Abstract
Sex hormone signaling regulates a variety of functions in the uterine endometrium essential for embryo implantation and immunity. Epithelial cells of the uterine endometrium are the target of the coordinated actions of estradiol (E2) and progesterone. However, little information exists regarding the interplay of estrogens with glucocorticoids in this tissue. Using the human uterine epithelial cell line ECC1, E2 was found to antagonize induction of the glucocorticoid-induced leucine zipper (GILZ) gene expression, which is associated with several of the immune-related functions of glucocorticoids. Interestingly, E2 antagonizes glucocorticoid regulated nascent RNA GILZ expression within 1 h of hormone treatment. Repression of glucocorticoid-induced GILZ expression requires the estrogen receptor (ER), because both treatment with the ER-antagonist ICI 182,780 and small interfering RNA knockdown of ERα block E2's ability to repress GILZ gene expression. Antagonism of glucocorticoid-induced GILZ expression may not be unique to ERα, as the ERβ agonist Liquiritigenin is also able to antagonize glucocorticoid signaling. Transcriptional regulation appears to be at the level of promoter binding. Both the glucocorticoid receptor and ERα are recruited to regions of the GILZ promoter containing glucocorticoid response elements and the transcriptional start site. Glucocorticoid receptor binding to these regions in the presence of dexamethasone decreases with E2 treatment. GILZ gene expression was also found to be repressed in the whole mouse uterus treated with a combination of dexamethasone and E2. Regulation of the antiinflammatory gene GILZ by glucocorticoids and E2 suggests cross talk between the immune modulating functions of glucocorticoids and the reproductive actions of estradiol signaling.
The coordinated actions of the female sex steroids regulate many essential functions in the uterine endometrium (reviewed in Ref. 1). Preovulatory increase in the secretion of estradiol (E2) promotes a wave of cell division in the luminal and glandular epithelium of the uterus that is required for efficient embryo implantation (2). In addition, the actions of progesterone and E2 regulate locally produced cytokines and growth factors to create a window, in which the generally immune hostile environment of the uterus becomes transiently permissive to embryo attachment and invasion (3, 4). The sex steroids E2 and progesterone not only alter the local environment in preparation for conception but also balance immune tolerance of the semiallogenic fetus while providing a network of protective immune mechanisms against microbial pathogens (5). The epithelial cells of the uterine endometrium are the target of many of the coordinated actions of female sex steroids within the uterus, acting as both the site of initial embryo contact and as the barrier to primary infection. Interestingly, the uterine epithelium also expresses the glucocorticoid receptor (GR), a known integrator of immune function (6).
Glucocorticoids regulate numerous physiological functions essential for life and play a fundamental role in the maintenance of both basal and stress-related homeostasis (7, 8). Although initially named for their role in glucose metabolism, the spectrum of functions attributed to glucocorticoids now includes several key biological processes important for growth, development, reproduction, and immune and inflammatory reactions (9). Due to the presence and activity of glucocorticoids in such a broad range of tissues and cell types, it is thought that changes in gene expression mediate the actions of glucocorticoids. Gene expression changes are regulated by signaling through intracellular GR, a member of the nuclear receptor superfamily of transcription factor proteins (10–12). Upon ligand binding, GR translocates to the nucleus, where it can regulate gene expression in both a positive and negative manner. Microarray studies performed in our lab on hormone-treated whole rat and mice uteri, as well as a human uterine fibroid cell line, demonstrate that glucocorticoids significantly regulate thousands of genes in this tissue and cells (13, 14). Moreover, treatment with glucocorticoids and E2 in the uterus results in a large subset of genes that are uniquely coregulated, suggesting some interplay between the two hormones.
