Abstract
Pregnancy-associated plasma protein-A (PAPP-A) is a large multidomain metalloprotease involved in cleavage of IGF binding protein (IGFBP)-4 and -5 thereby causing release of bioactive IGF. Individual domains of PAPP-A have been characterized in vitro, including the metzincin proteolytic domain important for IGFBP proteolytic activity, short consensus repeats critical for cell surface association, and Lin-12/Notch repeat module demonstrated to determine IGFBP substrate specificity. To test the hypothesis that specific cleavage of IGFBP-4 by PAPP-A in close proximity to the cell surface is required for development of lesions in a murine model of atherosclerosis, the following PAPP-A transgenic (Tg) mice were generated: TgE483A, which lacks all PAPP-A proteolytic activity; TgD1499A, which selectively lacks proteolytic activity against IGFBP-4; and TgK1296A/K1316A, in which cell surface binding is compromised. Following cross-breeding with apolipoprotein E (ApoE) knockout (KO) mice, ApoE KO/Tg mice were fed a high-fat diet to promote aortic lesion development. Lesion area was increased 2-fold in aortas from ApoE KO/Tg wild-type compared with ApoE KO mice (P < 0.001). However, there was no significant increase in the lesion area in any of the ApoE KO/Tg mutant mice. We conclude that PAPP-A proteolytic activity is required for the lesion-promoting effect of PAPP-A and that its specificity must be directed against IGFBP-4. Furthermore, our data demonstrate that cleavage of IGFBP-4 at a distance from the cell surface, and hence from the IGF receptor, is not effective in promoting the development of the atherosclerotic lesions. Thus, PAPP-A exerts its effect while bound to the cell surface in vivo.
Pregnancy-associated plasma protein-A (PAPP-A) is a complex, multidomain protein with the potential to be multifunctional. However, to date, the only identified function of PAPP-A is as an IGF binding protein (IGFBP) protease (see the review in Ref. 1). PAPP-A is the major, if not the only, physiological protease for IGFBP-4 in mice and humans (2–5). IGFBP-5 has also been identified as a substrate for PAPP-A (6), although the physiological significance of this has yet to be determined.
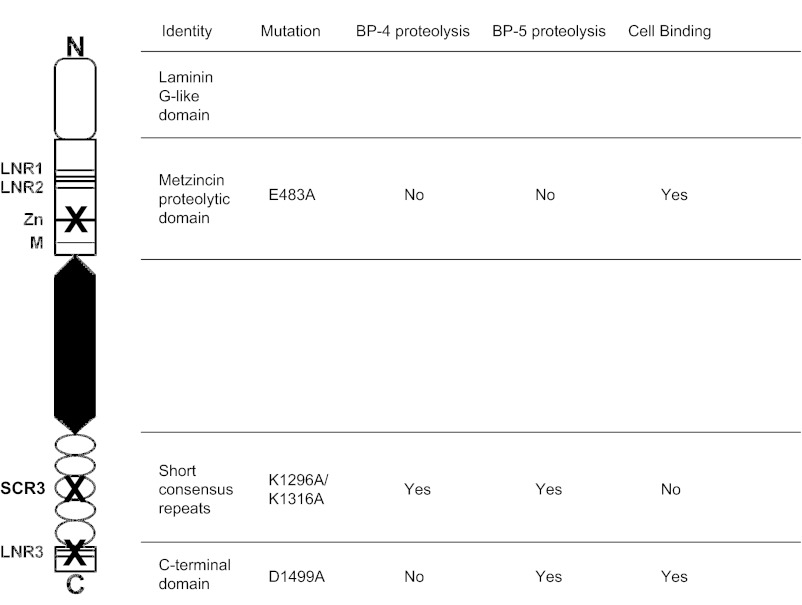
PAPP-A is composed of five major domains (see Fig. 1). The N-terminal domain is related to laminin G-like modules and has no known function (7). The proteolytic domain contains the two motifs that define the primary structures of members of the metzincin superfamily, an elongated zinc-binding consensus sequence and downstream Met-turn (8). Two Lin-12/Notch repeat (LNR) modules are present within the proteolytic domain, with a third present close to the C terminus. Head-to-tail dimerization of PAPP-A subunits through LNR1 and LNR2 interaction with LNR3 is obligatory for proteolytic activity against IGFBP-4 but not against IGFBP-5 (9, 10). PAPP-A also contains five sequential copies of short consensus repeats (SCRs) in its C-terminal domain. A PAPP-A variant with mutation of SCR3 retains proteolytic activity but is not able to bind to heparan sulfate proteoglycans present on cell surfaces, and it has been hypothesized that cell-associated PAPP-A serves to potentiate delivery of IGF to transmembrane signaling receptors (11). Although in vitro analyses provide clues to the structure/function roles of the different domains, there has been limited in vivo evaluation.
