Abstract
Glucosensing nodose ganglia neurons mediate the effects of hyperglycemia on gastrointestinal motility. We hypothesized that the glucose-sensing mechanisms in the nodose ganglia are similar to those of hypothalamic glucose excited neurons, which sense glucose through glycolysis. Glucose metabolism leads to ATP-sensitive potassium channel (KATP) channel closure and membrane depolarization. We identified glucosensing elements in the form of glucose transporters (GLUTs), glucokinase (GK), and KATP channels in rat nodose ganglia and evaluated their physiological significance. In vitro stomach-vagus nerve preparations demonstrated the gastric vagal afferent response to elevated glucose. Western blots and RT-PCR revealed the presence of GLUT1, GLUT3, GLUT4, GK, and Kir6.2 in nodose ganglia neurons and gastric branches of the vagus nerve. Immunocytochemistry confirmed the expression of GLUT3, GK, and Kir6.2 in nodose ganglia neurons (46.3 ± 3%). Patch-clamp studies detected glucose excitation in 30% (25 of 83) of gastric-projecting nodose ganglia neurons, which was abolished by GLUT3 or GK short hairpin RNA transfections. Silencing GLUT1 or GLUT4 in nodose ganglia neurons did not prevent the excitatory response to glucose. Elevated glucose elicited a response from 43% of in vitro nerve preparations. A dose-dependent response was observed, reaching maximum at a glucose level of 250 mg/dl. The gastric vagal afferent responses to glucose were inhibited by diazoxide, a KATP channel opener. In conclusion, a subset of neurons in the nodose ganglia and gastric vagal afferents are glucoresponsive. Glucosensing requires a GLUT, GK, and KATP channels. These elements are transported axonally to the gastric vagal afferents, which can be activated by elevated glucose through modulation of KATP channels.
Hyperglycemia significantly affects gastrointestinal motility. Bulatao and Carlson (1) discovered that hyperglycemia inhibited hunger contractions in fasted dogs, and conversely, insulin-induced hypoglycemia stimulated gastric motility. Similar observations have been made in humans. Barnett and Owyang (2) showed that acute hyperglycemia inhibited the gastric interdigestive migrating motor complex in healthy volunteers. During a 3-h hyperglycemia period, gastric contractions were completely inhibited at the serum glucose level of 250 mg/dl and significantly reduced at 175 and 140 mg/dl.
Little is known about the site(s) and mechanism(s) of action by which hyperglycemia modulates gastric motility. We have shown that hyperglycemia stimulates vagal afferent nerves, which act by way of the brainstem to stimulate the vagal efferent cholinergic pathway, synapsing with intragastric nitric oxide-containing neurons to mediate gastric relaxation (3). In vitro intracellular recording confirmed the presence of glucose-excited neurons in rat nodose ganglia (4). In these neurons, silencing Kir6.2 subunit expression blocks the depolarizing effects of hyperglycemia, indicating that the ATP-sensitive potassium channel (KATP) plays a critical role in the glucose-excitatory response (4). Furthermore, in vivo studies showed that inhibition of gastric motility by hyperglycemia is mediated by glucose-excited neurons in the nodose ganglia, which contain KATP channels (5). It is not clear how these neurons sense peripheral glucose. Previous studies report that glucose-responsive neurons in the hypothalamus sense glucose through glycolysis using a mechanism similar to that used by the pancreatic β-cells (6). We hypothesized that glucose-sensing mechanisms in the nodose are similar to those of hypothalamic glucose excited neurons. Unlike most neurons, glucosensing neurons in the nodose ganglia use glucose as a signaling molecule to regulate membrane potential. Glucose transporters (GLUTs) transport glucose into the neurons, in which it is metabolized by glucokinase (GK). This, in turn, raises the ATP to ADP ratio and inactivates KATP channels, leading to membrane depolarization. Glucose transport and glycolysis are therefore major regulators of glucosensing in the nodose ganglia.
We further hypothesized that changes in ambient glucose concentrations at vagal afferent terminals in the gastrointestinal mucosa are sufficient to inactivate KATP channels, leading to neuronal excitation. We tested these hypotheses with Western blots and immunocytochemistry to show the presence of GLUTs, GK, and KATP channels in the nodose ganglia. Western blot analysis of vagal trunks showed that these protein molecules are transported to the peripheral vagal afferents. Patch-clamp recordings using pharmacological tools and small inhibitory RNA (siRNA) technology revealed the physiological significance of GLUTs, GK, and KATP channels. Finally, electrophysiological recordings of in vitro isolated stomach-vagus nerve preparations demonstrated the response of gastric vagal afferents to changes in glucose concentration.
Materials and Methods
Animal preparation
All experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan, in compliance with National Institutes of Health guidelines. Male Sprague Dawley rats (250–300 g) were anesthetized with ketamine (250 mg/kg) and xylazine (25 mg/kg) (4). Nodose ganglia and the gastric branch of the vagus nerve were dissected as previously described (7) for Western blots, RT-PCR, immunohistochemistry, and electrophysiology. The segments of the ventral and dorsal gastric branch of the vagus nerve were exposed and isolated from the esophagus at the gastroesophageal junction.
Western blot analysis
Nodose ganglia and the vagus nerves were obtained from six rats for Western blots (4). Tissues were lysed and centrifuged at 14,000 × g for 10 min at 4 C. Protein samples (20–40 μg) were separated on Ready Gel 12% Tris-HCl (Bio-Rad, Hercules, CA) for 60 min at 80 V and transferred to nitrocellulose Hybond enhanced chemiluminescence membranes (GE Healthcare UK Limited, Buckinghamshire, UK) for 60 min at 80 V. The membranes were washed once in Tris-buffered saline with 0.1% Tween 20 and then blocked with StartingBlock T20 buffer (Thermo Scientific, Rockford, IL) for 1 h at room temperature. The membranes were probed with antibodies GLUT1 (Millipore, Temecula, CA), GLUT2 (Millipore; Alpha Diagnostic, San Antonio, TX), GLUT3 (Santa Cruz Biotechnology, Santa Cruz, CA), GLUT4 (α Diagnostic), GK (Abcam, Cambridge, MA) and Kir6.2 (LifeSpan BioSciences, Seattle, WA) at 1:500–1000 dilution in the blocking buffer and incubated overnight at 4 C. The membranes were washed three times in Tris-buffered saline with 0.1% Tween 20 within 5 min and incubated with the corresponding secondary antibodies in the blocking buffer for 1 h at room temperature. The membranes were exposed to enhanced chemiluminescence buffer for 1 min and then high-performance chemiluminescence film in the dark. The resulting bands were scanned and the density of the bands was analyzed using the ImageJ program (National Institutes of Health, Bethesda, MD).
RT-PCR
RT-PCR was performed as previously described (5). Total RNA was extracted from the nodose ganglia using TRIzol (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Reverse transcription was performed using 5 μg of total RNA. The resultant cDNAs were used for PCR with primer sets targeting GLUT1–4, GK (pancreatic form), sulfonylurea receptor (SUR1), and Kir6.2 are shown in Table 1. Glyceraldehyde-3-phosphate dehydrogenase served as an internal control. PCR was performed with Taq DNA polymerase (Promega, Madison, WI) through 30 cycles of denaturation (30 sec at 94 C), annealing (30 sec at 50 C), and extension (30 sec at 72 C), followed by final extension (10 min at 72 C). The PCR products were loaded in a 1.2% Tris-borate-EDTA-buffered agarose gel, and the bands were visualized after gel electrophoresis by ethidium bromide staining and UV light illumination. The resulting bands were scanned with an Epson Stylus Photo R2400 (Long Beach, CA) and analyzed using ImageJ [National Institutes of Health (NIH)].
Table 1.
