Abstract
In mice, GH levels rise in response to short-term fasting or starvation (food restriction to 40% of ad libitum intake), similar to that which occurs in humans in response to fasting or anorexia. Recent studies using acyl-ghrelin knockout mice have suggested that the rise in GH during food restriction is essential to support glucose levels. To directly test this hypothesis, adult-onset isolated GH deficient (AOiGHD) mice and their GH-replete littermate controls were provided 40% of ad libitum food intake for 11 d. As previously shown, food restriction increased GH levels in controls, and this response was not observed in AOiGHD mice. In both controls and AOiGHD, food restriction resulted in an initial decline in glucose, which stabilized to 82–85% of ad libitum-fed values by d 2. In addition, loss of lean mass in response to food restriction was not altered by GH status. However, the loss of fat mass and the associated rise in circulating free fatty acids and ketones was blunted in starved AOiGHD mice compared with controls. Taken together, these results suggest a rise of GH during starvation is not required to support glucose levels and muscle mass but may be important in supporting fat mobilization.
In humans, GH levels rise in response to fasting and are elevated in anorexia (1, 2). This rise in GH is thought to preserve glucose homeostasis by antagonizing the systemic actions of insulin and promoting lipolysis and lipid oxidation (3, 4), thus maintaining circulating glucose for central use and providing fatty acids for hepatic gluconeogenesis and muscle function. Similar to humans, GH levels rise in mice after short-term fasting as well as with severe food restriction (40% of ad libitum food intake) (5). In the case of severe food restriction, it was suggested that the rise in GH is critical in supporting glucose levels (5). Specifically, GH levels failed to rise in food restricted ghrelin-O-acyl transferase (GOAT) knockout mice, which was associated with severe hypoglycemia and morbidity, in which GH infusion prevented hypoglycemic events (5). However, in a more recent study using the same paradigm, circulating glucose levels were similar in mice with inactivating mutations in the GOAT, ghrelin, or ghrelin receptor (GHSR) genes compared with their respective controls (6).
The current study was designed to test the hypothesis that a rise in GH is required to support glucose levels during starvation. To directly test this hypothesis, adult-onset, isolated GH-deficient (AOiGHD) mice and their GH replete littermate controls were provided 40% of ad libitum food intake for 11 d. The AOiGHD model was generated by crossbreeding rat GH promoter driven Cre-recombinase mice (7) with mice carrying the inducible monkey diphtheria-toxin (DT) receptor (iDTR) transgene (8). Mice heterozygous for iDTR, with and without Cre-recombinase, were treated with DT at 10 wk of age, leading to the specific destruction of somatotropes in the Cre-positive mice, thereby lowering circulating GH concentrations to 25% of Cre-negative control mice (9).
Materials and Methods
Animals and diet
This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committees (IACUC) of Jesse Brown Veterans Affairs Medical Center and the University of Illinois at Chicago.
The AOiGHD mouse model was generated as previously reported (9). In brief, mice heterozygous for iDTR, with (Cre+/−, iDTR+/−) and without (Cre−/−, iDTR+/−) Cre-recombinase (in a C57BL/6 background), were treated with DT at 10 wk of age by continuous low-dose delivery via sc osmotic pumps (6 ng/h for 7 d). Pumps were surgically placed and removed under isoflurane anesthesia. Therefore, GH deficiency (GHD) is induced by DT-mediated destruction of GH-producing cells of the anterior pituitary (9). Although to date we are not aware of other factors secreted by GH-producing cells, we cannot completely rule out the possibility that loss of these factors may contribute in some way to the phenotype. However, it should be noted that currently this is the only model established with long-term adult-onset GHD, in which the presence and the function of other pituitary cell types remain intact (9).
All mice were housed on a 12-h light, 12-h dark cycle (0600 h lights on to 1800 h lights off) at 22–24 C and maintained on a standard rodent chow diet (17% calories from fat, 56% calories from carbohydrate, and 25% calories from protein; 7912 Teklad LM-485 mouse/rat irradiated diet; Harlan Laboratories, Madison, WI). Identical proportions of fat/carbohydrates/protein were used by Yi et al. (6) (17% calories from fat, 58% calories from carbohydrates, and 25% calories from protein) and similar proportions by Zhao et al. (5) (18% calories from fat, 49% calories from carbohydrates, and 33% calories from protein).