Fig. 1.
Model of the domain structure of PAPP-A protein indicating the mutations introduced and their consequences for IGFBP proteolysis and ability to bind to cells.
In a previous study, transgenic mice overexpressing wild-type PAPP-A in arterial smooth muscle exhibited enhanced lesion development in a murine model of atherosclerosis (12). In the present study, we generated transgenic mice expressing three PAPP-A mutants driven by the same targeting promoter to ascertain the structural and functional determinants of PAPP-A critical for its ability to enhance atherosclerotic plaque development. We hypothesized that the ability of PAPP-A to proteolyze specific IGFBPs and bind to vascular cells is essential for enhanced lesion development.
Materials and Methods
Transgene construct and mutagenesis
The 445-bp SM22α promoter engineered to continuously express during lesion development (ΔSM22α) was used to drive PAPP-A expression in arterial smooth muscle, as described previously (12). Mutations (see Fig. 1) were introduced by overlap extension PCR, using the plasmid encoding full-length human PAPP-A following the mutated vascular smooth muscle cell promoter. Two outer primers 5′-CTGAGGCCTTCAAGCAATAC-3′ and 5′-CCGGACCACCTTATACTTCA-3′ were used in combination with mutated primers 5′-ATGATCCATGCGATTGGTCACAGCCTGGGCC-3′ and 5′-GTGACCAATCGCATGGATCATGGTGTGGGTG-3′ (introduced mutation is underlined) to generate a mutated fragment subsequently digested with BspEI/ClaI restriction enzymes and cloned into pΔSM22α-PA to obtain the variant pΔSM22α-PA-E483A. Outer primers, 5′-CCAGGTGTGTCGAACCAAG-3′ and 5′-ACAGATGGCTGGCAACTAGA-3′, and mutated primers, 5′-CTGCAAATACGCATGCAAGCCTGGATACCATG-3′ and 5′-AGGCTTGCATGCGTATTTGCAGAAGGAGCCCA-3′, in combination with 5′-ACGGGCCTTCGCGACTCAGTGTACCCAGGATG-3′ and 5′-ACACTGAGTCGCGAAGGCCCGTTTCTTTGACT-3′ were used for construction of variant pΔSM22α-PA-K1296A/K1316A or mutated primers 5′-TGCAACTATGCCGGTGGGGATTGCTGCACCT-3′ and 5′-ATCCCCACCGGCATAGTTGCAAAAGGCTCGG-3′ for construction of variant pΔSM22α-PA-D1499A. The PCR fragments were swapped into pΔSM22α-PA using KpnI/ApaI. All PCRs were made using plaque-forming units of DNA polymerase (Promega, Madison, WI). All constructs were verified by sequence analysis and three independent clones of each construct were used for further analysis. Rat arterial smooth muscle cells (A7r5; American Type Culture Collection, Manassas, VA) were transfected with a similar amount of purified plasmid DNA per number of cells using TransFast transfection reagent (Promega), and effective expression of PAPP-A variants was confirmed for the mutated constructs.
Generation of transgenic mice
Expression constructs, with bacterial vector sequences removed, were linearized and purified before pronuclear microinjection by the Mayo Clinic Transgenic Core Facility. Embryos from FVB NHSD mice (Harlan, Indianapolis, IN) were used for injection. Positive founders for transgene integration were identified using two complementary PCR strategies targeting ΔSM22α and human PAPP-A sequences, as described (12). Founders demonstrating germline transmission when crossed with the wild-type FVB mice were characterized further: wild-type human PAPP-A (PAPP-A TgWT), proteolytically inactive PAPP-A (PAPP-A TgE483A), PAPP-A with proteolytic activity against IGFBP-5 but not IGFBP-4 (PAPP-A TgD1499A), and non-cell-binding PAPP-A (PAPP-A TgK1296A/K1316A). Two founders for each mutation were used to ensure that the results were not due to insertional effects of the transgene.
All animal procedures were approved by Mayo Clinic's Institutional Animal Care and Use Committee and complied with the standards stated in the Guide for the Care and Use of Laboratory Animals.
Primary smooth muscle cell cultures
Primary smooth muscle cells were isolated from mouse aorta (AoSMCs), as described previously (13). AoSMCs were used between passage 3 and 7 for experiments.
Enzyme-linked immunosorbent assays
Human PAPP-A was measured by an ultrasensitive PAPP-A ELISA from Beckman Coulter, Inc. (Brea, CA) or Ansh Labs (Webster, TX)). These ELISAs do not detect murine PAPP-A. Serum IGF-I was measured using a mouse IGF-I ELISA kit generously provided by ImmunoDiagnostic Systems, Inc. (Fountain Hills, AZ).
