Abstract
Estrogen receptor-α (ERα) expressed by hypothalamic proopiomelanocortin and steroidogenic factor-1 neurons largely mediates the antiobesity effects of estrogens in females. However, the critical molecular events that are coupled to ERα and mediate estrogenic effects on energy balance remain unknown. In the current study, we demonstrated that steroid receptor coactivator-1 (SRC1), a nuclear receptor coactivator, is abundantly expressed by both proopiomelanocortin and steroidogenic factor-1 neurons. We further showed that central administration of an ERα agonist, propyl pyrazole triol, acutely increases physical interaction between SRC1 and ERα in the hypothalamus. Finally, we demonstrated that the effects of estrogens on energy homeostasis are significantly blunted in female mice lacking SRC1 globally. Collectively our results indicate that SRC1 is functionally required to mediate the antiobesity effects of estrogen-ERα signals.
Estrogens play an important role in the regulation of energy homeostasis in females. Ovariectomy (OVX) in female animals leads to increased body weight (1–3), effects that are prevented by estrogen replacement (4). Therefore, estrogen replacement therapy may provide antiobesity benefits in postmenopausal women. However, current estrogen therapy is often associated with increased risks of heart disease and breast cancer (5). Understanding where and how estrogens act to produce antiobesity effects may facilitate the development of highly selective therapies that can treat obesity without causing detrimental side effects.
Women or mice with mutations in the estrogen receptor (ER)-α gene are obese (6, 7). In contrast, deletion of another estrogen receptor, ERβ, does not lead to obesity in mice (8). Consistently, a selective ERα agonist suppresses food intake, whereas pharmacological stimulation of ERβ does not influence feeding behavior (9, 10). GPR30 is a putative G protein-coupled estrogen receptor (11, 12). Although Haas et al. (13) reported increased body weight in GPR30 knockout mice, these findings were not reproduced by a number of other laboratories (14–18). Together these findings indicate that the estrogenic effects on energy balance are primarily mediated by ERα.
We showed that ERα is expressed by proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARH) and steroidogenic factor-1 (SF1) neurons in the ventromedial hypothalamic nucleus (VMH) (20). Using mouse models lacking ERα only in POMC and/or SF1 neurons, we recently demonstrated that ERα in these hypothalamic neurons largely mediates estrogenic actions on food intake and energy expenditure in females (20). Although we and others (20–22) have significantly narrowed down the action sites of estrogens, the intracellular mechanisms for their antiobesity effects are not fully understood.
Steroid receptor coactivator-1 (SRC1) is a member of the p160 protein nuclear receptor coactivator family. SRC1 can bind to ERα (23, 24). This SRC1-ERα interaction will facilitate recruitment of other common coactivators (e.g. cAMP-response-element-binding protein-binding protein and p300), which will ultimately initiate the transcription of the targeted genes (25). Importantly, SRC1 is widely expressed in the central nervous system. In particular, abundant SRC1 mRNAs are found in the ARH and VMH of rat brains (26). The SRC1 knockout (SRCKO) mice are found to be more sensitive to diet-induced obesity (27). Collectively these findings led us to hypothesize that intact SRC1 functions are required to mediate the antiobesity effects of estrogens.
In the present study, we first examined the cellular distribution of SRC1 in the ARH and VMH of mouse brains. We then assessed the hypothalamic SRC1-ERα interaction in response to an ERα agonist. Finally, we examined whether global deficiency in SRC1 would affect the antiobesity efficacy of estrogens in female mice.
Materials and Methods
Mice
Care of all animals and procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Mice were housed in a temperature-controlled environment at 22–24 C using a 12-h light, 12-h dark cycle. The mice were fed on standard chow with minimal phytoestrogens (6.5% fat, no. 2920; Harlan-Teklad, Madison, WI) and water was provided ad libitum.
POMC-EGFP mice (28) were obtained from Jackson Labs (Bar Harbor, ME) and bred to C57BL6J mice. Female offspring of this line were used in neuroanatomy studies described below. Roas26-tdTOMATO mice (29) were bred to SF1-Cre mice (30). Female offspring that were positive for both Rosa26-tdTOMATO allele and the SF1-Cre transgene were used in neuroanatomy studies described below.
SRC1KO mice (31) were generated by crossing males and females that were both heterozygous for the SRC1KO allele. These crosses produced mice that were homozygous for the SRC1KO allele (referred to as SRC1KO mice) and the WT littermates. These littermates were used in neuroanatomy and ovariectomy/estradiol replacement studies described below. The complete deletion of SRC1 in SRC1KO mice have been validated previously (31). All mice were on the C57BL/6J background.
