Abstract
Differentiation of endometrial stromal cells into decidual cells is a prerequisite for successful embryo implantation. Our previous studies in the mouse have shown that bone morphogenetic protein 2 (BMP2), a morphogen belonging to the TGFβ superfamily, is essential for this differentiation process. BMP2 is markedly induced in human primary endometrial stromal cells (HESCs) as they undergo differentiation in response to steroid hormones and cAMP. The present study was undertaken to identify the BMP2-mediated molecular pathways in primary cultures of HESCs during decidualization. Using gene expression profiling, we identified wingless-related murine mammary tumor virus integration site 4 (WNT4) as a target of BMP2 regulation during decidualization. Attenuation of WNT4 expression in HESCs by small interfering RNA administration greatly reduced BMP2-induced stromal differentiation. Additionally, adenovirus-mediated overexpression of WNT4 in HESCs markedly advanced the differentiation program, indicating that it is a key regulator of decidualization. The stimulatory effect of WNT4 was accompanied by the accumulation of active β-catenin in the nuclei of decidualizing stromal cells, indicating the involvement of the canonical WNT signaling pathway. Functional inhibition of WNT4/β-catenin pathway by Dickkopf-1, an inhibitor of the canonical WNT signaling, or small interfering RNA-mediated silencing of β-catenin expression, greatly reduced the BMP2- and WNT4-induced decidualization. Gene expression profiling revealed that Forkhead box protein O1, a forkhead family transcription factor and previously reported regulator of HESC differentiation, is a common downstream mediator of both BMP2 and WNT4 signaling. Taken together, these studies uncovered a linear pathway involving BMP2, WNT4/β-catenin, and Forkhead box protein O1 that operates in human endometrium to critically control decidualization.
In humans and rodents, a hallmark maternal response to embryo implantation is the formation of the decidua, a stromal cell-derived tissue that encases the growing products of conception. The primary components of this tissue, the maternal decidual cells, intertwine with the invading fetal trophoblasts and maternal capillaries at the feto-maternal interface to critically regulate the development and function of the placenta. Numerous functions are attributed to decidual cells, including secretion of growth factors and cytokines necessary for fetal development, control of trophoblast invasion into the endometrial bed, and modulation of the maternal immune response (1, 2). An aberrant decidual response is associated with various female reproductive disorders, such as infertility, recurrent miscarriage, preeclampsia, intrauterine growth restriction, and preterm delivery (3–8).
During decidualization, the steroid hormone progesterone (P) acts on the estrogen (E)-primed endometrium to transform fibroblastic stromal cells into large, epithelioid-like decidual cells. This morphological transition of stromal cells is accompanied by a marked rearrangement of the intracellular architecture, accumulation of glycogen, and secretion of various proteins, such as prolactin (PRL) and IGF-binding protein 1 (IGFBP1) (9, 10). In human endometrium, “predecidualization” starts in the perivascular stromal cells at the mid-late secretory phase of the menstrual cycle. If pregnancy ensues, the decidual reaction persists and spreads throughout the entire endometrial stroma (1, 2). Previous studies using primary cultures of human endometrial stromal cells (HESCs) have revealed that a synergistic action between progesterone receptor (PGR) and cAMP/protein kinase A-mediated signaling pathways is critical for the induction and maintenance of human decidualization (10–12). However to this date, only a handful of genes, including FOXO1 (forkhead box protein O1) (13, 14), HOXA10 (15), and IL-11 (16), have been implicated as the potential mediators of cAMP- and/or PGR-signaling in HESCs during decidualization.
Our previous studies have shown that bone morphogenetic protein 2 (BMP2), a member of the TGFβ superfamily, functions downstream of PGR and is required for stromal cell differentiation in both mouse and human endometrium (17). In this study we investigated the pathway that operates downstream of BMP2 to regulate transformation of HESC into decidual cells.
Materials and Methods
Reagents
Progesterone, 17β-estradiol, and 8-bromo-cAMP were purchased from Sigma Chemical Co. (St. Louis, MO). Antibodies against BMP2, IGFBP1, wingless-related MMTV integration site 4 (WNT4), FOXO1, and Calnexin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against active β-catenin, and p-SMAD 1/5/8 were purchased from Cell Signaling Technology (Danvers, MA).
HESC culture and in vitro decidualization
Our studies involving human endometrial biopsies and endometrial cell cultures adhere to the regulations set forth for the protection of human subjects participating in clinical research and are approved by the Institutional Review Board of Emory University, Wake Forest University and the University of Illinois at Urbana-Champaign. Endometrial samples from the early proliferative stage of the menstrual cycle were obtained by Pipelle biopsy at Emory University Medical Center from regularly cycling, fertile volunteers on no hormonal medications, after providing written informed consent.
HESCs were isolated from the biopsies and cultured in DMEM/F-12 (Invitrogen, Carlsbad, CA) containing 5% (vol/vol) fetal bovine serum (Hyclone Laboratories, Logan, UT), 50 μg/ml penicillin, and 50 μg/ml streptomycin (Invitrogen) as described previously (18, 19). To induce in vitro decidualization, the cells were treated with medium containing a hormonal cocktail: 10 nm 17ß-estradiol, 1 μm P, and 0.5 mm 8-bromo-cAMP (E+P+cAMP). For certain experiments 100 ng/ml of BMP2 (R&D systems, Minneapolis, MN) was added in the culture media as indicated in the figure legends. For each experiment, three or more batches of HESCs isolated from different individuals were examined.
Small interfering RNA (siRNA) transfection
HESCs were transfected with predesigned siRNA constructs targeted to mRNAs corresponding WNT4 (Ambion, AM16104), β-catenin encoded by CTNNB1 (Ambion, 4390824), or a negative control green fluorescent protein (Invitrogen, 46–5376). Briefly, SilentFect transfection reagent (Bio-Rad Laboratories, Hercules, CA) was mixed with siRNA (50 nm) and added to HESCs at 80% confluency in six-well culture plates. After 24 h, siRNA was removed and cells were treated with media containing E+P+cAMP to induce decidualization. Cells were harvested at various time points after the hormone treatment. Gene expression was examined by quantitative real-time PCR using gene-specific primers.