Forty-two weeks after DT treatment, female AOiGHD mice and GH replete littermate controls (n = 15–20/group) were subjected to whole-body nuclear magnetic resonance (NMR), and a subset of mice were killed under ad libitum-fed conditions (n = 7 AOiGHD and n = 10 controls). The remainder of the mice (n = 8 AOiGHD and n = 10 controls) were subjected to 60% food restriction as described below.
In vivo evaluation of basal metabolic status of AOiGHD mice
Glucose tolerance tests were performed at 31 wk of age (21 wk of GHD) after an overnight fast (2 g/kg glucose, ip) and insulin tolerance tests (ITTs) were performed at 26 wk of age (16 wk of GHD) under ad libitum-fed conditions (1 U/kg Novolin, ip, Novo Nordisk, Bagsvaerd, Denmark), beginning between 0800 and 0900 h. Tail vein blood samples were taken after application of anesthetic cream (2.5% lidocaine, 2.5% prolocaine). It should be noted that before any experimental procedure or euthanasia, mice were acclimated to personnel and handling to minimize stress.
Food restriction study
Food restriction studies were conducted based on the paradigm established previously by Zhao et al. (5) and Yi et al. (6). Before starting food restriction, mice were individually housed and daily ad libitum food intake was monitored for 3 consecutive days in 52-wk-old female AOiGHD (42 wk of GH deficiency) and control mice (n = 8–10). Body composition (by NMR; MiniSpec LF50; Bruker Optics, Manning Park, Billerica, MA), blood GH, IGF-I, nonesterified free fatty acids (NEFAs), ketones [3-hydroxybutyrate (3-HB)], and glucose concentration were measured in ad libitum-fed mice 1 d before food restriction. Subsequently, mice were provided 40% of the daily amount consumed by the same mouse during the 3 d of acclimation, during which the food was provided between 1600 and 1700 h. Body composition and blood glucose were measured daily at 1530–1600 h (just before providing food) because this is the only time point when hypoglycemia was observed in food restricted GOAT knockout (KO) mice (5). NEFAs and 3-HB levels were measured every other day. Mice were killed 11 d after starting food restriction, based on end point criteria set by the local IACUC, which specified that the experiment will be stopped if any animal shows clear morbidity (which includes minimal movement or response to touch). Circulating GH levels were assessed in single blood samples taken from the tail vein before food restriction and in trunk blood samples, following 11 d of food restriction. Although serial blood sampling would have been more robust in detecting differences in GH levels due to the pulsatile nature of GH release, given the stressful nature of this experimental paradigm, we (as well as the IACUC) deemed only single daily blood samples were justified for humane and scientific reasons (i.e. minimize hypovolemia).
Circulating hormones and metabolites
GH (Millipore, Bedford, MA) and IGF-I (Immunodiagnostic Systems, Fountain Hills, AZ) levels were assessed using commercial ELISA kits. NEFAs and 3-HB were determined using reagents and microtiter plate procedures from WAKO Diagnostics (Richmond, VA). Blood glucose was assessed by a glucometer (Alpha TRACK blood glucose monitoring system; Abbott, Abbott Park, IL). Performance parameters of the various assays used have been previously reported (10).
mRNA analysis
Total RNA was extracted from pituitaries using the Absolutely RNA RT-PCR miniprep kit (Stratagene, La Jolla, CA) and from livers using Trizol reagent (Life Technologies, Carlsbad, CA) with deoxyribonuclease treatment (Promega, Madison, WI), reversed transcribed (cDNA first strand synthesis kit; MRI Fermentas, Hanover, MD) and amplified by quantitative RT-PCR. Primer sequences, GenBank accession numbers, and product sizes have been previously reported (10), except for fibroblast growth factor (FGF)-21 (NM_020013; sense, CACACCGCAGTCCAGAAAGT, and antisense, GATCAAAGTGAGGCGATCCA; 135 bp) and phosphoenolpyruvate carboxykinase (PEPCK) (NM_011044; sense, TGGGGTGTTTGTAGGAGCAG, and antisense, CCAGGTATTTGCCGAAGTTG; 128 bp).