Neuroanatomy
To validate the specificity of SRC1 antibody, we performed immunohistochemistry in both WT and SRC1KO mouse brains. As we did previously (20), WT and SRC1KO (two per group) were deeply anesthetized with ip injections of ketamine (100 mg/kg)/xylazine (10 mg/kg). Mice were perfused with saline followed by 10% formalin, and coronal brain sections were cut at 25 μm (1:5 series). The sections were incubated in the primary rabbit anti-SRC1 serum (1:2000; catalog no. 2191; Cell Signaling, Beverly, MA) at room temperature overnight, followed by biotinylated antirabbit secondary antibody (1:1000; Vector Laboratories, Burlingame, CA) for 2 h. Sections were then incubated in the avidin-biotin complex (Vector Elite kit; 1:500) and incubated in 0.04% diaminobenzidine and 0.01% hydrogen peroxide. After dehydration through graded ethanol, the slides were then immersed in xylene and coverslipped. Images were analyzed using a Leica 5500 microscope configured with a bright-field camera (Leica, Heidelberg, Germany).
To examine the colocalization of SRC1 and POMC, three female POMC-EGFP mice were perfused and brain sections were collected as described above. Brain sections were incubated with primary rabbit anti-SRC1 serum (1:1000; catalog no. 2191; Cell Signaling) at room temperature overnight, followed by incubation in donkey antirabbit AlexaFluor594 (1:500; Invitrogen, Carlsbad, CA) for 1.5 h. After thorough washes, sections were incubated in chicken anti-green fluorescent protein (GFP; 1:5000; Aves Labs Inc., Tigard, OR) at room temperature overnight, followed by incubation in goat anti-chicken AlexaFluor 488 (1:250; Invitrogen) for 1.5 h. Sections were mounted on slides and coverslipped with 4′,6′-diamino-2-phenylindole mounting medium. To count the double-labeled neurons, at least five coronal sections containing the ARH were imaged from each mouse brain.
To examine the colocalization of SRC1 and SF1, three female SF1-Cre/rosa26-tdTOMATO mice were perfused and brain sections were collected as described above. Brain sections were incubated with primary rabbit anti-SRC1 serum (1:1000; catalog no. 2191; Cell Signaling) at room temperature overnight, followed by incubation in donkey antirabbit AlexaFluor 488 (1:500; Invitrogen) for 1.5 h. Sections were mounted on slides and coverslipped with 4′,6′-diamino-2-phenylindole mounting medium. Fluorescence images were taken using the Leica 5500 fluorescence microscope with OptiGrid structured illumination. SF1 neurons were identified by endogenous red fluorescence generated by tandem dimer tomato (tdTOMATO), and SRC1 signals were recognized as green fluorescence. The double-labeled neurons were counted similarly as described above.
Hypothalamic SRC1-ERα interaction in response to intracerebroventricular (i.c.v.) propyl pyrazole triol (PPT)
Female WT mice (6 months) were anesthetized with inhaled isoflurane. As previously described (20), bilateral OVX was performed. Under the same anesthesia, mice were mounted onto the stereotaxic frame, on which stainless steel cannulae (Plastics One, Roanoke, VA) were inserted into the lateral ventricles (0.34 mm caudal and 1 mm lateral from bregma; depth, 2.3 mm). Intracerebroventricular cannulation was confirmed by demonstration of increased thirst in response to administration of angiotensin II (10 ng, 3–4 d after surgeries), as we did before (32). Seven days after OVX and i.c.v. cannulations, mice were fasted overnight and then received i.c.v. injections of PPT (an ERα agonist; 500 pmol in 1 μl) or vehicle (1 μl). PPT (Sigma, St. Louis, MO) was first dissolved in dimethylsulfoxide (DMSO) and then diluted 10 times with saline to make the final concentration (500 pmol/μl). Ten percent of DMSO in saline solution was used as vehicle. Thirty minutes after the i.c.v. injections, mice were deeply anesthetized with inhaled isofluorane and immediately killed. Hypothalami were quickly isolated and immunoprecipitated with mouse anti-ERα monoclonal antibody (1:100; Ab-10; Lab Vision, Fremont, CA). Immunoprecipitates were then subjected to immunoblotting with mouse anti-ERα (1:1000; Lab Vision) or rabbit anti-SRC1 (1:2000; Cell Signaling). Three percent of the total lysates (20 μg) were used as input and immunoblotted with anti-SRC1 as above.
Responses to estrogens
Female SRC1KO and WT littermates (12 wk) were anesthetized with isoflurane. As previously described (20), bilateral OVX was performed, followed by sc implantations of pellets containing estradiol-17β (0.5 μg/d for 30 d, OVX+E) or containing vehicle (OVX+V). These pellets were purchased from Innovative Research of America (Sarasota, FL). After recovery from the surgery (7 d), body weight and food intake were monitored every other day. Changes in body weight (compared with presurgical body weight) and cumulative food intake were calculated. Energy expenditure was estimated by calculating feed efficiency (changes in body weight per cumulative food intake).
