Abstract
Obesity and a nondipping circadian blood pressure (BP) pattern are associated with diastolic dysfunction. Ectopic lipid accumulation is increasingly recognized as an important metabolic abnormality contributing to diastolic dysfunction. However, little is known about the contribution of different lipids and the composition of lipid analytes to diastolic dysfunction. We have performed functional and structural studies and analyzed cardiac lipid profile at two time points during progression to diastolic dysfunction in a genetic model of obesity. Serial cardiac magnetic resonance imaging and telemetric measures of BP between 12 and 15 wk of age in obese male db/db mice indicated a nondipping circadian BP pattern and normal diastolic function at 12 wk that progressed to a deteriorating nondipping pattern and onset of diastolic dysfunction at 15 wk of age. Lipidomic analysis demonstrated elevated fatty acids and ceramides in db/db at 12 wk, but their levels were decreased at 15 wk, and this was accompanied by persistent mitochondrial ultrastructural abnormalities in concert with evidence of increased fatty acid oxidation and enhanced production of reactive oxygen species. Triacylglyceride and diacylglyceride levels were elevated at both 12 and 15 wk, but their composition changed to consist of more saturated and less unsaturated fatty acyl at 15 wk. An increase in the lipid droplets was apparent at both time points, and this was associated with increases in phosphatidycholine. In conclusion, a distinct pattern of myocardial lipid remodeling, accompanied by oxidative stress, is associated with the onset of diastolic dysfunction in obese, insulin-resistant db/db mice.
Diastolic dysfunction is often the earliest functional cardiac abnormality associated with obesity (1–3). The obese population has a high incidence of insulin resistance, which is an important risk factor for progression to diabetes. Insulin resistance is associated with elevated blood pressure (BP) (4), and the development of hypertension may initially be manifested as a loss of nocturnal BP dipping in the absence of elevated BP in the awake, active state (5). Indeed, this state of nondipping is often associated with abnormal cardiac structural and functional changes (6, 7). Chronic overnutrition also promotes insulin resistance in adipose tissue that results in disruption of whole-body lipid homeostasis that is due, in part, to reductions in the capacity of adipose tissue to store excess fat. This leads to a condition of dyslipidemia and ectopic deposition of fat in peripheral tissues, including the heart (8–10). The abnormal accumulation of fat in cardiomyocytes, or cardiac steatosis, suggests alterations in local homeostatic mechanisms that may initially be protective yet toxic if the condition persists (11). Importantly, cardiac steatosis is associated with impaired substrate metabolism characterized by altered fatty acid β-oxidation (FAO) (11–13). Although cardiac steatosis has been linked to development of diastolic dysfunction in type 2 diabetic individuals (14, 15), the contribution of distinct lipid classes and the composition of lipid analytes in relation to diastolic dysfunction is not well known.
In the current investigation, we hypothesized that ectopic myocardial lipid deposition due to obesity-related insulin resistance would lead to quantitative and qualitative alterations in myocardial lipids. We further posited that changes in the myocardial lipid profile in concert with a nondipping circadian pattern of BP contribute to the onset of diastolic dysfunction. To test this notion, we examined the LepRdb/db mouse model (db/db) of diabetic cardiomyopathy that exhibits a mutation in the leptin receptor that results in hyperleptinemia. It also exhibits a nondipping blood pressure pattern and diastolic dysfunction; these features of metabolic disease are also observed in obese and insulin-resistant humans (16–20). After a preliminary cardiac function testing using high-resolution cine-magnetic resonance imaging (cMRI) to determine an approximate time course to onset of diastolic dysfunction, two time points were chosen, the first (12 wk of age) corresponding to normal diastolic function and the second (15 wk of age) corresponding to the first detection of diastolic dysfunction. We have integrated cardiac function parameters, radiotelemetric BP monitoring, cardiac lipidomics, and measures of oxidative/nitrosative stress to evaluate the transitional relationship between loss of BP dipping, abnormal cardiac lipid deposition, and diastolic dysfunction. Our investigation shows that quantitative changes in cardiac lipid levels and, perhaps most importantly, their fatty acid composition, in concert with an altered capacity for fatty acid use/oxidation, increased oxidative/nitrosative stress, and mitochondrial structural abnormalities, are collectively associated with progression to diastolic dysfunction.
Materials and Methods
All animal procedures were approved by the University of Missouri Institutional Animal Care and Use Committee and the Harry S. Truman Veterans Affairs Memorial Hospital subcommittee for animal safety. Eight-week-old male db/db (leptin receptor mutant) and db/wild-type (WT) were purchased from Jackson Labs (Bar Harbor, ME) and were housed under standard laboratory conditions in which the room temperature was 21–22 C and light and dark cycles were 12 h each.
In vivo cMRI
Noninvasive high-resolution MRI scans were performed on two cohorts of mice that were approximately 12 and 15 wk old using a 7T horizontal bore MRI (Agilent Technologies, Palo Alto, CA) equipped with a 38-mm birdcage radiofrequency coil as previously described (21).
Telemetric BP monitoring
To monitor ambulatory BP, heart rate (HR), BP dipping status, and spontaneous cage activity during light and dark cycles (counts of lateral movement per minute), we placed catheters attached to radiotransmitters (TA11PA-C40; Data Sciences International, St. Paul, MN) in the carotid artery of a subset of 10-wk-old WT and db/db mice as previously described (22, 23). It should be noted that although humans are diurnally active and exhibit nocturnal BP dipping, rodents are nocturnally active and normally exhibit diurnal BP dipping. Therefore, a normal BP dipping pattern for rodents is defined as a decrease in diurnal BP of 10% or greater relative to nocturnal BP. On the other hand, a nondipping circadian BP pattern occurs when the diurnal decrease in the BP is less than 10%.
Blood biochemistry
Before the animals were killed, a venous blood sample was collected under isoflurane anesthesia from a subset of 12- and 15-wk-old WT and db/db mice that had been fasted for 5 h. Plasma glucose levels were measured by an automated hexokinase glycerol-6-pyruvate dehydrogenase assay, and insulin levels were assessed using an ELISA kit specific for rat insulin using previously established protocols (21). Whole-body insulin resistance, as assessed by the homeostasis model of assessment for insulin resistance (HOMA-IR), was calculated by taking the product of the fasting values of glucose (millimoles per liter) and insulin (microunits per milliliter) and dividing by 22 (21).
Myocardial whole-cell extract and subcellular fractionation
Tissue samples were flash frozen at autopsy and stored at −80 C. Left ventricular (LV) samples were homogenized in ice-cold homogenization buffer containing 250 mm sucrose, 50 mm HEPES, 0.5 mm EDTA, 1 protease inhibitor cocktail tablet, 200 mm sodium orthovanadate, and 50 mm sodium pyrophosphate. The homogenates were centrifuged at 2500 × g for 10 min. Insoluble pellets were discarded, and supernatants were again ultracentrifuged at 19,000 × g for 60 min. The resulting pellets were collected as enriched mitochondrial fractions, and the supernatants were again ultracentrifuged at 33,000 × g for 90 min. The final supernatant was collected as the cytosolic fraction. Okadaic acid (Acros Organics, Morris Plains, NJ) was added to each mitochondrial and cystosolic sample at a final concentration of 0.1 μm. Protein concentrations were determined using a bicinchoninic assay protein assay kit (Thermo Scientific, Rockford, IL).
