revealed a correlation coefficient of −0.511 and a P value of 6×10−5.
This observation was in contrast to results obtained in BALB/c mice treated with oxazolone and ethanol. As previously observed in hairless mice, murine IgE levels remained unaffected in ethanol-treated mice and increased significantly in the oxazolone-treated group. In this study, BALB/c mice exhibited basal IgE levels of 495±252 ng/ml prior to treatment and reached values of 2671±830 ng/ml in the oxazolone-treated group (P<0.05, compared with untreated mice) and 721±384 ng/ml in the ethanol-treated group (P=0.5, compared with untreated mice) at day 23 post treatment.
FACS analysis of lymphocytes in blood, skin and spleen
In order to analyze engraftment levels and the ratio of CD4 to CD8 T cells in peripheral blood, skin and spleen, lymphocytes from engrafted mice were subjected to FACS analysis at day 30 post engraftment, which corresponded to day 23 post treatment. Engraftment levels varied considerably ranging from 0.2 to 73%, with a median engraftment level of 1.5%. As expected, there was a strong correlation of engraftment levels and hIgG levels (P=0.001); however, there was no correlation between engraftment levels and hIgE (P=0.15), engraftment levels and histological score (P=0.3) and hIgG levels and hIgE levels (P=0.32). In addition, there was no negative correlation between engraftment levels and weight changes. High and low level engrafted animals gained weight similarly, indicating that there was no onset of GVHD.
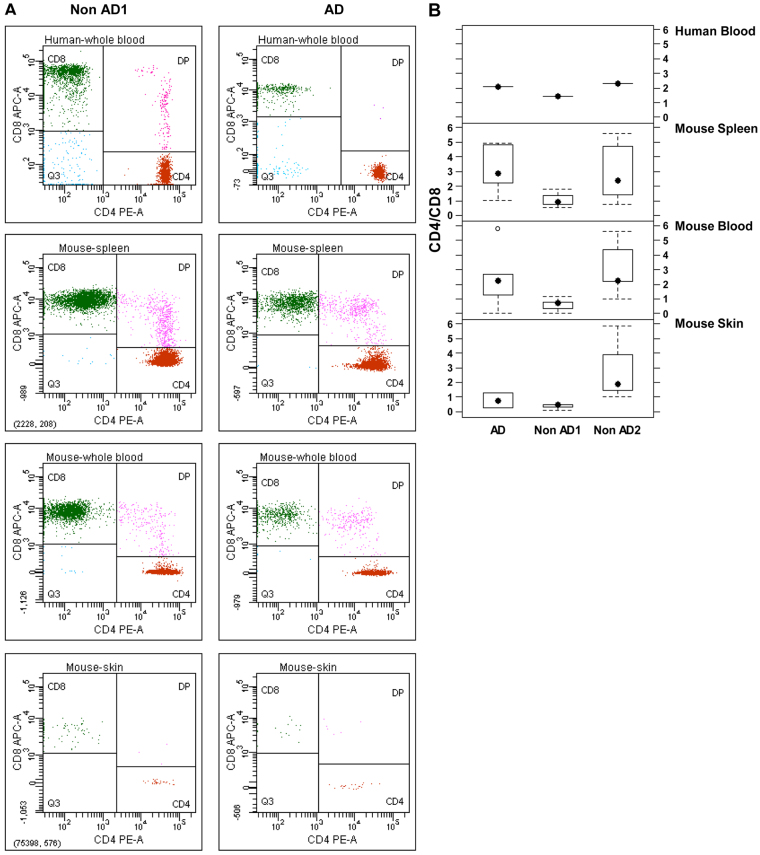
The ratio of CD4 to CD8 was compared to the original ratio in peripheral blood of the respective donors. The ratio was determined in three different donors. One donor suffered from AD and displayed a ratio of 2.1 at time of engraftment. The two healthy volunteers (non-AD1 and non-AD2) differed with respect to the capacity of the isolated PBMC to secrete IgE in vitro (Fig. 4B) and in their respective CD4:CD8 ratio at time of engraftment. PBMC from non-AD1 secreted low levels of IgE and displayed a CD4:CD8 ratio of 1.4, whereas PBMC from non-AD2 secreted IgE at similar levels to PBMC isolated from the AD patient and also exhibited a similar CD4:CD8 ratio of 2.4. As shown in Fig. 5, this pattern was preserved in engrafted mice. CD4:CD8 ratios of human lymphocytes in the spleen and peripheral blood reflect the ratio detected in the whole blood at time of engraftment. The challenge with oxazolone did not influence the ratio in mice engrafted with PBMC derived from non-AD1.