IGFBP protease assays
Conditioned media (serum free containing 0.1% BSA) or aortas homogenized in mammalian protein extraction buffer (Thermo Scientific, Rockford, IL) were incubated at 37 C for 6 h with equal starting amounts of 125I-IGFBP-4 (10,000 cpm) in the absence or presence of 5 nmol/liter recombinant IGF-II (Bachem Inc., Torrance, CA), as described (2, 13). IGF-II facilitates PAPP-A-mediated IGFBP-4 proteolysis in the assay by binding IGFBP-4 and likely changing its conformation (14). For IGFBP-5 proteolysis, conditioned media were incubated with equal starting amounts of 125I-IGFBP-5 without IGF, as described (15). Reaction products were separated by SDS-PAGE and visualized by autoradiography.
Atherosclerosis model
As in our previous study (12), PAPP-A Tg mice (FVB background) were crossed with apolipoprotein E (ApoE)-deficient mice (mixed C57BL/6 and 129 background), the latter being an established mouse model of atherosclerosis (16–18). Offspring from this mating, which were heterozygous for the ApoE gene and positive for PAPP-A transgene expression, were then intercrossed to produce ApoE knockout (KO) mice negative or positive for the transgene, thereby generating ApoE KO (control), ApoE KO/TgWT, ApoE KO/TgE483A, ApoE KO/TgD1499A, and ApoE KO/TgK1296A/K1316A mice. Littermates, males and females housed separately up to five per cage, were fed a high-fat, Western-style diet [21% by weight (42% of calories) fat and 0.15% by weight cholesterol (Harland Tekland, South Easton, MA) starting at 7 wk of age] for 10 wk, as previously described (12). At harvest, the mice were anesthetized and blood was collected from the retroorbital plexus. Hearts and aorta were perfused with 20 ml PBS containing 20 mmol/liter 2,6-Di-tert-butyl-4-methylphenol and 2 mmol/liter EDTA at the rate of 1 ml/min. After perfusion, the aorta was carefully dissected from the intercostal arteries to the iliac bifurcation, cleaned of extravesicular fat and connective tissue, opened longitudinally, and pinned in place on black wax for en face analysis. Lipid-rich regions were stained with Sudan IV and images captured using a Nikon SMZ80 dissecting scope (Tokyo, Japan), a Nikon DMX200F camera, and Nikon Act-1 software version 2.62. Image analyses were performed using Adobe Photoshop software version 6.0.1 (San Jose, CA). The lesion area was calculated for each animal as a percent of total aortic area.
RNA isolation and quantitative PCR
RNA was isolated from tissues and cultured cells using the ribonuclease minikit (QIAGEN Inc., Valencia, CA). One microgram of RNA from each sample was reverse transcribed with Taqman reverse transcription reagents, random hexamers, and Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA).
Quantitative PCRs were conducted using the primer sequences either previously published (12) or listed below and the iCycler iQ detection system. Amplification plots were analyzed with the iCycler iQ detection system analysis software, version 3.0.6070 (Bio-Rad Laboratories, Hercules, CA). Gene expression was normalized to mouse ribosomal protein L19 as an internal control. These include the following: human PAPP-A forward, 5′-TGACGCGCGAGCAGGTGG-3′, reverse, 5′-CCGTCGTGGCCCGTCAGC-3′; and mouse PAPP-A forward, 5′-CGGCTTCCTGTTGGAC-3′, reverse, 5′-CAATCTGTTGCCAGCTCA-3′.
Data analysis
An ANOVA and the Dunnett test were used to compare the means of multiple groups to a control. Means were considered significant at P < 0.05 and values presented are mean ± sem.
Results
Expression and characterization of PAPP-A variants
A variety of PAPP-A mutations that could be used to probe the structural determinants of PAPP-A function have been described (8, 9, 11, 19). Using mutagenesis, we chose to manipulate key functions of PAPP-A including general and differential IGFBP proteolysis as well as cell surface binding (Fig. 1). To express proteolytically inactive PAPP-A, we mutated glutamate 483 of PAPP-A to alanine. [The numbering of the mature PAPP-A polypeptide is used in this paper. Glu1 of mature PAPP-A is at position 82 of prepro-PAPP-A (Swiss-Prot accession no. Q13219).] This glutamate residue is part of the zinc-binding consensus sequence of the metzincins, HExxHxxGxxH, in PAPP-A specifically H482EIGHSLGLYH492. The glutamate functions to activate the zinc-bound water molecule involved in the hydrolysis of the peptide bond to be cleaved and is therefore critical for catalysis. The E483A mutant shows a complete loss of proteolytic activity (8).