Adenovirus transduction
Adenovirus expressing BMP2, WNT4, and DKK1 were kindly provided by Drs. R.T. Franceschi (University of Michigan, Ann Arbor, MI), P.J. Hornsby (University of Texas Health Science Center, San Antonio, TX), and C.J. Kuo (Stanford University School of Medicine, Stanford, CA), respectively. HESCs were transduced with the adenovirus expressing BMP2, WNT4, DKK1, and a control virus harboring empty vector at MOI 50:1 in 2 ml of culture medium. After transduction for 24 h, the viral particles were removed and the cells were treated with E+P or E+P+cAMP to induce decidualization. Overexpression of BMP2, WNT4, and DKK1 in response to adenovirus was confirmed by real-time PCR.
RNA isolation and gene expression profiling
Three independent primary cultures of HESCs were established from endometrial biopsies of women. Each culture was then seeded into wells, and the cells were transduced with adenovirus expressing BMP2, WNT4, and a control virus harboring empty vector. Total RNA was purified from each of these cultures, pooled (n = 3), and then subjected to microarray analysis, according to the Affymetrix protocol using Human Genome HG-U133 A2.0 Array (Affymetrix, Santa Clara, CA) as described previously (20). The gene expression profiling of the pooled samples was performed once, and the data have been submitted to the GEO repository. The genes the expression of which was altered at least 2-fold or more in response to BMP2 or WNT4 stimulation were subjected to Ingenuity Pathway Analysis program, which is used for analyzing and ranking the molecular or signaling pathways based on their known biochemical and molecular functions (www.ingenuity.com). Validation of gene expression changes (≥2 fold) predicted by the microarray analysis was performed by real-time PCR experiments. Briefly, total RNA was isolated from cells (n = 3) during the decidualization process, and mRNA levels of selected genes were quantified by real time RT-PCR (three replicates) using gene-specific primers (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) and SYBR Green PCR mix (Applied Biosystems, Warrington, UK). 36B4, encoding an acidic ribosomal phosphoprotein, was used as an internal control.
Western blot analysis
Whole-cell extracts were prepared from HESCs and subjected to Western blot analysis as described previously (21). Briefly, cells were lysed with the radioimmune precipitation assay buffer containing a cocktail of protease inhibitors (Sigma). After removal of the cell debris, 20 μg of the protein extract were loaded onto SDS-PAGE and transferred to polyvinylidene fluoride membrane (Amersham Biosciences, Inc., Piscataway, NJ). The membrane was blocked in 5% nonfat dry milk and probed with primary antibody against β-catenin (Santa Cruz Biotechnology Inc, SC7963, 1:200). The blot was then incubated with horseradish peroxidase-conjugate secondary antibody, and the signal was detected by chemiluminescence. For the loading control, the membrane was stripped and reprobed with an antibody against calnexin (Santa Cruz Biotechnology Inc, SC-11397, 1:200).
Immunohistochemistry/immunocytochemistry
Immunohistochemical and immunocytochemical analyses were performed as described previously (17, 20). Briefly, sections of paraffin-embedded human endometrial tissue were deparaffinized and rehydrated. After antigen retrieval, endometrial sections were incubated with a primary antibody against human WNT4 or active β-catenin. For immunocytochemistry, cultured cells were fixed and incubated with a primary antibody against active β-catenin (Millipore Corp., Bedford, MA; catalog no. 05–665, 1:200), p-SMAD 1/5/8 (Cell Signaling Technology; catalog no. 9516, 1:100), IGFBP1 (Santa Cruz Biotechnology, Inc; catalog no. SC-13097, 1:100), or FOXO1 (Epitomics, catalog no. 1874–1, 1:250). Immunostaining was performed using horseradish peroxidase-labeled Avidin-Biotin system (Vector Laboratories, Burlingame, CA) and 3-amino-9-ethylcarbazole (AEC) substrate solution. Cells were briefly counterstained with hematoxylin, mounted, and examined by microscopy. Red deposits indicate the sites of immunostaining.
Statistical analysis
The RNA and protein samples were prepared from at least three independent primary cultures subjected to the same experimental treatments. Differences in values between control groups and experimental groups were analyzed by one-way ANOVA followed by Dunnett's post hoc test when comparisons were made between control group and more than one experimental group (GraphPad Prism 4.0, GraphPad Software, Inc., San Diego, CA). Data are expressed as mean ± sem. Significance is defined as a two-tailed P < 0.05.