Primer sequences and GenBank accession numbers
| Gene | Direction | Sequence (5′–3′) | GenBank accession no |
|---|---|---|---|
| GLUT1 | Sense | CCGCTTCCTGCTCATCAATCG | NM_138827 |
| Antisense | GCCACGATACTCAGATAGGAC | ||
| GLUT2 | Sense | GGCGGAATGGTCGCCTCGTTC | NM_012879 |
| Antisense | GCAGATAGGCCAAGTAGGATGTG | ||
| GLUT3 | Sense | CCGGATGTGATCCAGGAGATC | NM_017102 |
| Antisense | GACCAAGATAGCCACAATACAG | ||
| GLUT4 | Sense | CACTCAACCAATTGGCCATCG | NM_012751 |
| Antisense | ACACATCAGCCCAGCCTGTCAGG | ||
| GK | Sense | GATGCAGAAGGAGATGGAC | M25807 |
| Antisense | GGTTCCTCCCAGGTCTAAG | ||
| SUR1 | Sense | CACATTCACCACAGCACCTG | NM_013039 |
| Antisense | CCAGCTGGCATGTATAAGTG | ||
| Kir6.2 | Sense | AGACCACCAGCCCGGAGGGCG | NM_031358 |
| Antisense | GGGCACTTTAACGGTGTTCCC | ||
| GAPDH | Sense | CACCACCATGGAGAAGGCTGG | AF106860 |
| Antisense | ATGGCATGGACTGTGGTCATG |
GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
Immunocytochemistry
Nodose ganglia were removed and placed in 4% paraformaldehyde for 2 h at room temperature and then in 25% sucrose in PBS overnight at 4 C. Longitudinal sections (10 μm) were mounted on gelatin-coated slides (4, 6) and stored at −70 C.
Single, double, or triple staining was performed using primary antibodies against Kir6.2 (1:500; LifeSpan Biosciences), GK (1:200, Abcam), and GLUT1 and GLUT2 (1:200; Santa Cruz Biotechnology). GLUT3 antibody (1:100; R&D Systems, Minneapolis, MN) is a mouse monoclonal antibody specific for human GLUT3, but it does not cross-react with GLUT1, GLUT2, or GLUT4. After incubation in a humid chamber for 16–48 h, sections were washed in PBS and incubated for 1 h at room temperature in species-specific fluorophore-conjugated secondary antibodies Cy3, aminomethylcoumarin acetate (both from Jackson ImmunoResearch Laboratories, West Grove, PA), and Alexa Fluor 488 (Molecular Probes, Life Technologies, Carlsbad, CA). Sections were rinsed in buffer, coverslipped with antifading gel mount (BioMeda, Foster City, CA), and examined with an Olympus BX-51 microscope (Tokyo, Japan) or a Zeiss KS400 LSM confocal laser scanning microscope (Thornwood, NY).
The immunohistochemical specificity was verified either by omitting one of the primary antibodies and the secondary antibodies or by adding blocking peptides.
Retrograde tracing of nodose ganglia
Sprague Dawley rats (age 2–4 wk) were deeply anesthetized with a 4% mixture of halothane in air, as previously described (8). After laparotomy, the retrograde tracer DiI (Molecular Probes) was applied to the gastric corpus. The wound was closed and the animals recovered for 15 d before being killed for nodose ganglia dissection.
Isolation and culture of nodose ganglia neurons
Nodose ganglia were dissected and placed in a 35-mm culture dish containing Ca2+- and Mg2+-free Hanks' balanced salt solution with penicillin and streptomycin, as previously described (4). Fragments of desheathed ganglia were placed in a 1.5-ml centrifuge tube containing digestion buffer. After a 60-min incubation at 37 C, cells were dispersed and washed in DMEM. The cells were resuspended in L-15 medium (Life Technologies) containing 10% fetal bovine serum, plated on poly-l-lysine-coated (100 μg/ml) coverslips for 30 min, and cultured in DMEM with 10% fetal calf serum at 37 C. Neurons were stuck to coverslips and cultured for 24–48 h at 37 C before recording.
Patch-clamp electrophysiology
Recordings were made from DiI retrograde-labeled nodose ganglia neurons identified using a Nikon E600 FN microscope (Tokyo, Japan) equipped with tetramethylrhodamine isothiocyanate epifluorescence filters. Measurements were made in a physiological saline solution comprising (in millimoles): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, 10 mannitol, and 10 HEPES (pH adjusted to 7.3 with NaOH). Mannitol was used to adjust the osmolarity of the solution, depending on the glucose concentration (5 or 10 mm). Whole-cell recordings were performed using borosilicate glass electrodes (resistance 3–6 mΩ) backfilled with a saline solution comprising (in millimoles): 130 potassium gluconate, 10 HEPES, 10 EGTA, 1.0 MgCl2, 2.5 CaCl2, 1.0 ATP, and 0.3 GTP. Current and voltage recordings were obtained from discrete nodose ganglia neurons using an Axopatch 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) filtered by a four-pole, low-pass Bessel filter at 2 kHz. The signals were digitized, stored, and analyzed by pCLAMP 9 software (Molecular Devices).
Transfection of nodose ganglia with GK and GLUT1, GLUT3, and GLUT4 short hairpin RNAs (shRNAs)
Construction of pLentilox3.7 shRNAs
shRNA sequences were designed with GK (M25807) and GLUT3 (NM_017102) nucleotides flanked with blunt T/A bp on the 5′ end and an XhoI sticky end on the 3′ end.
GK sense strand
The GK sense strand was tGCTACTATGAAGACCGCCAATttcaagagaATTGGCGGTCTTCATAGTAGCttttttc.
GK antisense strand
The GK antisense strand was aCGATGATACTTCTGGCGGTTAaagttctctTAACCGCCAGAAGTATCATCGaaaaaagagct.
GLUT3 sense strand
The GLUT3 sense strand was tGCCATGAGCTTTGTCTGTATTttcaagagaAATACAGACAAAGCTCATGGCttttttc.
GLUT3 antisense strand
The GLUT3 antisense strand was aCGGTACTCGAAACAGACATAAaagttctctTTATGTCTGTTTCGAGTACCGaaaaaagagct (Integrated DNA Technologies, Coralville, IA). Sense and antisense strands were generated by oligonucleotide synthesis with 5′-phosphate and PAGE purification. To introduce RNA interference stem-loop, complementary strands of GK and GLUT3 sequences were annealed and inserted into the HpaI and XhoI sites of shRNA-expressing vector pLL3.7, which was contained in the mouse U6 promoter upstream of a CMV-EGFP expression cassette (9). The following plasmids were used to construct the shRNA for GLUT1 and GLUT4: GLUT 1 sense strand, tCCTCTTTGTTAATCGCTTTttcaagagaAAAGCGATTAACAAAGAGGttttttc; GLUT1 antisense strand, tAAAGCGATTAACAAAGAGGttcaagagaCCTCTTTGTTAATCGCTTTttttttc; GLUT 4 sense strand, tCCTTCAGTTTGGCTATAACttcaagagaGTTATAGCCAAACTGAAGGttttttc; and GLUT 4 antisense strand, tGTTATAGCCTTTCTGAAGGttcaagagaCCTTCAGAAAGGCTATAACttttttc.
Generation of lentiviral vectors
pLL3.7-GK or pLL3.7-GLUT1, -GLUT3, or -GLUT4 shRNA plasmids and packaging plasmids (pMLDg/pRRE, pRSV-Rev, pCI-VSVG) were transfected into 293T cells by standard calcium phosphate precipitation methods. The supernatant was collected after 72 h and pelleted by centrifugation at 12,300 × g in a Beckman JA-17 rotor (Beckman Coulter, Fullerton, CA). The viral pellet was resuspended in DMEM at 10 times the original concentration. The resulting shRNA lentiviruses were quality tested in A549 cells. The lenti-shRNAs were shown to transduce greater than 60% of A549 cells, as determined by fluorescence-activated cell sorter analysis, which measures the enhanced green fluorescent protein (EGFP) marker in the recombinant lentiviral genome. siRNA-A (Santa Cruz Biotechnology) was used as the control.
Cell infection
Primary nodose ganglia neuronal cultures were supplemented with 20–100 × 106 transducing units of lenti-GK or lenti-GLUT1, -GLUT3, or -GLUT4 shRNAs or scrambled siRNA viral particles at 37 C. After 3 h, media containing lentiviral particles were aspirated and replaced with DMEM/F-12 containing 100 mg/dl glucose and 10% fetal bovine serum supplemented with gentamicine (100 U/ml).
In vitro stomach-vagus nerve preparations
Sprague Dawley male rats (250–300 g) were anesthetized with urethane (1.5 g/kg, ip). After laparotomy, an in vitro stomach-ventral gastric vagus nerve preparation was isolated, as previously described (10, 11), and pinned on the Sylgard-coated chamber of an organ bath. The bath was perfused with oxygenated modified Ringer solution (33 ± 1 C, 2.0–2.5 mL/min). The left gastric artery was cannulated with polyethylene tubing (PE-10) for intraarterial injection. A thin strand (∼10 μm) was teased from the ventral gastric vagus nerve and recordings were made from the distal cut end of the nerve. Single-fiber unit activity was recorded under euglycemic and hyperglycemic conditions.