Statistics
The data are presented as mean ± sem. Statistical analyses were performed using GraphPad Prism (GraphPad Inc., San Diego, CA). The temporal profile of body weight and feed efficiency was compared by two-way ANOVA with repeated measurements, followed by post hoc Bonferroni tests. Body weight before OVX was analyzed by Student t test. Cumulative food intake and changes in body weight on d 18 after OVX were compared by two-way ANOVA, followed by post hoc Bonferroni tests. P < 0.05 was considered to be statistically significant.
Results
SRC1 is abundantly expressed by hypothalamic POMC and SF1 neurons
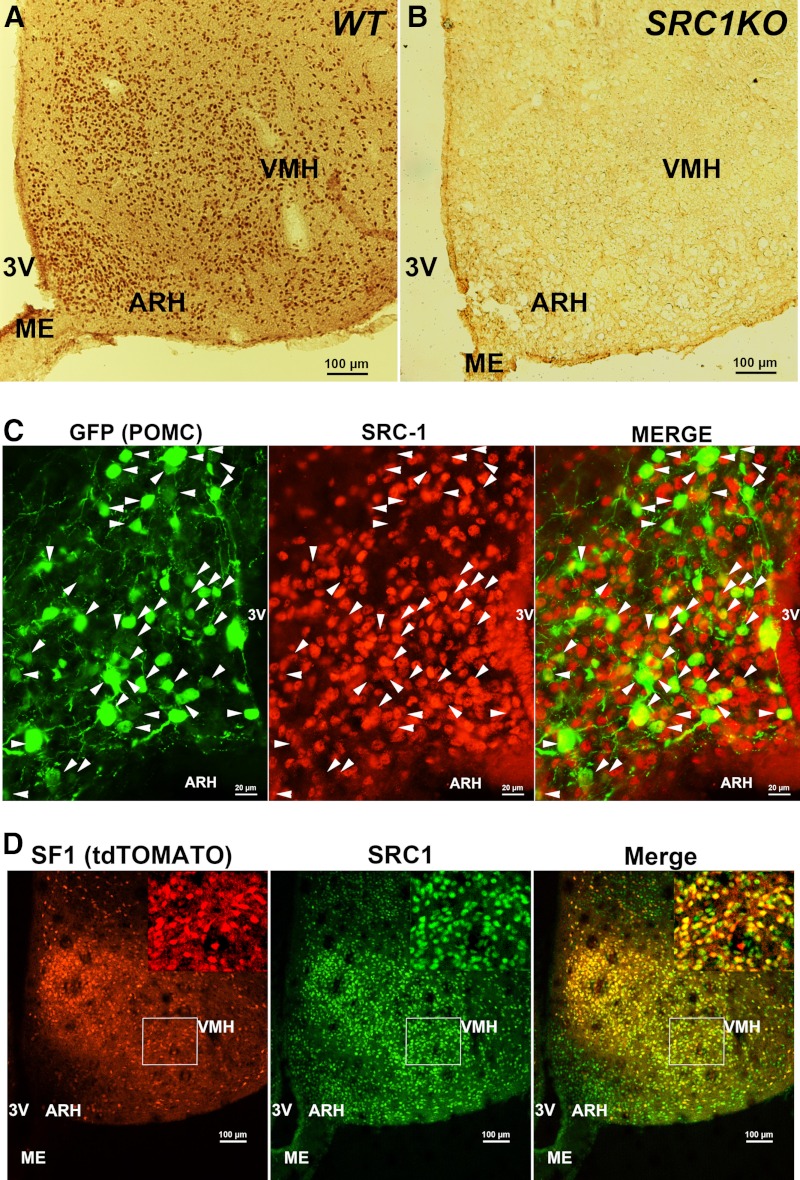
We performed immunohistochemistry to examine the expression of SRC1 in the mouse hypothalamus. In agreement with the known mRNA expression pattern in rat brains (26), we found abundant SRC1-postive neurons in the basomedial hypothalamus, including the ARH and VMH (Fig. 1A). The SRC1 antibody was validated to be specific because it did not produce any immunoreactivity in the hypothalamus from SRC1KO mice (Fig. 1B).
Fig. 1.
Distribution of SRC1 in mouse hypothalamus. A and B, Representative SRC1 immunohistochemical staining in coronal hypothalamic sections from WT (A) or SRC1KO (B) mice. Scale bars (A and B), 100 μm. C, A representative dual-immunofluorescent staining for GFP (green) and SRC1 (red) in POMC-EGFP mice. Arrowheads identify double-labeled neurons. Scale bar, 20 μm. D, A representative immunofluorescent staining for SRC1 (green) in SF1-Cre/rosa26-tdTOMATO mice. SF1 neurons were identified by tdTOMATO (red) fluorescence. Scale bars, 100 μm. Inserts are higher magnification of the region in the white box. 3V, Third ventricle; ME, median eminence.