Western blotting
Proteins were separated by SDS-PAGE, transferred to polyvinylidene fluoride membranes, and immunoblotted. Antibodies were purchased from either Abcam [Cambridge, MA) (hormone sensitive lipase (HSL), serine(660) phosphorylated HSL (Cell Signaling Technology, Beverly, MA), adipose triglyceride lipase (ATGL), serine(563) phosphorylated HSL] or Santa Cruz Biotechnology [Santa Cruz, CA; β-actin (sc-47778)]. The binding of the antibodies was detected by chemiluminescence, and images were recorded using a Bio-Rad ChemiDoc XRS image analysis system (Bio-Rad Laboratories, Santa Cruz, CA). The quantitation of protein band density, normalized to the density of total protein via amido black stain for enriched mitochondrial fractions or β-actin for cytosolic fractions, was performed using Image Lab software (Bio-Rad Laboratories).
Enzyme activities
Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities were measured from LV mitochondrial enriched extracts and expressed as nanomoles per minute−1 per milligram−1 using previously established procedures and all data are expressed as a percent of WT within each age group (23, 24).
Lucigenin-enhanced chemiluminescence
Superoxide anions were measured via the lucigenin-enhanced chemiluminescence method, as previously described (25), and all reactive oxygen species (ROS) data are expressed as a percent of WT within each age group.
3-Nitrotyrosine (3-NTY)
To assess the level of the oxidative damage to proteins, samples of LV were harvested, fixed, embedded in paraplast, sectioned, and evaluated for 3-NTY residue using immunofluorescence microscopy as previously described (23). Gray-scale intensity was measured within a fixed sized region of interest rectangle as previously described.
Tissue lipid analysis
LV plus septum was rinsed in chilled PBS, blotted dry, flash frozen in liquid nitrogen for storage at −80 C, and were subsequently pulverized at the temperature of liquid nitrogen. Lipids were sequentially extracted by the method of Bligh and Dyer (26) in the presence of an internal standard for fatty acids (FA), triacylglyceride (TAG), lysophosphatidylcholine (LPC), phosphatidylcholine (PC), sphingomyelin (SM), phosphatidylethanolamine (PE), ceramide (CE), diacylglyceride (DAG), and cholesteryl ester (CE) that included eicosanoic acid, triheptadecenoin, 1-0-heptadecanoyl-sn-glycero-3-phosphocholine, 1,2-dieicosanoyl-sn-glycero-3-phosphocholine, N-heptadecanoyl-sphingomyelin, 1,2-ditetradecanoyl-sn-glycero-3-phosphoethanolamine, N-heptadecanoyl-ceramide, 1,2-dieicosanoyl-sn-glycerol and cholesteryl heptadecanoate, respectively. Extracted lipids were resuspended and diluted in methanol/chloroform (4:1, by volume) before analysis by electrospray ionization-mass spectrometry using a Thermo Electron TSQ Quantrum Ultra instrument (San Jose, CA). Samples were analyzed as sodiated adduct positive ions and deprotonated negative ions using a shotgun lipidomics approach. For PC and SM, neutral loss (NL) scanning of 59.1 was monitored at collision energy of −28 eV in the positive ion mode. NL scanning of 368.5 was performed for CE molecular species at collision energy of −25 eV in positive ion mode. Precursor ion scanning for PE (m/z 196) and neutral loss scanning for ceramide (NL 256.2) was performed in the negative ion mode at collision energies of 50 and 32 eV, respectively.
FA and TAG were assessed by survey ion scans of negative- and positive-ion scans, respectively. DAG molecular species were quantified in positive ion mode using selected reaction monitoring of individual molecular species of the sodiated ion transitioning to the sodiated FA (with the saturated FA chosen as the stable product ion), using the paradigm previously used to assess lithiated DAG molecular ions (27). Individual molecular species were quantified by comparing the ion intensity of individual molecular species to that of the appropriate internal standards after corrections for type I and type II 13C isotope effects (28). Additional corrections were made from response curves for CE molecular species (27) and algorithms derived for TAG molecular species (29).
Statistical procedures
Results are reported as the mean ± se. Differences in outcomes between db/db and db/WT mice were determined using t tests or two-way ANOVA and were considered significant when P < 0.05. Repeated-measures ANOVA was used to analyze some telemetric derived BP data. All statistical analyses were performed using Sigma Plot (version 12) software (Systat Software, Point Richmond, CA).
Results
Experimental parameters
Body weight of db/db mice was approximately double that of age-matched WT mice in both age groups (P = 0.001; Table 1), and the percent increase in body weight between 12 and 15 wk of age was 6.2 ± 2.2 vs. 15.2 ± 3.0% for WT and db/db mice, respectively (P < 0.05). Heart weight normalized to tibia length did not differ among groups despite a slight but significant increase in tibia length with age (P = 0.001) that was mostly apparent within the db/db strain (P < 0.05). Fasting plasma glucose and insulin concentrations were elevated in db/db mice at both ages compared with WT (Table 1). Whole-body insulin resistance, as assessed by HOMA-IR, was evident at both ages in db/db mice. It should be noted that our use of a brief period of isoflurane anesthesia during acquisition of fasting plasma glucose levels results in above normal values for both strains of rats. Thus, the data should be interpreted in the context of relative rather than absolute values.
Table 1.
Baseline parameters in 12- and 15-wk-old (WT) and db/db mice
| Parameter | Main effects | P value | WT (10) | db/db (9) | WT (10) | db/db (9) |
|---|---|---|---|---|---|---|
| Age | 12.1 ± 0.1 | 12.1 ± 0.1 | 15.2 ± 0.1 | 15.2 ± 0.1 | ||
| Body mass (g) | Strain | 0.001 | ||||
| Age | 0.079 | |||||
| Interaction | 0.228 | 25.7 ± 0.5a | 48.8 ± 1.2 | 26.1 ± 0.6b | 51.0 ± 0.4 | |
| Heart weight (HW) (mg) | Strain | 0.434 | ||||
| Age | 0.773 | |||||
| Interaction | 0.415 | 133 ± 5 | 133 ± 3 | 130 ± 5 | 139 ± 5 | |
| Tibia length (TL) (mm) | Strain | 0.103 | ||||
| Age | 0.001 | |||||
| Interaction | 0.146 | 17.7 ± 0.2 | 16.8 ± 0.2c | 18.3 ± 0.3 | 18.3 ± 0.3 | |
| HW/TL (mg/mm−1) | Strain | 0.149 | ||||
| Age | 0.236 | |||||
| Interaction | 0.848 | 7.54 ± 0.24 | 7.91 ± 0.18 | 7.14 ± 0.32 | 7.62 ± 0.24 | |
| Fasting glucose (mmol/liter) | Strain | 0.001 | ||||
| Age | 0.724 | |||||
| Interaction | 0.024 | 12.6 ± 0.7a | 30.9 ± 1.0 | 17.2 ± 0.7b | 26.7 ± 3.4 | |
| Fasting insulin (μU/ml) | Strain | 0.001 | ||||
| Age | 0.491 | |||||
| Interaction | 0.373 | 5.5 ± 0.9a | 55.9 ± 6.7 | 2.3 ± 0.1b | 79.9 ± 28.9 | |
| HOMA-IR | Strain | 0.001 | ||||
| Age | 0.752 | |||||
| Interaction | 0.702 | 2.9 ± 0.6a | 78.5 ± 12.0 | 1.8 ± 0.1b | 65.9 ± 29.3 |
Data represent mean ± se. Numbers in parentheses are sample sizes.
P < 0.05, strain within 12 wk.
P < 0.05, strain within 15 wk.