Fig. 5.
Immunological background of the donor determines the CD4:CD8 ratio in organs of engrafted NOD-scid IL2R γ null mice. (A,B) CD4:CD8 ratio of human T cells in the spleen, blood and skin of mice engrafted with PBMC derived from a patient suffering from AD and two healthy volunteers (non-AD) was compared with the CD4:CD8 ratio in whole blood from the respective donors prior to engraftment. (A) Representative flow cytometric analysis of human T cells stained with anti-human CD4 and CD8. (B) Quantitative analysis of CD4:CD8 ratio. Sample sizes: AD n=4, non-AD1 n=4 and non-AD2 n=7.
DISCUSSION
In this study we describe the induction of oxazolone-mediated features of AD in immune-compromised NOD-scid IL2Rγnull mice engrafted with human PBMC derived from patients with AD. Upon treatment with oxazolone, engrafted mice developed the same characteristics as previously observed in immunocompetent mice, which include epithelial hyperplasia, infiltration of inflammatory cells into the dermis and epidermis and IgE secretion (Man et al., 2008) and effects also seen in our experiments using BALB/c mice. As shown by immunohistochemistry and FACS analysis, the cellular infiltrate was of human origin with T cells being the major constituent.
Of note, the presence of human lymphocytes was alone not sufficient to induce AD-like features. Mice engrafted with PBMC derived from patients suffering from AD without any further treatment neither developed any symptoms of AD spontaneously nor exhibited increased IgE levels. Additional stimulation by irritation of the skin by treatment with oxazolone or ethanol was necessary to produce an effect. Furthermore, treatment with oxazolone without engraftment did not induce any alterations in the skin architecture. Non-engrafted NOD-scid IL2Rγnull mice treated with oxazolone displayed no epithelial hyperplasia or keratosis. Thus, epithelial hyperplasia was strictly dependent on the presence of PBMC.
Impact of the immunological background of the donor
Our results strongly suggest that the immunological background and phenotype of the engrafted cells are important with respect to mounting an immunological response resulting in AD-like features. Mice engrafted with PBMC derived from individuals without any history of atopic diseases did not react to treatment. These mice were unresponsive to treatment with regard to IgG and IgE secretion, cellular infiltration and epithelial hyperplasia. Furthermore, an elevated CD4:CD8 ratio is not sufficient to induce atopic dermatitis like features. Engraftment with PBMC derived from a healthy donor who displayed a similar ratio as the AD donor did not result in the development of phenotypic symptoms in response to challenge with oxazolone. These results suggest that the immunological status of the donor was not influenced by the challenge but that the engrafted cells were only able to execute what they had previously ‘learned’. Thus, in these experiments the development of AD-like features was dependent on the presence of PBMC derived from patients with AD.
This is in contrast to our results from experiments with BALB/c mice and to previous results obtained in hairless mice (Man et al., 2008). Both are immunologically naïve strains with respect to AD and were able to elicit an immunological response upon challenge with oxazolone. Oxazolone is thought to have a dual function, acting as a haptenizing agent in addition to activating STAT6-mediated secretion of chemokines (Koeper et al., 2007). Because engrafted PBMC are incompetent to generate a robust immune response de novo (Shultz et al., 2007b), the function of oxazolone might be restricted to its role as an activator of previously primed lymphocytes via activation of keratinocytes. The absence of peripheral lymph nodes and dendritic cells, which constrain the development of a peripheral immune system, might limit the potential of PBMC in this experimental setting. Hence, the model depends on previously primed immune cells.