Results
WNT4 expression is induced downstream of BMP2 signaling during decidualization of HESCs
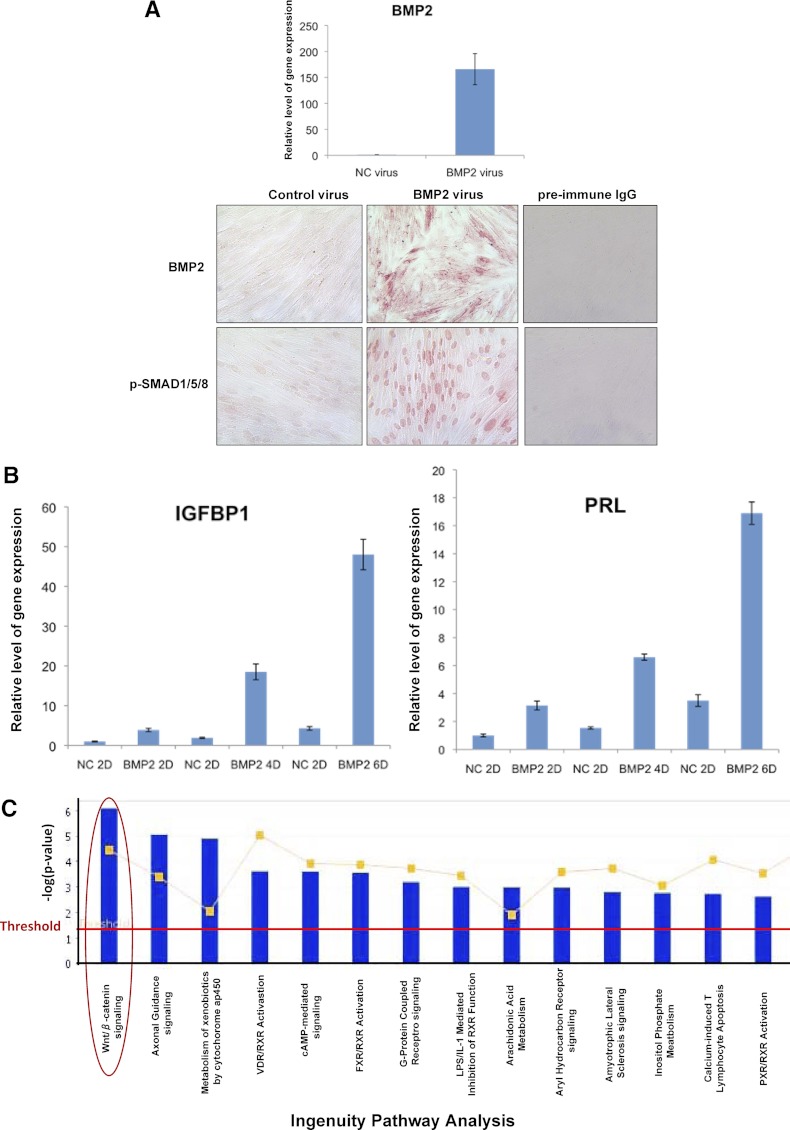
To assess the function of BMP2 during in vitro decidualization of HESCs, we elevated the level of BMP2 in these cells via an adenovirus-based vector (Fig. 1A). We first confirmed the overexpression of BMP2 mRNA (Fig. 1A, upper panel) and protein (Fig. 1A, lower panel) by real-time PCR and immunocytochemistry, respectively. We also confirmed that BMP2 signaling was markedly elevated in response to viral transduction as indicated by the expression of phosphorylated Smad 1/5/8 (Fig. 1A, lower panel). When the cells transduced with recombinant adenovirus expressing BMP2 or an empty vector (a negative control) were subjected to in vitro differentiation, we observed that BMP2 overexpression markedly advanced the stromal differentiation process as indicated by enhanced expression of mRNAs corresponding to IGFBP1 and PRL, well-known biomarkers of endometrial decidualization (Fig. 1B). To identify the molecular networks regulated by BMP2, we performed gene expression profiling during in vitro decidualization as described in Materials and Methods. Of the 7525 transcripts that showed altered expression (≥2 fold) in response to BMP2, 3312 transcripts were up-regulated whereas 4213 transcripts were down-regulated (GEO accession no. GSE39949). Ingenuity Pathway Analysis revealed that the genes associated with WNT/β-catenin signaling constitute the biological category mostly affected upon BMP2 stimulation of HESCs. Interestingly, expressions of genes involved in axonal guidance signaling as well as those involved in metabolism mediated by cytochrome p450 were also affected in response to BMP2 stimulation (Fig. 1C). In this study we focused on the role of WNT/β-catenin signaling in the regulation of HESC differentiation.
Fig. 1.
Overexpression of BMP2 in HESCs via an adenovirus-based vector. HESCs were transduced with recombinant adenovirus expressing BMP2 or green fluorescent protein (NC, negative control) and subjected to in vitro decidualization. A (upper), Cells were harvested at 72 h, and the expression of BMP2 mRNA was assessed by quantitative real-time PCR. A (lower), The cells were fixed and subjected to immunocytochemistry using antibodies against BMP2 and phospho-SMAD1/5/8. B, Total RNA was isolated from these cells at 2, 4, and 6 d (indicated by 2D, 4D, and 6D in the panel) after exposure to BMP2- or control (NC) virus, and the expression of mRNAs corresponding to IGFBP1 and PRL was monitored by real time RT-PCR. The relative expression of these genes at different times was determined by normalizing with respect to the level in NC cells at 2 d. C, Total RNA was purified at 72 h, and gene expression profiling was performed. Genes differentially regulated by BMP2 (≥2 fold) were subjected to Ingenuity Pathway Analysis. The top 15 signaling pathways regulated by BMP2 are shown. Blue bars represent the analysis of the actual dataset, and the red horizontal line indicates the threshold for significant differences, with bars above that line deemed significant. The red circle indicates the WNT/β-catenin signaling pathway.
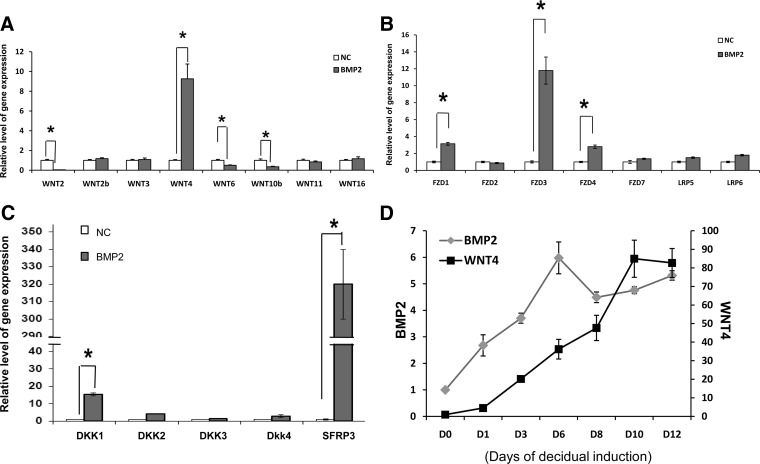
Real-time RT-PCR was performed to validate gene expression changes predicted by the microarray analyses. We confirmed that the expression of WNT4 was markedly enhanced in BMP2-stimulated HESCs, whereas the expression levels of WNT2, WNT2b, WNT3, WNT6, WNT10b, WNT11, or WNT16 were either unchanged or down-regulated in these cells (Fig. 2A). We also observed that the expression of several Frizzled proteins, which serve as WNT receptors, FZD1, FZD3, FZD4, and FZD5, and WNT inhibitors, DKK1, DKK2, and SFRP3 was significantly elevated in response to BMP2 (Fig. 2, B and C). When we examined the temporal expression profiles of BMP2 and WNT4 mRNAs in HESCs undergoing differentiation in response to steroids and cAMP stimulation, they exhibited overlapping patterns of expression, consistent with the regulation of WNT4 by BMP2 (Fig. 2D). Collectively, these results indicated that WNT4 is the major WNT ligand that is induced downstream of BMP2 signaling during HESC decidualization.