Uterine events, such as menstruation, implantation, and parturition, parallel an inflammatory event, and thus, it is likely that glucocorticoids, key immune regulators, play a substantial role in reproductive processes. Regulation of gene expression by glucocorticoids is one mechanism by which GR may regulate signaling in the uterus. However, interestingly in both the rat uterus and in a human uterine fibroid cell line, treatment with glucocorticoids and E2 have similar effects on the expression on many genes, with few genes displaying antagonistic regulation (13, 14). This finding does not reflect the antagonism of biological changes in the uterus often ascribed to combinations of glucocorticoids and estrogens (13, 15). Here, estrogens are able to induce rapid morphological changes similar to an acute inflammatory response, including edema of the stroma and myometrium, increases in vascular permeability, infiltration of immune cells, and enhanced bactericidal activity in the uterine luminal fluid (13, 15, 16). Glucocorticoids coadministered with E2 are able to block these immune-modulatory proinflammatory effects. Furthermore, increased synthesis of prostaglandins by E2, involved in both inflammation and parturition, is reduced in the uterus with glucocorticoid treatment (17). Despite the clear effects of glucocorticoids on biological responses induced by estrogens in the uterus, relatively little is known regarding the molecular basis of this antagonism and whether glucocorticoid antagonism on target tissue can be identified in the uterine epithelium, a critical cell type in the uterus for inflammatory events.
Although E2 and progesterone have been identified as key regulators in the uterus during pregnancy and as members of the local immune response, the search remains to identify other signaling networks important for embryo implantation in the uterine epithelium, a highly immunogenic event. Glucocorticoids, key immune regulators, provide an interesting possibility as a nonsex steroid reproductive hormone that may be involved in this process. With receptors present throughout the uterus and known antagonistic interplay with estrogens, it is a viable physiological mechanism presently understudied. Here, we show that GR is present and hormone responsive in a human uterine epithelial cell line (ECC1). Treatment of ECC1 cells with glucocorticoids and E2 has a distinct effect on gene expression that is mediated by direct transcriptional regulation. Specifically, we investigate the cellular mechanism by which glucocorticoids and E2 regulate gene expression in the uterine epithelium, focusing on a specific an immune effector, the glucocorticoid-induced leucine zipper (GILZ).
Materials and Methods
Reagents
RPMI 1640 was purchased from Invitrogen (Life Technologies, Inc., Carlsbad, CA). Heat inactivated fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Charcoal-stripped heat inactivated FBS was purchased from HyClone (Logan, UT). Dexamethasone (Dex) and E2 were purchased from Steraloids, Inc. (Newport, RI). Cycloheximide was purchased from EMD4 Biosciences (Gibbstown, NJ). Actinomycin D and Fulvestrant were purchased from Sigma-Aldrich (St. Louis, MO). Small interfering RNA (siRNA) targeted to estrogen receptor (ER) was purchased from Thermo Scientific (Waltham, MA). Liquiritigenin (Liq) was purchased from Tocris Bioscience (Minneapolis, MN). 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). TaqMan RT-PCR primer-probes were purchased from Applied Biosystems (Foster City, CA).
Culture of human ECC1 cells
An immortalized uterine human endometrial cancer cell line (ECC1) was obtained from the American Type Culture Collection (Manassas, VA) and grown in a standard tissue culture incubator at 37 C, with 95% humidity and 5% carbon dioxide. ECC1 cells were maintained in RPMI 1640 (Invitrogen; Life Technologies, Inc.) supplemented with 5% FBS and 24 h before treatment, media were changed to phenol red-free RPMI 1640 containing 5% charcoal dextran-treated (stripped) FBS. Cells were treated with 100 nm Dex (1,4-pregnadien-9-fluoro-16-methyl-11β,17,21-triol-3,20-dione) prepared in PBS or 10 nm E2 [1,3,5(10) Estratrien-3,17β-Diol] prepared in ethanol.
Animals and hormone treatment
Female 8-wk-old wild-type C57BL/6J mice [intact and ovariectomized (OVX)] were purchased from Charles River (Wilmington, MA). Mice were treated according to a protocol approved by the Institutional Animal Care and Use Committees at National Institute of Environmental Health Sciences. Hormones were prepared before injection as follows: 1) Dex was prepared by dissolving in saline with sonication until into solution, and 2) E2 was prepared by first dissolving in 100% ethanol and then diluting in saline. Eight-week-old virgin female mice were subjected to exogenous hormone treatment through ip injection with saline, 1 mg/kg of Dex, 10 μg/kg of E2, or 1 mg/kg of Dex and 10 μg/kg E2. Mice were killed by cervical dislocation and their uteri dissected and weighed at 4 or 24 h. For each mouse, one uterine horn was processed for RNA extraction and the other for protein extraction.