The FACS analysis of T cells in peripheral blood, splenic and skin lymphocytes corroborates the results obtained from the analysis of hIgE secretion and the histological score. In either organ the ratio of CD4 to CD8 cells reflected the disease status of the donor. In T cell populations of peripheral blood from the healthy donor, the ratio remained unaffected in splenic lymphocytes upon engraftment. Challenge with oxazolone did not affect this ratio, suggesting that the status of the cell remained unchanged and that, unlike BALB/c mice, the engrafted immune cells were resistant to immunological stimulation.
The CD4:CD8 ratio of peripheral blood from the patient or of engrafted T cells isolated from spleen, peripheral blood and skin from engrafted mice indicated a CD4-dominated immunological background vigilant to mount an allergic response. However, we would not conclude from these results that only PBMC primed by a history of AD have the capacity to induce these features. Further experiments are required to investigate whether an atopic background such as, for example, found in allergies is sufficient to elicit similar responses.
Under these conditions, stimulation with IL-4 in vivo did not result in the development of symptoms or hIgG and hIgE secretion in engrafted mice. This is in contrast to previous results that showed induction of IgE secretion in response to IL-4 in vivo (Spiegelberg et al., 1994; Mayer et al., 2000). Both protocols involve the intraperitoneal application of 10 μg/day for 5 consecutive days. The results in both experiments differed significantly with respect to detected IgE levels (11.915 ng/ml and 200–1000 ng/ml, respectively). The clearance in mice is very fast. In normal mice, the IL-4 serum level was ∼600 ng at 5 hours after injection of 10 μg IL-4 and declined with a half-life of 20 minutes (Spiegelberg et al., 1994), indicating that approximately after 6–7 hours the serum level was below the required concentration. The lack of response in our experiments was not due to the incapability of the engrafted PBMC to respond to IL-4, as shown by in vitro experiments. Here, the same cells that failed to respond to IL-4 in vivo, secreted IgE upon exposure to IL-4. We observed, however, that a single incubation with 50 ng IL-4 for 24 hours was not sufficient to induce IgE secretion. Without continuous exposure to IL-4 the cells were unable to secrete IgE (data not shown). Because the group of IL-4-treated mice displayed the same pattern as untreated engrafted mice and did not display IgG levels beyond basal levels, the irresponsiveness is most probably due to the short half-life of IL-4 and the limited exposure to IL-4 in vivo. One explanation for the discrepancy of our results to those of Spiegelberg and Mayer might be that the required exposure times to IL-4 are different in different mouse strains or dependant on the number of engrafted cells. In previous experiments, CB.17 scid mice were used for in vivo induction of hIgE and six to ten times more PBMC were engrafted.
The fact that Biedermann et al. observed the activation of dendritic cells in BALB/c mice in response to a single application of 0.1 or 1 μg of IL-4 (Biedermann et al., 2001) suggests that long-term exposure might only be required for the IgM-IgE class switch. Bioavailability of IL-4 is still an unsolved problem in the use of IL-4 and IL-4 muteins as therapeutic agents in diseases like psoriasis and AD (Röcken, 2010) and slow release formulations might improve the in vivo induction of IgE in our experimental setting.
Differences in responses of immunocompetent BALB/c mice
Although the NOD-scid IL2Rγnull mice engrafted with PBMC derived from patients suffering from AD showed the same features of AD as observed in immunocompetent mice, there were some differences in the results obtained in BALB/c mice. First of all, the immunological response in BALB/c mice was restricted to the oxazolone-treated group. Levels of IgE increased throughout challenge with oxazolone and hardly changed upon ethanol challenge. In engrafted NOD-scid IL2Rγnull mice, however, an immunological response could also be observed in ethanol-treated mice. At the end of the experiment, the level of IgE expression varied considerably in both groups (oxazolone- or ethanol-treated), reaching from levels below detection level up to 1286 ng/ml regardless of treatment. The observation that treatment with ethanol did statistically change IgE levels might reflect the milder capacity of ethanol as an irritant. Even shaving and depilation seemed to sufficiently irritate the skin in some cases. In addition, in every cohort there were mice that did not respond at all to treatment. In 4 out of 12 animals, levels of hIgE remained below detection level, as seen in the IL-4-treated or untreated groups, suggesting that either the stimulus was not sufficient under these conditions or that the response was delayed. Because the response in BALB/c mice was also not as pronounced as observed in hairless mice, differences in the robustness of hairy skin as compared with hairless skin might also lower susceptibility.