P < 0.05, age within db/db.
cMRI evaluation of LV function
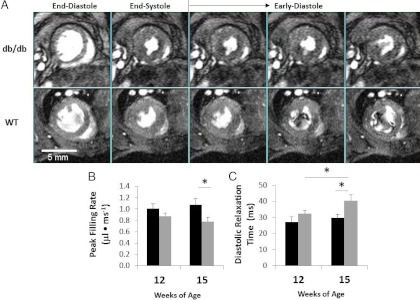
Cardiac MRI was performed at 12 and 15 wk of age, and results are listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org (supplemental material available online with this article). The LV of 15-wk-old db/db mice exhibited a reduction in peak filling rate (P < 0.05), but not initial (P = 0.07) filling rate, as well as prolonged diastolic relaxation time (P < 0.05) indicative of diastolic impairment (Fig. 1, A–D). Peak ejection rate, a systolic parameter, was slightly decreased in db/db mice at 15 wk of age compared with WT (P < 0.05). These abnormalities were not apparent at 12 wk of age. Collectively these data indicate no differences in LV diastolic and systolic function between WT and db/db mice at 12 wk of age but do show that 15-wk-old db/db mice exhibit abnormalities in both diastolic and systolic function compared with WT.
Fig. 1.
A, Representative mid-ventricle short-axis cMRI images illustrate enddiastole, end-systole, and early diastole phases (frame 1 and 9–12 of 16 captured) in a cardiac cycle. The top row demonstrates delayed LV diastolic relaxation and decreased early filling rate in the 15-wk-old db/db mice compared to that of WT, shown in the bottom row. Bar graphs show the mean ± SE for the diastolic parameters, peak filling rate (B), and diastolic relaxation time (C). Statistical analysis was by two-way ANOVA and Holm-Sidak post hoc test for paired comparisons or by Student's t test (*, P < 0.05).
BP telemetric parameters
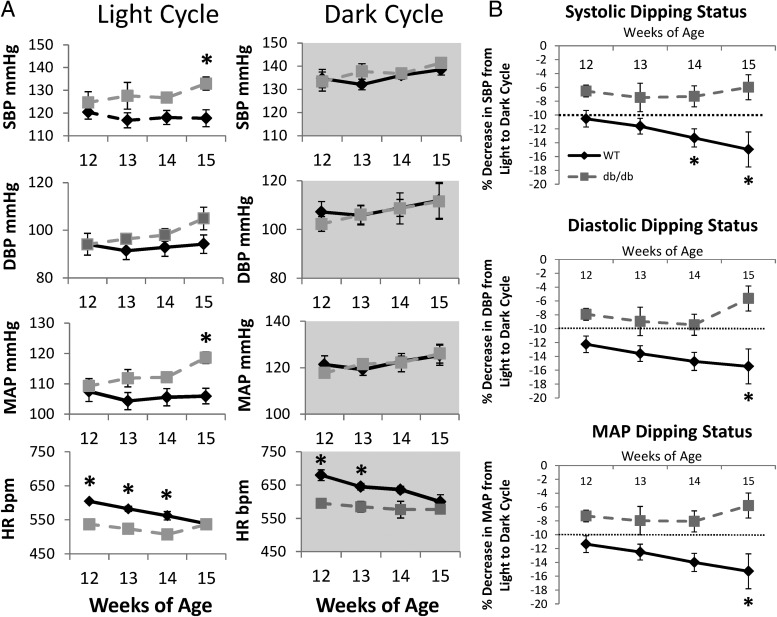
Between 12 and 15 wk of age, there were age-related trends, indicating graded increases in systolic BP (SBP), diastolic BP, and mean arterial pressure (MAP) during the light cycle in the db/db; however, the differences in light cycle BP within the db/db strain between 12 and 15 wk of age were statistically significant for MAP only (P < 0.05). No such BP age-related trends were observed within WT mice. At 15 wk of age, SBP and MAP during the light cycle were higher in db/db compared with 15-wk-old WT mice (P < 0.05) (Fig. 2A). Upon evaluation of BP dipping status, i.e. the percent decrease in BP from the dark to the light cycle, we observed that the WT mice exhibited a normal pattern of systolic BP, diastolic BP, and MAP dipping during the light cycle (Fig. 2B). On the other hand, SBP, diastolic BP, and MAP dipping occurred in db/db mice at all ages studied, but the magnitude of changes at 15 wk in these outcomes were lower, although not statistically significant. These data suggest that a nondipping BP pattern already exists in 12-wk-old db/db mice and deteriorates further at 15 wk of age. HRs during the light and dark cycles were significantly higher in WT mice between 12 and 14 wk of age but were not different at 15 wk of age. HR was higher for both strains during the dark cycle. HR decreased with age in WT but not db/db mice.
Fig. 2.
Radiotelemetry-derived SBP and diastolic BP (DBP) pressures, MAP, and HR of 12- to 15-wk-old WT and db/db mice recorded during the light and dark cycles. B, Line graphs show systolic, diastolic, and MAP dipping status for 12- to 15-wk-old WT and db/db mice. Each point or bar represents the mean ± SE for four WT or three db/db mice. Statistical analysis was by repeated-measures ANOVA or Student's t test (*, P < 0.05, WT vs. db/db within age).
Metabolic shifting, myocardial oxidative capacity, and mitochondrial remodeling
Capacity for FAO
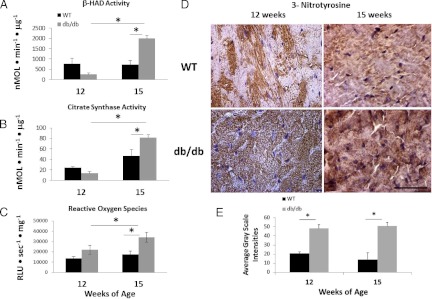
To evaluate the oxidative capacity of myocardial mitochondria, we measured the activities of β-HAD and citrate synthase, respectively, in mitochondrial extracts. β-HAD activity, a marker of FAO, and citrate synthase activity, a marker of trichloroacetic acid cycle activity used to assess the aerobic capacity of mitochondria, were elevated in the myocardium of 15-wk-old db/db mice (Fig. 3, A and B). It is noteworthy that the magnitude of increase in β-HAD activity at 15 wk of age in the db/db heart is greater than the increase in citrate synthase activity (i.e. 3-fold vs. 2-fold).
Fig. 3.
Myocardial β-HAD and citrate synthase activities (A, B) from LV of 12- and 15-wk-old WT and db/db mice (n = 5–6/group). Oxidative and nitrosative stress are elevated in the db/db myocardium. C, Increased reactive oxygen species in the 15-wk db/db heart. D, Representative sections show 3-NTY immunostaining in the panels, and the accompanying bar graph (E) indicates increased 3-NTY levels in the db/db heart at 12 and 15 wk of age compared to age-matched WT. *, P < 0.05.
Myocardial oxidative stress
There was a significant increase in ROS in the db/db heart compared with WT (strain effect, P = 0.005), and this was most evident at 15 wk of age (P = 0.004) (Fig. 3C). There was a nearly significant effect of age on the level of ROS (P = 0.06), and this was primarily due to an increase in ROS in 15-wk-old db/db hearts compared with 12-wk-old db/db hearts (P = 0.04). 3-NTY immunofluorescence did not vary with age in the LV of WT mice; however, the 3-NTY level was similarly elevated in db/db strain compared with WT at both 12 and 15 wk of age (Fig. 3, D and E). There were no differences in nicotinamide adenine dinucleotide phosphate oxidase activity in myocardial plasma membrane extracts between the mouse strains at either time point (not shown), suggesting that the burden of oxidants is mostly derived from mitochondria.