Because the antiobesity effects of estrogens are largely mediated by ERα in hypothalamic POMC and SF1 neurons (20), we then tested whether SRC1 is coexpressed by these hypothalamic populations. Dual immunofluorescence for GFP and SRC1 was performed in POMC-EGFP mice (28) to examine the colocalization of SRC1 and POMC (GFP). We found that 86.6 ± 3.5% POMC neurons in the ARH coexpressed SRC1 (Fig. 1C). In parallel, we generated SF1-Cre/rosa26-tdTOMATO mice, which express tdTOMATO (a strong red fluorescent protein) exclusively in SF1 cells. Therefore, SF1 neurons in the VMH can be identified as red fluorescence-filled cells. Immunofluorescence for SRC1 (green) was performed to examine the colocalization of SRC1 and SF1. Strikingly, we found that almost all SF1 neurons in the VMH coexpressed SRC1 (Fig. 1D).
Hypothalamic SRC1 interacts with ERα in a ligand-dependent manner
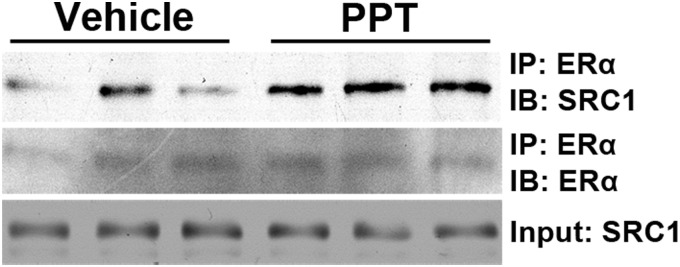
It has been previously reported that hypothalamic SRC1 can form a protein complex with ERα in vitro (23). But the hypothalamic SRC1-ERα interaction has not been demonstrated in vivo. Here we detected this SRC1-ERα interaction in the mouse hypothalamus in vivo. Importantly, we demonstrated that this hypothalamic SRC1-ERα interaction was significantly enhanced by central administration of an ERα agonist (PPT) (Fig. 2). These results indicate that activation of ERα in hypothalamic neurons leads to recruitment of SRC1 to ERα.
Fig. 2.
PPT enhances hypothalamic SRC1-ERα interaction. Female OVX WT mice were fasted overnight, followed by i.c.v. injections of PPT (500 pmol per 1 μl) or vehicle (10% DMSO in saline). Hypothalami were isolated and were pulled down with anti-ERα antibody. The associated SRC1 was detected with anti-SRC1 antibody. ERα in the immunoprecipitates was measured with immunoblotting. Input was measured by immunoblotting for SRC1 in cell lysates. IP, Immunoprecipitation; IB, immunoblotting.
Global deficiency in SRC1 blunts anti obesity effects of estrogens
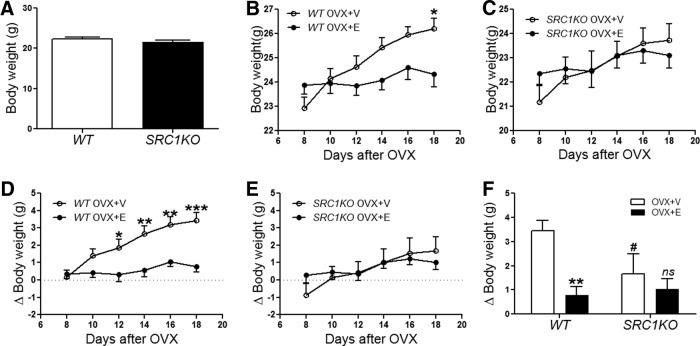
To determine the roles of SRC1 in body weight control in females, we monitored body weight of chow-fed female SRC1KO mice and their WT controls. However, no significant difference in body weight was observed up to 12 wk of age (Fig. 3A). To further determine whether SRC1 is functionally required for the antiobesity effects of estrogens, these female SRC1KO mice and their WT controls were ovariectomized under anesthesia and then interscapularly implanted with pellets containing 17β-estradiol (0.5 μg/d, 30 d, sc; OVX+E) or placebo pellets (OVX+V). After a 7-d recovery, food intake and body weight were monitored. Consistent with previous reports (4, 33–35), we found that OVX+V WT mice showed significant body weight gain, whereas this OVX-induced body weight gain was significantly prevented in OVX+E WT mice. These are demonstrated both by absolute body weight (Fig. 3B) and relative body weight change (Fig. 3D). We also monitored body weight responses of SRC1KO mice in OVX+V and OVX+E conditions. Interestingly, OVX+V SRC1KO mice showed modest body weight gain to the depletion of estrogens (Fig. 3, C and E), and the body weight gain on d 18 after OVX was significantly smaller than that seen in OVX+V WT mice (Fig. 3F). More importantly, estrogen replacement (OVX+E) failed to induce significant body weight loss in SRC1KO mice (Fig. 3, C, E, and F).
Fig. 3.