The secretion of hIgE was paralleled by the secretion of hIgG. Most mice with hIgG levels beyond basal levels also expressed hIgE. Basal levels were similar to those previously observed (King et al., 2008). In this analysis we also observed a high variability in each cohort, ranging from 18 μg/ml to a maximum of even 6680 μg/ml regardless of the treatment. It might therefore be expected that increases in histological scores would have been observed in both the oxazolone- and ethanol-treated groups. Indeed, in some mice treated with ethanol alone we observed an influx of inflammatory cells and alterations of the skin architecture similar to the oxazolone-challenged mice, albeit milder, suggesting that exposure to ethanol might be sufficient to elicit an immunological response in PBMC bearing a lifelong history of exposure to allergens.
A further important discrepancy between the results obtained in BALB/c mice and those obtained in NOD-scid IL2Rγnull mice was the observed high variability of the histological score and levels of IgE secretion in the oxazolone-challenged group. Unexpectedly, treatment resulted in the development of symptoms or left mice unaffected. In every cohort of mice engrafted with PBMC derived from patients, we observed mice with basal IgG levels and IgE levels below detection limit.
In summary, we have shown that in engrafted NOD-scid IL2Rγnull mice human lymphocytes perform similar functions as resident lymphocytes in immunocompetent BALB/c and hairless mice. Thus, this model might be useful for studying the efficacy of therapeutics targeting human lymphocytes in vivo. Furthermore, it might be a useful substitute for studies that have to be performed in primates due to the requirement for a high homology of protein structures. In light of the immunological and phenotypic background of engrafted PBMC produced by exposure to allergens and/or disease, we feel confident that this model could eventually be developed into a model with greater similarity and translatability to the human disease, to potentially include elucidation of cellular mechanisms inducing and sustaining flares of the disease.
METHODS
Isolation and engraftment of human PBMC
Peripheral blood was collected from three patients suffering from AD and two healthy volunteers. All donors gave informed written consent and the study was approved by the ethic commission of the University of Munich.
PBMC were isolated following a protocol established at the Helmholtz Zentrum Munich. 30 ml of blood in trisodium citrate solution was diluted with 30 ml of HANKS Balanced Salt Solution (Sigma-Aldrich, Deisenhofen, Germany) and loaded on Leucosep Tubes (Greiner Bio One, Frickenhausen, Germany). Cells were separated at 800 g for 15 minutes according to the manufacturer’s instruction. PBMC were isolated, washed in HANKS Balanced Salt Solution supplemented with 2500 IE Heparin Natrium (Braun) and resuspended in PBS at a concentration of 20×106/ml.
At least 6-week-old NOD-scid IL2Rγnull mice were engrafted with 200 μl of the cell suspension by intravenal injection. The animals were rested for 7 days prior to first challenge with oxazolone. The mean age was 15.5±5.51 weeks and the mean weight 25.2±3.3 g. Animals were randomized for age and sex.
Cell culture and stimulation assay
PBMC (4×106 cells) were resuspended in 2 ml RPMI containing 10% FCS, 1% sodium pyruvate, 1% penicillin/streptomycin and 1% glutamine (Sigma) and incubated for 14 days in 24-well flat-bottom cell culture plates (Greiner Bio-One) with IL-4 (50 ng/ml) and 1 μl anti-CD40 (1 μg/ml; BD Biosciences, Heidelberg, Germany) in a humidified incubator at 37°C with 5% CO2 as previously described (Kobayashi et al., 2009).
