Abstract
Previously we obtained compelling evidence that the fetus provides a critical signal for the initiation of term labor through developmental induction of surfactant protein (SP)-A expression by the fetal lung and secretion into amniotic fluid (AF). We proposed that interactions of AF macrophage (Mφ) Toll-like receptors (TLRs) with SP-A, at term, or bacterial components, at preterm, result in their activation and migration to the pregnant uterus. Herein the timing of labor in wild-type (WT) C57BL/6 mice was compared with mice homozygous null for TLR2, SP-A, SP-D, or doubly deficient in SP-A and SP-D. Interestingly, TLR2−/− females manifested a significant (P < 0.001) delay in timing of labor compared with WT as well as reduced expression of the myometrial contraction-associated protein (CAP) gene, connexin-43, and Mφ marker, F4/80, at 18.5 d postcoitum (dpc). Whereas in first pregnancies, SP-A−/−, SP-D−/−, and SP-A/D−/− females delivered at term (∼19.5 dpc), in second pregnancies, parturition was delayed by approximately 12 h in SP-A−/− (P = 0.07) and in SP-A/D−/− (P <0.001) females. Myometrium of SP-A/D−/− females expressed significantly lower levels of IL-1β, IL-6, and CAP genes, connexin-43, and oxytocin receptor at 18.5 dpc compared with WT. F4/80+ AF Mφs from TLR2−/− and SP-A/D−/− mice expressed significantly lower levels of both proinflammatory and antiinflammatory activation markers (e.g. IL-1β, IL-6, ARG1, YM1) compared with gestation-matched WT AF Mφs. These novel findings suggest that the pulmonary collectins acting via TLR2 serve a modulatory role in the timing of labor; their relative impact may be dependent on parity.
Approximately 15 million babies are born prematurely each year throughout the world (1). Preterm birth, defined as birth at less than 37 wk of gestation, is the leading cause of neonatal morbidity and mortality in developed countries and the second leading cause of death in children under the age of 5 yr worldwide (1). It is estimated that 20–30% of preterm labor is caused by an underlying infection, 25–30% results from premature rupture of membranes, whereas 40–45% is idiopathic (2–4). In the United States, the incidence of preterm birth has risen to approximately 13% within the last 2 decades (www.marchofdimes.com/peristats); its impact on the health care system is reflected by the approximately $30 billion spent annually to care for children born prematurely.
Murine models of infection-induced preterm labor have been established in an attempt to extrapolate the critical molecular events and molecules common to term and preterm labor in humans (5, 6). Although infection induced preterm labor and spontaneous labor at term share common signaling mechanisms leading to uterine contraction and birth, the initiating events are distinct. This fact cannot be overlooked because current drug treatments, focused on suppression of uterine contractions, have met with modest success; once labor is initiated, the process is essentially irreversible (www.marchofdimes.com/peristats).
The signal(s), cellular, and molecular mechanisms that promote labor at term are complex, multifactorial, and redundant. Both term and preterm labor are associated with an inflammatory response (7, 8), exemplified by increased levels of proinflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α, in reproductive tissues, amniotic fluid (AF) and maternal serum (9). This occurs upon infiltration of myometrium, cervix, and fetal membranes by neutrophils and macrophages (Mφs) (10, 11) and results in the activation of proinflammatory transcription factors, such as nuclear factor-κB (NF-κB), which enhance expression of contraction-associated protein (CAP) genes that promote the transformation of the quiescent myometrium to a contractile state. CAP genes include the gap junction protein, connexin-43 (CX43), the oxytocin receptor (OXTR), and cyclooxygenase 2 (COX-2), the critical enzyme in synthesis of contractile prostaglandins (8, 12).
While it is likely that infection associated with chorioamnionitis provides an important inflammatory stimulus for enhanced leukocyte activation and proinflammatory cytokine production leading to preterm labor (13), the signals for the increased inflammatory response associated with labor at term are less well defined. There is increasing evidence to suggest that the fetus may generate signals that contribute to the initiation of labor at term. In this regard, we (14) and others (15) have suggested that augmented surfactant production by the maturing fetal lung may serve as a fetal signal for the initiation of labor.
Pulmonary surfactant, a glycerophospholipid-rich, surface-active lipoprotein produced by alveolar type II cells, is essential for breathing. Surfactant production is developmentally regulated in the fetal lung and is detectable in AF only after approximately 80% of gestation is complete. There are four essentially lung-specific surfactant proteins (SP), SP-A, SP-B, SP-C, and SP-D (16–19). SP-A is the most abundant protein component of surfactant; its developmental regulation in concert with surfactant phospholipid synthesis provides an excellent marker of fetal lung maturity (20, 21). SP-A and SP-D are structurally related glycoproteins that belong to the C-type lectin/collectin superfamily, which also includes mannose-binding protein and conglutinin (19, 22–24). SP-A and SP-D play a critical role in the innate immunity of the lung, whereby interaction of lung collectins with bacteria, viruses, and fungi result either in agglutination or opsonization by immune cells (25). SP-A and SP-D modulate cellular functions and pulmonary immunity through direct and indirect interactions with a number of different receptors on immune cells (24, 26). These include SP-R210, signal-inhibitory regulatory protein-α, CD91-calreticulin, Toll-like receptor (TLR)-2 and TLR4 (for review, see Ref. 27). Receptor binding can elicit either a proinflammatory or antiinflammatory response, depending on the cell type, identity of the cell surface receptor, presence and type of pathogen or stimulus, orientation of the collectin oligomer, activation state of the cell, and period of ligand exposure (27–32).
We previously reported that the developmental increase in SP-A expression in mouse fetal lung and its secretion into the AF after 17 d postcoitum (dpc) was associated with enhanced expression of IL-1β in AF Mφs and activation of NF-κB in the maternal uterus (14). Purified SP-A also stimulated IL-1β and NF-κB expression in cultured AF Mφs. Studies using Rosa26 Lac-Z mice revealed that fetal-derived AF Mφ migrate to the uterus with the gestational increase in AF SP-A. Intraamniotic injection of purified SP-A at 15.5 dpc caused preterm delivery of fetuses within 6–24 h. By contrast, injection of an SP-A antibody or NF-κB inhibitor (SN50) into the AF compartment delayed labor by more than 24 h (14). Based on these and other data, it was suggested that AF Mφ interaction with SP-A at term, or with bacterial components at preterm, may initiate changes in Mφ phenotypic properties, resulting in their activation and infiltration of the maternal uterus in which their local production of cytokines, such as IL-1β, contribute to the induction of the inflammatory response by activating the NF-κB pathway. This, in turn, promotes increased uterine contractility by activation of CAP gene expression and/or by blocking progesterone receptor function (14).
Because of the inflammatory hallmarks of term and preterm labor and the reported interactions of SP-A and SP-D with TLR2 and TLR4 (28, 29, 33–36), we postulated involvement of these receptors in the initiation of labor. TLRs, a family of ancient pattern recognition receptors (PRRs) that are expressed in all vertebrate species, recognize specific molecular patterns unique to bacterial, viral, and fungal pathogens. Binding of PRRs to pathogen-associated molecular patterns signal infection and activate molecular cascades that control transcription of a cadre of inflammatory genes responsible for resolving or limiting tissue invasion (37, 38). Upon binding, TLR2 and TLR4 orchestrate a signaling cascade directing the expression of inflammatory genes, such as IL-1, IL-6, IL-8, and TNF-α, chemokines [i.e. monocyte chemoattractant protein-1 (MCP-1)], and type I interferons.
In light of the potential role of SP-A in the initiation of labor, its structural and functional relatedness to SP-D, and SP-A and SP-D interactions with TLRs, it was of interest to define the functional roles of these surfactant proteins and their putative receptors in the timing of labor using gene targeted mice. The current studies were undertaken to determine the following: 1) whether mice deficient in SP-A, SP-D, and in SPA/D manifest parturition defects, 2) whether mice homozygous null for TLR2 have altered parturition timing, and 3) whether deficiency in the SP-A/D or TLR2 alters the activation profile of AF Mφs near term and the expression of CAP genes in the pregnant myometrium.
Materials and Methods
Mice
All animal studies were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center. Cells and tissues were obtained from female mice that were euthanized by inhalation of isofluorane anesthetic (Baxter Healthcare Corp., Guayama, Puerto Rico) and cervical dislocation.
Timed-pregnant mice from commercial sources
Outbred timed-pregnant CD1/ICR mice were purchased from Harlan Laboratories (Harlan USA, Houston, TX). Mice were time mated by placing 8- to 10-wk-old males and females together between 1700 and 0700 h. Pregnancy was determined by the presence of a vaginal plug the following morning; gestational age was designated at that time as 0.5 dpc. Pregnant mice were received either at 12.5 or 14.5 dpc and housed under pathogen-free conditions during which they were maintained on a 12-h light, 12-h dark cycle with access to a standard pellet chow.
Timing of labor in gene-targeted and wild-type (WT) B6 mice
To examine the effects of deficiencies in SP-A (SP-A−/−), SP-D (SP-D−/−), SP-A and SP-D (SP-A/D−/−), and TLR2 (TLR2−/−) on the timing of labor, homozygous knockout breeding pairs were housed together overnight and separated in the morning (designated 0.5 dpc). The time of labor was documented upon delivery of the first pup or by the presence of a litter. Timing of parturition in WT C57BL/6 (B6) mice (Mouse Breeding Core Facility, University of Texas Southwestern) was carried out in a similar fashion. First pregnancies were generated by breeding virgin-female mice to genetically like males. Second pregnancies were generated in mice previously bred to genetically like males. All mice were housed under pathogen-free conditions, maintained on a 12-h dark, 12-h light cycle, and allowed free access to a standard pellet chow.
