Abstract
That a knock-in mouse harboring a dominant-negative thyroid hormone receptor (TR)-β (Thrb) mutation develops metastatic thyroid cancer strongly suggests the involvement of TRβ in carcinogenesis. Epigenetic silencing of the THRB gene is common in human cancers. The aim of the present study was to determine how DNA methylation affected the expression of the THRB gene in differentiated thyroid cancer (DTC) and how reexpression of the THRB gene attenuated the cancer phenotypes. We used methylation-specific PCR to examine the expression and promoter methylation of the THRB gene in DTC tissues. Thyroid cancer cells with hypermethylated THRB were treated with the demethylating agents 5′-aza-2′-deoxycytidine (5′-aza-CdR) and zebularine to evaluate their impact on the cancer cell phenotypes. THRB mRNA expression in DTC was 90% lower than in normal controls, and this decrease was associated with a higher tumor/lymph node staging. The promoter methylation level of the THRB gene had a significant negative correlation with the expression level of the THRB gene. Treatment of FTC-236 cells with 5′-aza-CdR or zebularine induced reexpression of the THRB gene and inhibited cell proliferation and migration. FTC-236 cells stably expressing TRβ exhibited lower cell proliferation and migration through inhibition of β-catenin signaling pathways compared with FTC-236 without TRβ. 5′-Aza-CdR also led to suppression of tumor growth in an in vivo xenograft model using FTC-236 cells consistent with the cell-based studies. These finding indicate that TRβ is a tumor suppressor and could be tested as a potential therapeutic target.
Thyroid hormone receptors (TRs) are members of the nuclear receptor superfamily that mediate the biological activities of the thyroid hormone (T3) in development, growth, differentiation, and metabolism (1, 2). Two TR genes, THRA and THRB, encode four T3-binding receptor isoforms (TRα1, TRβ1, TRβ2, and TRβ3). Previous studies have shown TR mutations are associated with human cancers, including hepatocellular carcinoma, renal cell carcinoma, breast cancer, and pituitary tumors (3–6). That a knock-in mouse model harboring a potent dominant-negative THRB mutation (denoted PV) spontaneously develops metastatic thyroid cancer indicates that the loss of normal functions of TRβ by mutation contributes to thyroid carcinogenesis (7). Silencing of the THRB expression by hypermethylation of the promoter region of the THRB gene is frequent in breast, lung, colon, acute lymphoblastic leukemia, and thyroid cancers (8–13). Recent studies have provided evidence that the expression of the THRB gene could also be repressed through a microRNA regulatory mechanism in papillary thyroid carcinoma (14). These findings raised the possibility that TRβ could act as a tumor suppressor and potentially be a therapeutic target.
The incidence of thyroid cancer, the most common malignancy in the endocrine organs, has greatly increased in the past two decades around the world (15). Recent advances in the understanding of molecular genetics of thyroid cancer have revealed that molecular abnormalities accumulate by genetic and epigenetic mechanisms during thyroid carcinogenesis (16, 17). Aberrant DNA methylation of tumor-suppressor genes occurred in the early phases of tumorigenesis and plays an important role in cancer development and progression (17, 18). Several cell-based studies have shown beneficial effects by reactivating the expression of hypermethylated genes with the use of demethylating agents (19–22). These cell-based studies raised the possibility for a novel potential treatment modality for thyroid cancers. Although hypermethylation of the THRB gene was previously reported in differentiated thyroid cancer (DTC) (11), functional consequences of inactivation of the THRB gene by hypermethylation in thyroid cancer have not been elucidated.
In the present study, we first evaluated promoter methylation and the expression of the THRB gene in tissue specimens from patients with DTC and in several human thyroid cancer cell lines. We found a positive correlation between the extent of promoter hypermethylation of the THRB gene and the progression of DTC. When human thyroid cancer cell lines in which the THRB gene was silenced by hypermethylation were treated with demethylation agents such as 5′-aza-2′-deoxycytidine (5′-aza-CdR) and zebularine (a newer demethylating agent with more stable and less toxic properties), the expression of the THRB gene was reactivated concurrently with inhibition of cancer cell proliferation, migration, and tumor growth in xenograft model. Reexpression of THRB in thyroid cancer cell lines inhibited cell proliferation and migration through suppressing the activation of β-catenin signaling pathway. The present study demonstrated that TRβ could act as a tumor suppressor in thyroid cancer and could be tested as a potential therapeutic target.
Materials and Methods
Human thyroid tissue specimens from patients with DTC and normal controls
Thyroid cancer tissue samples, patient demographics, and clinical and pathological information were collected after written informed consent under an Institutional Review Board-approved clinical protocol (NCI-09-C-0242 and NCT01005654). Samples were obtained at the time of thyroidectomy, snap frozen in liquid nitrogen, and stored at −80 C. The clinicopathological information of the patients with DTC is summarize in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). We enrolled 17 patients with papillary thyroid carcinoma and four patients with follicular thyroid carcinoma (FTC); the mean age of these 21 patients (eight male and 13 female) was 50.2 yr. The mean maximal diameter of tumor was 3.6 cm; 12 of 21 patients (57%) were classified in T1/T2 stage by the American Joint Committee on Cancer/International Union Against Cancer tumor-node-metastases classification staging classification (sixth edition). Cervical lymph node metastasis was present in 11 patients (52%), and distant metastasis was found in two patients (10%). In 11 patients undergoing thyroidectomy for benign disease, normal thyroid tissue was obtained from the contralateral thyroid lobe. Tissues with nodular hyperplasia and follicular adenoma were also obtained from patients for analysis.
Quantitative real-time RT-PCR
Total RNA from human thyroid tissues was extracted by TRIzol (Invitrogen, Carlsbad, CA), followed by ribonuclease-free deoxyribonuclease treatment and column purification (RNeasy Mini kit; QIAGEN, Valencia, CA). Total RNA from FTC-236 cells after treatment with 3 μm 5′-aza-CdR, 10 μm zebularine, or vehicle for 10 d was isolated using TRIzol as indicated by the manufacturer's protocol. For real-time RT-PCR, one-step RT-PCRs were performed with 100–200 ng total RNA using a QuantiTect SYBR green RT-PCR kit (QIAGEN) in a Roche LightCycler PCR instrument (Roche, Indianapolis, IN) according to the manufacturer's instructions. Specific primer sequences are listed in Supplemental Table 2. The reaction conditions were 50 C for 20 min, 95 C for 15 min, followed by 40–45 cycles of 94 C for 15 sec, 59 C for 25 sec, 72 C for 25 sec, 65–95 C with a heating rate of 0.1 C/sec, and finally a cooling step to 40 C.