Production of wild-type IL-4
Wild-type human IL-4 was produced in Escherichia coli as described (Kruse et al., 1991). Briefly, the cDNA encoding for the mature part of human IL-4 was cloned into the E. coli expression vector RBSIIPN25x/o (Stueber et al., 1984). Transformed E. coli cells of the strain BL21(DE3) were grown in Luria-Bertani (LB) medium to an optical density of 0.6 at 600 nm. Protein expression was induced by addition of 1 mM isopropyl-β-thiogalactoside (IPTG), expression was continued for further 3 hours. Cells were harvested by centrifugation and lysed by ultrasonication. IL-4 was expressed in insoluble form as inclusion bodies, which were dissolved in 20 volumes (v/w) of 6 M guanidinium hydrochloride (GuHCl), 50 mM Tris-HCl pH 8.0. The denatured protein was refolded by a two-step protocol, the first step being a rapid fivefold dilution of the protein solution in 6 M GuHCl in ice-cold water. The solution was stirred for 15 minutes and then dialyzed against 20 volumes PBS (20 mM sodium phosphate, 120 mM NaCl and 2 mM KCl, pH 7.4) for 24 hours at 4°C. Insoluble protein precipitate was removed by centrifugation and clear supernatant was loaded onto an cation exchange column (CM Sepharose FF; GE Healthcare). IL-4 was eluted by applying a linear gradient of 0–1 M sodium chloride in 20 mM Tris-HCl, pH 8.0. IL-4-containing fractions were pooled and subjected to a second purification employing reversed phase HPLC chromatography. High purity IL-4 protein was eluted using a linear gradient of 0.1% trifluoroacetic acid to 100% acetonitrile. Purity was checked by SDS-PAGE and ESI-FT-ICR mass spectrometry. Biological activity and receptor binding properties were examined by measuring the IL-4 dose dependency of TF-1 cell proliferation as described (Tony et al., 1994) and by determining the binding affinity of the recombinant IL-4 protein to its high-affinity receptor IL-4Rα via surface plasmon resonance as published (Shen et al., 1996).
Study protocol
BALB/cJ@Rf mice were obtained from Janvier Europe. NOD/LtSz-scid (abbreviated as NOD) IL2Rγnull mice were obtained from Charles River Laboratories (Sulzfeld, Germany). The mice were kept under conventional SPF conditions in individually ventilated cages. The facility is controlled by FELASA guidelines. All animal studies were approved by the Ethics Committee for animal use of the government of Upper Bavaria, Germany and performed in compliance with German animal welfare laws and policies.
At least 6-week-old BALB/c mice or NOD-scid IL2Rγnull mice at 7 days post engraftment were treated as previously described (Man et al., 2008). Following anesthesia with isoflurane, a 2×2 cm of the regio lumbalis dexter and sinister was shaved and depilated on day 1 and animals were presensitized by topical application of 20 μl 5% oxazolone (4-ethoxymethylene-2-phenyl-oxazolin-5-one) (Sigma-Aldrich) in 100% ethanol. From day 8, mice were rechallenged with 200 μl 0.1% oxazolone in 100% ethanol by topical application every other day. Animals were sacrificed on day 23. Control groups were challenged according the same protocol using ethanol or isotonic sodium chloride solution. Mice were inspected daily and the weight was controlled on day 1, and from day 8 on every other day.
For analysis of IL-4 induction, 200 μl of IL-4 (50 μg/ml) in isotonic in sodium chloride solution was administered intraperitoneally from day 8 to day 12 post engraftment (Spiegelberg et al., 1994; Mayer et al., 2000).