Electrical recording of the isolated nerve strand was continued for 30 min to ensure a stable recording. Glucose and other chemicals were administered through a catheter into the cannulated gastric artery. Glucose (100, 150, 200, 250, and 300 mg/dl) was administered over 20 sec. To show that glucose must be metabolized to stimulate glucose-excited neurons, we examined the effect of 2-deoxyglucose (50, 100, and 250 mg/kg, 0.5 ml) (12).
In response to an adequate stimulus, an action potential with a unique waveform and amplitude presented in an all-or-none manner was considered to be a unit action potential traveling along a single fiber (10). Thin nerve strands (approximately one third the diameter of the recording electrode) usually display three or more unit action potentials of different amplitudes. Unit action potentials were recorded online on a digital tape recorder and simultaneously logged to a personal computer equipped with an A/D board for off-line software processing. The average of a 5-min firing rate was used to represent basal activity. Data consisted of a 5-min total spike count before (baseline) and after each treatment, normalized by dividing the latter by the former. The quotient Q represented the response magnitude; whereby Q greater than 1 indicated that the treatment caused an excitatory effect, Q less than 1 indicated an inhibitory effect, and Q close to 1 indicated no effect.
Analysis of data
Results were expressed as means ± se. Statistical analysis was performed using one-way ANOVA, followed by the Kruskal-Wallis test or the Student t test, depending on the study design. Significance was accepted at the level of P < 0.05.
Results
Expression of glucosensing elements in nodose ganglia neurons and vagal trunks
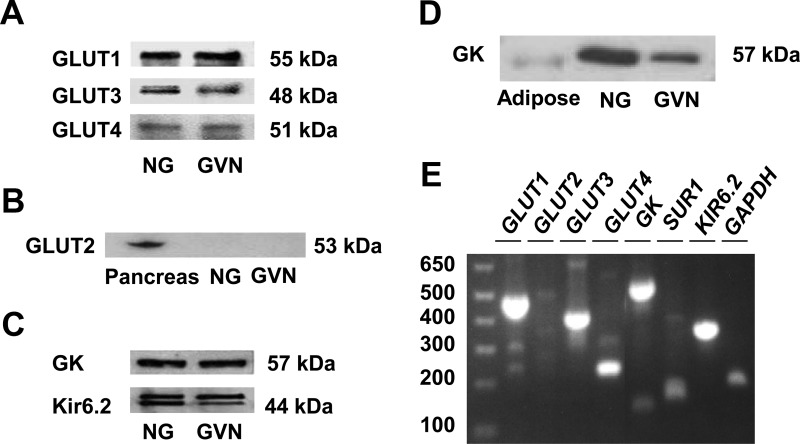
Western blots (Fig. 1, A–D) and RT-PCR (Fig. 1E) revealed that GLUT1, GLUT3, and GLUT4 were the predominant GLUT proteins and genes. GLUT2 was not detected. As expected, the nodose ganglia also expressed GK, Kir6.2, and SUR1 proteins and genes. As a control, GK was not detected in the mesenteric adipose tissue, which is devoid of GK, suggesting the specificity of the antibody (Fig. 1D). Western blots showed that GLUT1, GLUT3, and GLUT4 as well as GK and Kir 6.2 were present in the gastric branch of the vagal trunks (Fig. 1), confirming that these glucosensing elements are transported axonally.
Fig. 1.
Protein and gene expression of GLUT1-4, GK, and Kir6.2 in rat nodose ganglia and gastric branch of vagus nerve. A, Western blot analysis of rat nodose ganglia (NG) and gastric branch of the vagus nerve (GVN) with antibodies to GLUT1, GLUT2, GLUT3, and GLUT4. Representative gels show that the nodose ganglia and the gastric branch of the vagus nerve primarily expressed GLUT1, GLUT3, and GLUT4. B, GLUT2 was present in the pancreas but not detectable in the nodose ganglia or the gastric vagal nerve. C, Protein expression of GK and Kir6.2 immunoreactivity in the nodose ganglia and gastric branch of vagus nerve. Gel presented is representative of n = 5. D, The GK antibody failed to detect any significant amount of GK in the mesenteric adipose tissue, which is devoid of GK, suggesting specificity of the antibody. E, RT-PCR analysis of GLUT1-4, GK, SUR1, Kir6.2 mRNA in the rat nodose ganglia. mRNA expression was analyzed using PCR primers (see Table 1) designed for GLUT1, GLUT2, GLUT3, and GLUT4, and GK, SUR1, and Kir6.2. Gel presented is representative of n = 6. Note that GLUT2 mRNA was barely detected in the nodose ganglia.
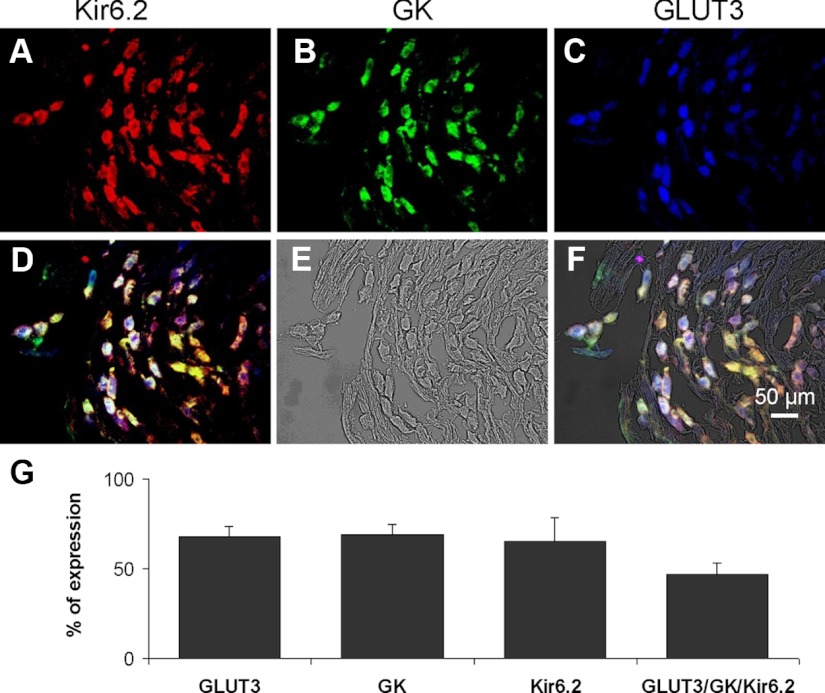
We speculated that GLUT3 is the principal GLUT used by glucose-excited neurons because of its high capacity and high affinity for glucose (13, 14). Triple immunofluorescence studies of histological sections of nodose ganglia clarified GLUT3 distribution in relation to Kir6.2 and GK (Fig. 2). Kir6.2, GK, and GLUT3 immunoreactivity (IR) varied from weak to strong and was observed throughout the nodose ganglia, and 84.3 ± 5.7% of total neurons were immunoreactive to Kir6.2, GK, or GLUT3 antibodies. These neurons were intermixed with nonimmunoreactive cells, which were 14.0 ± 5.5% of the total counted neurons in the nodose ganglia. Among the total nodose ganglia neurons, 65.5 ± 6.3, 68.7 ± 6.5, and 67.8 ± 12.8% expressed Kir6.2-IR, GK-IR, and GLUT3-IR, respectively (Fig. 2); 46.3 ± 3% of the total population of nodose ganglia neurons expressed all three glucose-sensing elements: Kir6.2, GK, and GLUT3 (Fig. 2).
Fig. 2.
Immunoreactivity of glucosensing elements Kir6.2, GK, and GLUT3 in rat nodose ganglia. Upper panel, Triple immunofluorescence studies demonstrated the distribution of Kir6.2-, GK-, and GLUT3-IR in the rat nodose ganglia. A, Immunostaining of Kir6.2, a subunit of the KATP channel in red (Cy3). B, Immunostaining of GK in green (Alexa Fluor 488). C, Immunostaining of GLUT3 in blue (aminomethylcoumarin acetate). D, Triple immunohistochemical staining shows Kir6.2-, GK-, and GLUT3-IR in many nodose ganglia neurons. E, Nomarski differential interference contrast image of the same section of the nodose ganglia. F, Superimposed triple immunostaining of Kir6.2, GK, and GLUT3 with the phase-contrast image. Calibration bar, 50 μm. Lower panel, Histogram shows the percentage of the total number of nodose ganglion neurons that exhibited immunoreactivities for GLUT3, GK, and Kir6.2. G, Percentage of nodose ganglia neurons that expressed only GLUT3, GK, or Kir6.2 (n = 5). Triple immunofluorescence studies indicated that 46% of the nodose ganglia neurons contain all the three glucosensing elements: GLUT3, GK, and Kir6.2.