DNA extraction and detection of DNA methylation
Genomic DNA from tissues and cells was isolated using a QIAamp DNA Micro kit (QIAGEN) according to the manufacturer's instructions. An isolated 100 ng of genomic DNA was bisulfite modified using a BisulFlash DNA modification kit (Epigentec, Brooklyn, NY) according to the manufacturer's instructions. Methylation-specific PCR was performed on 10 ng of bisulfite-treated DNA. The methylation-specific THRB primer sequences are listed in Supplemental Table 2. The PCR was performed for 40 cycles and the conditions were denaturation at 94 C for 15 sec, annealing at 59 C for 25 sec, and extension at 72 C for 25 sec, followed by a final 5-min extension at 72 C. The PCR products were separated by electrophoresis onto a 2% Tris-acetate-ethylenediaminetetraacetic acid agarose gel, stained with ethidium bromide, and visualized under UV illumination. The relative ratios of methylated to unmethylated DNA were determined by measuring the intensities of the amplified bands visualized on DNA gel.
Western blot
Preparation of whole-cell lysates from human thyroid tissues or FTC-236 cells and nuclear extracts of FTC-236 cells was carried out as previously described (23). The protein samples (40 μg of cell lysates from human tissues and 20–30 μg of lysates from FTC-236 cells) were loaded and separated by SDS-PAGE. After electrophoresis, the protein was electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA). Anti-TRβ C4 antibodies were used for detection of TRβ expression (24). The antibodies to phosphorylated β-catenin (p-β-catenin; 1:1000 dilution), β-catenin (1:1000 dilution), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1000 dilution) were purchased from Cell Signaling Technology (Danvers, MA). Antibody to cyclin D1 (1:500 dilution) was from Thermo Scientific (Waltham, MA), and antibody to α-tubulin (1:1000 dilution) was from Sigma-Aldrich (St. Louis, MO). Antibody to poly-(adenosine-diphosphate ribose) polymerase (PARP) (1:1000 dilution) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The blots were stripped with Re-Blot Plus (Chemicon, Temecula, CA) and reprobed with antibodies to GAPDH and PARP. Band intensities were quantified by using NIH ImageJ software version 1.44o (Wwayne Rasband, National Institutes of Health, Bethesda, MD).
Cell lines and treatment with demethylating agents
FTC-133 and FTC-236 cells, kindly provided by Dr. Orlo H. Clark (UCSF Mount Zion Medical Center, San Francisco, CA), were derived from a primary human follicular thyroid carcinoma and neck lymph node metastases, respectively. These cells were maintained in DMEM/Ham's F12 (1:1) medium (Invitrogen) supplemented with 10% fetal bovine serum, 10 μg/ml bovine insulin (Sigma-Aldrich), 1 mIU/ml bovine TSH (Sigma-Aldrich), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Culture media for other cell lines were described in Supplemental Table 3. The FTC-236 cell line was treated with 3 μm 5′-aza-CdR and 10 μm zebularine (Sigma-Aldrich). Media and reagents were changed every 3 d. Human THRB cDNA was cloned into pFH-IRESneo plasmid (a generous gift from R. G. Roeder, Rockefeller University, New York, NY) (25) to obtain Flag-hemagglutinin (FH)-tagged TRβ cells. The preparation of FTC-236 cells stably expressing FH-TRβ (FTC-236-TRβ) and control cells (FTC-236-Neo) was similar to what was described for HeLa cells (26). FTC-236 cells were transfected with FH-TRβ or the empty vector and selected with 200 μg/ml G418 (Invitrogen). G418-resistant colonies expressing FH-tagged proteins were expanded for subsequent experiments. The expression of TRβ protein was verified by Western blot analysis using anti-TRβ C4 antibodies (24).
Cell proliferation assay
After 7 d of treatment with 3 μm 5′-aza-CdR, 10 μm zebularine, or vehicle, 5 × 104 cells were plated in six-well plates and cultured for 24 h. Nonattached cells were removed by gently washing twice with 2 ml PBS. After trypsin treatment and resuspension, the number of attached cells was counted every 24 h from d 1–6 by using a cell and particle counter (Beckmann Coulter, Indianapolis, IN).
Wound-healing assay
After 7 d of treatment with 5′-aza-CdR, zebularine, or vehicle, 1×106 cells were seeded in six-well plates. The wound was applied with a pipette tip on confluent cells, and plates were washed with PBS followed by the addition of complete culture medium. At regular intervals, a camera system with an inverted microscope visualized cell migration at ×100 magnification. The migration rate was quantified by measuring the distance between the edges of wound, and the percentage of migration was determined as the ratio between migrated distance and initial distance of wound.
Immunohistochemistry of cultured FTC-236 cells
Cell pellets from 5 × 106 cells were fixed in 10% neutral-buffered formalin (Sigma-Aldrich) and subsequently embedded in paraffin. Five-micrometer-thick sections were prepared for staining. Immunohistochemistry was conducted as previously described (27). Rabbit anti-Ki-67 (1:300 dilution, NeoMarker; Thermo Scientific) was used for primary antibody.
In vivo mouse xenograft study
The National Cancer Institute Animal Care and Use Committee approved the protocols for animal care and handling in the present study. Four-week-old male athymic NCr-nu/nu mice were obtained from NCI-Frederick animal facility. FTC-236 cells (5 × 106 cells) in 200 μl suspension mixture with Matrigel basement membrane matrix (BD Biosciences, San Jose, CA) were sc inoculated into the right flank of mice. Mice were randomly divided into two groups (six mice for each). Weekly 5′-aza-CdR (5 mg/kg) or vehicle treatment by ip injection started 2 d after cell inoculation. Tumor size was measured with calipers, and tumor volume was calculated as (length × width2)/2.
Statistics
Data are presented as mean ± se or as median/range. Student's t test or Mann-Whitney U test was used to compare continuous variables. Fisher's exact test was used for comparison of categorical variables. The pattern of relative expression of mRNA and relative methylation ratios in different groups were evaluated by examining the P value for trend via linear regression analysis. P values were two sided throughout, and P < 0.05 was considered statistically significant. Data were analyzed using SPSS statistics version 19.0 (SPSS Inc., Chicago, IL). GraphPad Prism version 4.0a (GraphPad Software, San Diego, CA) was used to draw graphs.