Histological score
Skin regions in the regio lumbalis dexter and sinister of the mice, which had direct contact with the agents, were fixed for 24 hours in formalin, followed by preservation in 70% ethanol prior to paraffin embedding. Sections were stained with hematoxilin and eosin (HE) and Giemsa. Inflammation was scored as follows: (0) infiltration of few inflammatory cells into dermis, (2) major infiltration of inflammatory cells into the dermis and (3) infiltration of the epidermis. Alterations of skin architecture were scored as follows: (0) no alteration, (1) epithelial hyperplasia, (0) no fibrosis, (1) fibrosis. The resulting score parameters were added in a total histological score ranging from 0 (healthy) to 5 (maximal histological damage). The determination of the score was supported by a trained pathologist ignorant of the treatment status of the animals.
Serum IgG and IgE levels
Samples were blinded and human serum IgG levels were measured turbidimetrically with COBAS INTEGRA 800 (Roche, Penzberg, Germany). Human serum IgE levels were measured by the Elecsys 2010 Immunoassay (Roche). Murine IgE levels were analyzed by ELISA (BD Biosciences).
Immunohistochemistry
Tissue samples of the skin were fixed for 24 hours in 4% neutral buffered formalin, and embedded in paraffin. Antigen retrieval was performed by incubating slides for 30 minutes at 95–100°C in Tris-EDTA buffer (10 mM Tris, 0.5 mM EDTA, pH 9). Non-specific binding was blocked with normal goat serum (1:10; MP Biomedicals, Solon, OH). Sections were incubated with rabbit anti-human CD3 (1:100; Dako, Hamburg, Germany) for 1 hour at room temperature in TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.6) and rabbit anti-human CD45 (1:400 in TBS; Antibody Online, Aachen, Germany) for 1 hour at room temperature. A biotin-conjugated goat anti-rabbit IgG was used as secondary antibody (1:100 in TBS; Dako), DAB served as chromogen. Sections were analyzed by a Leitz microscope (10×) and micrographs were taken with a Leitz camera DFC 295.
Analysis of lymphocytes by flow cytometry
Anti-human CD3-FITC (clone SK7), anti-human CD4-PE (clone SK3), anti-human CD8-APC (clone SK1), anti-human CD45-APC-H7 (clone 2D1) and anti-mouse CD45-PE-Cy7 (clone 30-F11) were purchased from BD Biosciences. To analyze human and murine lymphocytes, multicolor cytometric analysis was performed using a FACS Canto (BD Biosciences) and FACS Diva software.
Human peripheral blood was taken from the vein in EDTA tubes. At time of sacrifice peripheral mouse blood was collected in EDTA tubes from the anesthetized mouse by cardiocentesis. Blood (100μl) was stained with appropriated antibodies and incubated for 15 minutes at room temperature and in darkness. The erythrocytes were removed from the samples using 1×BD FACS Lysing Solution (BD Biosciences) for 10 minutes at room temperature and in darkness.
Single cell suspensions were prepared from the spleen in RPMI-1640 containing 10% FCS by mincing with a metal mesh followed by a 100 μm cell strainer (BD Biosciences). The cells were treated by 1× BD Pharm Lyse (BD Biosciences) for 10 minutes at room temperature. After a washing process in 2% RPMI-1640 (Sigma-Aldrich), 106 cells/ml were labeled with appropriated antibodies.
Skin lymphocytes were isolated as previously described (Gebhardt et al., 2009). Shaved mouse skin (1–2 cm) was cut into small pieces and incubated for 1.2–2 hours at 37°C in Eagle’s minimum essential medium containing 2% FCS (Gibco BRL), collagenase type I (6 mg/ml; Worthington) and DNase (5 mg/ml; Sigma-Aldrich) with occasional swirling, followed by filtering through a 70-μm cell strainer (BD Biosciences). The cells were collected in 10% RPMI-1640 and were stained with appropriate antibodies.
After staining, the cells were washed in FACS buffer (PBS containing 2% FCS) and resuspended in 500 μl of the same buffer. For all analyses, anti-mouse CD45 staining was performed to exclude murine host cells and only human CD45+ cells were included in the analyses. At least 10,000 events were measured using FACS Canto (BD Biosciences). Post-acquisition data was analysed using the FlowJo 7.6.5 software (Tree Star, Ashland, OR).