In contrast, compromising functionality in either of the three LNR modules causes a complete loss of proteolytic activity against IGFBP-4, whereas proteolytic activity against IGFBP-5 is unaffected (9). To obtain a PAPP-A mutant with such selective loss of IGFBP-4 proteolysis, aspartate 1499 was substituted with alanine.
The ability of PAPP-A to bind to cells was previously mapped to SCR3–4 (11, 19). Two patches of basic residues were defined, and the patch in SCR3 was found to be the most important. The most dramatic effects from mutation of single residues on cell surface binding were observed with K1296A and K1316A. These residues are located in two different clusters within SCR3 of PAPP-A. We hypothesized that the mutation of both K1296A and K1316A would produce a PAPP-A variant that has no cell surface binding but shows wild-type proteolytic activity against both IGFBP substrates.
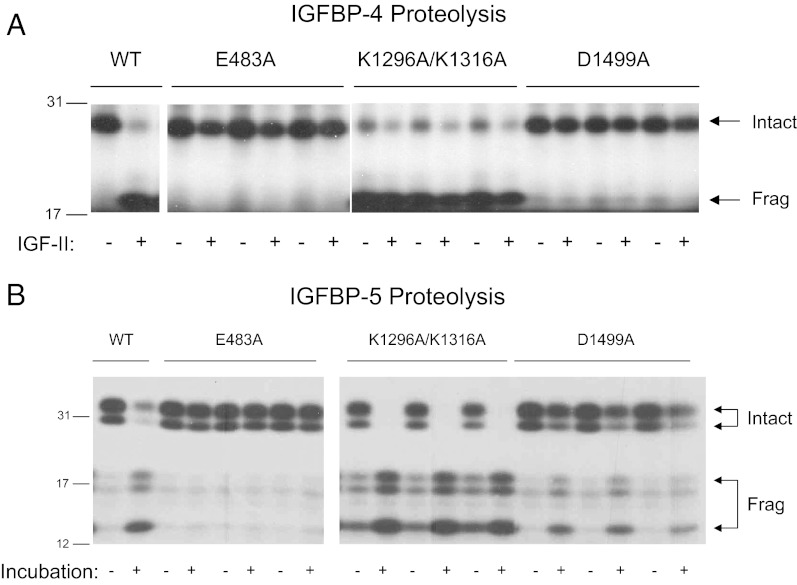
The mutations outlined above were introduced in the ΔSM22α-PA construct, which drives PAPP-A expression in arterial smooth muscle (12). After the transient transfection in rat arterial smooth muscle cells, three individual clones of each PAPP-A mutant were characterized with respect to protein expression levels (Table 1) and IGFBP protease activity (Fig. 2). IGFBP-4 proteolysis was inhibited in conditioned medium from cells expressing the proteolytically inactive (E483A) and selectively inactive (D1499A) PAPP-A mutant clones (Fig. 2A). Inhibition of PAPP-A cell binding caused by the K1296A/K1316A mutations resulted in a 6-fold increase in PAPP-A protein in the conditioned medium relative to WT (Table 1), explaining the increased IGFBP-4 proteolytic activity in the protease assays for this mutant (Fig. 2A). This proteolysis also occurred in the absence of IGF-II, although its addition increased the degree of proteolysis. IGF-independent IGFBP-4 proteolysis has been reported previously at high PAPP-A concentrations (6). In a similar manner, conditioned medium from cells expressing the K1296A/K1316A mutant clones showed increased IGFBP-5 proteolysis (Fig. 2B). Only the E483A mutant clones had inhibited IGFBP-5 proteolysis. These data confirm the characteristics of the PAPP-A mutant clones as outlined in Fig. 1. PAPP-A transgenic mice were then generated based on these constructs.
Table 1.
PAPP-A protein levels in aortic smooth muscle cell-conditioned medium
| PAPP-A mutants | PAPP-A (mIU/ml) |
|---|---|
| WT | 1.8 ± 0.10 |
| E483A | 4.3 ± 0.60a |
| D1499A | 1.7 ± 0.13 |
| K1296A/K1316A | 11.8 ± 1.92a |
E483A, Proteolytically inactive PAPP-A; D1499A, PAPP-A with proteolytic activity against IGFBP-5 but not IGFBP-4; K1296A/K1316A, non-cell-binding PAPP-A.
P < 0.05 vs. WT.
Fig. 2.