Results
The expression of the THRB gene is decreased in thyroid cancer and is inversely correlated with cancer progression
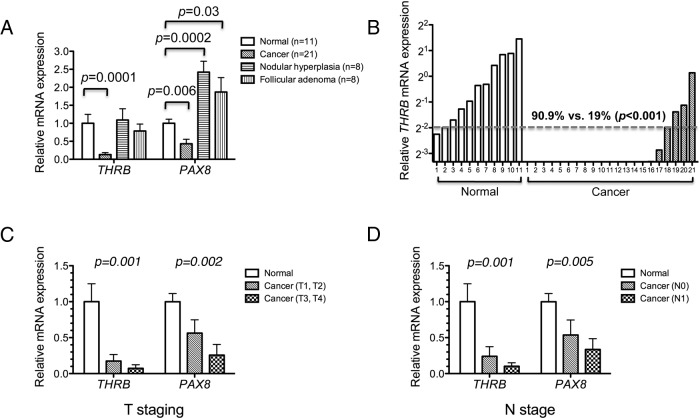
To understand the roles of the TRβ in human DTC, we first examined the expression of the THRB mRNA levels in 21 DTC, eight nodular hyperplasia, eight follicular adenoma, and 11 normal thyroid tissues. Concurrently, we also determined the mRNA levels of a thyroid-specific transcription factor, the PAX8 gene. The expression of the PAX8 gene is known to be repressed in human thyroid cancers (28). Therefore, we used PAX8 mRNA expression as a positive control and also for the validation of the thyroid cancer tissues. The THRB mRNA expression was 90% lower in cancer tissues than in normal controls (P = 0.0001, Fig. 1A). The THRB mRNA in nodular hyperplasia and follicular adenoma was higher than that in cancer tissues and was not significantly different from that in normal tissues (Fig. 1A). Consistent with reports by others (28), the expression of PAX8 mRNA was decreased by 60% in cancer tissues as compared with normal controls (P = 0.006, Fig. 1A) but was higher than normal tissues in nodular hyperplasia and follicular adenoma (P = 0.03 and P = 0.0002, respectively). The extent of the THRB mRNA expression for each normal thyroid and cancer tissue is shown in Fig. 1B, indicating more normal tissues expressed THRB mRNA above one fourth of the mean (90.9%) than those of cancer tissues (19%; P = 0.001).
Fig. 1.
Down-regulation of THRB gene in DTC tissues. A, Relative mRNA expression of THRB and PAX8 in DTC (n = 21), nodular hyperplasia (n = 8), follicular adenoma (n = 8), and normal thyroid tissue (n = 11). The data are presented as mean ± se and were analyzed by Student's t test. B, Relative THRB mRNA expression of individual tissue samples from DTC and normal thyroid determined by real-time RT-PCR assay. C, Relative THRB and PAX8 mRNA expression according to lower (n = 12) and higher (n = 9) tumor (T) staging in DTC tissues. The relative expression of mRNA in different groups was evaluated by examining the P value for trend via linear regression analysis. D, Relative THRB and PAX8 mRNA expression according to the absence (n = 10) or presence (n = 11) of lymph node (N) metastasis in DTC tissues. T staging and N staging were defined according to the sixth edition of the American Joint Committee on Cancer/International Union Against Cancer tumor-node-metastases (TNM) classification.
To correlate the clinical relevance of the THRB expression with the aggressiveness of DTC, we analyzed the THRB mRNA expression according to the subgroups of tumor (T) and lymph node (N) stage of cancer (see Supplemental Table 1). Figure 1C shows that the more advanced the thyroid cancer was, on the basis of T staging, the lower THRB and PAX8 mRNAs were [normal (n = 11), T1/T2 cancer (n = 12); T3/T4 (n = 9)] according to trend analysis (P = 0.001 and P = 0.002, respectively). A similarly significant decreasing trend was detected when lymph node staging was used (P = 0.001; Fig. 1D). These data indicated that the expression of the THRB gene was lower in cancer tissue than in normal, and the decreased THRB expression was inversely correlated with the severity in the progression of DTC.
The expression of the THRB gene is repressed by hypermethylation of the THRB promoter in DTC
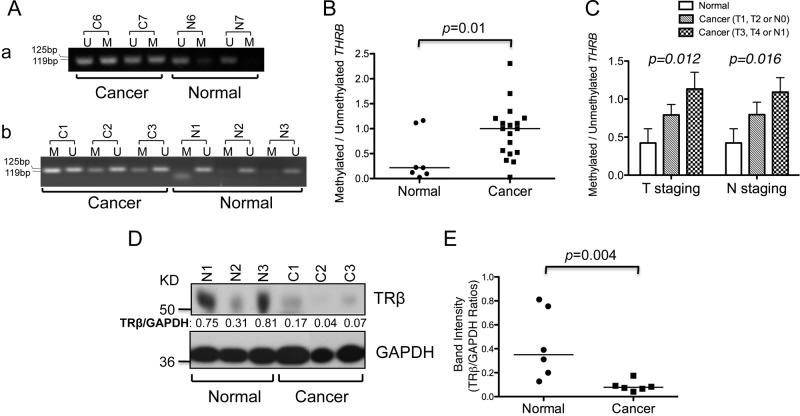
Epigenetic inactivation of the THRB gene through promoter hypermethylation has been reported in many human cancers (8–13). Using methylation-specific PCR, we examined the methylation status of the THRB promoter region in human thyroid tumors (n = 18). Figure 2A shows that the extent of THRB methylation was greater in thyroid cancer tissues than in normal controls. The ratio of methylated to unmethylated THRB (i.e. the relative THRB methylation ratio) was significantly increased by 4.6-fold in cancer tissues (P = 0.01, Fig. 2B; n = 18 for cancers and n = 7 for normal tissues). To understand the consequences of the repressed expression of the THRB gene by promoter methylation, we analyzed the relative THRB methylation ratios in normal and lower- and higher-stage cancers. As shown in Fig. 2C, the relative THRB methylation ratio was lower in normal tissues, higher in T1/T2 cancers and highest in T3/T4 cancer shown by T staging (P value by trend analysis = 0.012). The THRB methylation ratios of cancer categorized by lymph node staging also showed significant increasing trend comparing with normal tissues (P value by trend analysis = 0.016). The findings of significantly lower THRB methylation ratios in normal tissues and higher ratios of THRB methylation in aggressive disease strongly suggest that methylation of the promoter region is critical in regulating the expression of the THRB gene in thyroid cancer.