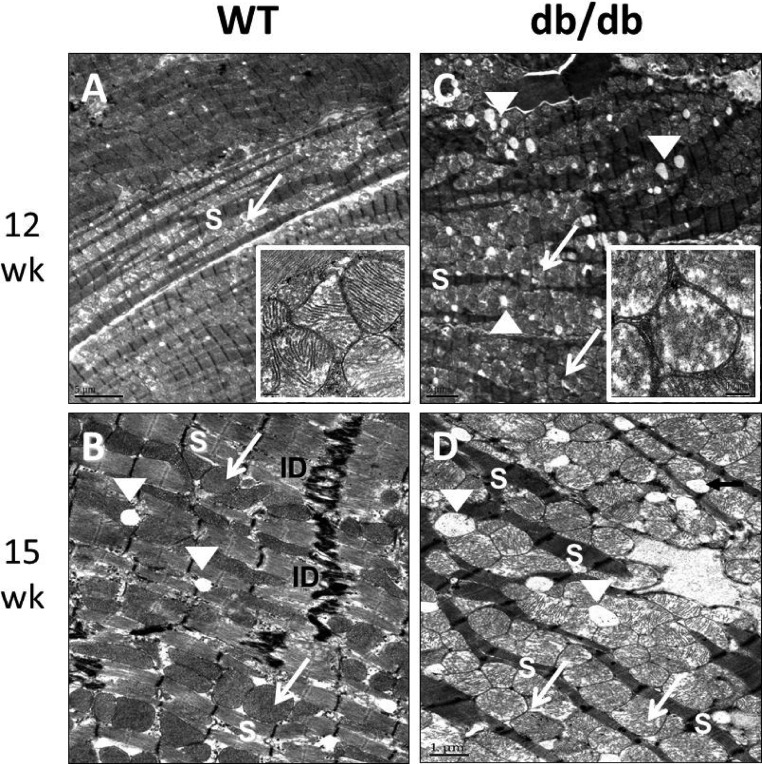
Mitochondrial ultrastructural remodeling
The ultrastructural arrangement in LV cardiomyocytes consists of a row of sarcomeres alternating with a row of intermyofibrillar mitochondria (imf-Mt) with well-defined cristae matrix structure and few cytosolic lipid droplets as observed in our WT controls using transmission electron microscopy (Fig. 4, A and B). This ultrastructural arrangement was disrupted in the db/db mice (Fig. 4, C and D). Specifically we observed an increase in imf-Mt and lipid droplet numbers in the db/db myocardium at 12 and 15 wk that was associated with the disruption of the normal cardiomyocyte sarcomere arrangement. In the db/db heart, the imf-Mt morphology was markedly deranged and there were numerous breaks and loss of mitochondrial cristae associated with the loss of mitochondrial matrix (Fig. 4C, inset).
Fig. 4.
Abnormalities in sarcomere and mitochondrial ultrastructure in 12- and 15-wk-old db/db mice compared to age-matched WT mice. Transmission electron micrographs (12 wk = 1000× and 15 wk = 1500× magnification) show that LV myocardial ultrastructure of db/db hearts at both time points exhibit increased numbers of lipid droplets (white arrowheads) and increased numbers of intramyofibrillar mitochondria (white arrows) and disrupted sarcomeric structure (white S). The insets show disrupted mitochondrial cristae structure and loss of matrix in the 12-wk db/db heart compared to WT.
Pattern of myocardial lipid accumulation in db/db mice
Changes in individual lipid classes
We used electrospray ionization-mass spectrometry to identify and quantify individual lipid species in LV tissue from 12- and 15-wk-old WT and db/db mice (Figs. 5–7 and Supplemental Figs. 1 and 2). Total FA content was 53% (P < 0.01) and 48% (P < 0.05) greater in db/db hearts at 12 and 15 wk of age, respectively, compared with age-matched WT mice (Fig. 5A). However, compared with the 12-wk-old hearts, FA content in 15-wk-old WT and db/db hearts declined by 61 and 62%, respectively, compared with those at 12 wk of age (P < 0.05 in both strains). Total TAG content was 277% (P < 0.0001) and 126% (P < 0.01) greater in db/db hearts at 12 and 15 wk of age, respectively, compared with WT at each age (Fig. 5B). Unlike FA, there was no age-dependent change in TAG content in db/db hearts between 12 and 15 wk of age; however, total TAG content of 15 wk old WT hearts was 91% greater compared with 12-wk-old WT (P < 0.05).
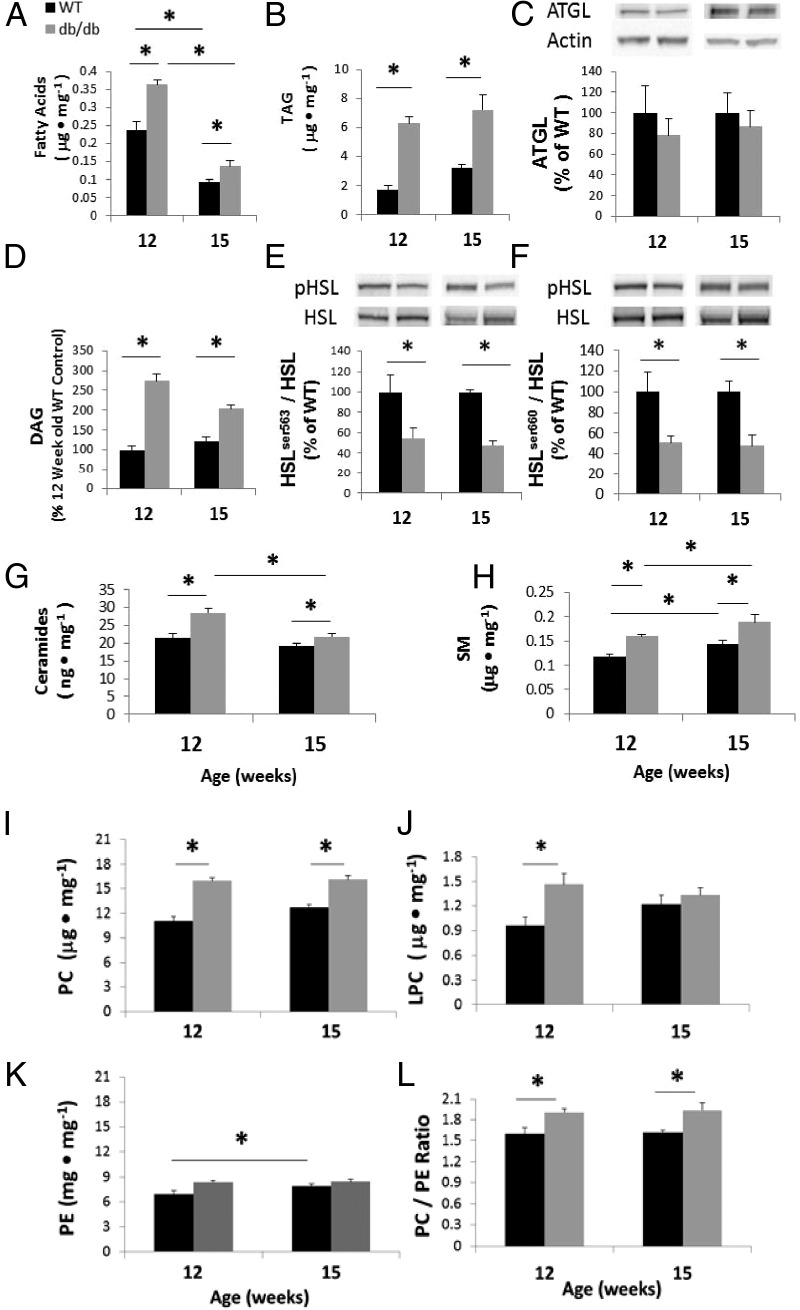
Fig. 5.