Effect of mutations in PAPP-A on IGFBP-4 (A) and IGFBP-5 (B) proteolysis. Conditioned medium from AoSMCs transfected with plasmids encoding WT and mutated PAPP-A was incubated with 125I-labeled IGFBP. Intact and fragmented IGFBPs are indicated by arrows. IGFBP-4 fragments run as a single band because PAPP-A-mediated IGFBP-4 proteolysis is IGF dependent. −/+ indicates incubation without and with IGF-II. For IGFBP-5 proteolysis, −/+ indicates without or with incubation. IGFBP-5 is O-glycosylated, giving rise to the cleavage products of different sizes. Conditioned medium from transfection experiments using three individual clones of each mutant was used for analysis. Molecular mass markers (in kilodaltons) are indicated on the left. Frag, fragmented.
Characterization of PAPP-A transgenic mice
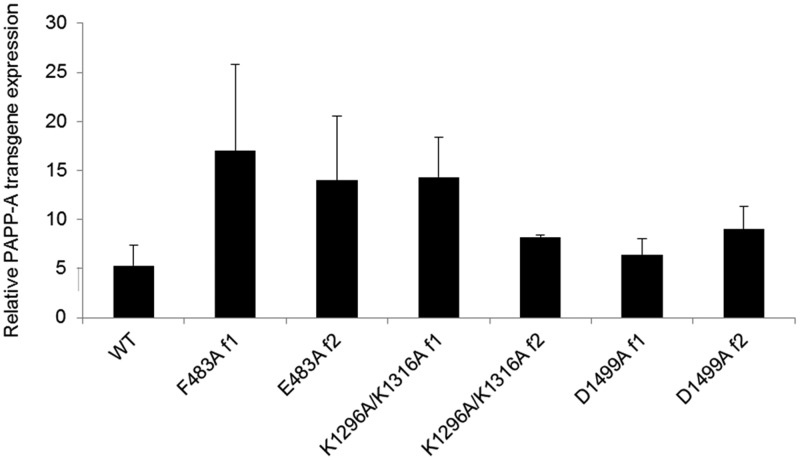
Transgene overexpression of human PAPP-A was examined in cultures of aortic smooth muscle cells from founders showing germline transmission (Fig. 3). Two founders for each of the PAPP-A Tg mutants and a previously characterized PAPP-A TgWT founder with low to moderate PAPP-A overexpression (designated Tg-4 in Ref. 12) were selected for further study. In addition to these cell culture studies, human PAPP-A (1.07 ± 0.149 ng per 5 μg protein) was detected in fresh aortic homogenates from PAPP-A Tg mice using an ultrasensitive PAPP-A ELISA that recognizes human (limit of detection 0.037 ng) but not murine PAPP-A.
Fig. 3.
PAPP-A transgene overexpression in primary cultures of aortic smooth muscle cells. The level of PAPP-A overexpression in aortic smooth muscle cells of each transgene founder (f) was established by real-time PCR. Results (means ± se, n = 3–5) are expressed as human PAPP-A transgene expression relative to endogenous mouse PAPP-A expression, as described previously (12).
Effect of PAPP-A expression on the development of atherosclerotic lesions
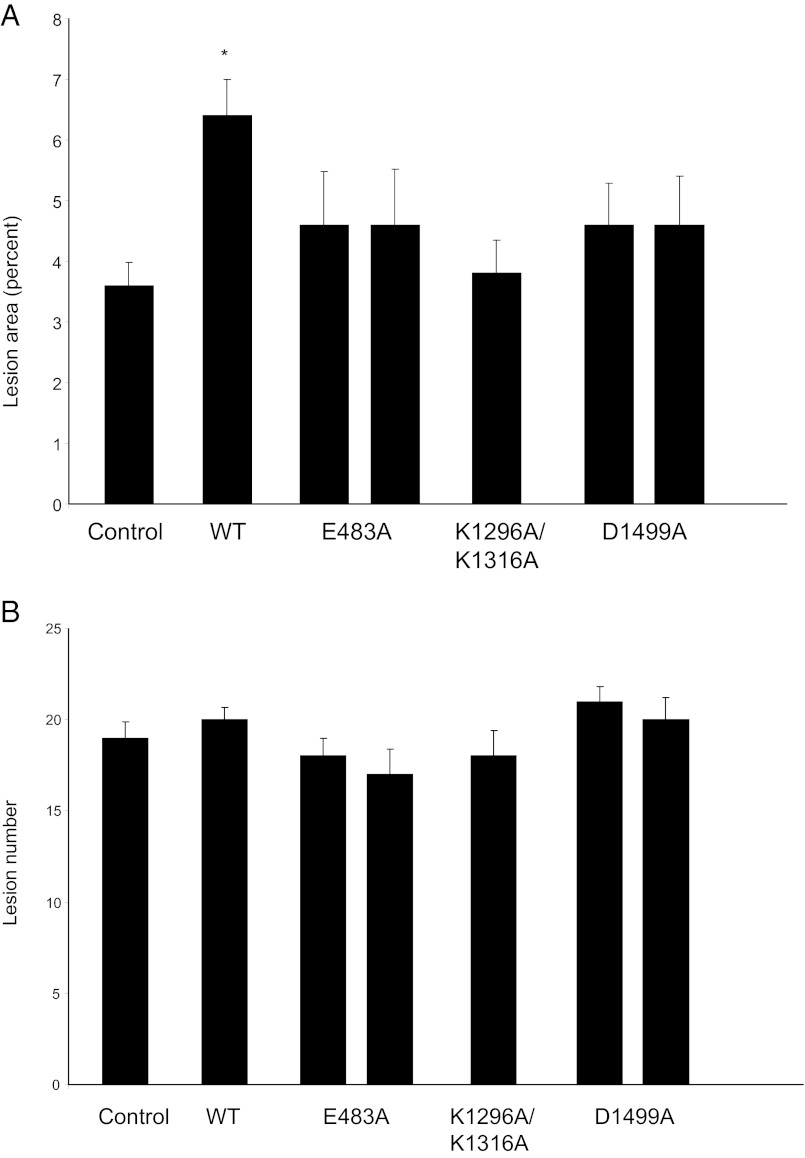
PAPP-A Tg mice were crossed with ApoE KO mice to generate mice overexpressing various PAPP-A mutants on an ApoE-null background. These mice were fed a high-fat, Western-style diet beginning at 7 wk of age. All mice gained weight over the 10 wk, serum IGF-I levels were not significantly different among the different groups, and there was no detectable human PAPP-A in the circulation of these mice (data not shown). After 10 wk on the diet, lesions in the descending aorta to the iliac bifurcation were stained with Sudan IV and evaluated for lesion area and number (Fig. 4). As shown in Fig. 4A, ApoE KO/TgWT mice had a 2-fold increase in lesion area compared with control ApoE KO mice (P < 0.001). On the other hand, there was no significant effect of overexpression of PAPP-A TgE483A, TgD1499A, or TgK1296A/K13116A on lesion area in the aorta. There was no significant difference in lesion number in the aorta among the different PAPP-A Tg mice (Fig. 4B), in