Fig. 2.
THRB is hypermethylated in DTC. A, Representative results of THRB promoter methylation in cancer and normal thyroid tissue. The amounts of methylated (M) or unmethylated (U) THRB promoter from bisulfite-treated genomic DNA of each tissue were determined by specific PCR analysis. B, The ratio of methylated to unmethylated THRB promoter from normal (n = 7) and cancer tissues (n = 18). The data are presented as medians and analyzed by the Mann-Whitney U test. C, The relative THRB methylation ratios of normal and DTC tissues according to lower (n = 10) and higher (n = 8) tumor (T) staging. The relative THRB methylation ratios of normal and DTC tissues are shown according to lymph node (N) staging [the absence (n = 9) or presence (n = 9) of lymph node (N) metastasis]. The relative ratio of methylated and unmethylated THRB in different groups was evaluated by examining the P value for trend via linear regression analysis. D, Representative results of Western blot analysis of TRβ and GAPDH as the loading control in normal and cancer tissues. E, Quantification of relative protein expression of TRβ after normalization via use of GAPDH as the loading control. The data are presented as medians and analyzed by the Mann-Whitney U test.
We also examined the TRβ protein level by Western blot analysis in DTC tissue (n = 6) and normal controls (n = 6). Representative examples show that the TRβ protein abundance in DTC was decreased (Fig. 2D). The band intensities were quantified, and the data show that the TRβ protein levels were significantly lower (by 78%) in cancer tissues than in normal tissues (P = 0.004, Fig. 2E). These findings further support the notion that methylation of the THRB promoter region inhibits THRB expression in thyroid cancer.
The demethylating agents 5′-aza-CdR and zebularine induce the expression of the THRB gene in human thyroid cancer cell lines
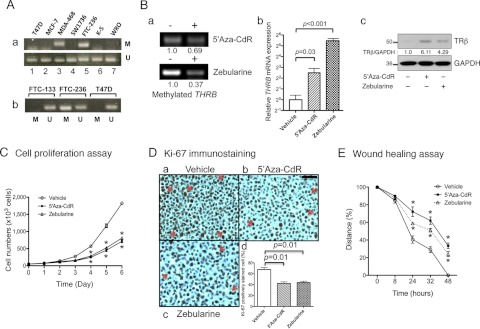
To elucidate the functional consequences of the silencing of the THRB gene by hypermethylation, we turned to the use of human thyroid cancer cell lines. We tested five thyroid tumor cell lines and three breast cancer cell lines for detection of inactivation of the THRB gene by hypermethylation. Figure 3A shows that the THRB gene in T47D and MCF-7 breast cancer cells was not methylated, but it was methylated in MDA468 cells (lane 3), as previously reported (8). Increased methylation of the THRB gene was detected in FTC-236 but not in four other thyroid cancer cell lines: SW1736, K-5, WRO, and FTC-133 cells (Fig. 3A, a and b). We therefore treated FTC-236 cells with the demethylating agents 5′-aza-CdR and zebularine to test whether they could demethylate the THRB gene. Indeed, Fig. 3B shows that after treatment, the extent of methylation of the THRB gene was decreased by 31% with 5′-aza-CdR and by 63% with zebularine (Fig. 3B, upper and lower panels). Importantly, the demethylation of the THRB gene led to the increased expression of the THRB mRNA by 5.6-fold (P = 0.03) and by 44-fold (P < 0.001) after treatment with 5′-aza-CdR and zebularine, respectively (Fig. 3Bb). We further showed that by Western blot analysis, TRβ protein was also detected by treatment of FTC-236 cells with 5′-aza-CdR or zebularine (Fig. 3C, upper panel). Therefore, these results indicate that the THRB gene was silenced by hypermethylation and that demethylation resulted in the reexpression of the THRB gene in FTC-236.
Fig. 3.
Demethylation agents 5′-aza-CdR and zebularine increase THRB expression through reduced THRB promoter methylation. A, The amounts of methylated (M) or unmethylated (U) THRB promoter determined by methylation-specific PCR analysis in human breast cancer cells (T47D, MCF-7, MDA-468) and thyroid cancer cells (SW1736, FTC-236, K-5, WRO, and FTC-133). Ba, Methylation of THRB promoter was decreased after treatment with the demethylating agents, 5′-aza-CdR (upper panel) and zebularine (lower panel) in the FTC-236 human thyroid cancer cell line; b, relative mRNA expression of THRB was increased by treatment with the demethylating agents in FTC-236 cells (data presented as mean ± sd and compared by Student's t test); c, protein abundance of TRβ was increased by treatment with demethylating agents in FTC-236 cells as shown by Western blot analysis of total cell lysates with or without treatment with demethylating agents as marked (upper panel). GAPDH was used as the loading control (lower panel). C, Relative to vehicle treatment, cell proliferation was inhibited by treatment with the demethylating agents in FTC-236 cells (data presented as mean ± sd). *, P < 0.01. D, Decreased Ki-67-positive cells after treatment with 5′-aza-CdR and zebularine in FTC-236 cells compared with vehicle treatment. Representative microphotographs of Ki-67 immunohistochemistry on paraffin-embedded cells after treatment with vehicle (a), 5′-aza-CdR (b), and zebularine (c). Cell proliferation index was determined by proportion of Ki-67-positive cells in FTC-236 cells (d). E, Relative to vehicle treatment, cell migration of FTC-236 was inhibited by treatment with demethylating agents, as determined by wound-healing assay (data presented as mean ± sd). *, P < 0.01.
Demethylating agents inhibit cancer cell proliferation and migration through down-regulation of the β-catenin pathway in FTC-236 cells
The finding that the THRB gene in FTC-236 cells was reactivated by treatment with the demethylating agents 5′-aza-CdR and zebularine prompted us to evaluate whether the reexpressed TRβ could lessen the cancer phenotype. As shown in Fig. 3C, FTC-236 cell proliferation was significantly reduced by treatment with 5′-aza-CdR or zebularine, beginning at d 3. On d 6, cell proliferation was 50% lower (Fig. 3C). Consistent with the decreased cell proliferation, Ki-67-positive cells were decreased by 37% (P = 0.01) and by 35% P = 0.01) after treatment with 5′-aza-CdR and zebularine, respectively (Fig. 3D).