Total fatty acids (A), TAG (B), DAG (D), Cer (G), SM (H), PC (I), LPC (J), and PE (K) in the left ventricles of 12- and 15-wk-old WT and db/db mice were quantified by electrospray ionization-mass spectrometry. C, Protein levels of ATGL, a lipase that hydrolyzes TAG to DAG; phosphorylated-HSLser563 normalized to total HSL (HSLser563/HSL) (E); and phosphorylated-HSLser660 normalized to total HSL (HSLser660/HSL) (F). Protein levels are expressed as a percentage of WT. L, Increases in the ratio of PC/PE in the left ventricles of 12- and 15-wk-old db/db mice, compared to their WT counterparts. Each bar represents the mean ± SE for five to six WT or db/db mice. Statistical analysis was by two-way ANOVA and Holm-Sidak post hoc test for paired comparisons or Student's t test (*, P < 0.05).
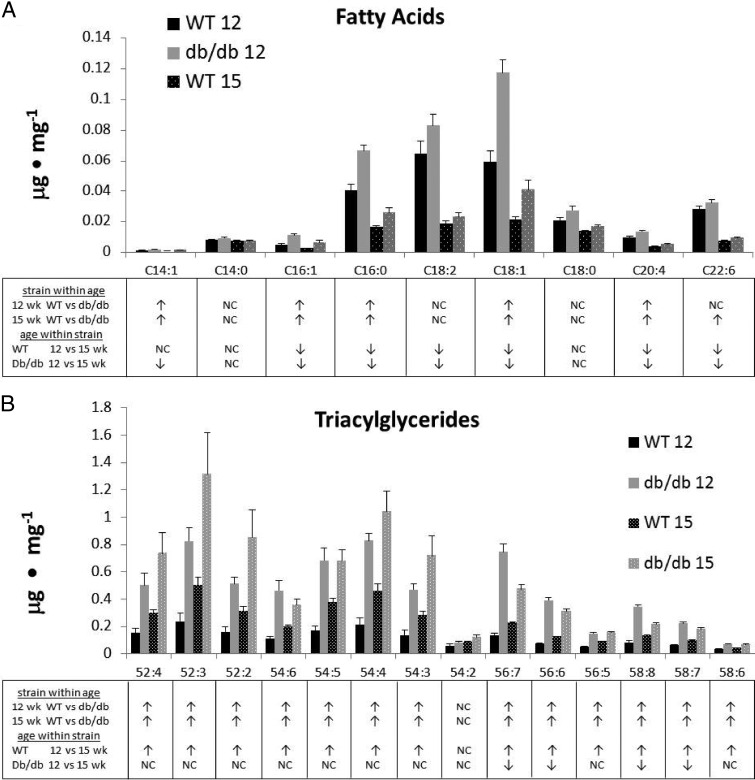
Fig. 6.
A, Fatty acid and TAG (B) accumulation in the hearts of 12- and 15-wk-old db/db mice compared to age-matched WT mice. Each bar represents the mean ± SE for 5–6 WT or db/db mice. Statistical analysis was by Student's t test for each lipid subspecies and differences (P < 0.05), i.e., increase, no change (NC) or decrease, are indicated by ↑, NC, and ↓, respectively, in the table below the graph.
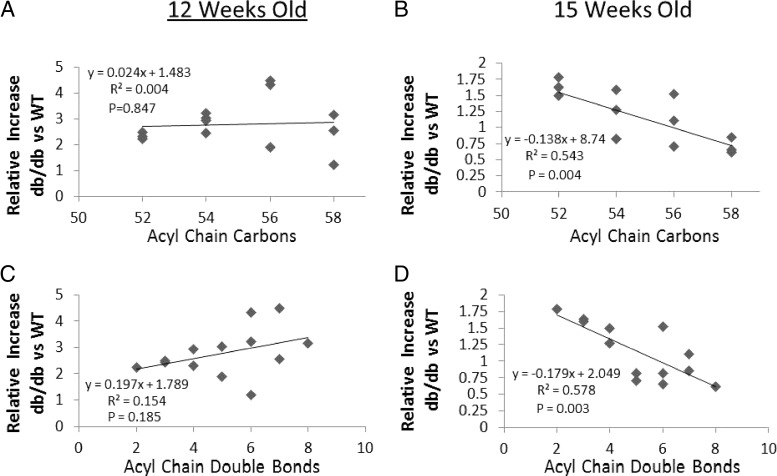
Fig. 7.
Myocardial TAG patterns between 12- and 15-wk-old WT and db/db mice (A–D). The content of each lipid species (μg · mg−1 tissue) in db/db mouse hearts was normalized to the corresponding lipid species in WT hearts to obtain a ratio representing the relative content of each lipid. Each data point represents the lipid ratio plotted as a function of either acyl-chain carbon number or acyl-chain double-bond number. Statistical analysis was by linear regression and data from five to six WT and db/db mice were used to generate regressions.
To examine whether there is evidence to support impaired lipolysis in the db/db myocardium, we measured protein levels of ATGL and HSL. The protein levels of ATGL, the enzyme that hydrolyzes TAG to DAG, did not differ between strains at either time point (Fig. 5C). We also examined the levels of DAG, which have been implicated in obesity-induced cardiac insulin resistance (30). Total content of DAG was 275 and 168% greater (both P < 0.001) in db/db hearts at 12 and 15 wk of age (Fig. 5D), respectively, although the DAG content decreased by 26% between 12 and 15 wk old db/db hearts (P < 0.001). We also determined protein levels of total and phosphorylated (activated) HSL, the enzyme that hydrolyzes DAG to monoacylglyceride, in 12- and 15-wk-old hearts. The relative levels of serine(563) phosphorylated HSL and serine(660) phosphorylated HSL were decreased (P < 0.05) in db/db mice at both 12 and 15 wk of age (Fig. 5, E and F).
Total Cer content varied significantly between WT and db/db mice (Fig. 5G). Total Cer did not change with age in WT hearts but was 33% (P < 0.01) greater in db/db hearts at 12 wk of age compared with WT. However, Cer levels decreased in 15-wk-old db/db mice, resulting in no differences with WT mice at 15 wk of age. The SM content varied significantly between the strains and was elevated in older mice of both strains (Fig. 5H). SM was 35 and 30% higher (both P < 0.05) in db/db hearts at 12 and 15 wk of age, respectively, compared with their WT counterparts.
Myocardial PC content varied significantly between the strains and was elevated in older mice (Fig. 5I). Total PC content was 44% (P < 0.01) and 27% (P < 0.05) greater in db/db hearts at 12 and 15 wk of age, respectively. Total LPC was 53% (P < 0.01) greater in db/db hearts at 12 wk of age, whereas there was no strain-related difference in LPC content at 15 wk of age (Fig. 5J). Total PE content was elevated in 12-wk-old db/db mice (P < 0.05; Fig. 5K) but was not different at 15 wk in the db/db strain, although the strain effect did not reach significance (P = 0.08). The PC to PE ratio was 20% (P < 0.01) greater in db/db hearts at both 12 and 15 wk of age compared with WT (Fig. 5L).