Estrogenic effects on body weight are blunted in SRC1KO mice. A, Body weight of female WT and SRC1KO littermates at the age of 12 wk. B–F, Twelve-week-old female WT (B and D) and SRC1KO (C and E) mice received bilateral OVX followed by sc implantations of pellets containing estradiol-17β (0.5 μg/d for 30 d, OVX+E) or containing vehicle (OVX+V). After a 7-d recovery, absolute body weight (B and C) and changes in body weight (D and E) were monitored every other day (n = 7–9/group). Data are presented as mean ± sem. Data were analyzed in two-way ANOVA analysis repeat measurement [RM ANOVA interaction treat × time: F (15, 135) = 9.117, P < 0.01]. Post hoc Bonferroni tests: *, P < 0.05, **, P < 0.01, and ***, P < 0.001 between OVX+V and OVX+E with the same genotype. E, Changes in body weight on d 18 after OVX surgery. Data were analyzed in two-way ANOVA analysis [ANOVA main effect OVX V/E: F (1, 27) = 9.849, P < 0.01]. Post hoc Bonferroni tests: **, P < 0.01 between WT OVX+E and WT OVX+E; #, P < 0.05 between SRC1KO OVX+V and WT OVX+V; ns, P > 0.05 between SRC1KO OVX+E and SRC1KO OVX+V.
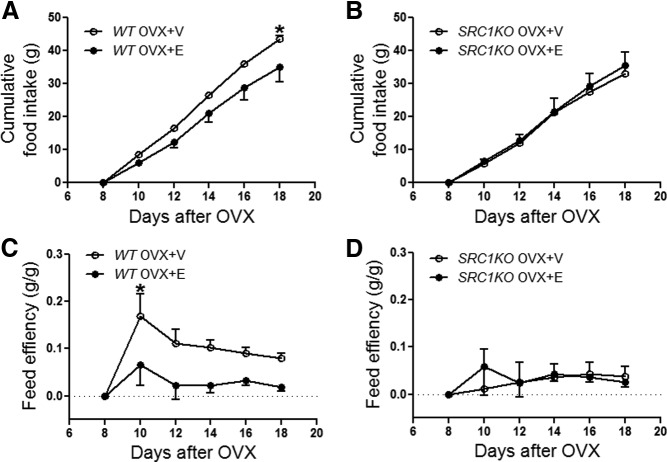
Estrogens prevent body weight gain partly via their anorexigenic effects to suppress food intake (4, 34, 35). Consistently, we found that cumulative food intake was significantly reduced in OVX+E WT mice when compared with OVX+V WT mice (Fig. 4A). However, food intake in OVX+E SRC1KO mice and OVX+V SRC1KO mice was not significantly different (Fig. 4B).
Fig. 4.
Estrogenic effects on food intake and feed efficiency are blunted in SRC1KO mice. A–D, Twelve-week-old female WT (A and C) and SRC1KO (B and D) mice received bilateral OVX followed by sc implantations of pellets containing estradiol-17β (0.5 μg/d for 30 d, OVX+E) or containing vehicle (OVX+V). After a 7-d recovery, food intake (A and B) were monitored every other day (n = 7–9/group). Data are presented as mean ± sem and analyzed in two-way ANOVA analysis [ANOVA main effect treat: F (3, 186) = 4.059, P < 0.01]. Post hoc Bonferroni tests: *, P < 0.05 between OVX+V and OVX+E with the same genotype. Feed efficiency (E and F) was calculated as changes in body weight per cumulative food intake to estimate energy expenditure (n = 7–9/group). Data are presented as mean ± sem and analyzed in two-way ANOVA repeat-measurement analysis [RM ANOVA main effect time: F (5, 135) = 5.320, P < 0.01]. Post hoc Bonferroni tests: *, P < 0.05 between OVX+V and OVX+E with the same genotype.
The body weight-reducing effects of estrogens are also partially due to the increased energy expenditure induced by the hormone (35, 36). Thus, we estimated the energy expenditure by calculating the feed efficiency (body weight gain per cumulative food intake) (37). We found that the feed efficiency was significantly reduced in OVX+E WT mice compared with OVX+V WT mice (Fig. 4C), suggesting that 17β-estradiol increased energy expenditure in OVX+E WT mice. Interestingly, the feed efficiency in OVX+E SRC1KO mice and OVX+V SRC1KO mice was comparable (Fig. 4D). Collectively these observations indicate that global deficiency in SRC1 blunts the antiobesity effects of estrogens in females.
Discussion
In the current study, we first demonstrated that SRC1 is abundantly expressed by POMC and SF1 neurons, hypothalamic populations that are known to largely mediate antiobesity effects of estrogens (20, 21). We further showed that hypothalamic SRC1 interacts with ERα in a ligand-dependent manner. Furthermore, we demonstrated that body weight gain induced by surgical depletion of endogenous estrogens is significantly attenuated in female mice lacking SRC1 globally. More importantly, the effects of estrogen replacement to suppress body weight, to decrease food intake, and to increase feed efficiency are significantly blunted in these mutant mice. Together these findings indicate that SRC1 plays a physiologically relevant role in mediating the antiobesity effects of estrogens.