Statistics
Differences in mean GH levels before and after 11 d of food restriction were evaluated by a Student's t test. Ranked plot analysis of GH was done by arranging GH values in order of magnitude as a rank plot (assigning to each GH value in the group the fraction of the mice that had a lower GH level), and the differences were compared by Wilcoxon signed-rank test, as previously reported (11). Student's t tests were also used to evaluate the expression of GH in the pituitary and IGF-I, FGF21, and PEPCK in the liver as well as the impact of AOiGHD on plasma IGF-I levels before and after food restriction, fat depot weight after food restriction, and body composition and organ weights in age-matched fed mice. Comparisons of the effect of GH status (AOiGHD vs. control) on body composition and circulating nutrients during food restriction were assessed by two-way ANOVA followed by Newman-Keuls post hoc test for multiple comparisons. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Inc., San Diego, CA) and SigmaPlot 11.0 (Systat Inc., San Jose, CA).
Results and Discussion
Impact of AOiGHD in ad libitum-fed female mice
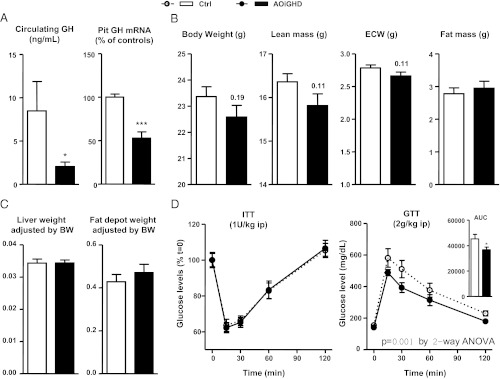
GHD was confirmed in ad libitum-fed, 42-wk-old, female AOiGHD by the clear reduction in plasma GH and pituitary GH mRNA levels (Fig. 1A). Body weights of ad libitum-fed, female AOiGHD mice tended to be smaller than controls (P = 0.19), with less lean mass (P = 0.11), whereas fat mass, fat depot weight, and liver weight were similar to GH replete controls (Fig. 1, B and C). In addition, the response to an ITT was not altered by GH status, but glucose clearance was slightly improved by AOiGHD (Fig. 1D). It should be noted that we previously observed that AOiGHD, in male mice, had a similar impact on lean mass but a more dramatic impact on fat mass (9). In addition, male AOiGHD mice exhibited improved insulin sensitivity, whereas glucose clearance remained unchanged when mice were maintained on a low-fat or chow diet (2–3 or 5–6 months after DT treatment, respectively) (9). Given the extensive data showing GH negatively impacts systemic insulin sensitivity (for review, see Ref. 12), these sex differences may be more apparent than real, in that wild-type female mice on a C57BL6 background (the background of the AOiGHD model) remain more insulin sensitive, when compared with male mice and are resistant to the age- and obesity-induced insulin resistance (13). Therefore, the dose of insulin used in the current study (1 U/kg, ip) may have been in excess to discern differences in systemic insulin sensitivity. However, fed and fasted insulin levels and glucose stimulated insulin release (30 min after the glucose injection) in female AOiGHD (performed in a separate cohort of mice) are similar to or slightly lower than controls (data not shown), in part serving to eliminate the possibility of enhanced insulin output to explain the improved glucose clearance observed in this study.
Fig. 1.
Characterization of AOiGHD females. Plasma GH levels and pituitary GH mRNA (A), body weight and body composition by NMR (lean mass, fat mass, and extracellular water) (B) and liver and adipose tissue weight (adjusted by total body weight) (C) were measured in 1-yr-old female AOiGHD and controls mice. ITTs (D, left panel) and glucose tolerance tests (GTTs) (D, right panel) were performed as described in Materials and Methods. Data represent mean ± sem of n = 15–20 mice/group. Asterisks (***, P < 0.001; *, P < 0.05) indicate values that significantly differ from controls. AUC, Area under the curve.