Changes in fatty acyl composition
Although myocardial steatosis involves increases in the total content of a number of lipid classes, this information may not be specific enough to identify toxic or salutary lipid intermediates that affect cardiac function. Changes in FA composition in db/db and WT hearts at 12 and 15 wk are shown in Fig. 6A. Although the magnitude of total FA content was lower at 15 wk of age in both strains (Fig. 5), five of the nine individual free FA molecular subspecies (C14:1, C16:1, C16:0, C18:1, and C20:4) were elevated (P < 0.05) in 12-wk-old db/db hearts and six of nine (C14:1, C16:1, C16:0, C18:1, C20:4, and C22:6) were elevated in 15-wk-old db/db hearts, respectively. In contrast to the pattern of FA distributions, TAG subspecies showed distinct changes in 15-wk db/db hearts compared with 12-wk db/db mice (Fig. 6B). The accumulation of TAG with lower acyl chain number was more pronounced in the 15-wk db/db hearts (Fig. 6B). To examine this further, we plotted the db/db to WT lipid ratio as a function of either acyl-chain carbon number or acyl-chain double-bond number (Fig. 7). In the LV of 12-wk-old db/db mice, there was no relationship between TAG ratio plotted as a function of acyl-chain carbon number (P = 0.847) and a slightly positive, but nonsignificant, relationship between TAG ratio and acyl-chain double-bond number (P = 0.185; Fig. 7, A and C). On the other hand, in 15-wk-old db/db hearts relative to WT, we observed significant negative linear relationships in which TAGs of relatively low acyl-chain carbon content (R2 = 0.54; P = 0.004) and double-bond number (R2 = 0.58; P = 0.003) were elevated (Fig. 7, B and D).
In addition to intracardiac TAG fatty acid composition, we also examined the composition of FAs in DAGs. To our knowledge, the significance of changes in the composition of DAG has not been examined during progression to diastolic dysfunction in cardiac tissue. Although DAG content decreased slightly, but significantly between 12 and 15 wk of age in the db/db heart, the total content of DAG at 15 wk of age was still higher in db/db mice compared with WT (Fig. 5). Moreover, the accumulation of FA species with lesser degree of unsaturation remained elevated, whereas DAG species with higher unsaturation were significantly decreased in the db/db hearts at 15 wk compared with 12-wk db/db (Supplemental Fig. 1). We also analyzed FA composition of Cer. Seven (C16:0, C18:0, C18:1, C20:0, C22:1, C23:0, and C24:1) of the nine individual Cer species were elevated (P < 0.05) in 12-wk-old db/db hearts and four of the nine (C16:0, C18:0, C18:1, and C23:0) were elevated at 15 wk of age in db/db (Supplemental Fig. 2).
Discussion
In humans, the earliest hemodynamic abnormality associated with obesity/overnutrition is a nondipping circadian BP pattern, and this is associated with diastolic dysfunction (1–3). In this investigation, we report quantitative and qualitative changes in the myocardial lipodomic profiles in a mouse model of obesity and insulin resistance before and after the development of diastolic dysfunction. Previous reports document in vivo cardiomyopathy in db/db mice using cardiac magnetic resonance imaging (20), echocardiography (31), and direct cardiac catheterization (32). Using high-resolution cMRI, we determined that db/db mice exhibit normal diastolic function at 12 wk of age with transition to impaired diastolic function shortly thereafter at 15 wk of age. Specifically we observed a reduction in peak filling rate, as well as prolonged diastolic relaxation time, both of which are indicative of diastolic dysfunction. Despite detection of several abnormal cardiac functional parameters, LV ejection fraction was preserved.
Abnormal circadian BP patterns have also been related to cardiac dysfunction in the setting of overweight/obesity (7, 18, 19, 33, 34). Although the changes in BP dipping status for the db/db mice did not vary significantly among the ages studied, the light cycle increases in SBP and MAP in db/db at 15 wk of age compared with WT mice suggest progressive deterioration of nondipping BP in db/db mice Our findings suggest an association between a worsening nondipping BP pattern and diastolic dysfunction. In this regard, the independent association between a nondipping BP pattern and cardiac diastolic dysfunction, in the absence of significant LV hypertrophy observed in the present study, is consistent with a recent report in normotensive humans with diastolic dysfunction who were nondippers (33).
Mitochondrial remodeling and dysfunction contribute to abnormalities in diastolic relaxation in the setting of overnutrition (35–37). The abnormal myocardial mitochondrial ultrastructure observed in 12- and 15-wk-old db/db mice, characterized by increased numbers of mitochondria with a high incidence of distortion of the cristae matrix, is consistent with our previous observations in insulin resistant Zucker obese rats with diastolic dysfunction (21). The initiating events contributing to mitochondrial dysfunction in the setting of obesity are thought to result from increases in myocardial FA uptake and oxidation, leading to a state of reduced metabolic (energy substrate) flexibility (38, 39). Herein we show that the db/db myocardium undergoes chronic lipid accumulation and concomitant elevations in nitrosative stress at both 12 and 15 wk; however, the burden of oxidative stress (ROS) is exaggerated at 15 wk of age and is associated with elevated capacity for FAO. Although β-HAD activity is not a direct measure of FAO but indicates the capacity for FAO, β-HAD activity in db/db heart was decreased at 12 wk (roughly one third that of WT) but increased at 15 wk of age (∼3-fold over WT). These changes were accompanied by increased myocardial content of FA at 12 wk and decreased FA levels at 15 wk of age in db/db hearts and suggest the possibility of impaired oxidation of FA at the earlier time point but enhanced oxidation at the later time point. Moreover, the magnitude of the increase in β-HAD activity relative to the increase in citrate synthase activity, an indicator of aerobic metabolism via trichloroacetic acid cycle activity, suggests excessive FAO also contributes to increased oxidative stress. Therefore, progression from low β-oxidation to increased β-oxidation and enhanced oxidative stress at 15 wk may contribute to diastolic dysfunction. Although increased myocardial FAO, oxidative stress, and diastolic dysfunction have been investigated in db/db mice (40, 41), in this investigation we report a novel association between a worsening nondipping BP pattern, diastolic dysfunction, and dysregulated FAO with enhanced oxidative stress in 15 wk old db/db mice.
Although experimental models demonstrate that heart-specific lipid accumulation impairs cardiac function (42, 43), the qualitative and quantitative changes in myocardial lipids during progression to cardiomyopathy has not been examined. In this study, we observed TAG accumulation in 12- and 15-wk-old db/db mice; nonetheless, the normal expression levels of ATGL in db/db mice at both ages suggests that the abnormal increase in TAGs cannot be ascribed to impaired ATGL-mediated hydrolysis to DAG. Moreover, we detected TAG-specific changes in patterns of saturated and unsaturated fatty acyl species in TAG at 15 wk, indicative of a significant decrease in unsaturated FA at 15 wk, whereas the TAG species containing less unsaturated species remained elevated. In this regard, a recent study reported that plasma lipid analytes associated with increased risk of diabetes are predominately associated with saturated and monounsaturated FA (44). The increase in myocardial TAG can give rise to the accumulation of putative toxic intermediates such as DAG and Cer (45), which are known to act as lipid second messengers that modify protein kinase C activity, which has been linked to cardiac hypertrophy, diastolic dysfunction, and heart failure (46, 47).