Fig. 2.
WNT4 is a target of BMP2 regulation in HESCs. HESCs were transduced with recombinant adenovirus expressing BMP2 (BMP2) or negative control virus (NC) and subjected to in vitro decidualization as described in Materials and Methods. Cells were harvested at 72 h, and the expression of WNT ligands (A), WNT receptors (B), WNT inhibitors (C) was assessed by quantitative real-time RT-PCR. D, HESCs were subjected to decidualization in vitro upon addition of E+P+cAMP. Total RNA was isolated from cells at different times for up to 12 d during the decidualization process. Real time RT-PCR was performed to monitor the expression of BMP2 and WNT4 mRNAs using gene-specific primers. Fold changes were calculated with respect to the expression levels of these genes in stromal cultures without hormone cocktail addition (0 h). All real-time RT-PCR data were normalized with respect to the mRNA level of 36B4. The values represent the average fold induction (±sem) in three independent treatments. *, P < 0.001.
WNT4 mediates the effects of BMP2 during HESC differentiation
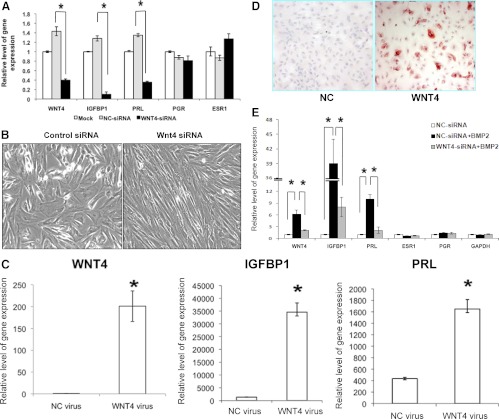
To investigate the role of WNT4 in HESC differentiation, we used the RNA interference technique to silence endogenous WNT4 expression in these cells. HESCs were transfected with siRNAs targeting WNT4 mRNA or a control siRNA for 24 h and then subjected to differentiation. As shown in Fig. 3, HESCs transfected with WNT4 siRNA exhibited more than 75% reduction in WNT4 mRNA expression compared with cells transfected with control siRNA (Fig. 3A). This down-regulation of WNT4 expression in HESCs resulted in a significant reduction in the expression of mRNAs corresponding to IGFBP1 and PRL, whereas the mRNA levels of PGR and estrogen receptor α (ESR1) remained unaltered (Fig. 3A). Furthermore, our studies revealed that whereas HESCs transfected with control siRNA exhibited cuboidal, epitheloid morphology characteristic of decidual cells, the cells transfected with WNT4 siRNA maintained their fibroblastic phenotype, indicative of their undifferentiated status (Fig. 3B).
Fig. 3.
Silencing of WNT4 inhibits differentiation, and overexpression of WNT4 promotes differentiation of HESCs. A and B, HESCs were transfected with a WNT4-specific siRNA (WNT4-siRNA) or a negative control siRNA (NC-siRNA) as described in Materials and Methods. Cells were subjected 24 h after transfection to in vitro decidualization by addition of E+P+cAMP and harvested 72 h after treatment. A, Total RNA was isolated and subjected to real time RT-PCR using gene-specific primers for genes encoding WNT4, PRL, IGFBP1, PGR, and ESR1. The values represent average fold change ± sem of three independent samples relative to mock-transfected controls. *, P < 0.001. B, The morphological transition of HESCs was assessed by the microscopic visualization of phase contrast images. Representative images from three independent experiments are shown and revealed more fibroblastic appearance of E+P+cAMP-treated HESCs in WNT4-siRNA-treated cells. C–E, HESCs were transduced with recombinant adenovirus expressing WNT4 or a negative control (NC) and cultured in a medium containing 2% CS-FBS supplemented with hormonal cocktail for 72 h as described in Materials and Methods. C, Quantitative RT-PCR was performed to analyze the expression of WNT4, IGFBP1, and PRL mRNAs. The values represent average fold change ± sem of three independent samples. The relative expression of these genes was determined by normalizing with respect to the level in NC-transduced cells. D, Immunocytochemical staining of IGFBP1 protein in HESC transduced with a negative control (NC) or WNT4 virus. The red deposits indicate the sites of immunostaining. E, HESCs were transfected with WNT4-specific siRNA or a control siRNA as described in Materials and Methods. Cells were cultured 24 h after transfection in a medium containing hormonal cocktail and supplemented with recombinant BMP2 protein for 72 h. Total RNA was isolated and subjected to real time RT-PCR using gene-specific primers for genes encoding WNT4, PRL, IGFBP1, PGR, and ESR1. The values represent average fold change ± sem of three independent samples. *, P < 0.001.
To further ascertain the role of WNT4 in differentiation of HESCs, we overexpressed WNT4 mRNA in HESC by an adenovirus-mediated expression vector. HESCs were transduced with recombinant adenovirus expressing either WNT4 or a negative control virus (NC) and subjected to differentiation in the presence of E+P+cAMP. As shown in Fig. 3C, WNT4 mRNA was markedly elevated in HESCs in response to viral transduction. Overexpression of WNT4 in HESCs resulted in a dramatic induction of IGFBP1 and PRL mRNAs compared with the cells transduced with control virus (Fig. 3C). We also observed a marked increase in the level of IGFBP1 protein in HESCs transduced with WNT4 (Fig. 3D). Collectively, these results established a critical role of WNT4 in the regulation of HESC decidualization.