SP-A, SP-D, and SP-A/D null mice
Mice homozygous for targeted disruption of the SP-A (39), SP-D (40) or both SP-A/D genes (41) were used. It should be noted that because SP-A and SP-D genes lie approximately 60 kb apart on mouse chromosome 14, they were sequentially targeted in embryonic stem cells to create the double knockout mice (41). Deletion of SP-A and SP-D genes was confirmed by PCR analysis of tail DNA using SP-A and SP-D primers as follows: SP-A, forward, 5′-GTGGGGTGGGATTAGATAAATGC-3′ (neomycin cassette detection); reverse: 5′-GCATTAGACGACAGAACTCCAGCC-3′; reverse, 5′-TACTGAGAGATGTGTGCTTGGTGAG-3′; SP-D, forward, 5′-TGGTTTCTGAGATGGAGTCGTG-3′; reverse, 5′-TGGGGCAGTGGATGGAGTGTGC-3′; reverse, 5′-GTGGATGTGGAATGTGTGCGAG-3′ (neomycin cassette detection). Amplification temperatures were 30 sec at 94 C, 30 sec at 63 C, and 31 sec at 72 C for 30 cycles after an initial denaturing step of 1 min at 94 C.
TLR2 null mice
Mice homozygous for targeted disruption of the TLR2tm/kir gene were obtained from Jackson Laboratories (Bar Harbor, ME). Deletion of TLR2 was confirmed by PCR analysis of tail DNA using primers for TLR2, forward, 5′-ACGAGCAAGATCAACAGGAGA-3′; reverse, 5′-CTTCCTGAATTTGTCCAGTACA-3′; and reverse, 5′-TAAGGGCCAGCTCATTCCTCC-3′ (neomycin cassette detection). Amplification times/temperatures were 30 sec at 94 C, 30 sec at 63 C, and 90 sec at 68 C for 35 cycles after an initial denaturing step of 3 min at 94 C.
Isolation of murine myometrium and fetal lungs at 18.5 dpc
Myometrium and fetal lungs were collected from timed-pregnant mice at approximately 1000 h on d 18.5 of the first pregnancy. Maternal myometrium was isolated by removing all fetal-derived tissues followed by gentle scraping and blotting of the endometrial layer. Intact fetal lungs were harvested on ice. All tissues were rinsed in ice-cold 1× PBS, flash frozen in liquid nitrogen, and stored at −80 C until analysis.
Amniotic fluid cell isolation and purification
Murine AF cell isolation
Amniotic fluid Mφs were isolated from 15.5, 17.5, and 18.5 dpc mice. Uteri were exposed and AF from individual amniotic sacs was carefully aspirated, avoiding maternal blood contamination, using a 20-gauge needle and a 1.0-ml syringe containing 0.1 ml of PBS (pH 7.4) supplemented with fetal bovine serum. The AF contents obtained from all individual sacs from a single pregnant mouse (e.g. n = 1) were pooled and incubated with hyaluronidase (SEIKAGAKU Corp., 0.2 U/ml) for 10 min at 37 C, followed by centrifugation for 5 min at 600 × g at 4 C. We found that because of increased AF viscosity near term, incubation with hyaluronidase was extremely important for efficient isolation of Mφ at late gestation. Limulus amebocyte lysate assay (Lonza, Switzerland) was used to assay for the presence of Gram-negative bacterial endotoxin and determine the lipopolysaccharide (LPS) concentration in the 1:500 hyaluronidase dilution used to incubate AF cells. Results demonstrated no detectable LPS at the dilution used to incubate AF cells. Red blood cells were removed by treatment with 400 μl of 1× red blood cell lysis buffer (eBioscience, San Diego, CA) for 4 min at room temperature. The total cell number contained within each single-cell suspension was determined using a hemacytometer and trypan blue (1:1). The cellular populations with greater than 95% viability were used for further analysis.
Flow cytometry and cell sorting
For identification of the AF Mφ population, cells from AF single-cell suspensions were incubated on ice for 10 min with rat-antimouse monoclonal antibody 2.4G2 (BD Bioscience, San Diego, CA) to block Fc-mediated binding of antibodies to mouse FcγIII/II receptors. Cells were washed twice using flow cytometry staining buffer (eBioscience) and stained for 30 min at 4 C using the following antibodies (eBioscience): anti-F4/80-phycoerythrin (anti-F4/80-PE), allophycocyanin (APC) antimouse CD11b (integrin aM, Mac-1a), phycoerythrin (PE) antimouse TLR2, and PE antimouse TLR4/MD-2 (molecule that physically complexes with TLR4 on the cell surface and confers LPS responsiveness). Samples were washed, fixed in 2% paraformaldehyde (Sigma Aldrich, St. Louis, MO), and analyzed using the FACSCalibur (BD Bioscience). A forward/side-scatter live gate was set and approximately 50,000–100,000 events were collected per sample. Analysis was carried out using FlowJo analysis software (Tree Star Inc., Ashland, OR) or Cellquest Pro analysis software (BD Bioscience). For isolation of AF Mφs, F4/80-stained populations were sorted immediately after the staining by FACSAria (BD Bioscience). Greater than 98% sample purity was consistently achieved as confirmed by the post-sort.
mRNA isolation and first-strand cDNA synthesis
Total RNA was extracted from myometrial tissues using TRIzol reagent (Invitrogen, Carlsbad, CA). Tissues were mechanically disassociated in QIAzol (QIAGEN Inc., Valencia, CA). Total RNA from myometrium was isolated using the QIAGEN RNeasy Mini Kit (QIAGEN) in conjunction with the QIAcube (QIAGEN). Superscript III reverse transcriptase system (Invitrogen) was used to transcribe 2 μg of deoxyribonuclease I-treated RNA (Invitrogen) as outlined by the manufacturer. RNA isolation from F4/80+ AF Mφs was carried out using TRIzol reagent and the one-step method of Chomczynski and Sacchi (42). RNA integrity was verified using Experion high-sensitivity chips (Bio-Rad Laboratories, Hercules, CA), and cDNA was synthesized from 500 ng of RNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories).
Quantitative real-time PCR and data analysis
Quantitative real-time PCR was performed using the Bio-Rad CFX384 real-time system (Bio-Rad Laboratories), SYBR Green PCR master mix (Applied Biosystems, Branchburg, NJ) and Taqman PCR master mix, No AmpErase UNG (Applied Biosystems). Taqman primers for IL-1β (Mm01336189_m1), IL-6 (Mm00446190_m1), arginase 1 (ARG1) (Mm00475990_m1), chitin 3-like 3 (YM1) (Mm00657889_mH), F4/80 (Mm00802529_m1), and ribosomal protein, large, PO (m36B4) (Mm0197446190_gh) were obtained from Applied Biosystems. Primers for CX43, OXTR, and m36B4 were as follows: CX43, forward, 5′-TCCAAGGAGTTCCACCACTT-3′, and reverse, 3′-TGGAGTAGGCTTGGACCTTG-5′; OXTR, forward, 5′-TTCTTCGTGCAGATGTGGAG-3′, and reverse, 3′-TGTAGATCCATGGGTTGC AG-5′; m36B4, forward, 5′-CACTGGTCTAGGACCCGAGAAG-3′, and reverse, 3′-GGTGCCTCTGGAGATTTTCG-5′. Optimal quantitative PCR conditions were standardized for each product. Reactions were performed in triplicate. The comparative cycle threshold (Ct) method was used to quantify gene expression (43). Normalized ΔCt values were calculated using endogenous housekeeping gene m36B4. Fold-change was calculated using the 2-ΔΔCt method.
SuperArray RT2 profiler array plate
SuperArray analysis of 84 gene transcripts associated with the inflammatory response was performed using the mouse inflammatory RT2 profiler PCR array plate PAMM-011E (SuperArray, Fredrick, MD) following the manufacturer's instructions. Briefly, cDNA amplification was performed using the RT2 first strand kit (SuperArray). A total of 25 μl of PCR mixture, containing cDNA and SYBR Green/ROX PCR master mix solution (SuperArray), was loaded into each well of the PCR array. Two-step cycling conditions were as follows: one cycle for 10 min at 95 C followed by 15 sec at 95 C and 1 min at 60 C for 40 cycles. Results were analyzed using SuperArray's RT2 Profiler PCR array data analysis software (www.sabiosciences.com/pcrarraydataanalysis.php). The average of five housekeeping genes (Gusb, Hrpt1, Hsp90ab1, Gapdh, actin b) was used to normalize sample Ct values. Time-matched AF Mφ from WT B6 mice served as calibrator or reference sample. Ct values above 35 were interpreted as below detectable limits. Analysis was based on the comparative Ct method. The Student's t test was used to determine statistical significance.
Statistical analysis
GraphPad Prism 5.0 software (GraphPad Prism, San Diego, CA) was used to determine statistical significance between groups via unpaired one-tailed Student's t test and by one-way ANOVA, followed by Tukey's analysis for determining differences among multiple groups. The data are expressed as mean ± sem. P < 0.05 was considered to be statistically significant.