Cancer cell migration is an important process involving local cancer invasion to adjacent tissues, infiltration of the lymphatic system, and hematogenous metastasis. We therefore used a wound-healing assay to examine migration in cells treated with 5′-aza-CdR, zebularine, or vehicle (Fig. 3E). Significant decreases of cancer cell migration were observed after treatment with the demethylating agents (Fig. 3E). These data suggest that reduced methylation of the THRB gene by the demethylating agents 5′-aza-CdR and zebularine could be therapeutically beneficial in the treatment of thyroid cancer.
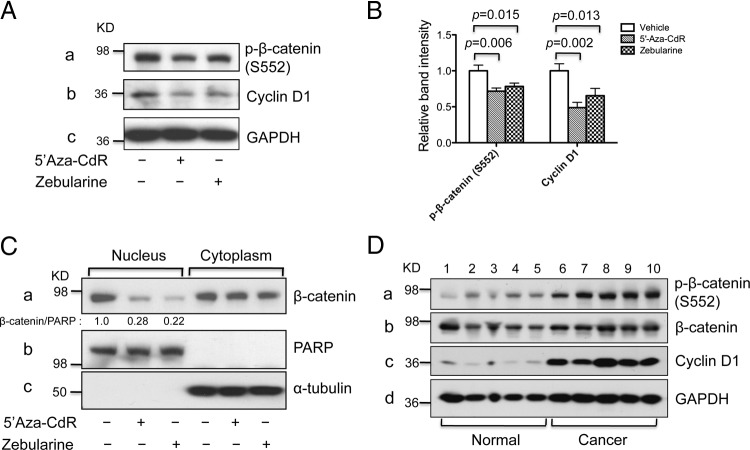
We further elucidated the molecular pathways affected by the treatment of FTC-236 cells with 5′-aza-CdR or zebularine. β-Catenin is a key regulator of the Wnt signaling pathway, which plays important roles in carcinogenesis. Aberrant activation of β-catenin has been reported in many human cancers, including thyroid cancer (29, 30). Previously, we reported that β-catenin signaling is repressed in TRβ-expressing cells but is activated in the thyroid tumors of ThrbPV/PV mice (31). We therefore examined whether the β-catenin signaling pathway was affected after treatment of FTC-236 cells with demethylating agents. As shown in Fig. 4A, activation of the β-catenin signaling pathway was inhibited by treatment of 5′-aza-CdR or zebularine as evidenced by a decrease of p-β-catenin (serine 552) which is critical for β-catenin transcriptional activation (Fig. 4A, panel a). The quantification of the band intensities was shown in Fig. 4B (the first three bars). The attenuated β-catenin transcriptional activity was supported by reduced cyclin D1 (Fig. 4A, panel b), which is a direct target of β-catenin signaling. Figure 4B shows that the protein abundance was decreased by 50 and 35% after treatment with 5′-aza-CdR and zebularine, respectively (see the last three bars). Moreover, we found that nuclear β-catenin abundance was decreased by 72 or 78% after treatment with 5′-aza-CdR or zebularine, respectively (Fig. 4C, panel a). No changes in the β-catenin abundance in the cytoplasmic fraction were observed. However, a lower nuclear β-catenin abundance was consistent with the notion of the attenuation of β-catenin signaling. The marker protein for the nuclear fraction, PARP, was not affected and was used as loading controls (Fig. 4C, panel b). These findings identified the down-regulation of β-catenin signaling as one of the pathways by which reexpression of TRβ by treatment with 5′-aza-CdR and zebularine inhibit cancer cell proliferation. We also evaluated the protein abundance of p-β-catenin (serine 552) and cyclin D1 in cancer and normal thyroid tissue samples. Activation of the β-catenin signaling pathway was evident by higher p-β-catenin (serine 552) levels in human thyroid cancer tissues than in normal tissues (Fig. 4D, panel a). The protein abundance of cyclin D1 was also increased in human thyroid cancer samples as compared with normal controls (Fig. 4D, panel c). These findings provided additional support for the association of suppressed THRB expression with activation of β-catenin signaling pathway in human thyroid cancers.
Fig. 4.
Demethylating agents 5′-aza-CdR and zebularine decrease cell proliferation by attenuating β-catenin signaling in FTC-236. A, Western blot analysis of p-β-catenin (serine 552), cyclin D1, and GAPDH as the loading control in whole-cell lysates from FTC-236 cells after treatment with demethylating agents. B, Quantification of relative protein abundance of p-β-catenin (serine 552) and cyclin D1 after normalization by using GAPDH as a loading control. C, Western blot analysis of protein abundance of β-catenin in the nuclear and cytoplasm cell fractions. PARP was used as loading control for nuclear fraction and α-tubulin was used as loading control for cytoplasmic fractions of FTC-236 cells. D, Western blot analysis of p-β-catenin (serine 552), total β-catenin, cyclin D1, and GAPDH as the loading control in human tissue lysates of normal thyroid and cancer samples.