agreement with previous studies that indicated an effect of PAPP-A on atherosclerotic lesion progression rather than initiation (12, 20). Of note, one of the two PAPP-A TgK1296A/K1316A lines showed a bimodal distribution with approximately half the mice (n = 11) having a mean plaque area of 19% (range 11–49%) and a mean plaque number of 31 (range 26–39). These numbers are outside the range of the other PAPP-A TgK1296/K1316A founder (plaque area 3.8 ± 0.55; plaque number 18 ± 1.4) and even the ApoE KO/TgWT (plaque area 6.5 ± 0.60; plaque number 20 ± 0.7).
Fig. 4.
Lesion area (A) and number in aortas (B) from ApoE KO mice and ApoE KO mice expressing WT and mutated PAPP-A transgenes after 10 wk on a high-fat diet. Results are means ± se (n = 10–20 mice). *, Significantly different from ApoE KO (control) (P < 0.05).
Discussion
In this study we generated transgenic mice expressing WT and mutant PAPP-A in arterial smooth muscle to determine the requirements of general proteolytic activity, specific proteolytic activity against IGFBP-4, and cell surface binding of PAPP-A for its effect on atherosclerotic lesion progression.
Proteolytic activity was critical for the effect of PAPP-A in this injury model because the ApoE KO/TgE483A did not accelerate lesion development beyond what was seen in the ApoE KO control mice. Similarly, overexpression of a PAPP-A mutant E483Q with impaired proteolytic activity had reduced effects on tumor growth relative to WT (21).
Results with ApoE KO/TgD1499A mice indicated the relative importance of IGFBP-4 and IGFBP-5 proteolysis by PAPP-A in atherosclerotic lesion development. IGFBP-4 is known to bind and inhibit IGF interaction with receptors. Based on our previous results indicating that proteolysis of IGFBP-4 is the primary consequence of PAPP-A activity on body size determination during fetal development in mice (2, 22), we anticipated that ApoE KO/TgD1499A mice would not exhibit accelerated lesion development. These were the first in vivo studies addressing possible physiological significance of PAPP-A-mediated IGFBP-5 proteolysis. Interestingly, there was no significant effect of PAPP-A D1499A overexpression on aortic lesion development. However, we cannot rule out the involvement of IGFBP-5 proteolysis by PAPP-A if colocalized IGFBP-4 in turn binds free IGF, also pointing to the importance of PAPP-A and IGFBP-4 as the primary pair of protease and substrate promoting lesion development in this model (23). Furthermore, the function of IGFBP-5 is controversial because it has been reported to have both IGF-dependent and IGF-independent actions, and some of its actions may be due to a proteolytically derived fragment (24). Whereas PAPP-A is the only known physiological protease for IGFBP-4, there are several proteases that have IGFBP-5 as substrate (25–28), so the impact of PAPP-A-specific IGFBP-5 proteolysis may be difficult to ascertain in vivo.