Effects of silencing GK and GLUT3 on glucose-excited nodose ganglia neurons
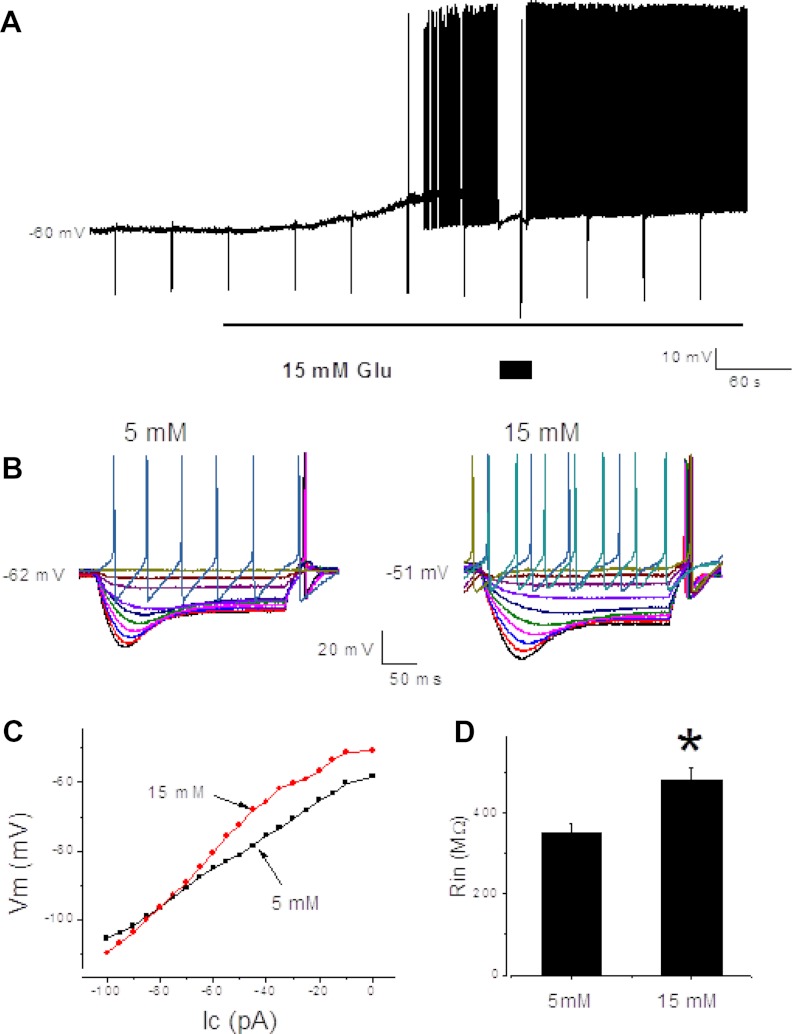
Whole-cell patch-clamp recordings were performed on retrograde-labeled nodose ganglia neurons whose projections to the stomach had been established using the retrograde fluorescent tracer DiI. Whole-cell recordings were obtained from 83 nodose ganglia neurons innervating the gastric corpus. In general, the glucose-excited neurons were quiescent under basal conditions. Spontaneous activity was observed in 31 of 83 neurons. Resting membrane potentials were between −54 and −67 mV. Overshooting action potential amplitudes ranged from 80 to 120 mV. Twenty-five of 83 neurons (30%) were glucose excited; the neuronal membrane potential was depolarized by increasing the extracellular glucose concentration from 5 to 15 mm, and this was associated with an increase in membrane input resistance. Current-voltage relationships from these neurons showed an increase in extracellular glucose concentration and increased the slope of the relationship; the effect reversed at approximately 105 mV, close to the estimated K+ equilibrium potential (Fig. 3).
Fig. 3.
A, Continued membrane potential recording depicts responses of a glucose-excited neuron. The depolarization in a glucose-excited neuron was associated with an increase in neuronal input resistance; the neuronal input resistance was tested every 40 sec by injecting 0.5 sec, 100 pA negative amplitude current pulses (negative membrane potential deflections). To evaluate changes in the membrane input resistance, the membrane potential was current clamped (box). B, The membrane potential hyperpolarization evoked by current pulses (from −100 to 100 pA, 10 pA step, 500 msec duration) at 5 and 15 mm extracellular glucose concentration (left panel). C, Current-voltage relationship constructed from the data shown in B demonstrates that the currents reversed approximately −105 mV, a theoretical K+ ion reversal potential for the conditions recorded. D, Summary of the data (n = 12) shows that the increase in extracellular glucose concentration significantly increased the neuronal input resistance (Rin) in the glucose excited neurons (right panel). *, P < 0.05 compared with control.
Ten of 83 neurons (12%) were glucose inhibited; an increase in extracellular glucose concentration from 5 to 15 mm hyperpolarized the membrane potential as we have reported earlier (4) (data not shown) as we focused our studies on glucose-excited neurons.
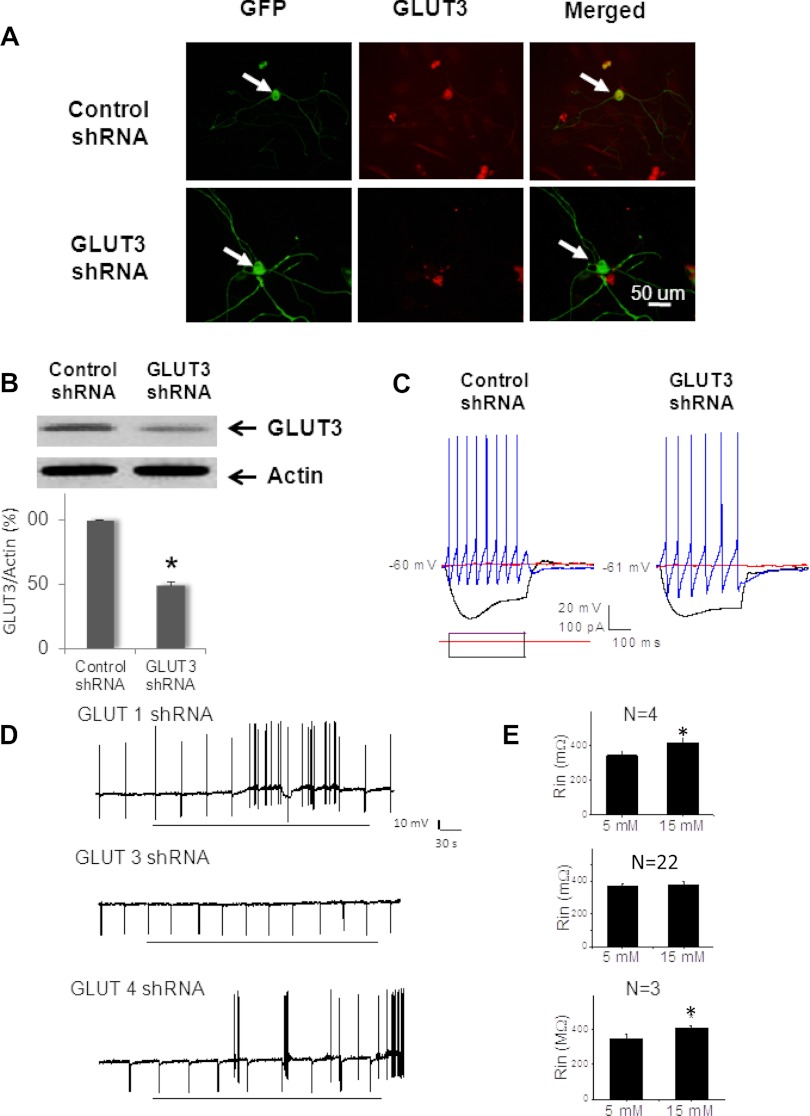
To demonstrate the role of GLUT3 in mediating glucose-evoked depolarization, primary nodose ganglia cultures were transfected with shRNA targeting GLUT3. Viral vectors are efficient gene delivery tools in mammalian cells (9). We constructed lentiviral vectors expressing GLUT3 shRNAs. For controls, we constructed lentiviral vectors expressing GLUT1 and GLUT4 shRNAs. These vectors were engendered to coexpress EGFP as a reporter gene, facilitating identification of infected cells. Western blot data showed a 61, 70, and 67% reduction in GLUT3 (Fig. 4), GLUT1, and GLUT4 protein expression in primary neuronal culture of nodose ganglia neurons infected with lentiviral vector. About 50–60% of the cultured neurons were EGFP positive and showed weak or no immunoreactivity for anti-GLUT3 (Fig. 4A). The results of anti-GLUT1 and anti-GLUT4 studies were similar (not shown). Conversely, nodose ganglia neurons transfected with scrambled siRNA were positive for both EGFP and anti-GLUT3 (Fig. 4) or anti-GLUT1 or anti-GLUT4. Thus, the lentiviral vector is capable of stably expressing transgenes that silence GLUT3, GLUT1, or GLUT4 in nodose ganglia neurons.