Results
SP-A/D double deficiency in mice results in delayed labor
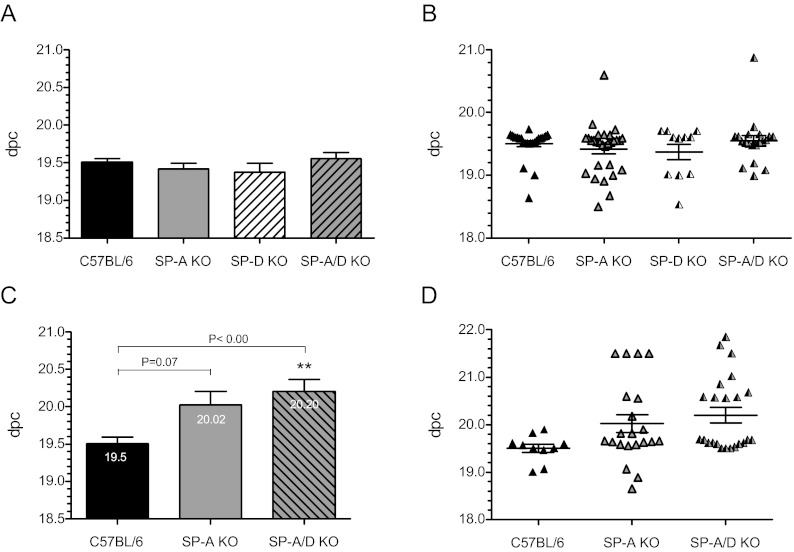
To define the roles of SP-A and SP-D in the timing of parturition, female mice singly deficient in SP-A (SP-A−/−), SP-D (SP-D−/−), or doubly deficient in SP-A and -D (SP-A/D−/−) were bred to genetically like males and the time to labor was assessed. During first pregnancies, no difference in the time to labor was evident among SP-A−/− (19.4 ± 0.07 dpc; n = 29), SP-D−/− (19.4 ± 0.12 dpc; n = 11), SP-A/D−/− (19.55 ± 0.08 dpc; n = 20) mice and WT C57BL/6 (B6) controls (19.50 ± 0.05; n = 25) (Fig. 1A). A scatter plot of these data show similar trends in parturition timing across all genotypes (Fig. 1B). By contrast, during second pregnancies, a statistically significant delay in the time to parturition was evident in SP-A/D−/− mice (20.20 ± 0.16 dpc; n = 23) (P < 0.001) compared with their WT B6 counterparts (19.5 ± 0.08 dpc; n = 10) (Fig. 1C). A similar delay was observed during third pregnancies as the average time to labor was 20.15 dpc (n = 4, data not shown). It is important to note that although the protracted time to labor in second pregnancies of SP-A−/− mice (20.02 ± 0.18, n = 21) did not reach statistical significance (P = 0.07), dysregulated parturition was evident in the wide variation of delivery times shown in the scatter plot (Fig. 1D). Furthermore, an increased incidence of dystocia among SP-A−/− mice during second pregnancies was confirmed by necropsy of mothers manifesting delayed labor and apparent distress. Pups delivered late by SP-A−/− and SP-A/D−/− mothers had anatomical features of enhanced maturity (i.e. whiskers, nails, and increased crown to rump length) and were significantly heavier than WT pups delivered at term (Table 1). To assess whether the observed delays in parturition were associated with defects in fecundity, we compared litter size among SP-A−/−, SP-A/D−/−, and WT B6 mice during second pregnancies. We found the average litter size across all genotypes to be similar (Table 1) and in agreement with published data for C57BL/6 mice (44). Litter viability was unaffected by the ablation of SP-A and/or SP-D genes. SP-D−/− mice were not further studied in second pregnancies. To determine whether age influences parturition timing, the age and time of labor among SP-A−/−, SP-A/D−/−, and WT B6 mice during first and second pregnancies were analyzed. Importantly, age had no significant influence on the time to labor for any of these genotypes (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Thus, parity, not age, influenced the time to labor in SP-A/D-deficient mice but had no effect on their WT counterparts.
Fig. 1.
SP-A and -SP-D doubly deficient mice manifest a significant delay in labor during second pregnancies. Timing of labor was assessed during first (A and B) and second (C and D) pregnancies in C57BL/6 (B6) WT mice and mice homozygous null for SP-A, SP-D, and for SP-A/SP-D genes. A, During first pregnancies B6 WT (n = 25), SP-A−/− (n = 29), SP-D−/− (n = 11), and SP-A/D−/− (n = 20) females bred to genetically like males delivered normally at 19.5 ± 0.05, 19.4 ± 0.07, 19.4 ± 0.12, and 19.55 ± 0.08 dpc, respectively. B, Scatter plots of the time to parturition illustrate similar parturition timing across all strains. C, During second pregnancies, SP-A−/− females bred to like males manifested a delay, albeit not insignificant (P = 0.07) in the average time to labor (20.02 ± 0.19 dpc, n = 21). Parturition was significantly (**, P < 0.001) delayed in SP-A/D−/− females (20.20 ± 0.16 dpc, n = 23) bred to like males compared with B6 WT mice (19.5 ± 0.09 dpc; n = 10). Gestational length is listed as dpc. Values are expressed as the mean ± sem of gestation length. Statistically significant differences were calculated by one-way ANOVA.
Table 1.
Effects of SP-A and SP-A/SP-D deficiency on parturition timing, pups per litter and pup weight
| Strain | Gestation time (d) | Pups/litter | Weight/pup (g) |
|---|---|---|---|
| C57BL/6 | 19.50 ± 0.08 (n = 10) | 8.00 ± 0.28 (n = 24) | 1.264 ± 0.08 (n = 49) |
| SP-A−/− | 20.02 ± 0.18 (n = 21) | 8.30 ± 0.58 (n = 18) | 1.55 ± 0.12 (n = 5)a |
| SP-A/D−/− | 20.20 ± 0.16 (n = 23)b | 7.72 ± 0.42 (n = 20) | 1.58 ± 0.03 (n = 8)a |
Parturition length was assessed as dpc, and data are presented as the mean ± sem. The total number of mice analyzed in each group is indicated in parentheses. Average litter size reflects the number of live and dead pups born to individual mothers. Only intact newborn pups were weighed to determine the average weight of pups per genotype. Weight is reported in grams. Statistically significant differences were determined using the Student's t test.
P < 0.05.
P < 0.001.
Delayed parturition in SP-A and SP-D doubly deficient mice is associated with decreased expression of myometrial inflammatory cytokines and CAP genes
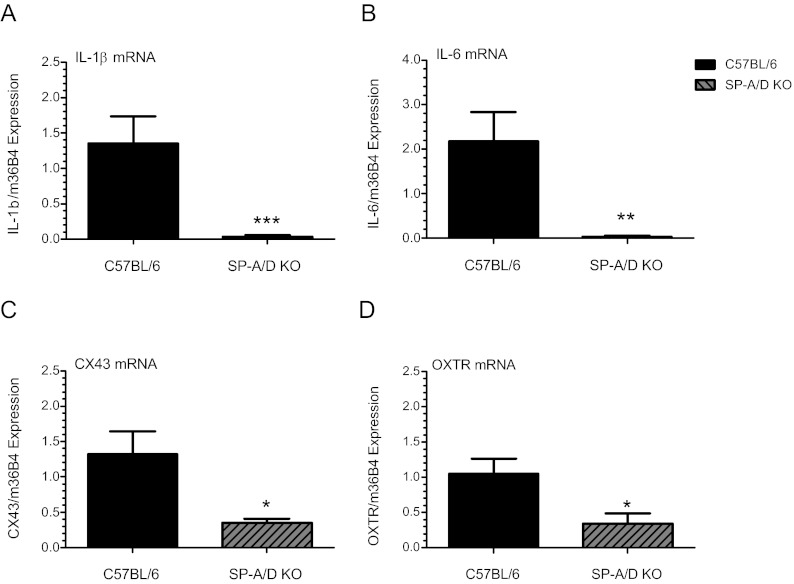
Proinflammatory cytokines, such as IL-1β and IL-6, contribute to the onset of uterine contractility, leading to term and preterm labor. This occurs, in part, via activation of NF-κB, which in turn, increases expression of COX-2 to stimulate prostaglandin synthesis and expression of CAP genes, such as OXTR and CX43 (8, 45–48). To investigate the basis for the delay in labor observed during SP-A/D−/− second pregnancies, we compared expression of IL-1β and IL-6 in myometrial tissues isolated from SP-A/D−/− and WT B6 mice at 18.5 dpc by quantitative RT-PCR (qRT-PCR). Analysis revealed significantly lower levels of IL-1β (P < 0.0001; n = 8) and IL-6 (P < 0.001; n = 8) expression in SP-A/D−/− mice compared with B6 WT mice (Fig. 2, A and B). qRT-PCR also revealed a coordinate reduction in CX43 (P < 0.05; n = 7) and in OXTR (P < 0.05; n = 7) (Fig. 2, C and D) mRNA expression in the doubly deficient mice compared with WT. Immunoblot analyses of CX43 protein in second pregnancy myometrial tissues revealed a coordinate and comparable reduction (∼60%) in CX43 protein in SP-A/D knockout mice compared with WT B6 (P < 0.05) (Supplemental Fig. 2).
Fig. 2.
Delayed parturition in SP-A/D doubly deficient mice is associated with reduced inflammatory and contraction-associated protein gene expression in myometrial tissues. SP-A/D knockout and B6 virgin-females were mated to genetically like males and allowed to deliver normally. Second pregnancies were generated in the same manner as described above. Myometrial tissues were harvested at 18.5 dpc from SP-A/D knockout and WT B6 mice and myometrial tissues were analyzed by qRT-PCR for IL-1β (A), IL-6 (B), CX43 (C), and OXTR (D) mRNA expression. Data were normalized to m36B4 housekeeping gene expression. Myometrial levels of IL-1β (A) (P < 0.0001; n = 8), IL-6 (B) (P < 0.001; n = 8), (C) CX43 (P < 0.05; n = 7), and (D) OXTR (P < 0.05; n = 7) mRNA were significantly decreased at 18.5 dpc in SP-A/D knockout mice compared with WT B6 controls. Data are the mean ± sem of each mRNA relative to m36B4 expression. Data were analyzed using the Student's t test. *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
Amniotic fluid Mφs isolated from SP-A/D null mice exhibit alterations in expression of proinflammatory (M1) and antiinflammatory (M2) Mφ activation markers at 18.5 dpc
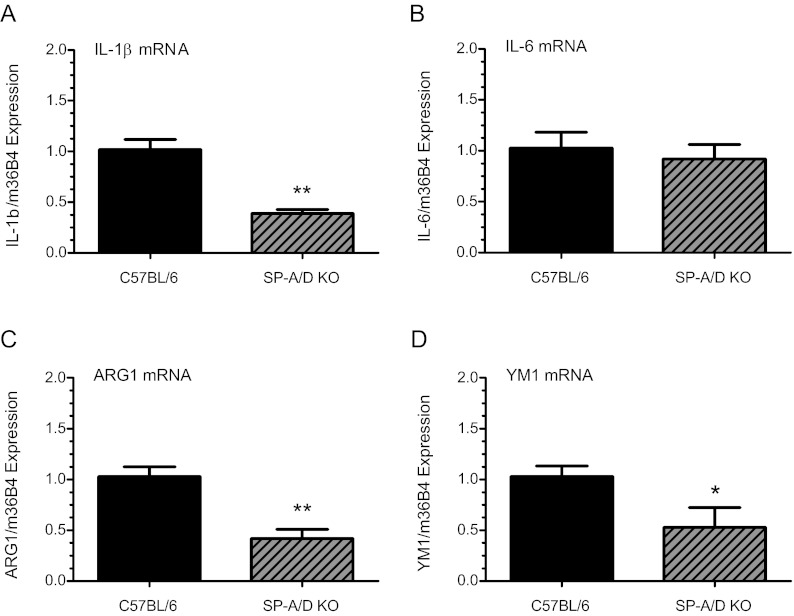
We previously suggested that SP-A may act via TLRs to stimulate and activate AF Mφs to a proinflammatory state near term (14). To investigate the consequences of SP-A/D deficiency on AF Mφ activation, fluid was harvested from amniotic sacs of SP-A/SP-D null and B6 WT fetuses at 18.5 dpc. F4/80+ AF Mφs were isolated by FACSAria (BD Bioscience)and analyzed for expression of proinflammatory/classical (M1) and antiinflammatory/alternative (M2) activation markers by qRT-PCR. Interestingly, we observed that the M1 marker, IL-1β, was significantly (P < 0.001; n = 4) down-regulated in AF Mφ from SP-A/d-deficient fetuses, compared with B6 WT (Fig. 3A). Whereas the M1 marker, IL-6 (Fig. 3B), was unaffected by SP-A/D deficiency (P < 0.6; n = 3), levels of the M2 markers, ARG1 (P < 0.001; n = 5) (Fig. 3C) and YM1 (P < 0.05; n = 4) (Fig. 3D) were significantly decreased in AF Mφs from SP-A/d-deficient fetuses, compared with B6 WT. Although we recognize the possible limitations of exclusively analyzing mRNA expression in these cells, analyses of protein levels in amniotic fluid Mφ was not possible in these studies or in those described below because of the very limited amounts of Mφ protein obtained.