Statistical analysis
Statistical analysis was performed using R, a free software environment for statistical computing and graphing. The Kruskal-Wallis test was performed to evaluate statistical significance. Correlations were analysed by Person-Product-moment analysis.
Acknowledgments
We thank Eric Whalley for critically reading the manuscript, Lisa Pichl for excellent technical assistance and Origenis GmbH for generous support.
Footnotes
COMPETING INTERESTS
The authors declare no competing or financial interests.
AUTHOR CONTRIBUTIONS
T.N. performed experiments and data analysis; M.Z.-K. performed experiments and data analysis; O.S. performed experiments; F.R. recruited patients and performed anamnesis; R.V. recruited patients and performed anamnesis; N.H. performed the histological scoring and edited the manuscript, R.W. edited the manuscript; A.W. analyzed data; T.M. provided and analyzed IL-4 and edited the manuscript; R.G. performed experiments, analyzed data, developed the approach and prepared the manuscript; E.W. and M.S. developed the approach and edited the manuscript.
FUNDING
This work was funded by the Bundesministerium für Forschung und Technik [grant number PTJ 0315466].
REFERENCES
- Biedermann T., Zimmermann S., Himmelrich H., Gumy A., Egeter O., Sakrauski A. K., Seegmüller I., Voigt H., Launois P., Levine A. D., et al. (2001). IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat. Immunol. 2, 1054–1060 [DOI] [PubMed] [Google Scholar]
- D’Eufemia P., Giardini O., Cantani A., Martino F., Finocchiaro R. (1992). Autoimmune thyroiditis in a case of tyrosinaemia type III. J. Inherit. Metab. Dis. 15, 861–862 [DOI] [PubMed] [Google Scholar]
- Davis L. S., Sackler M., Brezinschek R. I., Lightfoot E., Bailey J. L., Oppenheimer-Marks N., Lipsky P. E. (2002). Inflammation, immune reactivity, and angiogenesis in a severe combined immunodeficiency model of rheumatoid arthritis. Am. J. Pathol. 160, 357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Lee C. G., Zheng T., Ma B., Homer R. J., Zhu Z. (2003). New insights into the pathogenesis of asthma. J. Clin. Invest. 111, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P. M., Steinhoff M. (2008). “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J. Invest. Dermatol. 128, 1067–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P. M., Williams M. L., Crumrine D., Schmuth M. (2010). Inherited disorders of corneocyte proteins. Curr. Probl. Dermatol. 39, 98–131 [DOI] [PubMed] [Google Scholar]
- Gebhardt T., Wakim L. M., Eidsmo L., Reading P. C., Heath W. R., Carbone F. R. (2009). Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 [DOI] [PubMed] [Google Scholar]
- Grewe M., Gyufko K., Schöpf E., Krutmann J. (1994). Lesional expression of interferon-gamma in atopic eczema. Lancet 343, 25–26 [DOI] [PubMed] [Google Scholar]
- Kaminishi K., Soma Y., Kawa Y., Mizoguchi M. (2002). Flow cytometric analysis of IL-4, IL-13 and IFN-gamma expression in peripheral blood mononuclear cells and detection of circulating IL-13 in patients with atopic dermatitis provide evidence for the involvement of type 2 cytokines in the disease. J. Dermatol. Sci. 29, 19–25 [DOI] [PubMed] [Google Scholar]
- King M., Pearson T., Shultz L. D., Leif J., Bottino R., Trucco M., Atkinson M. A., Wasserfall C., Herold K. C., Woodland R. T., et al. (2008). A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin. Immunol. 126, 303–314 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Haruo N., Sugane K., Ochs H. D., Agematsu K. (2009). Interleukin-21 stimulates B-cell immunoglobulin E synthesis in human beings concomitantly with activation-induced cytidine deaminase expression and differentiation into plasma cells. Hum. Immunol. 70, 35–40 [DOI] [PubMed] [Google Scholar]