Fig. 4.
Lentivirus-based transfection of GLUT3 shRNA suppresses endogenous protein expression and causes the nodose ganglia neurons to lose glucose-excited properties. A, Photomicrographs of nodose ganglia neurons transfected with control lenti-shRNA (upper row) and lenti-shGLUT3 (lower row). Photomicrographs demonstrate that both lentivirus-control and -GLUT3 shRNAs transfected nodose ganglia neurons showing green EGFP staining (first column arrows). Immunostaining against GLUT3 (middle column, red Cy3 staining) revealed that the neuron transfected with lentivirus-control shRNA but not lentivirus-GLUT3 shRNA was immunoreactive for anti-GLUT3 (merged images) Bar, 50 μm. B, Western blot shows a significant reduction of GLUT3 protein 96 h after the primary nodose ganglia neuron cultures were transfected with GLUT3 shRNAs. Actin was used as a loading control (upper inset). Summary bar graph demonstrates that lentivirus-GLUT3 shRNA transfection significantly reduced the level of GLUT3 protein expression in primary cultures (49 ± 3% of control, n = 4, *, P < 0.05). C, Representative current-clamp responses recorded from lentivirus-control and -GLUT3 shRNA transfected (EGFP positive) neurons show that transfection did not change basic and firing neuronal properties, although the neurons transfected with GLUT3 shRNA lost their glucose-excited properties 5 (none of 22). D, Continuous membrane potential recordings depict responses of glucose-excited neurons after transfection with lenti-shGLUT1 (top panel), lenti-shGLUT3 (middle panel), and lenti-shGLUT3 (bottom panel). The depolarization in a glucose-excited neuron was associated with an increase in neuronal input resistance. The neuronal input resistance was tested every 40 sec by injecting 0.5 sec, 100 pA negative amplitude current pulses (negative membrane potential deflections). E, Summary of data show that the increase in extracellular glucose concentration significantly increased the neuronal input resistance (Rin) in glucose-excited neurons after transfection with lenti-shGLUT1 (n = 4) and lenti-shGLUT4 (n = 3). In contrast, after transfection with lenti-shGLUT3, none of the 20 EGFP-labeled neurons responded to elevation of ambient glucose from 5 to 15 mm. There was no change in neuronal input resistance (n = 22). *, P < 0.05 compared with the response to 5 mm.
Patch-clamp studies were performed in neurons transfected with GLUT3, GLUT1, or GLUT4 shRNAs, or with scrambled siRNA (control). None of the recorded neurons transfected with GLUT3 shRNA responded to the glucose transition from 5 to 15 mm with either excitation or inhibition (n = 22) (Fig. 4). On the other hand, three of 10 neurons transfected with scrambled siRNA responded to the glucose transition from 5 to 15 mm. This frequency of response was similar to that reported in nontransfected nodose ganglia neurons (4). For controls, we performed patch-clamp studies in neurons transfected with either GLUT1 or GLUT4 shRNA. We observed that four of 18 and three of 17 neurons transfected with GLUT1 or GLUT4 shRNA responded to the glucose transition from 5 to 15 mm (Fig. 4).
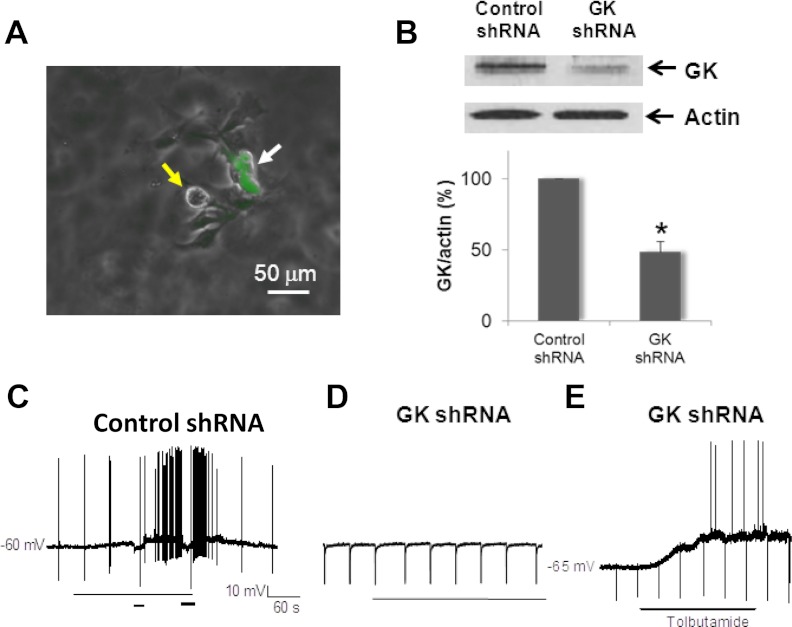
To demonstrate the role of GK in mediating glucose-evoked depolarization, we used primary nodose ganglia cultures transfected with scrambled siRNA or lentiviral vector that targeted GK mRNA expression. Western blot showed a 62% reduction in GK protein expression (Fig. 5). Only lentiviral vector-transfected EGFP-positive cells were recorded. None of the neurons transfected with shRNA for GK responded to the glucose transition from 5 to 15 mm with either excitation or inhibition (n = 25) (Fig. 5). In contrast, five of 18 of neurons transfected with scrambled siRNA maintained glucose-excited properties (Fig. 5) and two of 18 of the neurons showed inhibition (data not shown).
Fig. 5.
Lentivirus-based transfection of GK shRNA suppresses endogenous protein expression and excitatory response to elevated glucose. A, Superimposed phase-contrast and green fluorescent photomicrograhs of nodose ganglia neurons demonstrate unlabeled (yellow arrow) and EGFP-labeled (white arrow) neurons. B, Western blot demonstrates a significant reduction of GK protein 96 h after the primary nodose ganglion neuron cultures were transfected with lentivirus GK shRNAs. Actin was used as a loading control (upper inset). Summary bar graph demonstrates that lentivirus-GK shRNAs transfection significantly reduced the level of GK protein expression in primary cultures (48 ± 7% of control, n = 4). *, P < 0.05. C, Continuous membrane potential recording shows that lentivirus-control shRNA transfected neurons maintained glucose-excited properties (five of 18). D, Continuous membrane potential recording demonstrates that lentivirus-GK shRNA transfected neuron lost glucose-excited properties because it was confirmed in 25 recorded neurons. Bar marks the transition of glucose from 5 to 15 mm. E, Continuous membrane recording shows that lentivirus-GK shRNA transfection maintained responsiveness to tolbutamide (200 μm) in six of 20 neurons.
We have previously shown that neurons transfected with Kir6.2 shRNA fail to respond to the glucose transition from 5 to 15 mm or tolbutamide (4). Transfection did not affect the inhibitory response to increased concentration of glucose (4). However, six of 20 (30%) and five of 22(23%) neurons transfected with GK shRNA or GLUT3 shRNA, respectively, have a normal excitatory response to tolbutamide (200 μm) (Fig. 5), indicating that the transfected neurons were capable of an excitatory response to agents that directly modulate KATP channels. These observations, together with our previous demonstration that neurons transfected with Kir6.2 shRNA fail to respond to elevated ambient glucose (4), suggest that glucosensing requires the presence of GLUT3, GK, and KATP channels.