Fig. 3.
AF Mφs isolated from SP-A/D-deficient mice express decreased levels of M1 and M2 mφ activation markers near term. F4/80+ AF Mφs isolated from SP-A/D knockout mice during second pregnancies at 18.5 dpc were analyzed for the expression of M1 and M2 markers. Pooled Mφ mRNA (500 ng) from each timed-pregnant mouse was reverse transcribed, and expression of M1 and M2 markers was assayed by qRT-PCR. The Ct values were normalized to m36B4 and calculated as fold change over B6 WT controls using ΔΔCt. Analysis revealed significantly lower levels of the proinflammatory (M1) activation marker IL-1β (A) (P < 0.001; n = 4) and comparable levels of IL-6 mRNA (B) (P = 0.6; n = 3) in SP-A/D knockout AF Mφ compared with WT. Expression of the antiinflammatory activation (M2) markers ARG 1 (C) (P < 0.001; n = 5) and YM1 (D) (P = 0.049; n = 4) were significantly reduced in SP-A/D knockout AF Mφ compared with WT. Values are reported as mean ± sem. Statistically significant differences were calculated by the Student's t test. *, P < 0.05, **, P < 0.001.
Expression of TLR2 and TLR4/MD-2 increases significantly in CD11b+ AF Mφs during late gestation
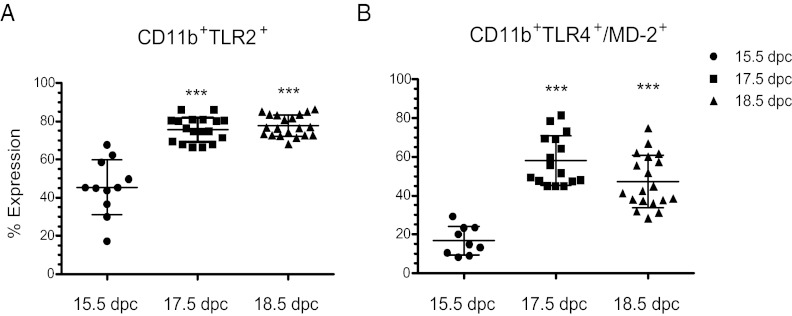
We next sought to characterize expression of TLR2 and TLR4/MD-2, known to bind the bacterial cell wall components, peptidoglygan (49), and LPS (50), respectively, in AF Mφs during late gestation when SP-A expression is up-regulated in the fetal lung. Whereas TLR2 and TLR4 expression in adult Mφs is well characterized, expression of these PRRs in fetal AF Mφs near term was heretofore unknown. To analyze TLR2 and TLR4/MD-2 expression in AF Mφ during late gestation, AF cells from all amniotic sacs of each pregnant CD1 mouse were pooled at 15.5, 17.5, and 18.5 dpc and stained using CD11b, a Mφ marker, in combination with antibodies against TLR2 or TLR4/MD-2. Cells were examined by flow cytometry. Expression of TLR2 (n = 11 samples from 11 timed pregnant mice) and TLR4/MD-2 (n = 9 samples from 9 timed pregnant mice) in CD11b+ AF Mφ at 15.5 dpc was readily detectable. A significant increase in cell surface expression of both TLR2 (P < 0.0001; n = 18 samples from 18 timed pregnant mice) and TLR4/MD-2 (P < 0.0001; n = 16 samples from 16 timed pregnant mice) was observed at 17.5 dpc. Importantly, this increase coincides with the developmental induction of SP-A expression by fetal lung and secretion into AF (14). Augmented levels of TLR2 (P < 0.0001; n = 20 samples from 20 timed pregnant mice) and TLR4/MD-2 complex (P < 0.0001; n = 19 samples from 19 timed pregnant mice) remained elevated through 18.5 dpc (Fig. 4, A and B).
Fig. 4.
Surface expression of TLR2 and TLR4/MD-2 is up-regulated in mouse AF Mφs near term. AF Mφs isolated from 15.5, 17.5, and 18.5 dpc CD1 mice were double stained for CD11b in combination with TLR2 or TLR4/MD-2. Expression by CD11b+ AF Mφs was assessed by flow cytometry using a FACSCalibur (Becton Dickinson). A live cell gate was set based on forward and side-scatter characteristics. A second gate was set based on previously established CD11b+F4/80+ double-positive AF Mφ populations at 15.5, 17.5, and 18.5 dpc. The number of events collected per sample ranged from 50,000 to 100,000. The CD11b+ population was analyzed for expression of TLR2 and TLR4/MD-2 using FlowJo (Tree Star) and Cellquest Pro software (BD Bioscience). The data are presented as the percentage of CD11b+ Mφ that express TLR2 and TLR4/MD-2 receptors. (A) TLR2 (P < 0.0001; n = 18) and (B) TLR4/MD-2 (P < 0.0001; n = 16) cell surface expression was significantly up-regulated between 15.5 and 17.5 dpc and remained elevated through 18.5 dpc (P < 0.0001; n = 19). Statistical significance between 15.5 dpc and later time points was determined by one-way ANOVA followed by Tukey's analysis. The data are expressed as mean ± sem. ***, P < 0.0001.
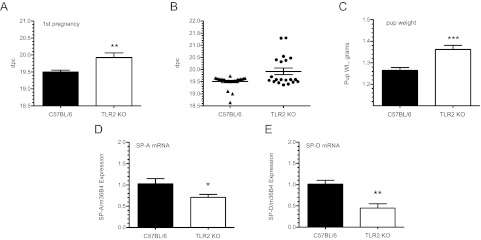
TLR2-deficient mice manifest a significant delay in the timing of labor that is associated with decreased expression of CX43 and F4/80 mRNA in the gravid myometrium and with significantly lower levels of SP-A and SP-D gene expression in fetal lung
TLR2 and TLR4 are expressed in numerous reproductive tissues including the decidua (51) and myometrium (52). In studies of human myometrial biopsies, TLR2 mRNA and protein were found to be significantly increased in tissues from laboring vs. nonlaboring women, whereas TLR4 protein levels remain unchanged (52, 53). Notably, SP-A has been reported to enhance expression of TLR2, but not TLR4, during differentiation of human monocytes to Mφs (54). Consequently, we decided to focus on the potential role of TLR2 in murine parturition. The time to labor was assessed in TLR2−/− mice during first pregnancies. Compared with WT B6 (19.5 ± 0.02 dpc, n = 25), TLR2−/− (19.93 ± 0.61 dpc) mice manifested a significant delay (P < 0.001; n = 20) in the timing of labor in first pregnancies (Fig. 5A). A scatter plot of these data reveals the wide range in the time to labor observed in the mutant mice (Fig. 5B). Accordingly, mice manifesting delayed labor also delivered pups whose average weight was significantly greater (P < 0.0001) than WT littermates (Fig. 5C). qRT-PCR analysis of SP-A and SP-D mRNA levels in fetal lungs at 18.5 dpc indicated a pronounced decrease in SP-A (P < 0.05; n = 12) and SP-D (P = 0.005; n = 12) expression in TLR2-deficient fetuses compared with WT B6 pups (Fig. 5, D and E).
Fig. 5.
TLR2-deficient mice manifest a significant delay in labor. Virgin TLR2−/− and B6 WT mice were bred to genetically like males and parturition timing was assessed in first pregnancies as d postcoitum (dpc). A, TLR2−/− mice manifested a statistically significant delay in parturition timing (19.93 ± 0.14 dpc) (P < 0.001; n = 20) compared with WT B6 (19.5 ± 0.05 dpc, n = 25). B, Scatter plot of the parturition data illustrates the degree of dysregulated parturition. C, TLR2−/− pups delivered by mothers that manifested delayed labor weighed significantly more than those born to B6 WT mothers at 19.5 dpc. Expression of SP-A (D) and SP-D (E) mRNA levels in TLR2−/− and B6 WT fetal lungs was evaluated at 18.5 dpc by qRT-PCR. Transcript expression was normalized to m36B4 expression and the comparative Ct (ΔΔCt) method was used to quantify expression levels. Results are expressed as fold change relative to gestation-matched WT controls. mRNA levels of (D) SP-A (P < 0.05; n = 12) and (E) SP-D (P = 0.005; n = 12) were significantly lower in fetal lungs of TLR2−/− pups. Values are expressed as mean ± sem. Statistically significant differences were calculated by the Student's t test. *, P < 0.05, **, P < 0.005, ***, P < 0.0001.