Impact of AOiGHD in aged female mice in response to severe food restriction
Growth hormone
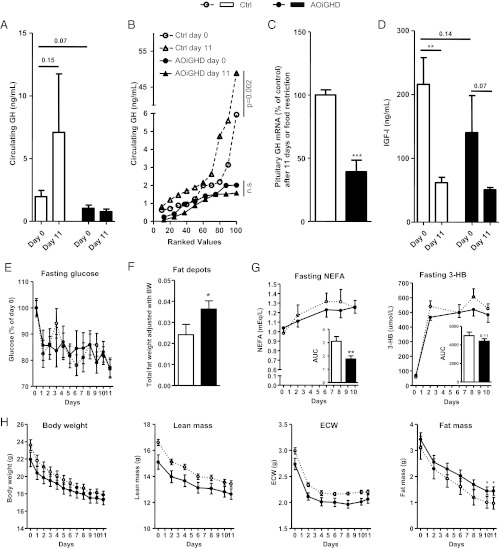
As previously shown in young C57BL/6 male mice (5, 6), 60% food restriction induced a marked rise in plasma GH levels in aged female C57BL/6 control mice (Fig. 2, A and B); however, the increase in average GH levels during food restriction did not reach statistical significance (P = 0.15), likely due to the pulsatility of GH release, coupled with the fact that it was only possible to obtain a single blood sample from each mouse, in accordance with IACUC restrictions. However, ranked plot analysis (11) of GH levels (Fig. 2B) revealed a significant elevation (P = 0.002) of GH levels, thus suggesting that both peak and nadir levels were elevated in control mice after 11 d of food restriction. In contrast, our results clearly demonstrate that GH levels did not rise during starvation in AOiGHD mice (Fig. 2, A and B). GHD was further confirmed in this group by a 60% reduction in pituitary GH mRNA, compared with controls (Fig. 2C). Although this is not a model of complete GHD, the fact that GH levels did not rise in AOiGHD female mice after 11 d of starvation supports the validity of this model to determine whether a rise in GH is required to support glucose levels during starvation.
Fig. 2.
Effect of food restriction on AOiGHD female mice. Plasma GH (A and B), IGF-I (D), glucose (E), and NEFAs and 3-HB (G) levels in female AOiGHD vs. controls mice during food restriction from tail vein samples taken right before feeding. Body composition by NMR (H) was measured right before blood sampling. Pituitary GH expression adjusted by a normalization factor (NF) (C) and fat depot weight adjusted by total body weight (F) were measured at the time the animals were killed after 11 d of food restriction. In A and B, line graphs show ranked plot analysis and bar graphs show mean of GH values. Data represent mean ± sem of n = 8–10 mice/group. Asterisks (***, P < 0.001; **, P < 0.01; *, P < 0.05) indicate values that significantly differ from controls.
Glucose
One day of food restriction reduced circulating glucose levels to a similar extent in both groups (17.6% reduction in controls and 14.4% in AOiGHD mice, P = 0.54), and these levels were essentially maintained, independent of GH status (Fig. 2E), which was reflected by a comparable rise in hepatic expression of PEPCK (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). These results are in contrast to that reported by Zhao et al. (5), who showed that food-restricted, 8-wk-old, male mice, which lacked the ghrelin-induced rise in GH, became hypoglycemic relative to controls. It should be noted that Zhao et al. emphasized that hypoglycemia was observed only when mice had less than 2% fat mass and circulating free fatty acids, ketones, pyruvate, lactate, leucine, and alanine were below fed levels, indicating these mice had completely depleted their fat stores and that proteolysis was severely blunted (14). Although end point criteria set by IACUC standards required us to kill the majority of mice before achieving less than 2% fat mass, fat mass in three of 10 control mice did fall below than 2% and, even in those mice, circulating glucose levels were maintained (Supplemental Fig. 2). The fact that the aged female mice used in this study were able to tolerate a more prolonged starvation period, compared with the young male mice used by Zhao et al. (5), may be in part due to the fact that starting fat mass was greater in our aged female mice. Also, it should be noted that young female mice have been reported to survive longer than young male mice under fasting conditions, in which estrogen may serve to support fatty acid oxidation (15).