Estrogens provide antiobesity benefits through both suppressing feeding and enhancing energy expenditure (35). Consistently, here we showed that 17β-estradiol treatment in WT OVX females decreased body weight and food intake and increased energy expenditure (demonstrated by decreased feed efficiency). We have previously shown that estrogenic actions on feeding vs. energy expenditure are largely mediated by anatomically distinct neural populations in the hypothalamus. Thus, estrogens act on ERα expressed by ARH POMC neurons only to suppress food intake (20). On the other hand, SF1 neurons in the VMH receive estrogen/ERα signals to stimulate energy expenditure without affecting feeding (20). Interestingly, we found that SRC1 is abundantly expressed in both POMC and SF1 neurons. These results provide the neuroanatomical basis that SRC1 in these hypothalamic populations could be involved in effects of estrogen-ERα signals on energy homeostasis.
This possibility is further supported by our observations that SRC1 interacts with ERα in the hypothalamus. This is consistent with an earlier report that SRC1 extracted from the rat hypothalamus interacts with ERα (23). Further supporting a possible role of SRC1 in mediating estrogenic effects, we showed that the hypothalamic SRC1-ERα interaction is enhanced by the central administration of an ERα agonist. These findings provide biochemical basis that hypothalamic SRC1 might be functionally relevant to estrogenic actions.
We further tested the functional involvement of SRC1 in antiobesity effects of estrogens using SRC1KO mice. First, we found that SRC1KO mice are less sensitive to OVX-induced body weight gain compared with WT controls. These suggest that antiobesity effects of endogenous estrogens in these SRC1KO mice may be attenuated due to the lack of SRC1. More importantly, we showed that effects of estrogen replacement on body weight, food intake, and energy expenditure are blunted in SRC1KO mice. Therefore, our results support a model that in response to estrogens, SRC1 is recruited to nuclear ERα complex. As a nuclear receptor coactivator, SRC1 facilitates ERα functions to suppress food intake and/or stimulate energy expenditure, which ultimately contributes to the maintenance of energy homeostasis.
It is important to note that our results, although suggesting a role of hypothalamic SRC1 in mediating estrogenic actions, do not rule out the potential roles of SRC1 in other estrogen-target sites in the context of body weight control. Indeed, it has been shown that estrogens also act in the brain stem to regulate satiation and food intake (34). Interestingly, SRC1 is also abundantly expressed by brain stem neurons (26). Dissection of the physiological relevance of SRC1 in the hypothalamus and other estrogen-target regions would warrant future studies.
Notably, the gonad intact SRC1KO females do not show significant body weight gain compared with their WT controls, at least when fed on chow. It appears to be a contradiction to our observations that the antiobesity effects of estrogens require SRC1 functions. One possibility is that SRC1 is also involved in other nuclear receptors and/or transcription factors that are relevant for body weight control. For example, SRC1 is known to interact with peroxisome proliferator-activated receptor-γ (38). Interestingly, peroxisome proliferator-activated receptor-γ actions, including those in the brain, have been shown to promote body weight gain (39, 40), which are opposite to those of estrogen/ERα. In addition, SRC1 is also shown to interact with signal transducer and activator of trascription-3 (41), which is essential to mediate the antiobesity effects of leptin (42). Therefore, the lack of metabolic phenotypes in gonad intact SRC1KO females might reflect the combined net effects from multiple nuclear receptors and/or transcription factors that recruit SRC1 as a coactivator.
Alternatively, the lack of body weight phenotype in gonad intact SRC1KO females also suggest that the antiobesity effects of estrogen/ERα are not solely mediated by SRC1-related pathways. Indeed, ERα-coupled intracellular events can be divided into several modes. Recent evidence indicates that subsets of intracellular ERα are concentrated on the cytomembrane and in the cytosol, in which it initiates rapid signaling pathways, including protein kinase A, MAPK, and phosphatidylinositol 3-kinase (PI3K) (43). These cytomembrane and/or cytosolic ERα-initiated rapid signals may be involved in estrogenic effects on body weight control. Indeed, estrogens have been shown to stimulate the rapid PI3K-Akt cascade in VMH neurons in mice (44). In addition, estrogens rapidly activate ARH POMC neurons, effects that can be blocked by PI3K inhibitors (45). Collectively these data suggest that the ERα-initiated PI3K pathway in these hypothalamic neurons may be physiologically relevant to mediate estrogenic actions on body weight. However, this view is challenged by observations from a transgenic MOER mouse model (46). In MOER mice, full-length ERα protein is replaced by the E domain of the receptor, which exists only on the cytomembrane and retains capacity of initiating rapid signalings (e.g. PI3K) (46). Importantly, no ERα activity is present in the cytosol or in the nucleus in MOER mice. Interestingly, MOER mice show similar obese phenotypes as ERα knockout mice, suggesting that rapid signals initiated by cytomembrane ERα is not sufficient to mediate antiobesity effects of estrogens (46). Nevertheless, because the rapid signals initiated by cytosolic ERα are also eliminated in these MOER mice, the possible contribution of the cytosolic ERα to energy homeostasis still remains unknown.