We next investigated whether WNT4 mediates the functional effects of BMP2 during differentiation. As shown in Fig. 3E, administration of recombinant BMP2 to HESCs treated with WNT4 siRNA failed to promote differentiation as evidenced by the lack of expression of mRNAs encoding IGFBP1 and PRL, indicating that WNT4 is a necessary mediator of BMP2 function during endometrial differentiation.
WNT4 regulates HESC differentiation via activation of β-catenin
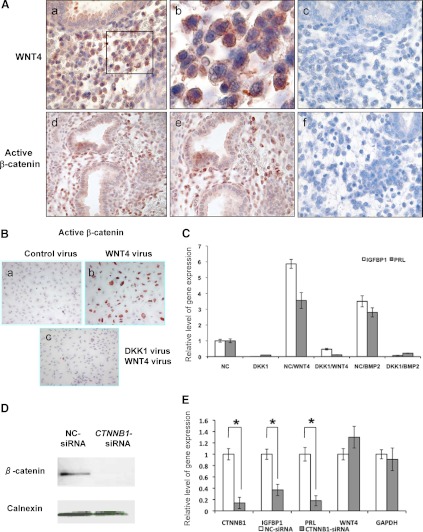
Signaling by the WNT factors is transduced via either the canonical WNT/β-catenin-dependent or the noncanonical β-catenin-independent pathway (22–24). We first examined by immunohistochemistry the expression of WNT4 and active β-catenin in secretory phase human endometrium when the stromal cells undergo decidualization in vivo (Fig. 4A). Representative staining of endometrial sections from the secretory phase revealed strong cytoplasmic staining of WNT4 (upper panels) and nuclear staining of active β-catenin (lower panels), predominantly in the stromal compartment. These results indicated regional WNT/β-catenin signaling in differentiating endometrial stromal cells during the menstrual cycle.
Fig. 4.
WNT4 regulates differentiation of HESCs via β-catenin-dependent canonical signaling pathway. A, Immunohistochemical analysis of WNT4 (upper panels) and active β-catenin (lower panels) in human endometrium during the secretory phase of the menstrual cycle (n = 4). Representative images are shown. Panels b (×40) and e (×20) represent magnified images of panels a (×20) and d (×10), respectively. Panels c and f indicate secretory phase endometrial sections subjected to IHC protocol replacing primary antibody with preimmune IgG. B, HESCs were first transduced with an adenovirus expressing DKK1 or a negative control (NC) for 24 h followed by overexpression of WNT4 by adenoviral transduction. Cells were cultured in the presence of E+P for 48 h, and immunocytochemical analysis was performed to examine the level of nuclear β-catenin. C, NC- or DKK1-transduced HESC were cultured in a medium containing the hormonal cocktail supplemented with WNT4 virus (WNT4 overexpression) or BMP2 recombinant protein (100 ng/ml) for 72 h. Quantitative RT-PCR was performed to monitor the levels of IGFBP1 and PRL after normalization to the level of internal control gene, 36B4. D and E, HESCs were transfected with 20 nm CTNNB1-specific or negative control siRNA oligos for 2 d. Transfected cells were subjected to decidualization upon addition of hormonal cocktail for 4 d. Protein extracts of these cells were subjected to Western blotting to examine the level of β-catenin protein. Calnexin served as a loading control (panel D). Quantitative RT-PCR was employed to examine the relative levels of gene expression. The values represent average fold change ± sem of three independent samples (panel E). *, P < 0.001. DKK, Dickkopf proteins; IHC, immunohistochemisty.
We next investigated whether the increased expression of WNT4 in HESCs leads to activation of β-catenin pathway. As shown in Fig. 4B, an intense nuclear accumulation of active β-catenin was observed in HESCs transduced with WNT4 adenovirus, but not in cells transduced with control adenovirus (compare panels a and b in Fig. 4B). We also cotransfected HESCs with a recombinant adenovirus expressing the DKK1, a well-characterized inhibitor of canonical WNT pathway (25). We first confirmed the overexpression of DKK1 in HESCs in response to viral transduction (Supplemental Fig. 1). Our studies revealed that overexpression of DKK1 led to a dramatic decline in the level of active β-catenin in HESCs (Fig. 4B, panel c). Our studies further revealed that the DKK1-mediated suppression of β-catenin activation resulted in a blockade of HESC differentiation as indicated by a significant decline in the levels of IGFBP1 and PRL mRNAs in these cells (Fig. 4C).
To obtain direct evidence that the β-catenin activation is indeed critical for HESC differentiation, we examined the effects of siRNA-mediated attenuation of β-catenin gene expression. Treatment with siRNA targeted to β-catenin mRNA (CTNNB1), which efficiently suppressed the level of this mRNA (Fig. 4E) and protein (Fig. 4D), but did not affect the level of WNT4 or glyceraldehyde 3-phosphate dehydrogenase mRNAs, resulted in a marked reduction in the expression of differentiation markers IGFBP1 and PRL. Collectively, these results indicated that the β-catenin-dependent signaling pathway is necessary for BMP2-WNT4-induced endometrial stromal decidualization.
FOXO1 is a downstream effector of the BMP2-WNT4 pathway during HESC differentiation
To identify the gene networks mediating WNT4 function during decidualization, we examined the alterations in mRNA expression profiles in HESCs transduced with WNT4 or a negative control adenovirus (NC) by subjecting these cells to in vitro decidualization followed by gene expression profiling using Affymetrix Human Genome U133 A2.0 arrays. We identified 4984 transcripts corresponding to known genes the expression of which was altered (≥2-fold) in the HESCs in response to WNT4 stimulation. Of these genes, the expression of 2882 genes was up-regulated whereas that of 2102 genes was down-regulated. When we compared the genes that are differentially regulated in response to WNT4 stimulation with those regulated by BMP2 stimulation, we identified 626 genes (up or down) that were common between these two data sets. These common pathways involved various biological categories, such as cell to cell signaling and interaction, lipid metabolism, and inflammatory response, all with plausible potential roles in decidualization.