Despite these discrepancies, the current results are in agreement with a more recent study that used the same food restriction paradigm to disregard the role of the ghrelin system in maintaining glucose levels in that severe hypoglycemic events were not observed in male and female mice of multiple ages with either ghrelin KO, GOAT KO, GHSR KO, or double-ghrelin and GHSR KO (6). These results, coupled with our current findings, strongly suggest that a GH rise is not essential to maintain glucose levels during severe food restriction, at least in aged female mice, even in conditions in which fat stores were virtually eliminated.
IGF-I and FGF21
Consistent with previous reports showing acute fasting reduces IGF-I levels in a variety of species including mice (16), plasma IGF-I levels (Fig. 2D) and hepatic IGF-I mRNA levels (Supplemental Fig. 1) were reduced to a similar extent in response to food restriction in both control and AOiGHD mice. This reduction in IGF-I is thought to be due in part to hepatic GH resistance mediated by a rise in hepatic FGF-21 (17), a response we observed in livers of both control and AOiGHD mice (Supplemental Fig. 1). Despite the fact that GH has been reported to directly stimulate FGF-21 gene expression (18), our data demonstrate the rise GH observed under severe food restriction cannot account for enhanced expression of hepatic FGF-21.
Body composition
Both control and AOiGHD mice steadily lost body weight over the course of food restriction (Fig. 2H). Controls lost relatively more lean and fat mass than AOiGHD mice [controls: 19.5 ± 0.7% of initial lean and 74 ± 5.5% of initial fat; AOiGHD: 16.2 ± 0.6% of initial lean and 58.2 ± 3.1% of initial fat (P = 0.006 and P = 0.033, respectively)]. The protection of total fat mass observed in AOiGHD mice (Fig. 2H) was reflected by higher postmortem fat depot weight compared with controls (Fig. 2F). This resistance to fat loss was associated with a more modest rise in circulating NEFAs compared with controls (Fig. 2G), consistent with previous reports showing GH enhances lipolysis during fasting (3, 4). Food restriction also induced a rapid increase in 3-HB (Fig. 2G). However, the increment in 3-HB in response to food restriction tended to be blunted in AOiGHD mice (area under the curve; P = 0.11), which is consistent with the fact that ketone production is largely regulated by substrate availability (free fatty acids) (19). In that GH is thought to maintain muscle mass under fasted conditions (20), the relative preservation of lean mass in AOiGHD mice was somewhat unexpected. However, several clinical studies have demonstrated that blocking the fasting induced rise in GH does not lead to enhanced proteolysis (3, 4). In our study, the possibility cannot be discounted that the method used to assess lean mass (NMR) does not accurately reflect specific loss of skeletal muscle mass. It could also be argued that the low levels of GH that remain in AOiGHD mice are sufficient to maintain lean mass.
Concluding remarks
Our findings do not disregard a crucial role of GH during food restriction because the low GH levels observed in AOiGHD could be sufficient to maintain glucose levels and lean mass. However, our data show that the GH elevation observed during severe food restriction is not essential to maintain glucose levels but may be important in promoting an appropriate response of adipose tissues, at least in aged, female C57BL6 mice.
Supplementary Material
Acknowledgments
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Merit Award, and National Institutes of Health Grants R21AG031465 and R01DK088133 (to R.D.K.); Fundacion Caja Madrid and “Sara Borrell” program (Grant CD11/00276) (to M.D.G.); Fundacion Alfonso Martin Escudero (to J.C.-C.); and Grants CTS-5051, BFU2010-19300, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y Nutrición, and Grant RYC-2007-00186 (to R.M.L.).
Disclosure Summary: The authors have nothing to disclose and no conflict of interest.
Footnotes
- AOiGHD
- Adult-onset isolated GH deficient
- DT
- diphtheria-toxin
- FGF
- fibroblast growth factor
- GHD
- GH deficiency
- GHSR
- ghrelin receptor
- GOAT
- ghrelin-O-acyl transferase
- 3-HB
- 3-hydroxybutyrate
- IACUC
- Institutional Animal Care and Use Committees
- iDTR
- inducible DT receptor
- ITT
- insulin tolerance test
- KO
- knockout
- NEFA
- nonesterified free fatty acid
- NMR
- nuclear magnetic resonance
- PEPCK
- phosphoenolpyruvate carboxykinase.