In addition to the rapid signaling pathways, ERα also acts as a classic nuclear receptor. In this mode, ERα translocates to the nucleus upon binding to estrogens. In the nucleus, ERα directly binds to the estrogen response elements (EREs) on the target genes and regulates gene transcription via the ERE-dependent manner. These ERE-dependent actions of ERα, however, do not appear to mediate the antiobesity effects of estrogens. This is evidenced by recent observations from a knock-in NERKI mouse model. In NERKI mice, E207A/G208A mutations are introduced in the DNA binding domain of ERα, which abolish ERα-ERE binding (47). Interestingly, when crossed to ERα knockout background, the NERKI allele was sufficient to rescue almost all obese phenotypes seen in ERα knockout mice, which suggests that the ERE-dependent ERα functions are not required to maintain body weight (44).
As a nuclear receptor, ERα is also shown to form complex with other transcription factors (TFs), which regulates gene transcription in an ERE-independent manner. Little is known, however, about whether the ERE-independent functions of ERα are involved in estrogenic effects on body weight control. Here we showed that ERα interacts with SRC1 (the nuclear receptor coactivator), and that intact SRC1 functions are required to mediate antiobesity effects of estrogens. These suggest that ERα functions as a nuclear receptor are required for estrogenic actions on energy homeostasis. Based on our observations and those from NERKI mice (44), we speculate that the ERE-independent ERα functions at least partly contribute to estrogenic effects on body weight control. Future studies, therefore, are warranted to identify the TFs that form complex with ERα and to search for transcriptional targets of this ERα-TF complex.
Although the antiobesity effects of estrogens have been well established in animal models (4, 19, 20, 34, 35, 48), estrogens also increase risks of heart diseases and breast cancer (5). Therefore, one priority of the field is to identify the specific molecular targets of estrogens that are critical for the antiobesity effects of the hormone. Our findings represent a significant advance in our understanding about molecular mechanisms for estrogenic actions on energy balance and may identify SRC1 as a potential target for development of novel therapeutic approaches for obesity.
Acknowledgments
This work was supported by Grants R01DK093587, R00DK085330, and P30 DK079638-03 (to Y.X.), Grant R01 CA112403 (to J.X.), and Grants R01HD008818 and P01DK059820 (to B.W.O.) from the National Institutes of Health; by the American Diabetes Association (to Y.X.); by the Klarman Family Foundation, the Naman Family Fund for Basic Research, and the Curtis Hankamer Basic Research Fund (to Y.X.); and by the National Natural Science Foundation of China 81200623 (to L.Z.).
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- ARH
- Arcuate nucleus of the hypothalamus
- DMSO
- dimethylsulfoxide
- ER
- estrogen receptor
- ERE
- estrogen response element
- GFP
- green fluorescent protein
- i.c.v.
- intracerebroventricular
- OVX
- ovariectomy
- PI3K
- phosphatidylinositol 3-kinase
- POMC
- proopiomelanocortin
- PPT
- propyl pyrazole triol
- SF1
- steroidogenic factor-1
- SRC1
- steroid receptor coactivator-1
- tdTOMATO
- tandem dimer tomato
- TF
- transcription factor
- VMH
- ventromedial hypothalamic nucleus.
References
- 1. Drewett RF. 1973. Sexual behaviour and sexual motivation in the female rat. Nature 242:476–477 [DOI] [PubMed] [Google Scholar]
- 2. Blaustein JD, Wade GN. 1976. Ovarian influences on the meal patterns of female rats. Physiol Behav 17:201–208 [DOI] [PubMed] [Google Scholar]
- 3. Wallen WJ, Belanger MP, Wittnich C. 2001. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr 131:2351–2357 [DOI] [PubMed] [Google Scholar]
- 4. Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. 2001. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142:4751–4757 [DOI] [PubMed] [Google Scholar]
- 5. Billeci AM, Paciaroni M, Caso V, Agnelli G. 2008. Hormone replacement therapy and stroke. Curr Vasc Pharmacol 6:112–123 [DOI] [PubMed] [Google Scholar]
- 6. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. 2000. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallagher CJ, Langefeld CD, Gordon CJ, Campbell JK, Mychaleckyj JC, Mychaleckyj JC, Bryer-Ash M, Rich SS, Bowden DW, Sale MM. 2007. Association of the estrogen receptor-α gene with the metabolic syndrome and its component traits in African-American families: the Insulin Resistance Atherosclerosis Family Study. Diabetes 56:2135–2141 [DOI] [PubMed] [Google Scholar]
- 8. Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. 2000. Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem Biophys Res Commun 278:640–645 [DOI] [PubMed] [Google Scholar]
- 9. Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. 2009. Divergent effects of estradiol and the estrogen receptor-α agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res 1268:88–96 [DOI] [PubMed] [Google Scholar]
- 10. Santollo J, Wiley MD, Eckel LA. 2007. Acute activation of ER α decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 293:R2194–R2201 [DOI] [PubMed] [Google Scholar]
- 11. O'Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, Kolakowski LF, Jr, George SR. 1998. Discovery of three novel G-protein-coupled receptor genes. Genomics 47:310–313 [DOI] [PubMed] [Google Scholar]
- 12. Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. 1997. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 45:607–617 [DOI] [PubMed] [Google Scholar]
- 13. Haas E, Bhattacharya I, Brailoiu E, Damjanovi M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. 2009. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104:288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Q, Li JG, Zheng XY, Jin F, Dong HT. 2009. Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast carcinomas. Chin Med J (Engl) 122:2763–2769 [PubMed] [Google Scholar]