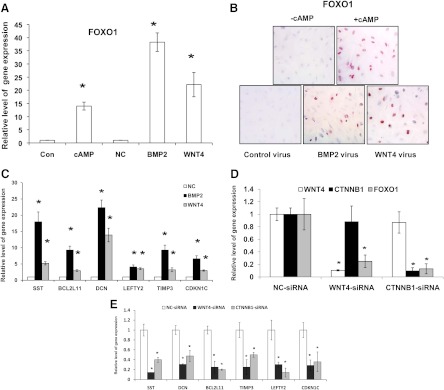
Further analysis of the microarray data revealed that the expression of the transcription factor FOXO1 is induced downstream of both BMP2 and WNT4 signaling. Previous studies have shown an important role of FOXO1 in HESC differentiation (26). We confirmed enhanced expression of FOXO1 downstream of BMP2 and WNT4 by real-time RT-PCR and immunocytochemistry. As shown in Fig. 5, the levels of both FOXO1 mRNA (panel A) and protein (panel B) were markedly elevated in response to BMP2 or WNT4 overexpression. Interestingly, several of the previously reported downstream targets of FOXO1 (26), such as somatostatin (SST), decorin (DCN), BCL2-like 11 (BCL2L11), (left-right determination factor 2 (LEFTY2)/Endometrial Bleeding-Association Factor (EBAF)/TGF-4), cyclin-dependent kinase inhibitor 1C (CDKN1C), and TIMP metallopeptidase inhibitor 3 (TIMP3) also showed enhanced expression in response to BMP2 or WNT4 stimulation. Consistent with the microarray data, real-time RT-PCR experiments indicated that the expression levels of somatostatin, decorin, BCL2-like 11, left-right determination factor 2/EBAF/TGF-4, cyclin-dependent kinase inhibitor 1C, and TIMP metallopeptidase inhibitor 3 (Fig. 5C) also increased in HESCs upon transduction with BMP2 or Ad-WNT4 adenovirus. In parallel experiments, silencing of WNT4 or CTNNB1 mRNA expression in HESCs also suppressed the levels of FOXO1 mRNA (Fig. 5D) and several FOXO1-regulated genes (Fig. 5E). Collectively, these results indicated that FOXO1 functions downstream of the BMP2-WNT4 signaling cascade to control the differentiation of HESCs.
Fig. 5.
FOXO1 is a downstream mediator of BMP2-WNT4 signaling in differentiating HESCs. HESCs were subjected to differentiation in response to cAMP, or BMP2 or WNT4 adenoviral-mediated overexpression for 72 h. The expression of FOXO1 mRNA was assessed by quantitative RT-PCR (A) and FOXO1 protein by immunocytochemistry (B). Panel C shows the expression of downstream targets of FOXO1 by quantitative RT-PCR analysis. D and E, HESCs were transfected with siRNA oligos targeted against WNT4, CTNNB1, or a negative control (NC) for 2 d, and then stimulated with hormonal cocktail for an additional 4 d. Quantitative RT-PCR was performed to examine the expression level of FOXO1 mRNA (D) and genes regulated by FOXO1 (E). The values represent average fold change ± sem of three independent samples. *, P < 0.001.
Discussion
We reported previously that BMP2 is induced in endometrial stromal cells at the onset of decidualization and promotes their differentiation into decidual cells (17). In this study, we present evidence that WNT4 mediates the function of BMP2 during HESC differentiation. Our study revealed that WNT4 operates via the canonical β-catenin-dependent signaling pathway to achieve its regulatory role during decidualization. These findings are consistent with our previous reports that conditional ablation of either Bmp2 or Wnt4 in the mouse uterus leads to female infertility primarily due to a defect in stromal decidualization (27, 28). The BMP2-WNT4-β-catenin signaling cascade, therefore, emerges as a unique conserved pathway in rodent and human endometrium that critically regulates stromal differentiation during early pregnancy.
WNT/β-catenin signaling has been implicated in regulation of cell proliferation, cell fate specification, and differentiation in diverse tissues (22–24). Aberrant activation of β-catenin is known to drive cell proliferation and tumorigenesis via up-regulation of cell cycle regulators, such as c-myc and cyclin D1 (22–24). In HESCs, however, inhibition of β-catenin activation results in the loss of the differentiation potential of these cells. Under the conditions of in vitro decidualization employed in this study, the primary HESCs cease to proliferate upon addition of E+P+cAMP and enter the differentiation program (W. Wang, Taylor R.N., Bagchi I.C., and Bagchi, M.K., unpublished data). The involvement of WNT/β-catenin signaling in the regulation of HESC differentiation is, therefore, independent of its effect on cell proliferation. This view finds support from our mouse knockout studies in which loss of uterine Bmp2 or Wnt4 expression led primarily to defects in stromal differentiation rather than proliferation (27, 28).
The expression of BMP2, WNT ligands, and their receptors in human endometrium has been reported previously (31–33). Stoikos et al. (34) have documented elevated expression of BMP2 in the human endometrium at the secretory phase of the menstrual cycle. Giudice and co-workers (32) reported the expression of certain members of WNT signaling pathway in endometrium during the menstrual cycle. Studies by Brosens and co-workers (35) showed that WNT4 mRNA production as well as nuclear β-catenin protein localization are induced downstream of PGR during in vitro differentiation of HESCs. Because our earlier studies showed that BMP2 is a mediator of PGR signaling in the differentiating endometrial stromal cells (17), it is plausible that BMP2 mediates PGR action in human secretory endometrium via induction of WNT4 expression. WNT4, in turn, signals via the Frizzled receptors to promote accumulation of active nuclear β-catenin that controls the downstream pathways involved in differentiation.