References
- 1. Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, Thorner MO. 1988. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest 81:968–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth J, Glick SM, Yalow RS, Bersonsa 1963. Hypoglycemia: a potent stimulus to secretion of growth hormone. Science 140:987–988 [DOI] [PubMed] [Google Scholar]
- 3. Moller L, Norrelund H, Jessen N, Flyvbjerg A, Pedersen SB, Gaylinn BD, Liu J, Thorner MO, Moller N, Lunde Jorgensen JO. 2009. Impact of growth hormone receptor blockade on substrate metabolism during fasting in healthy subjects. J Clin Endocrinol Metab 94:4524–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakharova AA, Horowitz JF, Surya S, Goldenberg N, Harber MP, Symons K, Barkan A. 2008. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J Clin Endocrinol Metab 93:2755–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. 2010. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 107:7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yi CX, Heppner KM, Kirchner H, Tong J, Bielohuby M, Gaylinn BD, Müller TD, Bartley E, Davis HW, Zhao Y, Joseph A, Kruthaupt T, Ottaway N, Kabra D, Habegger KM, Benoit SC, Bidlingmaier M, Thorner MO, Perez-Tilve D, Tschöp MH, Pfluger PT. 2012. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS One 7:e32100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD. 2007. Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology 148:1946–1953 [DOI] [PubMed] [Google Scholar]
- 8. Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. 2005. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2:419–426 [DOI] [PubMed] [Google Scholar]
- 9. Luque RM, Lin Q, Córdoba-Chacón J, Subbaiah PV, Buch T, Waisman A, Vankelecom H, Kineman RD. 2011. Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PLoS One 6:e15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gahete MD, Córdoba-Chacón J, Anadumaka CV, Lin Q, Brüning JC, Kahn CR, Luque RM, Kineman RD. 2011. Elevated GH/IGF-I, due to somatotrope-specific loss of both IGF-I and insulin receptors, alters glucose homeostasis and insulin sensitivity in a diet-dependent manner. Endocrinology 152:4825–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu J, Bekaert AJ, Dupont J, Rouve S, Annesi-Maesano I, De Magalhaes Filho CD, Kappeler L, Holzenberger M. 2011. Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. J Endocrinol 208:119–129 [DOI] [PubMed] [Google Scholar]
- 12. Yuen KC, Dunger DB. 2007. Therapeutic aspects of growth hormone and insulin-like growth factor-I treatment on visceral fat and insulin sensitivity in adults. Diabetes Obes Metab 9:11–22 [DOI] [PubMed] [Google Scholar]
- 13. Goren HJ, Kulkarni RN, Kahn CR. 2004. Glucose homeostasis and tissue transcript content of insulin signaling intermediates in four inbred strains of mice: C57BL/6, C57BLKS/6, DBA/2, and 129X1. Endocrinology 145:3307–3323 [DOI] [PubMed] [Google Scholar]
- 14. Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. 2012. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem 287:17942–17950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jikumaru M, Hiramoto K, Honma T, Sato EF, Sekiyama A, Inoue M. 2007. Effect of starvation on the survival of male and female mice. Physiol Chem Phys Med NMR 39:247–257 [PubMed] [Google Scholar]
- 16. Møller N, Jørgensen JO. 2009. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177 [DOI] [PubMed] [Google Scholar]
- 17. Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. 2008. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu J, Zhao L, Wang A, Eleswarapu S, Ge X, Chen D, Jiang H. 2012. Growth hormone stimulates transcription of the fibroblast growth factor 21 gene in the liver through the signal transducer and activator of transcription 5. Endocrinology 153:750–758 [DOI] [PubMed] [Google Scholar]
- 19. Miles JM, Haymond MW, Nissen SL, Gerich JE. 1983. Effects of free fatty acid availability, glucagon excess, and insulin deficiency on ketone body production in postabsorptive man. J Clin Invest 71:1554–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norrelund H, Nair KS, Jørgensen JO, Christiansen JS, Møller N. 2001. The protein-retaining effects of growth hormone during fasting involve inhibition of muscle-protein breakdown. Diabetes 50:96–104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.