- 15. Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. 2009. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80:34–41 [DOI] [PubMed] [Google Scholar]
- 16. Windahl SH, Andersson N, Chagin AS, Mårtensson UE, Carlsten H, Olde B, Swanson C, Movérare-Skrtic S, Sävendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C. 2009. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab 296:E490–E496 [DOI] [PubMed] [Google Scholar]
- 17. Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. 2009. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150:687–698 [DOI] [PubMed] [Google Scholar]
- 18. Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. 2009. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150:1722–1730 [DOI] [PubMed] [Google Scholar]
- 19. Asarian L, Geary N. 2002. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42:461–471 [DOI] [PubMed] [Google Scholar]
- 20. Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. 2011. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14:453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. 2007. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104:2501–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santollo J, Torregrossa AM, Eckel LA. 2011. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Horm Behav 60:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molenda-Figueira HA, Murphy SD, Shea KL, Siegal NK, Zhao Y, Chadwick JG, Jr, Denner LA, Tetel MJ. 2008. Steroid receptor coactivator-1 from brain physically interacts differentially with steroid receptor subtypes. Endocrinology 149:5272–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heery DM, Kalkhoven E, Hoare S, Parker MG. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733–736 [DOI] [PubMed] [Google Scholar]
- 25. Xu J, O'Malley BW. 2002. Molecular mechanisms and cellular biology of the steroid receptor coactivator (SRC) family in steroid receptor function. Rev Endocr Metab Disord 3:185–192 [DOI] [PubMed] [Google Scholar]
- 26. Meijer OC, Steenbergen PJ, De Kloet ER. 2000. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology 141:2192–2199 [DOI] [PubMed] [Google Scholar]
- 27. Picard F, Géhin M, Annicotte J, Rocchi S, Champy MF, O'Malley BW, Chambon P, Auwerx J. 2002. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 111:931–941 [DOI] [PubMed] [Google Scholar]
- 28. Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. 2001. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- 29. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. 2006. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- 31. Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. 1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922–1925 [DOI] [PubMed] [Google Scholar]
- 32. Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, Lowell BB, Zigman JM, Zhao JJ, Elmquist JK. 2010. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab 12:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Q, Horvath TL. 2008. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294:E817–E826 [DOI] [PubMed] [Google Scholar]
- 34. Asarian L, Geary N. 2007. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 148:5656–5666 [DOI] [PubMed] [Google Scholar]
- 35. Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. 2007. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13:89–94 [DOI] [PubMed] [Google Scholar]
- 36. Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. 2009. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150:2161–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. 2001. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4:605–611 [DOI] [PubMed] [Google Scholar]
- 38. Kurosaki E, Nakano R, Shimaya A, Yoshida S, Ida M, Suzuki T, Shibasaki M, Shikama H. 2003. Differential effects of YM440 a hypoglycemic agent on binding to a peroxisome proliferator-activated receptor γ and its transactivation. Biochem Pharmacol 65:795–805 [DOI] [PubMed] [Google Scholar]
- 39. Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, Webster NJ, Schwartz MW, Olefsky JM. 2011. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med 17:618–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. 2011. A role for central nervous system PPAR-γ in the regulation of energy balance. Nat Med 17:623–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giraud S, Bienvenu F, Avril S, Gascan H, Heery DM, Coqueret O. 2002. Functional interaction of STAT3 transcription factor with the coactivator NcoA/SRC1a. J Biol Chem 277:8004–8011 [DOI] [PubMed] [Google Scholar]
- 42. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr 2003. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- 43. Vasudevan N, Pfaff DW. 2008. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol 29:238–257 [DOI] [PubMed] [Google Scholar]
- 44. Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. 2011. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese ERα-null mutant mice. J Clin Invest 121:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malyala A, Zhang C, Bryant DN, Kelly MJ, Rønnekleiv OK. 2008. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol 506:895–911 [DOI] [PubMed] [Google Scholar]
- 46. Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EY, Luderer U, Levin ER. 2009. Developmental phenotype of a membrane only estrogen receptor α (MOER) mouse. J Biol Chem 284:3488–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. 2002. An estrogen receptor (ER) α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]
- 48. Asarian L, Geary N. 2006. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361:1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]