Our studies revealed that, in addition to the induction of WNT ligands and their receptors, the expression of several WNT inhibitors is modulated in HESCs in response to BMP2 stimulation. The expressions of DKK1 and SFRP3 were markedly up-regulated upon BMP2 stimulation. These findings are consistent with previous reports that DKK1 expression is elevated in HESCs during the secretory phase of the menstrual cycle, in first trimester deciduas, and during in vitro decidualization in response to P (36–38). A global knockout of Dkk1 has been generated (39). However, the embryonic lethality of the Dkk1-null mouse makes it impossible to investigate DKK1 function in adult uterine tissues. Although the significance of the higher expression of DKK1 during decidualization in human endometrium remains unclear, it is conceivable that BMP2 controls a delicate balance among WNT ligands, WNT receptors, and WNT inhibitors to govern optimal WNT signaling in the endometrial stromal compartment. This tightly controlled WNT signaling may be crucial for the normal physiological functions of human endometrium during decidualization. It is of interest to note that either ablation or overexpression of the active form of β-catenin in mouse uterus leads to a failure in stromal cell decidualization (30).
Most interestingly, our studies revealed that FOXO1 is a downstream mediator of BMP2-WNT4 signaling in HESCs during decidualization. Several previous reports indicated that FOXO1 is expressed in human endometrial stromal cells during the secretory phase of the menstrual cycle and is involved in endometrial decidualization (13, 14, 40–42). It was proposed that FOXO1 interacts with PGR or CCAAT/enhancer binding protein β to regulate the transcriptional activities of IGFBP1 and PRL promoters (26, 29, 42). In our study, using the gain-of-function and the loss-of-function approaches, we show that FOXO1 and its target genes function downstream of BMP2, WNT4, and β-catenin signaling in HESCs. Although there are no reports indicating that FOXO1 is directly regulated by WNT4-β-catenin signaling, we noted several ternary complex factor-response elements in the human FOXO1 promoter sequence. In future studies, we will address the functionality of these potential ternary complex factor-binding sites in the FOXO1 promoter. We also noted a previous report that indicated that down-regulation of FOXO1 expression in HESCs leads to a suppression of WNT4 mRNA expression (26). This raises the interesting possibility that FOXO1, once induced downstream of WNT4, sustains the decidual phenotype of HESCs by maintaining the persistent expression of WNT4 via a positive feedback loop.
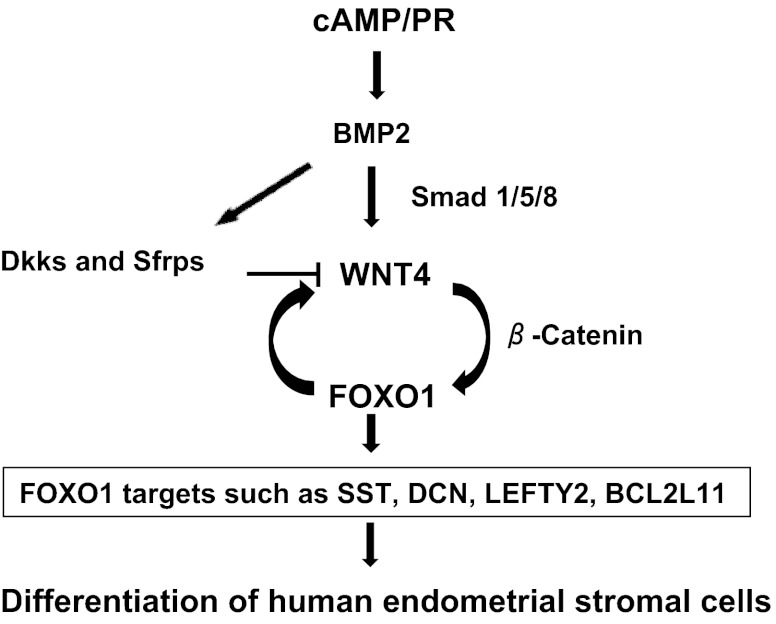
In summary, the present study has unraveled a molecular pathway that is initiated in response to steroid hormones and cAMP signaling in HESCs. We have shown previously that treatment with steroids and cAMP promotes the expression of BMP2 in HESCs. Signaling by BMP2 induces the expression of WNT4, which acts by activating the β-catenin pathway to control the expression of FOXO1 and its downstream genes during decidualization (Fig. 6). Undoubtedly, elucidation of the downstream gene networks governed by this molecular pathway will help us to understand better the various biological events, such as angiogenesis, immune cell trafficking, cell turnover, and tissue remodeling, that occur during decidualization, as well as explain their defects in decidualization-associated reproductive disorders of women.
Fig. 6.
BMP2-WNT4-FOXO1 pathway in HESC differentiation. BMP2 is induced in response to steroid hormones and cAMP in HESCs. BMP2 activates Smad1/5/8-mediated signaling pathway to promote WNT4 expression. WNT4 then functions via β-catenin-dependent signaling pathway to induce FOXO1 and its downstream genes to regulate differentiation of HESCs. FOXO1 also may be an essential maintenance factor for WNT4 expression. In addition, BMP2 controls expression of several WNT inhibitors including Dickkopf proteins and, secreted frizzled-related proteins to fine tune WNT signaling in HESCs.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants U54 HD055787 as part of the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH Centers Program in Reproduction and Infertility Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMP2
- Bone morphogenetic protein 2
- E
- estrogen
- FOXO1
- forkhead box protein O1
- HESC
- human endometrial stromal cells
- IGFBP1
- IGF-binding protein 1
- P
- progesterone
- PGR
- progesterone receptor
- PRL
- prolactin
- siRNA
- small interfering RNA
- WNT4
- wingless-related murine mammary tumor virus integration site 4.