Effects of hyperglycemia on activities of vagal afferent fibers
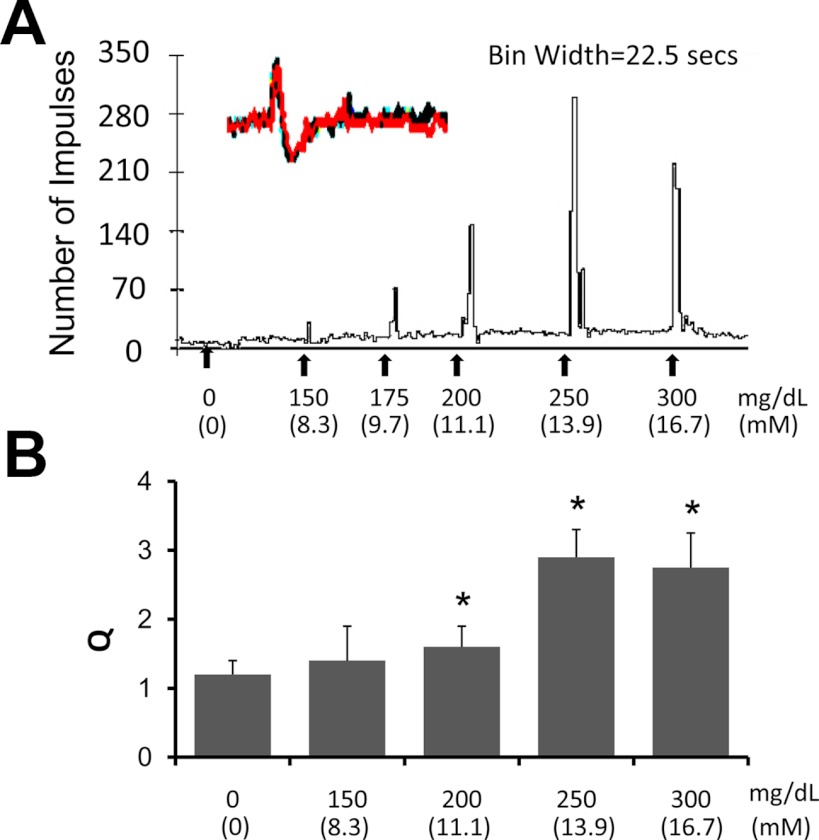
To determine whether gastric vagal afferents respond to changes in glucose concentration, in vitro isolated stomach-vagus nerve preparations were used for electrophysiological studies (10). Thin nerve trunks (∼10 μm) from the distal cut end of afferent fibers were sampled from the ventral branch of the gastric vagus nerve. From eight experiments, we examined the individual response patterns of 24 gastric vagus afferents (GVA) terminals. The basal activity ranged from 0 to 3.1 spikes/sec with an average of 1.1 spikes/sec. An example of a recording is presented in Fig. 6. The average of a 5-min total spike count before each treatment was normalized as 100% (Q = 1). Responses to intraarterial perfusion of PBS or glucose were recorded. There was no difference between basal activity and vehicle injection (PBS, Q = 1.15 ± 0.25, n = 8) (Fig. 6). Glucose perfusion (250 mg/dl, 20 sec) induced increased firing in nine of 21 U (43%). The average Q was 2.9 ± 0.4 (n = 9, P < 0.05). The dose-dependent response (150–300 mg/dl) reached maximum at 250 mg/dl (Fig. 6, A and B). There were no clear latency changes when the dose of glucose was increased from 150 to 250 mg/dl. To investigate whether the dose-related response was the result of an accumulative action of glucose sequential administration, an additional four experiments were performed. The same left gastric artery was injected with randomized doses of glucose. The same dose-related response was observed (data not shown). These findings indicate that a subset of gastric vagal afferents is glucoresponsive. In contrast to glucose, the nonmetabolizable 2-deoxyglucose (50, 100, and 250 mg/kg, 0.5 ml) elicited little response (Q = 1.05 ± 0.3, Q = 1.13 ± 0.3, and Q = 1.08 ± 0.2, respectively, n = 8, P < 0.05); all these units responded to glucose perfusion (250 mg/dl) with an average Q = 2.6 ± 0.3, n = 8, P < 0.05).
Fig. 6.
Responses of the gastric vagal afferents to changes in glucose concentration in an isolated stomach-vagus nerve preparation. A, Example of a glucose dose-response study obtained from gastric vagal afferent terminal recordings. The inset at the upper left shows the superimposed waveforms of the individual unit action potentials traced at the beginning and at the end of the recording. This ensured that the same unit was studied. B, Histogram shows a dose-response relationship with maximal response at a glucose level of 250 mg/dl. *, P < 0.05 compared with control.
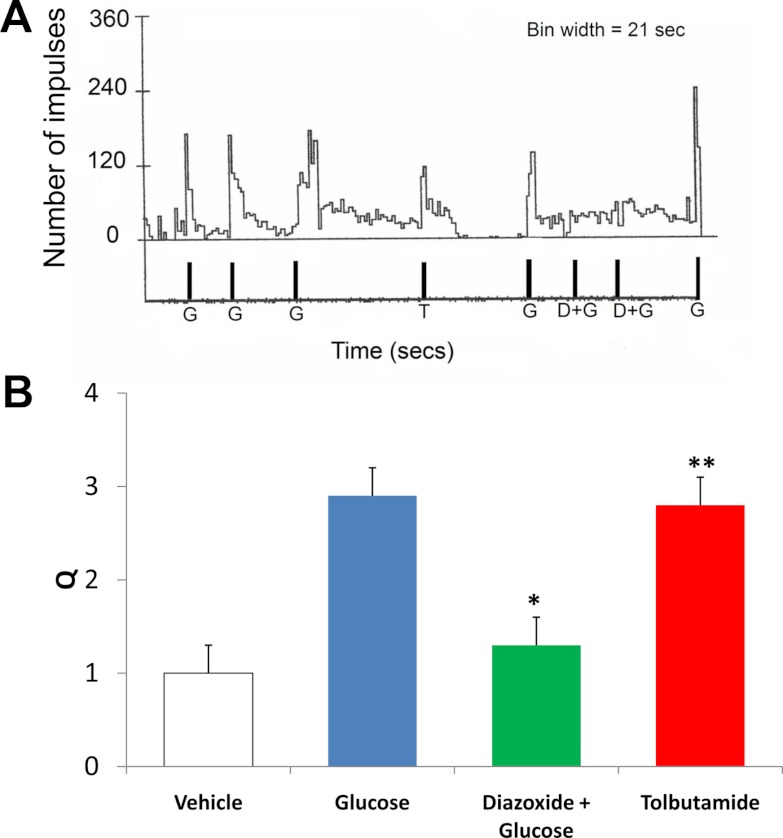
To demonstrate that the KATP channel mediated the stimulatory action of glucose, we showed that gastric vagal afferent responses to glucose (250 mg/dl) were inhibited by the KATP channel activator diazoxide (500 μm, Q = 1.3 ± 0.3, P < 0.05 compared with infusion of glucose alone, n = 5) (Fig. 7). In contrast, the KATP channel inactivator tolbutamide (500 μm) had excitatory effects (Q = 2.8 ± 0.5, P < 0.05, n = 6) (Fig. 7).
Fig. 7.
Effects of KATP activator (diazoxide) and inactivator (tolbutamide). A, Example of a recording obtained from gastric vagal fiber in response to glucose (G; 250 mg/dl), tolbutamide (T; 500 μm) and diazoxide (D; 500 μm) plus glucose (G; 250 mg/dl). Recordings showed repeated administration of glucose (G; 250 mg/dl) did not produce desensitization. The same unit also responded to tolbutamide (500 μm). Intraarterial administration of diazoxide (500 μm) inhibited the stimulatory action of glucose (250 mg/dl) on two occasions. The same unit subsequently responded to glucose stimulation. B, Histogram showing peak response of five GVA fibers (from three experiments) to intraarterial glucose injection (250 mg/dl). Diazoxide (500 μm) inhibited response to glucose perfusion (n = 5, *, P < 0.05, ANOVA for repeated measures). Tolbutamide (500 μm) stimulated GVA with a peak response of 2.8 ± 0.3 recorded from six GVA fibers (from three experiments). **, Q is significantly different from Q of vehicle (n = 6, *, P < 0.01, ANOVA from repeated measures).
Discussion
We have shown for the first time that a subset of neurons in the nodose ganglia express GLUT3. The same neurons also display immunoreactivity to GK and to the ATP-sensitive, inwardly rectifying K+ channel subunit Kir6.2. These findings, which were confirmed by Western blot analysis, showed that GLUT1, GLUT3, and GLUT4 are also present in the nodose ganglia. We also showed that GLUT3, GK, and KATP channels are transported axonally because these protein molecules are present in the gastric vagus nerve. Patch-clamp recordings using shRNA technology revealed that these molecules are critical in mediating the response of glucose-excited neurons to elevated ambient glucose concentrations. Nodose ganglia neurons appear to sense glucose using a mechanism similar to that used by pancreatic β-cells and hypothalamic glucose-excited neurons; that is, glucose must be transported into the neurons, in which it is metabolized through the action of GK, leading to increased ATP production and closure of the KATP channel, triggering a membrane depolarization. Electrophysiology studies using an in vitro isolated stomach-vagus nerve preparation showed that gastric vagal afferents responded to changes in glucose concentration.