In this study, we examined the levels and FA composition of DAG and Cer. DAG content was higher in db/db mice at both 12 and 15 wk compared with WT mice. The observed accumulation of DAG may be due, in part, to a combination of factors, including ATGL-mediated hydrolysis of TAG to DAG and reduced clearance of DAG caused by impaired activation of HSL (48). We found that DAG accumulation was accompanied by decreased activation of the lipase, HSL without any changes in ATGL. It should be noted that the DAG levels are increased at 12 and 15 wk compared with WT, but the magnitude of increase was lower at 15 wk. The changes in lipid composition observed in this study reflect whole-cell changes; however, it is possible that the intracellular compartmentalization of lipids is also important for their potential cytotoxic effects (49). Therefore, concentration of more saturated (toxic) DAG species could actually be increasing in critical cellular compartments, whereas the overall organ concentration of DAG decreases.
We also observed changes in the subspecies composition, indicating a relative increase in saturated and monounsaturated subspecies (Supplemental Fig. 1); thus, it is possible that decreases in the lipid aliphatic composition from polyunsaturated to saturated and monounsaturated aliphatic groups may have profound effects on membrane dynamics and cardiac function (50, 51). In contrast to DAG accumulation at both 12 and 15 wk, we observed increased Cer content in 12-wk-old db/db hearts compared with WT. With increasing age, Cer content decreased in the 15-wk-old db/db myocardium, whereas the Cer levels were similar in the young and older WT hearts. Only a few studies have examined the association of cardiac Cer levels with cardiac dysfunction in insulin-resistant states (52–54). Our studies showing increased myocardial Cer content are only partly in agreement with other studies showing similar results in obese rodents (55). In fact, previous studies show an increase, a decrease, or no change in Cer levels in either the heart or adipose tissue in obesity and diabetes (42, 43, 52, 55–57). In this regard, cardiomyocyte-specific deficiency of serine palmitoyltransferase subunit 2 in mice leads to cardiac dysfunction despite concomitant reduction in Cer levels (58). Finally, we observed an increase in PC as well as an increase in the PC to PE ratio at 12 and 15 wk in db/db hearts. This may be important, in part, in the context of the observed increases in numbers of lipid droplets (LDs) in the db/db myocardium at both ages. The TAG stored within LDs are surrounded by a phospholipid monolayer consisting largely of PC and a lesser amount of PE. It is possible that the relatively higher levels of myocardial PC may, in part, be a consequence of the greater need for packaging excess TAG in greater numbers of LDs. In this regard, recent studies have implicated an altered synthesis of PC and PE in the accumulation of TAG as well as contractile dysfunction (59, 60). Additional studies are needed to test whether the types of lipid changes described herein play a role in progression to diastolic dysfunction. Nonetheless, our results support the concept that overnutrition-induced cardiac steatosis, as indicated by a distinct changes in different lipid classes, results in a mismatch between myocardial lipid uptake/use that has deleterious consequences for diastolic function (43, 50).
In summary, the present study is the first to integrate detailed analyses of in vivo cardiac function, circadian BP dipping status, and myocardial lipid profiles using two distinct time points in obese db/db mice. Data from this investigation suggest that chronic loss of BP dipping, accompanied by lipid accumulation, increased capacity for myocardial mitochondrial FAO, and increased generation of ROS collectively may be the trigger for development of diastolic dysfunction in obesity. Lipodomics may help to identify lipid-driven cardiac metabolic, structural, and functional abnormalities contributed by factors (e.g. renin angiotensin aldosterone system and sympathetic activation) associated with diastolic dysfunction.
Supplementary Material
Acknowledgments
We gratefully acknowledge the technical support of Nathan T. Rehmer, Irina Mugerfeld, and Mona Garro. We also thank Brenda Hunter for her assistance in preparing the manuscript.
This work was supported by Grants HL-73101 and HL107910 (to J.R.S.) and Grants HL-074214 and HL-111906 (to D.A.F.) from the National Institutes of Health and grants from the Department of Veterans Affairs (a Merit Award to J.R.S. and a Career Development Award to A.W.-C.). Grants AG040638 and VA CDA-2 BB47 (to A.W.-C.) and the ASN-ASP Junior Development Grant in Geriatric Nephrology (to A.W.-C.) supported by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Society of Nephrology.
Disclosure Summary: The authors report no disclosures or conflicts of interest.
Footnotes
- ATGL
- Adipose triglyceride lipase
- BP
- blood pressure
- CE
- cholesteryl ester
- Cer
- ceramide
- cMRI
- cine-magnetic resonance imaging
- FA
- fatty acid
- FAO
- fatty acid β-oxidation
- β-HAD
- β-hydroxyacyl-CoA dehydrogenase
- HOMA-IR
- homeostasis model of assessment for insulin resistance
- HR
- heart rate
- HSL
- hormone-sensitive lipase
- imf-Mt
- intermyofibrillar mitochondria
- LD
- lipid droplet
- LPC
- lysophosphatidylcholine
- LV
- left ventricular
- MAP
- mean arterial pressure
- NL
- neutral loss
- 3-NTY
- 3-nitrotyrosine
- PE
- phosphatidylethanolamine
- ROS
- reactive oxygen species
- SBP
- systolic BP
- SM
- sphingomyelin
- TAG
- triacylglyceride
- WT
- wild type.
References
- 1. Santos JL, Salemi VM, Picard MH, Mady C, Coelho OR. 2011. Subclinical regional left ventricular dysfunction in obese patients with and without hypertension or hypertrophy. Obesity (Silver Spring) 19:1296–1303 [DOI] [PubMed] [Google Scholar]
- 2. Van Putte-Katier N, Rooman RP, Haas L, Verhulst SL, Desager KN, Ramet J, Suys BE. 2008. Early cardiac abnormalities in obese children: importance of obesity per se versus associated cardiovascular risk factors. Pediatr Res 64:205–209 [DOI] [PubMed] [Google Scholar]
- 3. Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. 2005. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 111:2073–2085 [DOI] [PubMed] [Google Scholar]
- 4. Kannel WB, Wilson PW, Zhang TJ. 1991. The epidemiology of impaired glucose tolerance and hypertension. Am Heart J 121:1268–1273 [DOI] [PubMed] [Google Scholar]
- 5. Lurbe E, Torro I, Aguilar F, Alvarez J, Alcon J, Pascual JM, Redon J. 2008. Added impact of obesity and insulin resistance in nocturnal blood pressure elevation in children and adolescents. Hypertension 51:635–641 [DOI] [PubMed] [Google Scholar]
- 6. Seo HS, Kang TS, Park S, Choi EY, Ko YG, Choi D, Ha J, Rim SJ, Chung N. 2006. Non-dippers are associated with adverse cardiac remodeling and dysfunction (R1). Int J Cardiol 112:171–177 [DOI] [PubMed] [Google Scholar]
- 7. Ivanovic BA, Tadic MV, Celic VP. 8 September 2011. To dip or not to dip? The unique relationship between different blood pressure patterns and cardiac function and structure. J Hum Hypertens 10.1038/jhh.2011.83 [DOI] [PubMed] [Google Scholar]
- 8. Bosma M, Kersten S, Hesselink MK, Schrauwen P. 2012. Re-evaluating lipotoxic triggers in skeletal muscle: relating intramyocellular lipid metabolism to insulin sensitivity. Prog Lipid Res 51:36–49 [DOI] [PubMed] [Google Scholar]
- 9. Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A. 2009. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis 19:146–152 [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Ren J. 2011. Role of cardiac steatosis and lipotoxicity in obesity cardiomyopathy. Hypertension 57:148–150 [DOI] [PubMed] [Google Scholar]
- 11. Lopaschuk GD, Folmes CD, Stanley WC. 2007. Cardiac energy metabolism in obesity. Circ Res 101:335–347 [DOI] [PubMed] [Google Scholar]
- 12. Luiken JJ, Arumugam Y, Dyck DJ, Bell RC, Pelsers MM, Turcotte LP, Tandon NN, Glatz JF, Bonen A. 2001. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem 276:40567–40573 [DOI] [PubMed] [Google Scholar]
- 13. Carley AN, Severson DL. 2005. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim Biophys Acta 1734:112–126 [DOI] [PubMed] [Google Scholar]
- 14. Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. 2008. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 52:1793–1799 [DOI] [PubMed] [Google Scholar]