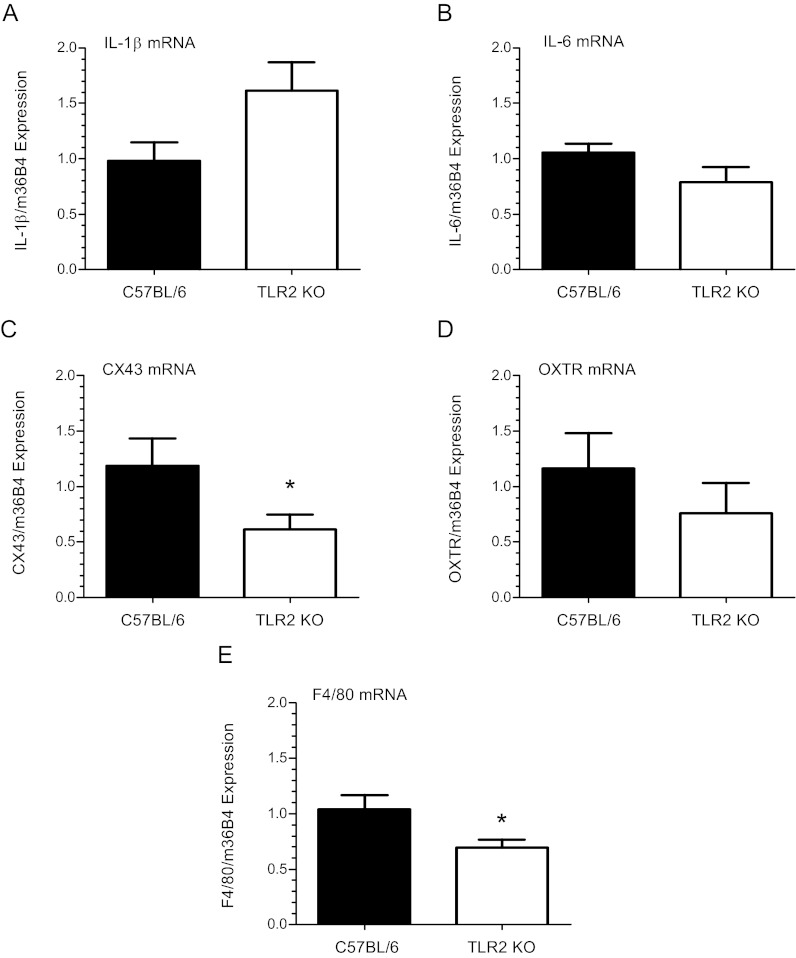
Analysis of myometrial tissues from TLR2-deficient females at 18.5 dpc revealed a trend toward increased expression of IL-1β (P = 0.06; n = 13) and no difference in IL-6 mRNA (P = 0.1; n = 10), compared with WT mice (Fig. 6, A and B). Notably, these cytokines are not downstream of TLR2. On the other hand, expression of the CAP gene, CX43, was significantly decreased (P < 0.05; n = 13) in TLR2 null vs. WT mice (Fig. 6C), whereas no significant difference in OXTR (P = 0.33; n = 10) mRNA levels (Fig. 6D) was detected. Interestingly, expression of the Mφ-specific marker, F4/80, was significantly decreased in the myometrium of TLR2−/− mice compared with WT at 18.5 dpc (Fig. 6E).
Fig. 6.
TLR2-deficient mice exhibit dysregulated expression of inflammatory and contraction-associated genes in myometrium near term. Myometrial tissues were isolated from TLR2−/− and B6 WT pregnant females at 18.5 dpc and analyzed for IL-1β, IL-6, CX43, and OXTR mRNA expression by qRT-PCR. Values were normalized to m36B4 and calculated as fold change over B6 WT control using the ΔΔCt method. Analyses revealed a trend for up-regulated, albeit insignificant (P < 0.06; n = 13), expression of IL-1β mRNA (A) and similar expression levels of IL-6 (B) in TLR2−/− (P = 0.1; n = 10) mice compared with WT controls. Expression of the CAP gene CX43 (C) was significantly decreased (P < 0.05; n = 13), whereas no difference in OXTR (D) (P = 0.33; n = 10) mRNA levels was detected. Significantly decreased levels of F4/80 mRNA (P < 0.05; n = 13) (E) in myometrial tissues of TLR2−/− mice were observed relative to B6 WT mice. Values are expressed as the mean ± sem. Statistically significant differences were calculated using the Student's t test. *, P < 0.05.
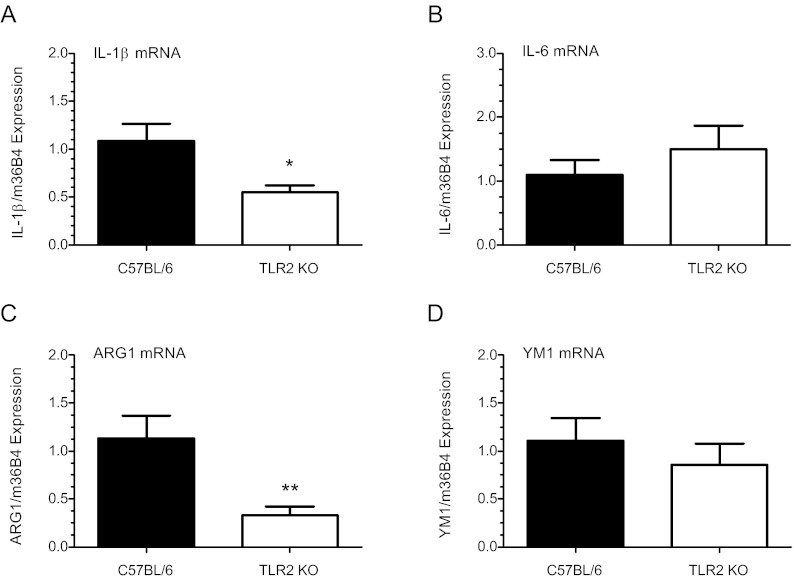
AF Mφs isolated from TLR2−/− mice exhibit aberrant changes in expression of proinflammatory and antiinflammatory activation markers at 18.5 dpc compared with B6 WT mice
To investigate the consequences of TLR2 deficiency in AF Mφ activation, F4/80+ AF Mφs were isolated at 18.5 dpc by FACSAria (BD Bioscience), and a SuperArray qRT-PCR-based inflammatory array was used to profile inflammation-associated gene transcription. Of the 84 genes assayed, 32 showed a significant decrease of 2-fold or greater (P < 0.05) compared with WT Mφ populations (Table 2). The top five down-regulated genes in cells isolated from TLR2-deficient mice included Ccl19 (21-fold), IL-13 (6-fold), IL-17b (5.5-fold), lymphotoxin-β (Ltb; 5.5-fold), and Ccr10 (5.4-fold). Ccl19/ELC/MIP-3β binds chemokine receptor (CCR)-7 and functions as a potent chemoattractant in T and B cell and mature dendritic cell recruitment (55). IL-13, produced primarily by activated Th2 cells, acts on a variety of cell types and is implicated as a key mediator in the pathogenesis of allergic inflammation (56). IL-17b stimulates release of proinflammatory cytokines from macrophages and monocytic cell lines. Ltb, a member of the TNF superfamily, plays a critical role in immune system development, regulation, and inflammation (57), whereas CCR10 binds CCL27 to stimulate intracellular calcium and promote chemotaxis (58). Importantly, expression of the proinflammatory activation (M1) marker, IL-1β, was also significantly decreased (P < 0.0001) in TLR2 null Mφs, as were numerous other inflammatory mediators and migration-associated genes (e.g. CCR2, receptor for the chemokine MCP-1). qRT PCR confirmed substantially lower levels of IL-1β (P < 0.05; n = 9) in independent samples of TLR2−/− AF Mφ (Fig. 7A). Analysis also revealed comparable levels of IL-6 (P = 0.4; n = 7) (Fig. 7B) in TLR2−/− vs. B6 WT Mφs. Examination of Mφ antiinflammatory activation (M2) markers indicated that ARG 1 (P < 0.001; n = 9) was significantly reduced, whereas YM1 (P = 0.4; n = 7) expression was unaffected by TLR2 deficiency (Fig. 7, C and D). Notably, the effects of TLR2 deficiency were similar to those observed in AF Mφs from SP-A/d-deficient mice (Fig. 3, A and D).
Table 2.