The cellular uptake of glucose is mediated by facilitative GLUTs, a family of membrane-spanning proteins. GLUT1 is relatively abundant in the vascular endothelium (15, 16). GLUT2 has been detected in the liver, kidney, and pancreatic β-cells (17, 18) and, in relatively small amounts, in specific brain nuclei (19). GLUT3 is expressed in the placenta, liver, kidney, and hypothalamus (12). GLUT4 is found mainly in the insulin-sensitive heart, skeletal muscles, and adipose tissues (20, 21). We demonstrated the presence of GLUT1, GLUT3, and GLUT4 in the nodose ganglia and gastric vagus nerve. GLUT2 is hardly detectable. Kang and colleagues (6) reported that although glucosensing neurons and nonglucosensing neurons express GLUT2 and GLUT4, single-cell RT-PCR showed that GLUT3, a high-capacity, high-affinity GLUT, is the primary transporter for the glucosensing neurons in the ventromedial hypothalamus of the rat. GLUT1 and GLUT4 have low affinity for glucose and thus are poor glucosensors (22, 23). We showed that 67.8 ± 12.8% of nodose ganglia neurons express GLUT3-IR and close to 50% of the total population of nodose ganglia neurons express GLUT3, GK, and Kir6.2. We speculated that GLUT3 is the principal GLUT used by glucose-excited neurons in the nodose ganglia because of its high capacity and high affinity for glucose. Transfection studies with GLUT3 shRNA to silence GLUT3 expression in the nodose ganglia abolished the response of glucose-excited neurons to elevated glucose (Fig. 4). On the other hand, silencing GLUT1 or GLUT4 expression did not affect the excitatory response to glucose in these neurons (Fig. 5). This confirms that GLUT3 is the primary GLUT responsible for glucose uptake in glucose-excited nodose ganglia neurons. These findings corroborate the report that GLUT3 exists in the peripheral nerves (24), mediating glucose uptake in the peripheral nervous system. It should be noted that kinetic studies in the rat cerebellar neurons indicate that GLUT3 is saturated at physiological glucose levels (23). The kinetic parameters of GLUT3 in the nodose neurons are unknown. Our studies suggest that GLUT3 is a necessary component of glucosensing in glucose-excited nodose ganglia neurons. However, it is conceivable the GLUT3 may not be functioning as the rate limiting molecule in the cascade of glucosensing.
The presence of the pancreatic form of GK (hexokinase IV) suggests that nodose ganglia neurons sense glucose through its metabolism. Unlike the hepatic form of GK (hexokinase I), which is saturated at physiological blood glucose concentrations and is subject to feedback inhibition by its primary product glucose-6-phosphate (25), the pancreatic form of GK is optimally effective at glucose levels of 5–20 mmol/liter (18) and is unaffected by end-product inhibition (23). This makes the pancreatic form of GK an ideal glucosensor (26–29). GK mRNA, which is expressed in approximately 70% of glucose-excited hypothalamic neurons (22), appears to be the critical regulatory step in their glucosensing ability (13, 26, 30–32), as it is for insulin secretion in β-cells (23, 27) and glucagon release (27) in α-cells. In our studies, knockdown of the pancreatic form of GK mRNA in cultured nodose ganglia neurons by transfection of shRNA for GK abolished the response of glucose-excited neurons to elevated glucose. Thus, the pancreatic form of GK appears to be the primary regulator for glucosensing in glucose-excited nodose ganglia neurons. In this manner, GK regulates glycolytic flux and intracellular ATP production. Glucose metabolism increases the ATP to ADP ratio. This causes ATP to bind to the KATP channel composed of a Kir 6.2 pore-forming unit for K+ and a sulfonylurea receptor (26). Binding of either ATP or sulfonylurea inactivates the channel and depolarizes the cell membrane.
Although nearly 50% of nodose ganglia neurons contain glucosensing elements, only 30% of the neurons exhibit glucose-excitatory properties. Similar findings have been made in the hypothalamus (33–35). These observations suggest that other elements may contribute to regulating the sensitivity of the KATP channel to glucose stimulation. A number of regulatory molecules may modulate the gating of KATP channels. Among these, phosphatidylinositol 4,5-bisphosphate (PIP2) has been shown to reduce the sensitivity of KATP channels to ATP (36, 37). In our previous patch clamp studies on isolated nodose ganglia, we showed that depleting intracellular PIP2 with wortmannin increases the fraction of glucose-excited neurons from 26 to 80%, which suggests that intracellular PIP2 levels affect the glucosensing ability of nodose ganglia neurons (4).
Although a necessary component of glucosensing in glucose-excited nodose ganglia neurons, the KATP channel is unlikely the sole determinant of glucose-excited neuronal glucosensing because it is present in many other neurons that have no apparent glucosensing ability (4). Similar findings have been reported in the hypothalamus (34). It is conceivable that in nonglucosensing neurons, KATP channel activation plays a neuroprotective role by hyperpolarizing the membrane as a defense against neurotoxic levels of glutamate during nerve injury (38).
All glucosensing elements, including GLUT3, GK, and KATP, are abundant in the gastric vagal afferents. These molecules likely are synthesized in nodose ganglia neurons and transported distally along the axon to the vagal afferent terminals, similar to other protein molecules, such as cholecystokinin-A receptors (39). Similar findings have been reported in rat peripheral nerves (24).
Isolated stomach-vagus nerve preparations (10, 11) were used to measure the responses of vagal afferents to changes in glucose concentrations and to examine the functional significance of the presence of KATP in the gastric vagal afferent terminals. This preparation eliminates any influence of the central nervous system and systemic factors and thus unequivocally shows the action of glucose at the vagal afferent level. Glucose perfusion (250 mg/dl) induced increased firing in nine of 21 units (43%). A dose-response relationship (Fig. 6) supports the specificity of the glucose-excited response. In contrast, 2-deoxyglucose elicited little or no response, suggesting that most glucosensing gastric vagal afferents are excited and not inhibited by glucose. This is consistent with our previous patch-clamp studies using gastric retrograde-labeled nodose neurons (4), and anticipated, as most glucose-inhibited nodose ganglia neurons are found in portal vein-projecting, not gastric-projecting, neurons. Consistent with our observations in cultured nodose ganglia neurons (4), the KATP channel opener diazoxide blocked the stimulatory action of glucose on vagal afferents. These observations clearly indicate that vagal axon terminals are able to sense changes in ambient glucose. This ability appears to be mediated by the closure of KATP channels, triggering membrane depolarization.
Because all the essential elements (GLUT3, GK and KATP) for glucosensing are present in the nodose cell bodies and gastric vagal afferent terminals, it is likely that both the cell bodies and the terminals can both respond to changes in blood glucose concentration. However, given that the vagal afferent terminals are in close proximities to the proximal gut mucosa, they may be strategically positioned to detect physiological changes in local glucose concentrations in the gut during the postprandial period.
Our demonstration that vagal axon terminals are capable of sensing changes in ambient glucose ranging between 150 and 250 mg/dl may have important physiological and clinical significance. In a human glucose clamp study, we showed that acute hyperglycemia dose dependently reduced gastric contractions at 140 and 175 mg/dl. Gastric contractions were almost absent at 250 mg/dl (2). Similar findings were observed in rats (3). This may explain previous findings that blood glucose elevation slowed gastric emptying (40). This physiological phenomenon may be important to prevent sudden influx of large quantities of glucose into the circulation. Our findings may also have clinical implications. It suggests that hyperglycemia alone, in the absence of underlying neuropathy, can alter gastric motor function. This may explain the common clinical observation that diabetic patients with stable motor defects often exhibit wide day-to-day variations in symptoms, depending on blood glucose controls (41).
In summary, we have demonstrated that a subset of neurons in the nodose ganglia and gastric vagal afferents are glucoresponsive. Glucosensing requires the presence of a GLUT, GK, and KATP channels. These characteristics are similar to those attributed to glucosensing neurons in the hypothalamus (13, 14). These glucosensing elements are transported axonally to the gastric vagal afferents, which can be activated by elevated glucose through the modulation of KATP channels.
Acknowledgments
We thank Dr. Jen Yu Wei for his generous support in vagal afferent recording.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1 DK48419, RO1 DK58913, and P30 DK34933 and the American Diabetes Association Awards I-06-JF-58 and I-09-IN-44.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EGFP
- Enhanced green fluorescent protein
- GK
- glucokinase
- GLUT
- glucose transporter
- GVA
- gastric vagus afferents
- IR
- immunoreactivity
- KATP
- ATP-sensitive potassium channel
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- shRNA
- short hairpin RNA
- siRNA
- small inhibitory RNA
- SUR1
- sulfonylurea receptor.