References
- 1. Irwin JC, Giudice D. 1999. In: Knobil E, Neill JD, eds. Encyclopedia of reproduction. New York: Academic Press; 823–835 [Google Scholar]
- 2. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. 2010. Endometrial decidualization: of mice and men. Semin Reprod Med 28:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waite LL, Atwood AK, Taylor RN. 2002. Preeclampsia, an implantation disorder. Rev Endocr Metab Disord 3:151–158 [DOI] [PubMed] [Google Scholar]
- 4. Norwitz ER. 2006. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online 13:591–599 [DOI] [PubMed] [Google Scholar]
- 5. Fazleabas AT. 2007. Physiology and pathology of implantation in the human and nonhuman primate. Semin Reprod Med 25:405–409 [DOI] [PubMed] [Google Scholar]
- 6. Tantbirojn P, Crum CP, Parast MM. 2008. Pathophysiology of placenta ac?creta: the role of decidua and extravillous trophoblast. Placenta 29:639–645 [DOI] [PubMed] [Google Scholar]
- 7. Cakmak H, Taylor HS. 2011. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update 17:242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel BG, Lessey BA. 2011. Clinical assessment and management of the endometrium in recurrent early pregnancy loss. Semin Reprod Med 29:491–506 [DOI] [PubMed] [Google Scholar]
- 9. Dunn CL, Kelly RW, Critchley HO. 2003. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online 7:151–161 [DOI] [PubMed] [Google Scholar]
- 10. Gellersen B, Brosens IA, Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- 11. Maruyama T, Yoshimura Y. 2008. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J 55:795–810 [DOI] [PubMed] [Google Scholar]
- 12. Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. 1997. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 6:301–307 [DOI] [PubMed] [Google Scholar]
- 13. Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ. 2006. FOXO1A differentially regulates genes of decidualization. Endocrinology 147:3870–3876 [DOI] [PubMed] [Google Scholar]
- 14. Grinius L, Kessler C, Schroeder J, Handwerger S. 2006. Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J Endocrinol 189:179–187 [DOI] [PubMed] [Google Scholar]
- 15. Lu Z, Hardt J, Kim JJ. 2008. Global analysis of genes regulated by HOXA10 in decidualization reveals a role in cell proliferation. Mol Hum Reprod 14:357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dimitriadis E, Robb L, Salamonsen LA. 2002. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod 8:636–643 [DOI] [PubMed] [Google Scholar]
- 17. Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. 2007. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282:31725–31732 [DOI] [PubMed] [Google Scholar]
- 18. Tang B, Guller S, Gurpide E. 1993. Cyclic adenosine 3′,5′-monophosphate induces prolactin expression in stromal cells isolated from human proliferative endometrium. Endocrinology 133:2197–2203 [DOI] [PubMed] [Google Scholar]
- 19. Ryan IP, Schriock ED, Taylor RN. 1994. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 78:642–649 [DOI] [PubMed] [Google Scholar]
- 20. Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. 2011. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331:912–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Das A, Li Q, Laws MJ, Kaya H, Bagchi MK, Bagchi IC. 2012. Estrogen-induced expression of Fos-related antigen 1 regulates uterine stromal differentiation and remodeling. J Biol Chem 287:19622–19630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacDonald BT, Tamai K, He X. 2009. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clevers H. 2006. Wnt/β-catenin signaling in development and disease. Cell 127:469–480 [DOI] [PubMed] [Google Scholar]
- 24. Logan CY, Nusse R. 2004. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810 [DOI] [PubMed] [Google Scholar]
- 25. Niehrs C. 2006. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25:7469–7481 [DOI] [PubMed] [Google Scholar]
- 26. Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, Unterman TG, Brosens JJ, Kim JJ. 2007. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349 [DOI] [PubMed] [Google Scholar]
- 27. Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. 2007. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27:5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ. 2011. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25:1176–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christian M, Zhang X, Schneider-Merck T, Unterman TG, Gellersen B, White JO, Brosens JJ. 2002. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein β in differentiating human endometrial stromal cells. J Biol Chem 277:20825–20832 [DOI] [PubMed] [Google Scholar]
- 30. Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. 2009. β-Catenin mediates glandular formation and dysregulation of β-catenin induces hyperplasia formation in the murine uterus. Oncogene 28:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonderegger S, Pollheimer J, Knöfler M. 2010. Wnt signalling in implantation, decidualization and placental differentiation–review. Placenta 31:839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC. 2003. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab 88:3860–3866 [DOI] [PubMed] [Google Scholar]
- 33. Cheng CW, Smith SK, Charnock-Jones DS. 2008. Transcript profile and localization of Wnt signaling-related molecules in human endometrium. Fertil Steril 90:201–204 [DOI] [PubMed] [Google Scholar]
- 34. Stoikos CJ, Harrison CA, Salamonsen LA, Dimitriadis E. 2008. A distinct cohort of the TGFβ superfamily members expressed in human endometrium regulate decidualization. Hum Reprod 23:1447–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkilä M, Ho KK, Teklenburg G, Lavery S, Jones MC, Trew G, Kim JJ, Lam EW, Cartwright JE, Poutanen M, Brosens JJ. 2008. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 149:4462–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. 2006. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 91:1453–1461 [DOI] [PubMed] [Google Scholar]
- 37. Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng EH, Yeung WS, Ho PC, Lee KF. 2010. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod 25:479–490 [DOI] [PubMed] [Google Scholar]
- 38. Macdonald LJ, Sales KJ, Grant V, Brown P, Jabbour HN, Catalano RD. 2011. Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium. Mol Hum Reprod 17:626–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Izpisúa Belmonte JC, Westphal H. 2001. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1:423–434 [DOI] [PubMed] [Google Scholar]
- 40. Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG. 2003. Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod 68:24–30 [DOI] [PubMed] [Google Scholar]
- 41. Brar AK, Handwerger S, Kessler CA, Aronow BJ. 2001. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 7:135–148 [DOI] [PubMed] [Google Scholar]
- 42. Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam EW, Brosens JJ. 2006. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol 20:35–44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.