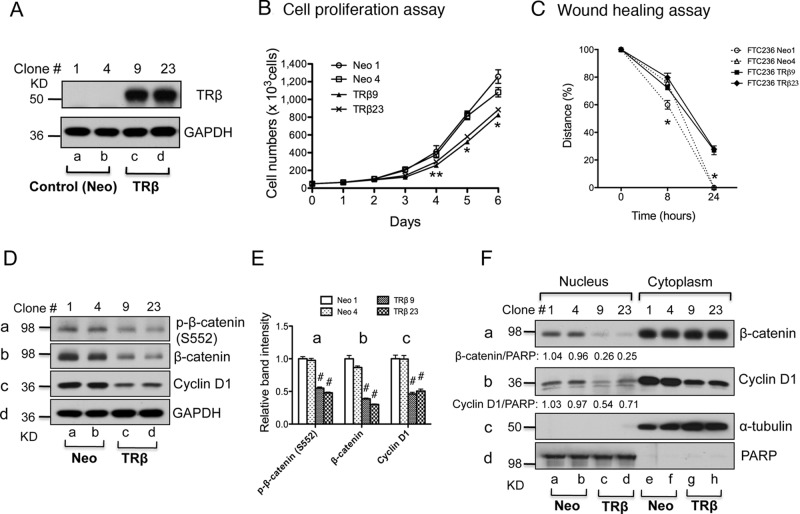
TRβ reexpression in FTC-236 cells reduces cancer cell proliferation and migration through down-regulation of the β-catenin pathway
To further support the idea that TRβ acts as a tumor suppressor in thyroid carcinogenesis, we generated FTC-236 cells stably expressing TRβ (TRβ9 and TRβ23) or only the neomycin gene as controls (Neo1 and Neo4). The expression of TRβ at the protein level in FTC-236-TRβ9 and FTC-236-TRβ23 was confirmed by Western blot analysis (Fig. 5A, lanes c and d), whereas no TRβ expression was observed in control Neo cells (Fig. 5, lanes a and b). The expression of TRβ in FTC-236-TRβ9 and FTC-236-TRβ23 led to a decreased cell proliferation, as shown in Fig. 5B. The effect of the expressed TRβ in FTC-236-TRβ9 and FTC-236-TRβ23 on cancer cell motility was evaluated by wound-healing assays. A decrease in cell motility was evident in that after 24 h, the cell migration was significantly impeded in FTC-236-TRβ9 and FTC-236-TRβ23 cells as compared with the control Neo cells (Fig. 5C). Activation of β-catenin signaling pathway was also inhibited in TRβ-expressing FTC-236 cells shown by decreased p-β-catenin (serine 552) and total β-catenin as compared with control Neo cells (Fig. 5D, panels a and b, respectively; see quantification of band intensities in Fig. 5E). The protein abundance of downstream target of β-catenin, cyclin D1, was also decreased by about 50% in TRβ-expressing FTC-236 cells as compared with control Neo cells (Fig. 5D, panel c; see quantification of band intensities in Fig. 5E). The nuclear abundance of β-catenin protein was clearly reduced, whereas no apparent changes in the cytoplasmic fraction were seen (Fig. 5F, panel a). Importantly, the protein abundance of a direct target of β-catenin signaling, cyclin D1, was lower in both the nuclear fraction (Fig. 5F, panel b; lanes c and d vs. lanes a and b) as well as cytoplasmic fraction (Fig. 5F, panel b; lanes g and h vs. lanes e and f) than those in control Neo cells, providing direct evidence to indicate the attenuation of the β-catenin signaling. Taken together, these data indicate that TRβ could act as a tumor suppressor in thyroid carcinogenesis through down-regulation of the β-catenin pathway and that epigenetic modulation by reactivation of the silenced THRB gene expression could be a therapeutic target for treatment of thyroid cancer.
Fig. 5.
Stable reexpression of TRβ in the FTC-236 thyroid cancer cells reduces cell proliferation and migration by attenuating β-catenin signaling. A, Western blot analysis of expression of TRβ in stable cells after transfection of control (Neo) or THRB-expressing vector in FTC-236 cells. B, Cell proliferation was lower in TRβ-expressing FTC-236 cells (TRβ9 and TRβ23) than in control FTC-236 cells (Neo1 and Neo4). Data are presented as mean ± se. **, P < 0.05; *, P < 0.01. C, Wound-healing assay showed that reexpressing of the TRβ inhibited cell migration of FTC-236 cells. Data are presented as mean ± se. *, P < 0.01. D, Western blot analysis of p-β-catenin (serine 552), total β-catenin, cyclin D1, and GAPDH in whole-cell lysates from TRβ-expressing FTC-236 cells and control FTC-236 cells. E, Quantification of relative protein abundance of p-β-catenin, total β-catenin, and cyclin D1 after normalization using GAPDH as a loading control. Data presented as mean ± se. #, P < 0.001. F, Western blot analysis of protein abundance of β-catenin and cyclin D1 in the nuclear and cytoplasm cell fractions. PARP was used as loading control for nuclear fraction, and α-tubulin was used as loading controls for cytoplasmic fraction from FTC-236 cells.
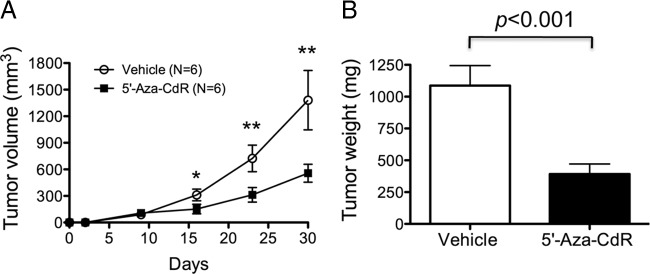
Demethylating agent 5′-aza-CdR inhibits growth of tumors induced by FTC-236 cancer cells in mouse xenograft models
To further validate our cell-based studies, we evaluated the effect of 5′-aza-CdR on in vivo mouse xenograft models. FTC-236 cells were sc inoculated into the right flanks of mice that were treated with or without 5′-aza-CdR (weekly 5 mg/kg body weight). As shown in Fig. 6A, tumor volume in mice treated with 5′-aza-CdR was significantly reduced as compared with vehicle-treated mice. The weights of FTC-236-induced tumors were measured at the end point (a month after cancer cell inoculation). As shown in Fig. 6B, tumor weight was significantly reduced (∼75%) in the 5′-aza-CdR-treated group as compared with the vehicle-treated group (Fig. 6B). These findings are consistent with the cell-based studies in that demethylation of the THRB gene in vivo by 5′-aza-CdR led to suppression of tumor growth.
Fig. 6.
Demethylating agent 5′-aza-CdR inhibits growth of tumors induced by FTC-236 cells in mouse xenograft models. A, Growth curves of FTC-236-induced tumors. FTC-236 cancer cells were sc inoculated into the right flank of athymic NCr-nu/nu mice. Mice were treated by weekly ip injection of 5′-aza-CdR (5 mg/kg; n = 6) or vehicle control (n = 6) for 30 d. Data are presented as mean ± se. *, P < 0.01; **, P < 0.001. B, Comparison of the weight of FTC-236 cell-induced tumors after treatment of 5′-aza-CdR (n = 6) or vehicle control (n = 6) at study endpoint. The data are represented as mean ± se. The differences are significant (P < 0.001).
Discussion
The reduced expression resulting from hypermethylation, deletion, or truncation of the THRB gene in human cancers suggests that TRβ could act as a tumor suppressor (8–13). A close association of somatic mutations of the THRB gene with several types of human cancer supports the hypothesis that loss of the normal suppressor functions of TRβ could lead to increased cell proliferation and aberrant growth (3–6). The fact that mice deficient in total functional TRs (Thra1−/−Thrb−/− mice) or with a targeted homozygous mutation of the Thrb gene (ThrbPV/PV mice) spontaneously develop metastatic thyroid carcinoma provides strong evidence of the critical roles of TRs in human cancer (7, 32).