References
- 1. Bulatao E, Carlson AJ. 1924. Influence of experimental changes in blood sugar level on gastric hunger contractions. Am J Physiol 69:107–115 [Google Scholar]
- 2. Barnett JL, Owyang C. 1988. Serum glucose concentration as a modulator of interdigestive gastric motility. Gastroenterology 94:739–744 [DOI] [PubMed] [Google Scholar]
- 3. Zhou SY, Lu YX, Owyang C. 2008. Gastric relaxation induced by hyperglycemia is mediated by vagal afferent pathways in the rat. Am J Physiol Gastrointest Liver Physiol 294:G1158–G1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grabauskas G, Song I, Zhou S, Owyang C. 2010. Electrophysiological identification of glucose-sensing neurons in rat nodose ganglia. J Physiol 588:617–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou SY, Lu Y, Song I, Owyang C. 2011. Inhibition of gastric motility by hyperglycemia is mediated by nodose ganglia KATP channels. Am J Physiol Gastrointest Liver Physiol 300:G394–G400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. 2004. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53:549–559 [DOI] [PubMed] [Google Scholar]
- 7. Zarbin MA, Wamsley JK, Innis RB, Kuhar MJ. 1981. Cholecystokinin receptors: presence and axonal flow in the rat vagus nerve. Life Sci 29:697–705 [DOI] [PubMed] [Google Scholar]
- 8. Yoshida-Yoneda E, O-Lee TJ, Wei JY, Vigna SR, Taché Y. 1996. Peripheral bombesin induces gastric vagal afferent activation in rats. Am J Physiol 271:R1584–R1593 [DOI] [PubMed] [Google Scholar]
- 9. Wu XY, Zhu JX, Gao J, Owyang C, Li Y. 2005. Neurochemical phenotype of vagal afferent neurons activated to express C-FOS in response to luminal stimulation in the rat. Neuroscience 130:757–767 [DOI] [PubMed] [Google Scholar]
- 10. Grabauskas G, Moises HC. 2003. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol 549:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 33:401–406 [DOI] [PubMed] [Google Scholar]
- 12. Wang YH, Taché Y, Sheibel AB, Go VL, Wei JY. 1997. Two types of leptin responsive gastric vagal afferent terminals: an in vitro single unit study in rats. Am J Physiol 273:R833–R837 [DOI] [PubMed] [Google Scholar]
- 13. Wei JY, Wang YH. 2000. Effect of CCK pretreatment on the CCK sensitivity of rat polymodal gastric vagal afferent in vitro. Am J Physiol Endocrinol Metab 279:E695–E706 [DOI] [PubMed] [Google Scholar]
- 14. Niijima A. 1984. The effect of D-glucose on the firing rate of glucose-sensitive vagal afferents in the liver in comparison with the effect of 2-deoxy-D-glucose. J Auton Nerv Syst 10:255–260 [DOI] [PubMed] [Google Scholar]
- 15. Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. 2004. Neuronal glucosensing: what do we know after 50 years? Diabetes 53:2521–2528 [DOI] [PubMed] [Google Scholar]
- 16. Harik SI, Kalaria RN, Andersson L, Lundahl P, Perry G. 1990. Immunocytochemical localization of the erythroid glucose transporter: abundance in tissues with barrier functions. J Neurosci 10:3862–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takata K, Hirano H, Kasahara M. 1997. Transport of glucose across the blood-tissue barriers. Int Rev Cytol 172:1–53 [DOI] [PubMed] [Google Scholar]
- 18. Sweet IR, Matschinsky FM. 1995. Mathematical model of β-cell glucose metabolism and insulin release. I. Glucokinase as glucosensor hypothesis. Am J Physiol 268:E775–E788 [DOI] [PubMed] [Google Scholar]
- 19. Thorens B, Cheng ZQ, Brown D, Lodish HF. 1990. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol 259:C279–C285 [DOI] [PubMed] [Google Scholar]
- 20. Leloup C, Arluison M, Lepetit N, Cartier N, Marfaing-Jallat JP, Ferré P, Pénicaud L. 1994. Glucose transporter 2 (GLUT 2): expression in species brain nuclei. Brain Res 638:221–226 [DOI] [PubMed] [Google Scholar]
- 21. Charron MJ, Brosius FC, 3rd, Alper SL, Lodish HF. 1998. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci USA 86:2535–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. James DE, Strube M, Mueckler M. 1989. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature 338:83–87 [DOI] [PubMed] [Google Scholar]
- 23. Maher F, Davies-Hill TM, Simpson IA. 1996. Substrate specificity and kinetic parameters of GLUT3 in rat cerebellar granule neurons. Biochem J 315:827–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manolescu AR, Witkowska K, Kinnaird A, Cessford T, Cheeseman C. 2007. Facilitated hexose transporters: new perspective on form and function. Physiology 22:234–240 [DOI] [PubMed] [Google Scholar]
- 25. Magnani P, Cherian PV, Gould GW, Greene DA, Sima AA, Brosius FC., 3rd1996. Glucose transporters in rat peripheral nerve: paranodal expression of GLUT1 and GLUT3. Metab Clin Exp 45:1466–1473 [DOI] [PubMed] [Google Scholar]
- 26. Roncero I, Alvarez E, Vázquez P, Blázquez E. 2000. Functional glucokinase isoforms are expressed in rat brain. J Neurochem 74:1848–1857 [DOI] [PubMed] [Google Scholar]
- 27. Matschinsky FM. Banting Lecture 1996 A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45:223–241 [DOI] [PubMed] [Google Scholar]
- 28. Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. 2002. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51:2056–2065 [DOI] [PubMed] [Google Scholar]
- 29. Jetton TL, Liang Y, Pettepher CC, Zimmerman EC, Cox FG, Horvath K, Matschinsky FM, Magnuson MA. 1994. Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J Biol Chem 269:3641–3654 [PubMed] [Google Scholar]
- 30. Maekawa F, Toyoda Y, Torii N, Miwa I, Thompson RC, Foster DL, Tsukahara S, Tsukamura H, Maeda K. 2000. Localization of glucokinase-like immunoreactivity in the rat lower brain stem: for possible location of brain glucose-sensing mechanisms. Endocrinology 141:375–384 [DOI] [PubMed] [Google Scholar]
- 31. Moriyama R, Tsukamura H, Kinoshita M, Okazaki H, Kato Y, Maeda K. 2004. In vitro increase in intracellular calcium concentrations induced by low or high extracellular glucose levels in ependymocytes and serotonergic neurons of the rat lower brainstem. Endocrinology 145:2507–2515 [DOI] [PubMed] [Google Scholar]
- 32. Matschinsky FM, Ellerman JE. 1968. Metabolism of glucose in the islets of Langerhans. J Biol Chem 243:2730–2736 [PubMed] [Google Scholar]
- 33. Yang XJ, Kow LM, Funabashi T, Mobbs CV. 1999. Hypothalamic glucose sensor: similarities to and differences from pancreatic β-cell mechanisms. Diabetes 48:1763–1772 [DOI] [PubMed] [Google Scholar]
- 34. Yang XJ, Kow LM, Pfaff DW, Mobbs CV. 2004. Metabolic pathways that mediate inhibition of hypothalamic neurons by glucose. Diabetes 53:67–73 [DOI] [PubMed] [Google Scholar]
- 35. Dunn-Meynell AA, Rawson NE, Levin BE. 1998. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res 814:41–54 [DOI] [PubMed] [Google Scholar]
- 36. Karschin C, Ecke C, Ashcroft FM, Karschin A. 1997. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett 401:59–64 [DOI] [PubMed] [Google Scholar]
- 37. Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. 1998. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282:1141–1144 [DOI] [PubMed] [Google Scholar]
- 38. Hilgemann DW, Ball R. 1996. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science 273:956–959 [DOI] [PubMed] [Google Scholar]
- 39. Zawar C, Neumcke B. 2000. Differential activation of ATP-sensitive potassium channels during energy depletion in CA1 pyramidal cells and interneurones of rat hippocampus. Pflug Arch 439:256–262 [DOI] [PubMed] [Google Scholar]
- 40. Aylett P. 1962. Gastric emptying and change of blood glucose level as affected by glucagon and insulin. Clin Sci 22:171–178 [PubMed] [Google Scholar]
- 41. Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, Shearman DJ. 1989. Gastric and esophageal emptying in patients with type II (non-insulin dependent) diabetes mellitus. Diabetologia 32:151–159 [DOI] [PubMed] [Google Scholar]