- Koeper L. M., Schulz A., Ahr H. J., Vohr H. W. (2007). In vitro differentiation of skin sensitizers by cell signaling pathways. Toxicology 242, 144–152 [DOI] [PubMed] [Google Scholar]
- Kruse N., Lehrnbecher T., Sebald W. (1991). Site-directed mutagenesis reveals the importance of disulfide bridges and aromatic residues for structure and proliferative activity of human interleukin-4. FEBS Lett. 286, 58–60 [DOI] [PubMed] [Google Scholar]
- Man M. Q., Hatano Y., Lee S. H., Man M., Chang S., Feingold K. R., Leung D. Y., Holleran W., Uchida Y., Elias P. M. (2008). Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J. Invest. Dermatol. 128, 79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. J., Bolognese B. J., Al-Mahdi N., Cook R. M., Flamberg P. L., Hansbury M. J., Khandekar S., Appelbaum E., Faller A., Marshall L. A. (2000). Inhibition of CD23 processing correlates with inhibition of IL-4-stimulated IgE production in human PBL and hu-PBL-reconstituted SCID mice. Clin. Exp. Allergy 30, 719–727 [DOI] [PubMed] [Google Scholar]
- Mueller T. D., Zhang J. L., Sebald W., Duschl A. (2002). Structure, binding, and antagonists in the IL-4/IL-13 receptor system. Biochim. Biophys. Acta 1592, 237–250 [DOI] [PubMed] [Google Scholar]
- Obara W., Kawa Y., Ra C., Nishioka K., Soma Y., Mizoguchi M. (2002). T cells and mast cells as a major source of interleukin-13 in atopic dermatitis. Dermatology 205, 11–17 [DOI] [PubMed] [Google Scholar]
- Reichle M. E., Chen L., Lin S. X., Chan L. S. (2011). The Th2 systemic immune milieu enhances cutaneous inflammation in the K14-IL-4-transgenic atopic dermatitis model. J. Invest. Dermatol. 131, 791–794 [DOI] [PubMed] [Google Scholar]
- Röcken M. (2010). Interleukin 4 or cytokine antagonists? Time to change the search for novel psoriasis therapies. Dermatology 221, 27–29 [DOI] [PubMed] [Google Scholar]
- Shen B. J., Hage T., Sebald W. (1996). Global and local determinants for the kinetics of interleukin-4/interleukin-4 receptor alpha chain interaction. A biosensor study employing recombinant interleukin-4-binding protein. Eur. J. Biochem. 240, 252–261 [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Ishikawa F., Greiner D. L. (2007a). Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Pearson T., King M., Giassi L., Carney L., Gott B., Lyons B., Rossini A. A., Greiner D. L. (2007b). Humanized NOD/LtSz-scid IL2 receptor common gamma chain knockout mice in diabetes research. Ann. N. Y. Acad. Sci. 1103, 77–89 [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Beck L., Kocher H. P., Fanslow W. C., Lucas A. H. (1994). Role of interleukin-4 in human immunoglobulin E formation in hu-PBL-SCID mice. J. Clin. Invest. 93, 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. (1984). A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 3, 3143–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe H., Silverman G. J., Kozin F., Tucker R., Gulizia R., Peebles C., Lotz M., Rhodes G., Machold K., Mosier D. E., et al. (1990). Autoantibody production by severe combined immunodeficient mice reconstituted with synovial cells from rheumatoid arthritis patients. Eur. J. Immunol. 20, 1843–1848 [DOI] [PubMed] [Google Scholar]
- Tony H. P., Shen B. J., Reusch P., Sebald W. (1994). Design of human interleukin-4 antagonists inhibiting interleukin-4-dependent and interleukin-13-dependent responses in T-cells and B-cells with high efficiency. Eur. J. Biochem. 225, 659–665 [DOI] [PubMed] [Google Scholar]
- Wollenberg A., Kraft S., Oppel T., Bieber T. (2000). Atopic dermatitis: pathogenetic mechanisms. Clin. Exp. Dermatol. 25, 530–534 [DOI] [PubMed] [Google Scholar]