In the present study, we first showed the correlation between reduced mRNA expression of the THRB gene and progression of DTC. We also found evidence showing that promoter methylation is one of the key regulatory mechanisms in the repression of THRB expression in DTC. Cell-based studies further showed that the demethylating agents 5′-aza-CdR and zebularine induced THRB reexpression, leading to the inhibition of cancer cell proliferation and migration. Importantly, when TRβ was stably expressed in FTC-236 cells, the cancer phenotype was markedly lessened. The cell-based findings were further validated by the observations of in vivo xenograft models. Thus, the present study supports the notion that TRβ can act as a tumor suppressor in thyroid carcinogenesis.
DNA methylation plays critical roles in the regulation of normal physiology during embryogenesis and cell division, but this process is also involved in the initiation and progression of cancer as well as in genetic abnormalities. During the past two decades, numerous drugs targeting DNA methylation have been developed to increase the efficacy and stability and to decrease the toxicity of cancer treatments (33). At present, 5′-aza-CdR (decitabine) is approved for the treatment of myelodysplastic syndromes (33). It is currently under investigation in a phase II clinical trial of metastatic, refractory DTC (NCT00085293). However, the toxicity and a short in vivo half-life (7–35 min) of 5′-aza-CdR could pose major obstacles to this drug's use in clinical settings (33, 35). Zebularine [1-(βd-ribofuranosyl)-1,2-dihydropyrimidin-2-1] is a new cytidine analog that forms a covalent complex with DNA methyltransferases, selectively inhibits human DNA methyltransferase 1 and has shown a preferential response in cancer cells (35, 36). However, little information is currently available for the effect of zebularine on thyroid cancer cells. In the present study, we showed that zebularine was as effective as 5′aza-CdR in reactivation of the expression of the THRB gene, leading to decreasing cell proliferation and motility (Fig. 3). It also exhibited similar molecular actions in dampening the β-catenin signaling pathway to decrease cell proliferation. These preclinical data could be useful in paving the way for further investigations of the benefits of zebularine, a less toxic methylating agent, on the treatment of DTC.
5′-Aza-CdR has been used in the redifferentiation of thyroid carcinoma cell lines by reexpression of sodium iodide symporter, thyroid transcription factor-1, thyroglobulin, and retinoic acid receptor β2 (21, 22). The present study shows that 5′-aza-CdR and a newer demethylating agent, zebularine, can reactivate the expression of the THRB gene. It is not clear whether different demethylating agents have different selectivity toward specific target genes or whether this class of demethylating agents has general effects on most target genes. However, the present findings that reactivating the expression of the THRB gene by 5′-aza-CdR and zebularine lessened the cancer phenotype by decreasing cell proliferation and motility suggest that demethylating agents are promising candidates in the treatment of advanced DTC.
Acknowledgments
We thank Dr. Francesco S. Celi of National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, for some of the cancer tissues used in the present studies.
This work was supported by the Intramural Research Program at the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- 5′-Aza-CdR
- 5′-Aza-2′-deoxycytidine
- DTC
- differentiated thyroid cancer
- FH
- Flag-hemagglutinin
- FTC
- follicular thyroid carcinoma
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- PARP
- poly-(adenosine-diphosphate ribose)
- p-β-catenin
- phosphorylated β-catenin
- TR
- thyroid hormone receptor.
References
- 1. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 2. Cheng SY, Leonard JL, Davis PJ. 2010. Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin KH, Shieh HY, Chen SL, Hsu HC. 1999. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol Carcinog 26:53–61 [DOI] [PubMed] [Google Scholar]
- 4. Ando S, Sarlis NJ, Oldfield EH, Yen PM. 2001. Somatic mutation of TRβ can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. J Clin Endocrinol Metab 86:5572–5576 [DOI] [PubMed] [Google Scholar]
- 5. Kamiya Y, Puzianowska-Kuznicka M, McPhie P, Nauman J, Cheng SY, Nauman A. 2002. Expression of mutant thyroid hormone nuclear receptors is associated with human renal clear cell carcinoma. Carcinogenesis 23:25–33 [DOI] [PubMed] [Google Scholar]
- 6. Silva JM, Domínguez G, González-Sancho JM, García JM, Silva J, García-Andrade C, Navarro A, Muñoz A, Bonilla F. 2002. Expression of thyroid hormone receptor/erbA genes is altered in human breast cancer. Oncogene 21:4307–4316 [DOI] [PubMed] [Google Scholar]
- 7. Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S. 2000. Mice with a targeted mutation in the thyroid hormone β receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA 97:13209–13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Meng ZH, Chandrasekaran R, Kuo WL, Collins CC, Gray JW, Dairkee SH. 2002. Biallelic inactivation of the thyroid hormone receptor β1 gene in early stage breast cancer. Cancer Res 62:1939–1943 [PubMed] [Google Scholar]
- 9. Iwasaki Y, Sunaga N, Tomizawa Y, Imai H, Iijima H, Yanagitani N, Horiguchi K, Yamada M, Mori M. 2010. Epigenetic inactivation of the thyroid hormone receptor β1 gene at 3p24.2 in lung cancer. Ann Surg Oncol 17:2222–2228 [DOI] [PubMed] [Google Scholar]
- 10. Ling Y, Xu X, Hao J, Ling X, Du X, Liu X, Zhao X. 2010. Aberrant methylation of the THRB gene in tissue and plasma of breast cancer patients. Cancer Genet Cytogenet 196:140–145 [DOI] [PubMed] [Google Scholar]
- 11. Joseph B, Ji M, Liu D, Hou P, Xing M. 2007. Lack of mutations in the thyroid hormone receptor (TR) α and β genes but frequent hypermethylation of the TRβ gene in differentiated thyroid tumors. J Clin Endocrinol Metab 92:4766–4770 [DOI] [PubMed] [Google Scholar]