- 15. Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. 2003. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 144:3483–3490 [DOI] [PubMed] [Google Scholar]
- 16. Hummel KP, Dickie MM, Coleman DL. 1966. Diabetes, a new mutation in the mouse. Science 153:1127–1128 [DOI] [PubMed] [Google Scholar]
- 17. Coleman DL. 1983. Lessons from studies with genetic forms of diabetes in the mouse. Metabolism 32:162–164 [DOI] [PubMed] [Google Scholar]
- 18. da Costa Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, Luft FC, Jordan J, Gross V. 2009. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension 53:387–392 [DOI] [PubMed] [Google Scholar]
- 19. Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. 2008. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol 295:H1634–H1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari Z, Zhang C. 2010. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress. Am J Physiol Heart Circ Physiol 299:H985–H994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR. 2009. Nebivolol improves diastolic dysfunction and myocardial tissue remodeling through reductions in oxidative stress in the Zucker Obese rat. Hypertension 55:880–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson MS, DeMarco VG, Heesch CM, Whaley-Connell AT, Schneider RI, Rehmer NT, Tilmon RD, Ferrario CM, Sowers JR. 2011. Sex differences in baroreflex sensitivity, heart rate variability, and end organ damage in the TGR(mRen2)27 rat. Am J Physiol Heart Circ Physiol 301:H1540–H1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeMarco VG, Johnson MS, Ma L, Pulakat L, Mugerfeld I, Hayden MR, Garro M, Knight W, Britton SL, Koch LG, Sowers JR. 2012. Overweight female rats selectively bred for low aerobic capacity exhibit increased myocardial fibrosis and diastolic dysfunction. Am J Physiol Heart Circ Physiol 302:H1667–H1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump C, Ferrario CM, Sowers JR. 2007. Angiotensin-II mediated oxidative stress promotes myocardial tissue remodeling in the transgenic TG (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293:E355–E363 [DOI] [PubMed] [Google Scholar]
- 25. DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. 2008. Oxidative Stress Contributes to Pulmonary Hypertension in the Transgenic (mRen2) 27 Ren2 Rat. Am J Physiol Heart Circ Physiol 294:H2659–H2668 [DOI] [PubMed] [Google Scholar]
- 26. Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917 [DOI] [PubMed] [Google Scholar]
- 27. Bowden JA, Albert CJ, Barnaby OS, Ford DA. 2011. Analysis of cholesteryl esters and diacylglycerols using lithiated adducts and electrospray ionization-tandem mass spectrometry. Anal Biochem 417:202–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han X, Gross RW. 2005. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev 24:367–412 [DOI] [PubMed] [Google Scholar]
- 29. Han X, Gross RW. 2001. Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem 295:88–100 [DOI] [PubMed] [Google Scholar]
- 30. Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C, Lopaschuk GD. 2011. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res 89:148–156 [DOI] [PubMed] [Google Scholar]
- 31. Semeniuk LM, Kryski AJ, Severson DL. 2002. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol 283:H976–H982 [DOI] [PubMed] [Google Scholar]
- 32. Van den Bergh A, Flameng W, Herijgers P. 2006. Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur J Heart Fail 8:777–783 [DOI] [PubMed] [Google Scholar]
- 33. Soylu A, Duzenli MA, Yazici M, Ozdemir K, Tokac M, Gok H. 2009. The effect of nondipping blood pressure patterns on cardiac structural changes and left ventricular diastolic functions in normotensives. Echocardiography 26:378–387 [DOI] [PubMed] [Google Scholar]
- 34. Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. 2009. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol 94:648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boudina S, Abel ED. 2010. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ren J, Pulakat L, Whaley-Connell A, Sowers JR. 2010. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med 88:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bugger H, Abel ED. 2010. Mitochondria in the diabetic heart. Cardiovasc Res 88:229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. 2005. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112:2686–2695 [DOI] [PubMed] [Google Scholar]
- 39. Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. 2007. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56:2457–2466 [DOI] [PubMed] [Google Scholar]
- 40. Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. 2005. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146:5341–5349 [DOI] [PubMed] [Google Scholar]
- 41. Aasum E, Hafstad AD, Severson DL, Larsen TS. 2003. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 52:434–441 [DOI] [PubMed] [Google Scholar]
- 42. Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. 2005. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 96:225–233 [DOI] [PubMed] [Google Scholar]
- 43. Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. 2001. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O'Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE. 2011. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 121:1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Young ME, McNulty P, Taegtmeyer H. 2002. Adaptation and maladaptation of the heart in diabetes: part II: potential mechanisms. Circulation 105:1861–1870 [DOI] [PubMed] [Google Scholar]
- 46. Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. 1999. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation 99:384–391 [DOI] [PubMed] [Google Scholar]
- 47. Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW., 2nd 2003. Protein kinase Cα negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res 93:1111–1119 [DOI] [PubMed] [Google Scholar]
- 48. Abel ED. 2011. A new twist in the function of the cardiac lipid droplet. Nat Med 17:1045–1046 [DOI] [PubMed] [Google Scholar]
- 49. O'Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. 2006. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am J Physiol Endocrinol Metab 290:E448–E455 [DOI] [PubMed] [Google Scholar]
- 50. Harmancey R, Wilson CR, Wright NR, Taegtmeyer H. 2010. Western diet changes cardiac acyl-CoA composition in obese rats: a potential role for hepatic lipogenesis. J Lipid Res 51:1380–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dowhan W, Bogdanov M, Mileykovskaya E. 2008. Functional roles of lipids in membranes. In: Vance DE, Vance JE, eds. Biochemistry of lipids, lipoproteins and membranes. 5th ed Amsterdam: Elsevier; 1–37 [Google Scholar]
- 52. Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. 2008. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res 49:2101–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Summers SA. 2006. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45:42–72 [DOI] [PubMed] [Google Scholar]
- 54. Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM. 2006. TNF-α contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 26:475–480 [DOI] [PubMed] [Google Scholar]
- 55. Torre-Villalvazo I, Gonzalez F, Aguilar-Salinas CA, Tovar AR, Torres N. 2009. Dietary soy protein reduces cardiac lipid accumulation and the ceramide concentration in high-fat diet-fed rats and ob/ob mice. J Nutr 139:2237–2243 [DOI] [PubMed] [Google Scholar]
- 56. Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. 2007. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J Clin Invest 117:2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. 2006. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 55:2579–2587 [DOI] [PubMed] [Google Scholar]
- 58. Lee SY, Kim JR, Hu Y, Khan R, Kim SJ, Bharadwaj KG, Davidson MM, Choi CS, Shin KO, Lee YM, Park WJ, Park IS, Jiang XC, Goldberg IJ, Park TS. 2012. Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J Biol Chem 287:18429–18439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Birse RT, Bodmer R. 2011. Lipotoxicity and cardiac dysfunction in mammals and Drosophila. Crit Rev Biochem Mol Biol 46:376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. 2011. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473:528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.