Although cell-associated PAPP-A has been characterized in vitro (29, 30), its importance in vivo had not been investigated as of yet. It has been hypothesized that cell surface association of PAPP-A is necessary for the effective concentration and localization of the proteolytically released IGF in the microenvironment (11). This appears to be the case because the loss of the ability of PAPP-A to bind to the surface of vascular cells, even though it is secreted by the arterial SMCs and retains proteolytic activity, resulted in attenuated effectiveness on lesion development in this model (ApoE KO/TgK1296A/K1316A vs. ApoE KO/TgWT). However, firm conclusions could not be drawn because one of the two founders showed a bimodal distribution by which approximately half the mice had dramatic increases in mean plaque area and number. Although active PAPP-A distributed over a broader area could have additional effects on the microenvironment, these anomalies are more likely explained by an insertional effect, a major limitation of transgenic analyses.
In summary, this study shows that the accelerated atherosclerotic lesion development from overexpression of PAPP-A is directly related to its specific proteolytic activity against IGFBP-4 in proximity to the cell surface, improving our understanding of the structure/function relationship of PAPP-A that is critical for potential future studies manipulating PAPP-A for therapeutic benefit.
Acknowledgments
We thank Suban Chakraborty, Sally West, Emily Mason, Jessica Mader, and Jacquelyn Grell for their excellent technical assistance.
This work was supported by National Institutes of Health Grant HL-74871 (to C.A.C.).
Current address for H.B.B.: Centre for Molecular Biology and Neuroscience, Department of Anatomy, University of Oslo, P.O. Box 1105 Blindern, N-0317 Oslo, Norway.
Disclosure Summary: H.B.B., L.K.B., Z.T.R., C.O., M.T.O., and C.A.C. have nothing to disclose.
Footnotes
- AoSMC
- Smooth muscle cells isolated from mouse aorta
- ApoE
- apolipoprotein E
- IGFBP
- IGF binding protein
- KO
- knockout
- LNR
- Lin-12/Notch repeat
- PAPP-A
- pregnancy-associated plasma protein-A
- SCR
- short consensus repeat
- Tg
- transgenic.
References
- 1. Conover CA. 2012. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab 23:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Füchtbauer EM, Oxvig C, van Deursen J. 2004. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development 131:1187–1194 [DOI] [PubMed] [Google Scholar]
- 3. Phang D, Rehage M, Bonafede B, Hou D, Xing W, Mohan S, Wergedal JE, Qin X. 2010. Inactivation of insulin-like growth factors diminished the anabolic effects of pregnancy-associated plasma protein-A (PAPP-A) on bone in mice. Growth Horm IGF Res 20:192–200 [DOI] [PubMed] [Google Scholar]
- 4. Byun D, Mohan S, Yoo M, Sexton C, Baylink DJ, Qin X. 2001. Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J Clin Endocrinol Metab 86:847–854 [DOI] [PubMed] [Google Scholar]
- 5. Conover CA, Oxvig C, Overgaard MT, Christiansen M, Giudice LC. 1999. Evidence that the insulin-like growth factor binding protein-4 protease in human ovarian follicular fluid is pregnancy associated plasma protein-A. J Clin Endocrinol Metab 84:4742–4745 [DOI] [PubMed] [Google Scholar]
- 6. Laursen LS, Overgaard MT, Søe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. 2001. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 504:36–40 [DOI] [PubMed] [Google Scholar]
- 7. Boldt HB, Glerup S, Overgaard MT, Sottrup-Jensen L, Oxvig C. 2006. Definition, expression, and characterization of a protein domain in the N-terminus of pregnancy-associated plasma protein-A distantly related to the family of laminin G-like modules. Protein Expr Purif 48:261–273 [DOI] [PubMed] [Google Scholar]
- 8. Boldt HB, Overgaard MT, Laursen LS, Weyer K, Sottrup-Jensen L, Oxvig C. 2001. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J 358:359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boldt HB, Kjaer-Sorensen K, Overgaard MT, Weyer K, Poulsen CB, Sottrup-Jensen L, Conover CA, Giudice LC, Oxvig C. 2004. The Lin12-notch repeats of pregnancy-associated plasma protein-A bind calcium and determine its proteolytic specificity. J Biol Chem 279:38525–38531 [DOI] [PubMed] [Google Scholar]
- 10. Weyer K, Boldt HB, Poulsen CB, Kjaer-Sorensen K, Gyrup C, Oxvig C. 2007. A substrate specificity-determining unit of three Lin12notch repeat modules is formed in trans with the pappalysin-1 dimer and requires a sequence stretch C-terminal to the third module. J Biol Chem 282:10988–10999 [DOI] [PubMed] [Google Scholar]