Differentially expressed proinflammatory genes in AF Mφs from TLR2−/− mice relative to B6 WT
| Reference sequence | Gene symbol | Function | Fold change | P value | |
|---|---|---|---|---|---|
| 1 | Mm.424740 | Ccl19 | Potent leukocyte chemoattractant | −21.26 | 0.006 |
| 2 | Mm.1284 | IL13 | Mediator of allergic inflammation | −6.22 | 0.020 |
| 3 | Mm.59313 | IL17b | Stimulates TNF and IL-1β from monocytic cell lines | −5.53 | 0.005 |
| 4 | Mm.1715 | Ltb | Immune system development, regulation, amd inflammation | −5.48 | 0.018 |
| 5 | Mm.8021 | Ccr10 | Regulates chemotaxis in various leukocytes | −5.41 | 0.097 |
| 6 | Mm.10116 | Cxcl13 | Stimulates Ca2+ influx, B cell migration | −4.89 | 0.003 |
| 7 | Mm.234466 | Cxcr2 | Binds KC and MIP-2 | −4.72 | 0.002 |
| 8 | Mm.1349 | IL1r2 | Decoy receptor inhibits IL-1α, IL-1β, and IL-1R type 1 | −4.59 | 0.013 |
| 9 | Mm.390241 | Xcr1 | Increases intracellular Ca2+ levels | −4.36 | 0.002 |
| 10 | Mm.64326 | Cxcl15 | Major mediator of the inflammatory response | −4.19 | 0.000 |
| 11 | Mm.766 | Cxcl9 | T cell trafficking | −3.71 | 0.001 |
| 12 | Mm.14302 | Ccr5 | Binds MCP-2, MIP-1α, and MIP-1β | −3.56 | 0.004 |
| 13 | Mm.288474 | Spp1 | Involved in IFNg and IL-12 production | −3.56 | 0.011 |
| 14 | Mm.6272 | Ccr2 | Receptor for MCP-1, MCP-3, and MCP-4 | −3.44 | 0.008 |
| 15 | Mm.42029 | Ccl8 | Chemoattractant for monocytes, lymphocytes, basophiles | −3.24 | 0.016 |
| 16 | Mm.103794 | IL20 | Stimulates kerotinocyte proliferation and TNF synthesis | −3.21 | 0.020 |
| 17 | Mm.19131 | C3 | Activation of the complement system | −3.12 | 0.006 |
| 18 | Mm.57050 | Ccr3 | Binds eotaxin, eotaxin-3, MCP-3, MCP-4, RANTES, and MIP-1Δ | −3.08 | 0.007 |
| 19 | Mm.4392 | IL15 | Stimulates growth of T and NK cells | −2.99 | 0.001 |
| 20 | Mm.2856 | IL6ra | Binds IL-6, may lead to acute phase reaction | −2.89 | 0.025 |
| 21 | Mm.274927 | Ccr1 | Mediates recruitment of immune cells to inflammatory sites | −2.84 | 0.008 |
| 22 | Mm.347398 | Bcl6 | Transcriptional repressor, B cells | −2.75 | 0.019 |
| 23 | Mm.1410 | IL18 | Induces IFNγ production in T cells | −2.68 | 0.005 |
| 24 | Mm.222830 | IL1b | Macrophage and T cell activation | −2.55 | 0.017 |
| 25 | Mm.262106 | Itgam | Implicated in adhesive interactions | −2.54 | 0.013 |
| 26 | Mm.137 | Ccl6 | Chemotactic factor for T cells and monocytes | −2.47 | 0.006 |
| 27 | Mm.235328 | Tnfrsf1b | Mediates numerous metabolic effects of TNF | −2.41 | 0.002 |
| 28 | Mm.24208 | IL13ra1 | Forms receptor complex with IL4RA | −2.33 | 0.004 |
| 29 | Mm.35814 | IL11 | Proliferation of stem cells | −2.33 | 0.032 |
| 30 | Mm.10137 | IL16 | chemoattractant for CD4+ cells, monocytes, and eosinophils | −2.22 | 0.004 |
| 31 | Mm.4861 | Cd40lg | Regulates B cell function via CD40 binding | −2.18 | 0.008 |
| 32 | Mm.4154 | IL10rb | Inhibits inflammatory cytokine synthesis in Mf | −2.03 | 0.000 |
F4/80+ AF Mφs were isolated from TLR2-deficient and B6 WT mice at 18.5 dpc by FACSAria (BD Bioscience). RNA (500 ng) was reverse transcribed and expression of 84 inflammation associated genes was evaluated using SuperArray's mouse inflammatory RT2 profiler PCR array plate. Data were analyzed using PCR array data analysis template (SABioscience). The comparative Ct method (ΔΔCt) was used for quantification of gene expression. Genes down-regulated 2-fold or greater in TLR2−/− AF Mφs relative to WT are listed from largest to smallest differences. Samples were analyzed in duplicate. Statistical significance was determined by the Student's t test.
Fig. 7.
Amniotic fluid Mφs isolated from TLR-2-deficient mice exhibit decreased expression of M1 and M2 activation markers. F4/80+ AF Mφs isolated from TLR2−/− and WT B6 mice were isolated by FACSAria (BD Bioscience) at 18.5 dpc. Pooled Mφ mRNA (500 ng) from each timed-pregnant mouse was reverse transcribed and the expression of IL-1β and IL-6 was analyzed by qRT-PCR. Expression was normalized to m36B4 and calculated as fold change over WT control using the ΔΔCt method. Data indicate decreased levels of the classical activation (M1) marker IL-1β (A) (P < 0.05; n = 9) and comparable levels of IL-6 (B) (P = 0.4; n = 7) in TLR2−/− AF Mφ compared with WT. Analysis of alternative activation (M2) markers revealed significantly lower levels of ARG1 (C) mRNA (P < 0.001; n = 9) in TLR2−/− Mφs, whereas YM1 (D) (P = 0.4; n = 7) expression was similar to WT. Values are the mean ± sem. Statistically significant differences were analyzed by the Student's t test. *, P < 0.05, **, P < 0.001.
Discussion
In light of our previous findings, which suggested a role of SP-A produced by the fetal lung in inflammatory signaling leading to labor (14), it was of interest to functionally characterize the roles of SP-A, and the related C-type lectin, SP-D, in the timing of parturition in mice. To accomplish this, the timing of labor was assessed during first and second pregnancies in C57BL/6 WT mice and in mice homozygous null for SP-A, SP-D, and doubly deficient in SP-A and SP-D. SP-A−/−, SP-D−/−, and SP-A−/−/D−/− female mice bred to genetically-like males delivered normally at term during first pregnancies. However, during second pregnancies, SP-A and SP-A/SP-D null females bred to genetically like males manifested a delay in parturition timing. Although the delay did not reach statistical significance for the SP-A gene-targeted mice (P = 0.07), a similar delay in labor in the SP-A/SP-D null mice was highly significant. The pups born to both SP-A and SP-A/SP-D null mice were significantly heavier than WT, further indicating their advanced maturity at the time of birth. Because SP-A and SP-D genes lie only 60 kb apart on the mouse chromosome 14, to generate mice deficient in both SP-A and SP-D, it was necessary to sequentially target these genes (41). Deletion of SP-A and SP-D resulted in decreased expression of the mannose binding lectin 1 (MBL1) gene, which lies between them (41). Interestingly, a polymorphism in the SP-D gene has been associated with spontaneous preterm birth in a Finish cohort; however, none was noted in the genes for SP-A or MBL1 in this population (59). We reasoned that the normal timing of parturition observed in the SP-A deficient and the SP-A/D doubly deficient mice in first pregnancies is potentially due to multifactorial regulation of parturition timing and the dominant role of uterine stretch as a signal for parturition (12, 60, 61) in the nonadapted uterus. However, in subsequent pregnancies, the prior mechanical adaptation of the uterus to stretch, resulting in increased elasticity (62) may allow other signals (e.g. surfactant proteins) to play a more significant signaling role.
Up-regulation of cytokine production, NF-κB activation, and prostaglandin signaling pathways within the myometrium are proposed to serve an important role in the initiation of parturition at term. Similarly, during pathogen-induced preterm labor, robust inflammatory cytokine production in fetal and maternal reproductive tissues promote the expression of secondary mediators responsible for enhancing myometrial contractile activity and birth (2, 63). Our finding in this study that SP-A/SP-D doubly deficient mice express significantly lower levels of IL-1β and IL-6 in myometrium suggests that interaction of SP-A and SP-D with their cognate receptor(s) promote the induction of proinflammatory cytokines leading to parturition.
IL-1β and IL-6 promote increased myometrial contractility by direct activation of NF-κB, COX-2 and prostaglandin signaling pathways (64–66). IL-1β is highly expressed in amnion, chorion, isolated decidua, and myometrium, and IL-1β levels are increased in AF and in AF Mφs near term (9, 11, 14, 67, 68). The finding that mice deficient for the IL-1 receptor (type 1) apparently deliver normally (69) is likely due to functional redundancy of parturition-associated cytokines because administration of IL-1 to nonhuman primates and rodents triggers preterm labor (70, 71). Although it was previously reported that IL-6 was not necessary or required for bacterially induced preterm labor in mice (72), Robertson et al. (73) observed a delay in labor at term of approximately 24 h in IL-6-deficient mice. Interestingly, this delay was associated with a 24 h latency in up-regulation of COX-2 and OXTR mRNA expression in myometrial tissues (73). Moreover, chronic administration of IL-6 restored normal parturition in these mice. Although IL-6 infusion did not alter maternal progesterone levels, pronounced changes in the expression of genes associated with uterine contractile activity were observed (73). The reduced levels of IL-1β and IL-6 mRNA in myometrium of SP-A/D−/− mice at 18.5 dpc, compared with WT, observed in the present study, were associated with a pronounced reduction in expression of the CAP genes, OXTR and CX43. This serves to underscore the critical role of SP-A and SP-D early in the inflammatory signaling pathway and in the subsequent activation of CAP genes leading to parturition. Thus, the delay in labor in the SP-A/SP-D null mice is likely attributed to the interruption of the inflammatory cascade at the level of binding of these pulmonary collectins to their cognate receptors.
Our previous studies suggested that augmented production of SP-A by fetal lung serves as a hormonal signal for the initiation of labor that is transmitted to the maternal uterus by fetal AF Mφs (14). We observed that the gestational increase in SP-A secretion by mouse fetal lung was associated with increased expression of IL-1β in AF Mφs and increased Mφ infiltration, IL-1β expression, and NF-κB activation in the maternal uterus (14). Moreover, SP-A treatment of AF Mφs caused an up-regulation of IL-1β, a proinflammatory marker. In the present study, we found that AF Mφs surrounding SP-A/d-deficient fetuses manifested significantly decreased expression of the Mφ proinflammatory M1 activation marker, IL-1β, and the antiinflammatory M2 activation markers, ARG1 and YM1. These findings are in accord with a growing body of evidence that SP-A and SP-D proteins act as modulators of both proinflammatory and antiinflammatory immune cell function (74).
Due to the similar inflammatory hallmarks of term and preterm labor and the association of preterm labor with underlying bacterial infection (13), a role for TLR2 and/or TLR4 in spontaneous labor at term has been postulated. As mentioned, SP-A, and SP-D are known ligands for TLR2 and TLR4. During ontogeny in mice, TLR2 and TLR4 mRNA levels within the fetal lung increase approximately 7-fold between 15 dpc and term (75). Whether these increases are due to enhanced expression in lung cells or in resident Mφs was not determined. Our present findings reveal that expression in TLR2 and TLR4/MD-2 was up-regulated between 15.5 and 18.5 dpc in AF Mφs, which likely arise from the fetal lung. These findings are consistent with studies demonstrating that SP-A selectively increases surface expression of TLR2 in human monocyte-derived Mφs (54). Importantly, in the present study, we also observed a significant delay in the timing of parturition in mice deficient in TLR2 during first pregnancies. TLR2-deficient mothers with delayed labor delivered significantly larger pups, supporting the observation of protracted gestation length. Scatter plots of data for parturition timing in TLR2-deficient mice suggest a perturbation in parturition timing and further emphasize the existence of compensatory mechanisms (e.g. uterine stretch) during first pregnancies.