- 12. Hörkkö TT, Tuppurainen K, George SM, Jernvall P, Karttunen TJ, Mäkinen MJ. 2006. Thyroid hormone receptor β1 in normal colon and colorectal cancer-association with differentiation, polypoid growth type and K-ras mutations. Int J Cancer 118:1653–1659 [DOI] [PubMed] [Google Scholar]
- 13. Dunwell TL, Hesson LB, Pavlova T, Zabarovska V, Kashuba V, Catchpoole D, Chiaramonte R, Brini AT, Griffiths M, Maher ER, Zabarovsky E, Latif F. 2009. Epigenetic analysis of childhood acute lymphoblastic leukemia. Epigenetics 4:185–193 [DOI] [PubMed] [Google Scholar]
- 14. Jazdzewski K, Boguslawska J, Jendrzejewski J, Liyanarachchi S, Pachucki J, Wardyn KA, Nauman A, de la Chapelle A. 2011. Thyroid hormone receptor β (THRB) is a major target gene for microRNAs deregulated in papillary thyroid carcinoma (PTC). J Clin Endocrinol Metab 96:E546–E553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y. 2009. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 20:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kondo T, Ezzat S, Asa SL. 2006. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 6:292–306 [DOI] [PubMed] [Google Scholar]
- 17. Russo D, Damante G, Puxeddu E, Durante C, Filetti S. 2011. Epigenetics of thyroid cancer and novel therapeutic targets. J Mol Endocrinol 46:R73–R81 [DOI] [PubMed] [Google Scholar]
- 18. Sigalotti L, Fratta E, Coral S, Cortini E, Covre A, Nicolay HJ, Anzalone L, Pezzani L, Di Giacomo AM, Fonsatti E, Colizzi F, Altomonte M, Calabrò L, Maio M. 2007. Epigenetic drugs as pleiotropic agents in cancer treatment: biomolecular aspects and clinical applications. J Cell Physiol 212:330–344 [DOI] [PubMed] [Google Scholar]
- 19. Kondo T, Nakazawa T, Ma D, Niu D, Mochizuki K, Kawasaki T, Nakamura N, Yamane T, Kobayashi M, Katoh R. 2009. Epigenetic silencing of TTF-1/NKX2–1 through DNA hypermethylation and histone H3 modulation in thyroid carcinomas. Lab Invest 89:791–799 [DOI] [PubMed] [Google Scholar]
- 20. Zuo H, Gandhi M, Edreira MM, Hochbaum D, Nimgaonkar VL, Zhang P, Dipaola J, Evdokimova V, Altschuler DL, Nikiforov YE. 2010. Downregulation of Rap1GAP through epigenetic silencing and loss of heterozygosity promotes invasion and progression of thyroid tumors. Cancer Res 70:1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vivaldi A, Miasaki FY, Ciampi R, Agate L, Collecchi P, Capodanno A, Pinchera A, Elisei R. 2009. Re-differentiation of thyroid carcinoma cell lines treated with 5-aza-2′-deoxycytidine and retinoic acid. Mol Cell Endocrinol 307:142–148 [DOI] [PubMed] [Google Scholar]
- 22. Miasaki FY, Vivaldi A, Ciampi R, Agate L, Collecchi P, Capodanno A, Pinchera A, Elisei R. 2008. Retinoic acid receptor β2 reexpression and growth inhibition in thyroid carcinoma cell lines after 5-aza-2′-deoxycytidine treatment. J Endocrinol Invest 31:724–730 [DOI] [PubMed] [Google Scholar]
- 23. Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, Willingham MC, Cheng SY. 2005. An unliganded thyroid hormone β receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol 25:124–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhat MK, McPhie P, Cheng SY. 1995. Interaction of thyroid hormone nuclear receptor with antibody: characterization of the thyroid hormone binding site. Biochem Biophys Res Commun 210:464–471 [DOI] [PubMed] [Google Scholar]
- 25. Martinez E, Palhan VB, Tjernberg A, Lymar ES, Gamper AM, Kundu TK, Chait BT, Roeder RG. 2001. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol Cell Biol 21:6782–6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ying H, Furuya F, Zhao L, Araki O, West BL, Hanover JA, Willingham MC, Cheng SY. 2006. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone β receptor inhibits mitotic progression. J Clin Invest 116:2972–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim WG, Guigon CJ, Fozzatti L, Park JW, Lu C, Willingham MC, Cheng SY. 2012. SKI-606, an Src inhibitor, reduces tumor growth, invasion, and distant metastasis in a mouse model of thyroid cancer. Clin Cancer Res 18:1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ambroziak M, Pachucki J, Stachlewska-Nasfeter E, Nauman J, Nauman A. 2005. Disturbed expression of type 1 and type 2 iodothyronine deiodinase as well as titf1/nkx2-1 and pax-8 transcription factor genes in papillary thyroid cancer. Thyroid 15:1137–1146 [DOI] [PubMed] [Google Scholar]
- 29. Polakis P. 2007. The many ways of Wnt in cancer. Curr Opin Genet Dev 17:45–51 [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Rostan G, Tallini G, Herrero A, D'Aquila TG, Carcangiu ML, Rimm DL. 1999. Frequent mutation and nuclear localization of β-catenin in anaplastic thyroid carcinoma. Cancer Res 59:1811–1815 [PubMed] [Google Scholar]
- 31. Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng SY. 2008. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol Cell Biol 28:4598–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu XG, Zhao L, Willingham MC, Cheng SY. 2010. Thyroid hormone receptors are tumor suppressors in a mouse model of metastatic follicular thyroid carcinoma. Oncogene 29:1909–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang X, Lay F, Han H, Jones PA. 2010. Targeting DNA methylation for epigenetic therapy. Trends Pharmacol Sci 31:536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Groeningen CJ, Leyva A, O'Brien AM, Gall HE, Pinedo HM. 1986. Phase I and pharmacokinetic study of 5-aza-2′-deoxycytidine (NSC 127716) in cancer patients. Cancer Res 46:4831–4836 [PubMed] [Google Scholar]
- 35. Cheng JC, Yoo CB, Weisenberger DJ, Chuang J, Wozniak C, Liang G, Marquez VE, Greer S, Orntoft TF, Thykjaer T, Jones PA. 2004. Preferential response of cancer cells to zebularine. Cancer Cell 6:151–158 [DOI] [PubMed] [Google Scholar]
- 36. Zhou L, Cheng X, Connolly BA, Dickman MJ, Hurd PJ, Hornby DP. 2002. Zebularine: a novel DNA methylation inhibitor that forms a covalent complex with DNA methyltransferases. J Mol Biol 321:591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]