- 11. Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C. 2002. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats. J Biol Chem 277:47225–47234 [DOI] [PubMed] [Google Scholar]
- 12. Conover CA, Mason MA, Bale LK, Harrington SC, Nyegaard M, Oxvig C, Overgaard MT. 2010. Transgenic overexpression of pregnancy-associated plasma protein-A in murine arterial smooth muscle accelerates atherosclerotic lesion development. Am J Physiol Heart Circ Physiol 299:H284–H291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Resch ZT, Simari RD, Conover CA. 2006. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology 147:5634–5640 [DOI] [PubMed] [Google Scholar]
- 14. Qin X, Byun D, Lau KH, Baylink DJ, Mohan S. 2000. Evidence that the interaction between insulin-like growth factor (IGF)-II and IGF binding protein (IGFBP)-4 is essential for the action of the IGF-II-dependent IGFBP-4 protease. Arch Biochem Biophys 379:209–216 [DOI] [PubMed] [Google Scholar]
- 15. Conover CA, Boldt HB, Bale LK, Clifton KB, Grell JA, Mader JR, Mason EJ, Powell DR. 2011. Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice. Endocrinology 152:2837–2844 [DOI] [PubMed] [Google Scholar]
- 16. Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. 1994. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 14:133–140 [DOI] [PubMed] [Google Scholar]
- 17. Reddick RL, Zhang SH, Maeda N. 1994. Atherosclerosis in mice lacking ApoE evaluation of lesional development and progression. Arterioscler Thromb 14:141–147 [DOI] [PubMed] [Google Scholar]
- 18. Smith JD, Breslow JL. 1997. The emergence of mouse models of atherosclerosis and their relevance to clinical research. J Intern Med 242:99–109 [DOI] [PubMed] [Google Scholar]
- 19. Weyer K, Overgaard MT, Laursen LS, Nielsen CG, Schmitz A, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C. 2004. Cell surface adhesion of pregnancy-associated plasma protein-A is mediated by four clusters of basic residues located in its third and fourth CCP module. Eur J Biochem 271:1525–1535 [DOI] [PubMed] [Google Scholar]
- 20. Harrington SC, Simari RD, Conover CA. 2007. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res 100:1696–1702 [DOI] [PubMed] [Google Scholar]
- 21. Boldt HB, Conover CA. 2011. Overexpression of pregnancy-associated plasma protein-A in ovarian cancer cells promotes tumor growth in vivo. Endocrinology 152:1470–1478 [DOI] [PubMed] [Google Scholar]
- 22. Ning Y, Schuller AG, Conover CA, Pintar JE. 2008. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol 22:1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laursen LS, Kjaer-Sorensen K, Andersen MH, Oxvig C. 2007. Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol Endocrinol 21:1246–1257 [DOI] [PubMed] [Google Scholar]
- 24. Schneider MR, Wolf E, Hoeflich A, Lahm H. 2002. IGF-binding protein-5: flexible player in the IGF system and effector on its own. J Endocrinol 172:423–440 [DOI] [PubMed] [Google Scholar]
- 25. Busby WH, Jr, Nam TJ, Moralez A, Smith C, Jennings M, Clemmons DR. 2000. The complement component C1s is the protease that accounts for the cleavage of insulin-like growth factor binding protein-5 in fibroblast medium. J Biol Chem 275:37638–37644 [DOI] [PubMed] [Google Scholar]
- 26. Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. 2001. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem 276:21849–21853 [DOI] [PubMed] [Google Scholar]
- 27. Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. 2002. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry 41:15394–15403 [DOI] [PubMed] [Google Scholar]
- 28. Hemers E, Duval C, McCaig C, Handley M, Dockray GJ, Varro A. 2005. Insulin-like growth factor binding protein-5 is a target of matrix metalloproteinase-7: implications for epithelial-mesenchymal signaling. Cancer Res 65:7363–7369 [DOI] [PubMed] [Google Scholar]
- 29. Sun IY, Overgaard MT, Oxvig C, Giudice LC. 2002. Pregnancy-associated plasma protein A proteolytic activity is associated with the human placental trophoblast cell membrane. J Clin Endocrinol Metab 87:5235–5240 [DOI] [PubMed] [Google Scholar]
- 30. Conover CA, Harrington SC, Bale LK, Oxvig C. 2007. Surface association of pregnancy-associated plasma protein-A accounts for its colocalization with activated macrophages. Am J Physiol Heart Circ Physiol 292:H994–H1000 [DOI] [PubMed] [Google Scholar]