The finding of significantly lower levels of SP-A and SP-D expression in the lungs of TLR2−/− fetal mice, supports the concept that Mφ-type II cell interactions play an important role in lung surfactant production and in parturition timing. The intraamniotic administration of endotoxin (76) or IL-1 (77) to pregnant rabbits was previously observed to increase fetal lung expression of SP-A and to induce preterm birth. A similar induction of SP-A expression in fetal lung was observed after the intraamniotic administration of endotoxin to fetal sheep (78). Moreover, the incidence of respiratory distress syndrome was reported to be decreased in infants born prematurely to women with chorioamnionitis (79), suggesting a role for increased AF cytokines in fetal lung maturation and surfactant synthesis. Notably, the finding that LPS treatment increased SP-A gene expression in A549 human lung adenocarcinoma cells through TLR2-mediated sequential activation of the MYD88-MAPK kinase-4-c-Jun N-terminal kinase 1-activator protein-1 pathway, further suggests a role for TLR2 signaling in the regulation of SP-A expression (80). Thus, in the setting of sterile inflammation, as that found within the AF compartment near term, SP-A, and SP-D, and TLR2 may function in a positive feed-forward loop, resulting in further increases in SP-A and SP-D and enhanced Mφ activation. Interruption of this pathway in TLR2-deficient mice would therefore be expected to result in decreased expression of SP-A and SP-D by the fetal lungs.
In TLR2-deficient mice, IL-1β and IL-6 were expressed in myometrial tissues at levels similar to WT. This is likely due the fact that IL-1 and IL-6 are not components of the TLR signaling pathway. The lack of an effect of TLR2 deficiency on OXTR expression may reflect the dominant role of uterine stretch in activating expression of this CAP gene (12) during first pregnancies. However, the significantly reduced levels of the Mφ marker, F4/80, and of CX43 expression in myometrium of TLR2−/− mice indicates that Mφ migration and gap junction formation were disrupted by TLR2 deficiency. The reduced expression of CX43 may contribute to the prolonged gestation in TLR2−/− mice because conditional deletion of myometrial CX43 was observed to significantly delay labor (81). Indeed, gap junction formation in human airway and intestinal epithelial cell lines was reported to be mediated by TLR2-dependent transcriptional regulation and posttranslational modification of CX43 (82, 83). The significantly decreased levels of the Mφ marker, F4/80, in myometrium of TLR2-deficient mice indicates that Mφ migration also was markedly affected. These findings are in accord with the recent report that TLR2 activation is necessary for SP-A-stimulated chemotaxis of murine macrophages (84).
We therefore postulate that the delay in labor observed in TLR2-deficient mice might be attributed, in part, to a disruption in AF Mφ activation. SuperArray analysis of F4/80+ AF Mφs isolated from TLR2−/− mice (18.5 dpc) supported this notion in light of the large number of proinflammatory and migration-associated genes that were strongly down-regulated in TLR2-deficient AF Mφs, compared with WT at 18.5 dpc. Thus, TLR2 deficiency prevented the up-regulation of the proinflammatory and migratory genes in AF Mφ near term, which may contribute to the observed decrease in levels of F4/80 in the myometrium. Importantly, the altered M1 and M2 molecular profile observed in TLR2-deficient mice was mirrored in SP-A/D−/− AF Mφs because they too expressed significantly reduced levels of these same markers, in addition to YM1, at 18.5 dpc. Together these findings suggest that SP-A and TLR2 are essential for normal AF Mφ activation and potentially for subsequent Mφ infiltration of the myometrium at term.
In conclusion, our findings suggest that the signals leading to the initiation of labor are multifactorial and their relative impacts may be dependent on parity. Whereas in the first pregnancy, uterine stretch may function as the primary and overriding signal, during subsequent pregnancies, signals, such as SP-A and SP-D acting via TLRs may play a more critical role. Our data further indicate that interaction of SP-A and SP-D with TLR2 may cause activation of the AF Mφ population, which, in turn, contributes to the inflammatory cascade within the myometrium that culminates in increased CAP gene expression leading to labor at term. Importantly, these findings may provide insight into the novel therapeutic strategies for the prevention of preterm birth.
Supplementary Material
Acknowledgments
We thank Dr. Robert Hammer (University of Texas Southwestern Medical Center) for expert advice, and Mr. Ryan Norcross and Ms. Jo Smith for their assistance in this project.
This research was funded, in part, by National Institutes of Health Grants R01-HL050022, P01-HD011149 and Prematurity Research Grant No. 21-FY11-30 from the March of Dimes Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AF
- Amniotic fluid
- ARG1
- arginase 1
- CAP
- contraction-associated protein
- CCR
- chemokine receptor
- COX-2
- cyclooxygenase 2
- Ct
- cycle threshold
- CX43
- connexin-43
- dpc
- d postcoitum
- LPS
- lipopolysaccharide
- Ltb
- lymphotoxin-β
- Mφ
- macrophage
- MCP
- monocyte chemotactic protein
- MD-2
- molecule that physically complexes with TLR4 on the cell surface and confers LPS responsiveness
- NF-κB
- nuclear factor-κB
- OXTR
- oxytocin receptor
- PE
- phycoerythrin
- PRR
- pattern recognition receptor
- qRT-PCR
- quantitative RT-PCR
- SP
- surfactant protein
- TLR
- Toll-like receptor
- WT
- wild type
- YM1
- chitin 3-like 3.
References
- 1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. 2012. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379:2162–2172 [DOI] [PubMed] [Google Scholar]
- 2. Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. 1988. Infection in the pathogenesis of preterm labor. Semin Perinatol 12:262–279 [PubMed] [Google Scholar]
- 3. Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, Merkatz IR, Greene MF, Schwarz RH. 2005. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol 193:626–635 [DOI] [PubMed] [Google Scholar]
- 4. Goldenberg RL, Culhane JF, Iams JD, Romero R. 2008. Epidemiology and causes of preterm birth. Lancet 371:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirsch E, Wang H. 2005. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig 12:145–155 [DOI] [PubMed] [Google Scholar]
- 6. Elovitz MA, Mrinalini C. 2004. Animal models of preterm birth. Trends Endocrinol Metab 15:479–487 [DOI] [PubMed] [Google Scholar]
- 7. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. 2009. Inflammation and pregnancy. Reprod Sci 16:206–215 [DOI] [PubMed] [Google Scholar]
- 8. Mendelson CR. 2009. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 23:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cox SM, Casey ML, MacDonald PC. 1997. Accumulation of interleukin-1β and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum Reprod Update 3:517–527 [DOI] [PubMed] [Google Scholar]
- 10. Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. 1999. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 14:229–236 [PubMed] [Google Scholar]
- 11. Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 9:41–45 [DOI] [PubMed] [Google Scholar]
- 12. Shynlova O, Tsui P, Jaffer S, Lye SJ. 2009. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 144(Suppl 1):S2–S10 [DOI] [PubMed] [Google Scholar]
- 13. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. 2007. The role of inflammation and infection in preterm birth. Semin Reprod Med 25:21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Condon JC, Jeyasuria P, Faust JM, Mendelson CR. 2004. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA 101:4978–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. López Bernal A, Newman GE, Phizackerley PJ, Turnbull AC. 1988. Surfactant stimulates prostaglandin E production in human amnion. Br J Obstet Gynaecol 95:1013–1017 [DOI] [PubMed] [Google Scholar]
- 16. Mendelson CR, Boggaram V. 1990. Hormonal and developmental regulation of pulmonary surfactant synthesis in fetal lung. Baillieres Clin Endocrinol Metab 4:351–378 [DOI] [PubMed] [Google Scholar]
- 17. Whitsett JA, Weaver TE. 2002. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 347:2141–2148 [DOI] [PubMed] [Google Scholar]
- 18. Hawgood S, Shiffer K. 1991. Structures and properties of the surfactant-associated proteins. Annu Rev Physiol 53:375–394 [DOI] [PubMed] [Google Scholar]
- 19. Hawgood S, Poulain FR. 2001. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol 63:495–519 [DOI] [PubMed] [Google Scholar]
- 20. Snyder JM, Kwun JE, O'Brien JA, Rosenfeld CR, Odom MJ. 1988. The concentration of the 35-kDa surfactant apoprotein in amniotic fluid from normal and diabetic pregnancies. Pediatr Res 24:728–734 [DOI] [PubMed] [Google Scholar]
- 21. Mendelson CR, Condon JC. 2005. New insights into the molecular endocrinology of parturition. J Steroid Biochem Mol Biol 93:113–119 [DOI] [PubMed] [Google Scholar]
- 22. Drickamer K. 1999. C-type lectin-like domains. Curr Opin Struct Biol 9:585–590 [DOI] [PubMed] [Google Scholar]
- 23. Seaton BA, Crouch EC, McCormack FX, Head JF, Hartshorn KL, Mendelsohn R. 2010. Review: structural determinants of pattern recognition by lung collectins. Innate Immun 16:143–150 [DOI] [PubMed] [Google Scholar]
- 24. Kuroki Y, Takahashi M, Nishitani C. 2007. Pulmonary collectins in innate immunity of the lung. Cell Microbiol 9:1871–1879 [DOI] [PubMed] [Google Scholar]
- 25. Crouch E, Wright JR. 2001. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol 63:521–554 [DOI] [PubMed] [Google Scholar]
- 26. Kingma PS, Whitsett JA. 2006. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol 6:277–283 [DOI] [PubMed] [Google Scholar]
- 27. Wright JR. 2005. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68 [DOI] [PubMed] [Google Scholar]
- 28. Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. 2002. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 168:5989–5992 [DOI] [PubMed] [Google Scholar]
- 29. Murakami S, Iwaki D, Mitsuzawa H, Sano H, Takahashi H, Voelker DR, Akino T, Kuroki Y. 2002. Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-α secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J Biol Chem 277:6830–6837 [DOI] [PubMed] [Google Scholar]
- 30. Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. 2003. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115:13–23 [DOI] [PubMed] [Google Scholar]
- 31. Sano H, Kuroki Y. 2005. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol Immunol 42:279–287 [DOI] [PubMed] [Google Scholar]
- 32. Mukherjee S, Giamberardino C, Thomas J, Evans K, Goto H, Ledford JG, Hsia B, Pastva AM, Wright JR. 2012. Surfactant protein A integrates activation signal strength to differentially modulate T cell proliferation. J Immunol 188:957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. 2003. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-κB activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J Immunol 171:417–425 [DOI] [PubMed] [Google Scholar]
- 34. Ohya M, Nishitani C, Sano H, Yamada C, Mitsuzawa H, Shimizu T, Saito T, Smith K, Crouch E, Kuroki Y. 2006. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry 45:8657–8664 [DOI] [PubMed] [Google Scholar]
- 35. Tsan MF, Gao B. 2004. Endogenous ligands of Toll-like receptors. J Leukoc Biol 76:514–519 [DOI] [PubMed] [Google Scholar]
- 36. Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. 2006. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem 281:21771–21780 [DOI] [PubMed] [Google Scholar]
- 37. Janeway CA, Jr, Medzhitov R. 2002. Innate immune recognition. Annu Rev Immunol 20:197–216 [DOI] [PubMed] [Google Scholar]
- 38. Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145 [DOI] [PubMed] [Google Scholar]
- 39. Li G, Siddiqui J, Hendry M, Akiyama J, Edmondson J, Brown C, Allen L, Levitt S, Poulain F, Hawgood S. 2002. Surfactant protein A-deficient mice display an exaggerated early inflammatory response to a β-resistant strain of influenza A virus. Am J Respir Cell Mol Biol 26:277–282 [DOI] [PubMed] [Google Scholar]
- 40. Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, Clements J, Carlson E, Gillespie AM, Epstein C, Hawgood S. 1998. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci USA 95:11869–11874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hawgood S, Ochs M, Jung A, Akiyama J, Allen L, Brown C, Edmondson J, Levitt S, Carlson E, Gillespie AM, Villar A, Epstein CJ, Poulain FR. 2002. Sequential targeted deficiency of SP-A and -D leads to progressive alveolar lipoproteinosis and emphysema. Am J Physiol Lung Cell Mol Physiol 283:L1002–L1010 [DOI] [PubMed] [Google Scholar]
- 42. Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- 43. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 44. Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. 2010. Mouse gestation length is genetically determined. PLoS One 5:e12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yan X, Sun M, Gibb W. 2002. Localization of nuclear factor-κB (NF-κB) and inhibitory factor-κB (IκB) in human fetal membranes and decidua at term and preterm delivery. Placenta 23:288–293 [DOI] [PubMed] [Google Scholar]
- 46. Chow L, Lye SJ. 1994. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol 170:788–795 [DOI] [PubMed] [Google Scholar]
- 47. Ou CW, Chen ZQ, Qi S, Lye SJ. 1998. Increased expression of the rat myometrial oxytocin receptor messenger ribonucleic acid during labor requires both mechanical and hormonal signals. Biol Reprod 59:1055–1061 [DOI] [PubMed] [Google Scholar]
- 48. Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. 2010. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci U S A 107:20828–20833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and Gram-positive bacterial cell wall components. Immunity 11:443–451 [DOI] [PubMed] [Google Scholar]
- 50. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088 [DOI] [PubMed] [Google Scholar]
- 51. Canavan TP, Simhan HN. 2007. Innate immune function of the human decidual cell at the maternal-fetal interface. J Reprod Immunol 74:46–52 [DOI] [PubMed] [Google Scholar]
- 52. Youssef RE, Ledingham MA, Bollapragada SS, O'Gorman N, Jordan F, Young A, Norman JE. 2009. The role of toll-like receptors (TLR-2 and -4) and triggering receptor expressed on myeloid cells 1 (TREM-1) in human term and preterm labor. Reprod Sci 16:843–856 [DOI] [PubMed] [Google Scholar]
- 53. O'Brien M, Morrison JJ, Smith TJ. 2008. Upregulation of PSCDBP, TLR2, TWIST1, FLJ35382, EDNRB, and RGS12 gene expression in human myometrium at labor. Reprod Sci 15:382–393 [DOI] [PubMed] [Google Scholar]
- 54. Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. 2008. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol 180:7847–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. 2002. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 169:424–433 [DOI] [PubMed] [Google Scholar]
- 56. Hershey GK. 2003. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol 111:677–690; quiz 691 [DOI] [PubMed] [Google Scholar]
- 57. Locksley RM, Killeen N, Lenardo MJ. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501 [DOI] [PubMed] [Google Scholar]
- 58. Homey B, Alenius H, Müller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bünemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A. 2002. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med 8:157–165 [DOI] [PubMed] [Google Scholar]
- 59. Karjalainen MK, Huusko JM, Tuohimaa A, Luukkonen A, Haataja R, Hallman M. 2012. A study of collectin genes in spontaneous preterm birth reveals an association with a common surfactant protein D gene polymorphism. Pediatr Res 71:93–99 [DOI] [PubMed] [Google Scholar]
- 60. Shynlova O, Tsui P, Dorogin A, Lye SJ. 2008. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol 181:1470–1479 [DOI] [PubMed] [Google Scholar]
- 61. Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. 2004. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod 10:109–113 [DOI] [PubMed] [Google Scholar]
- 62. Wu X, Morgan KG, Jones CJ, Tribe RM, Taggart MJ. 2008. Myometrial mechanoadaptation during pregnancy: implications for smooth muscle plasticity and remodelling. J Cell Mol Med 12:1360–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. 2003. Cytokines, prostaglandins and parturition—a review. Placenta 24(Suppl A):S33–S46 [DOI] [PubMed] [Google Scholar]
- 64. Hardy DB, Janowski BA, Corey DR, Mendelson CR. 2006. Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- 65. Dong YL, Gangula PR, Fang L, Yallampalli C. 1996. Differential expression of cyclooxygenase-1 and -2 proteins in rat uterus and cervix during the estrous cycle, pregnancy, labor and in myometrial cells. Prostaglandins 52:13–34 [DOI] [PubMed] [Google Scholar]
- 66. Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. 2001. Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the 'functional progesterone withdrawal.' Mol Hum Reprod 7:581–586 [DOI] [PubMed] [Google Scholar]
- 67. Dudley DJ, Collmer D, Mitchell MD, Trautman MS. 1996. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig 3:328–335 [PubMed] [Google Scholar]
- 68. Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP, Mitchell MD. 1990. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 35:235–238 [PubMed] [Google Scholar]
- 69. Abbondanzo SJ, Cullinan EB, McIntyre K, Labow MA, Stewart CL. 1996. Reproduction in mice lacking a functional type 1 IL-1 receptor. Endocrinology 137:3598–3601 [DOI] [PubMed] [Google Scholar]
- 70. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. 2006. Preterm labor is induced by intraamniotic infusions of interleukin-1β and tumor necrosis factor-α but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 195:1578–1589 [DOI] [PubMed] [Google Scholar]
- 71. Romero R, Mazor M, Tartakovsky B. 1991. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 165:969–971 [DOI] [PubMed] [Google Scholar]
- 72. Yoshimura K, Hirsch E. 2003. Interleukin-6 is neither necessary nor sufficient for preterm labor in a murine infection model. J Soc Gynecol Investig 10:423–427 [DOI] [PubMed] [Google Scholar]
- 73. Robertson SA, Christiaens I, Dorian CL, Zaragoza DB, Care AS, Banks AM, Olson DM. 2010. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology 151:3996–4006 [DOI] [PubMed] [Google Scholar]
- 74. Nayak A, Dodagatta-Marri E, Tsolaki AG, Kishore U. 2012. An insight into the diverse roles of surfactant proteins, SP-A and SP-D in innate and adaptive immunity. Front Immunol 3:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Harju K, Glumoff V, Hallman M. 2001. Ontogeny of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr Res 49:81–83 [DOI] [PubMed] [Google Scholar]
- 76. Bry K, Lappalainen U. 2001. Intra-amniotic endotoxin accelerates lung maturation in fetal rabbits. Acta Paediatr 90:74–80 [DOI] [PubMed] [Google Scholar]
- 77. Bry K, Lappalainen U, Hallman M. 1997. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest 99:2992–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, Possmayer F, Hallman M, Ikegami M. 2000. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol 182:401–408 [DOI] [PubMed] [Google Scholar]
- 79. Tsuda H, Takahashi Y, Iwagaki S, Kawabata I, Hayakawa H, Kotani T, Shibata K, Kikkawa F. 2010. Intra-amniotic infection increases amniotic lamellar body count before 34 weeks of gestation. J Matern Fetal Neonatal Med 23:1230–1236 [DOI] [PubMed] [Google Scholar]
- 80. Chuang CY, Chen TG, Tai YT, Chen TL, Lin YH, Tsai CH, Chen RM. 2011. Toll-like receptor 2-mediated sequential activation of MyD88 and MAPKs contributes to lipopolysaccharide-induced SP-A gene expression in human alveolar epithelial cells. Immunobiology 216:707–714 [DOI] [PubMed] [Google Scholar]
- 81. Döring B, Shynlova O, Tsui P, Eckardt D, Janssen-Bienhold U, Hofmann F, Feil S, Feil R, Lye SJ, Willecke K. 2006. Ablation of connexin43 in uterine smooth muscle cells of the mouse causes delayed parturition. J Cell Sci 119:1715–1722 [DOI] [PubMed] [Google Scholar]
- 82. Martin FJ, Prince AS. 2008. TLR2 regulates gap junction intercellular communication in airway cells. J Immunol 180:4986–4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ey B, Eyking A, Gerken G, Podolsky DK, Cario E. 2009. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem 284:22332–22343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Foley JP, Lam D, Jiang H, Liao J, Cheong N, McDevitt TM, Zaman A, Wright JR, Savani RC. 2012. TLR2, TGFβ, hyaluronan and RHAMM are required for surfactant protein A-stimulated macrophage chemotaxis. J Biol Chem 287:37406–37419 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.