Western blot analysis
ECC1-treated cells grown in vitro or dissected mouse uteri were washed twice with ice-cold PBS, treated with 350-μl radioimmunoprecipitation buffer with the addition of protease inhibitor cocktail tablets (Roche, Indianapolis, IN) and phosphatase inhibitor cocktail set II (Calbiochem, La Jolla, CA), scraped off the plate, and incubated at 4 C for 30 min. Cellular debris was removed by centrifugation at 13,200 rpm (16,100 relative centrifugal force) for 20 min in a tabletop Eppendorf 5415R Centrifuge (Eppendorf International, Hauppauge, NY) at 4 C and supernatant collected. Protein concentration was determined using a BCA protein quantitation kit (Pierce, Rockford, IL). Samples containing 40 μg of protein were heated to 95 C for 5 min and separated on a 4-20% ReadyGel Tris-Gly gels (Bio-Rad, Hercules, CA). Proteins were then transferred onto a nitrocellulose membrane. Membranes were blocked for 1 h with 10% skim milk in Tris-buffered saline with 0.1% Tween 20 and subsequently probed with polyclonal anti-GR antibodies (1:1000) (18), monoclonal anti-ER antibodies (1:750; Immunotech, Marseille, France), polyclonal anti-GILZ antibodies (1:500; Santa Cruz Biotechnology, Inc.), or monoclonal anti-β-actin antibodies (1:10,000; Millipore, Temecula, CA). Immunoreactivity was visualized by incubation with horseradish peroxidase-linked secondary antibodies (GE Healthcare, Buckinghamshire, UK) and treatment with enhanced chemiluminescence reagents. Alternatively, quantification of immunoreactivity was determined by incubation with a mixture of goat antirabbit Alexa Fluorophore 680-conjugated (Molecular Probes, Carlsbad, CA) and goat antimouse IRDye 800-conjugated secondary (Rockland Immunochemicals, Gilbertsville, PA) antibodies for 1 h at room temperature and visualization using the Odyssey LI-COR imaging system (LI-COR Biosciences, Lincoln, NE).
RNA isolation
Total RNA was extracted from ECC1 cells and mice uteri using the QIAGEN RNeasy mini kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol. Deoxyribonuclease treatment was performed on-column using a ribonuclease-free deoxyribonuclease kit (QIAGEN) according to the manufacturer's instruction. Samples were quantified by Nanodrop ND-1000 (Thermo Scientific, Wilmington, DE) spectrophotometer, and purity was analyzed by the 260:280 absorbance ratio.
Quantitative real-time PCR (QRT-PCR)
QRT-PCR was performed by using the 7900HT sequence detection system and predesigned primer/probe sets obtained from Applied Biosystems and used according to the manufacturer's instructions. QRT-PCRs were performed on 100 ng of total RNA using the One-Step QRT-PCR Universal Master Mix reagent. Standard curves were generated by serial dilution of a preparation of total RNA isolated from ECC1 cells and mice uteri. The signal obtained from each gene primer-probe set was normalized to that of the unregulated housekeeping gene peptidylprolyl isomerase B (Cyclophilin B) primer-probe set (Applied Biosystems). Each primer-probe set was analyzed with at least three different sets of RNA.
RNA interference
ER and nontarget control (NTC) siRNA SMARTpools were purchased from Thermo Scientific. For each RNA interference experiment, cells were plated in six-well plates at approximately 70% confluency 1 d before transfection; 50 nm each siRNA was transfected into cells with DharmaFECT transfection reagent (Thermo Scientific) following manufacturer's instructions. After 48 h of siRNA treatment, cells were induced with 100 nm Dex, 10 nm E2, or Dex and E2.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed using the Magna ChIP A ChIP kit (Millipore) according to the manufacturer's protocol. Briefly, cells were plated in 150-mm dishes in RPMI 1640. After 24 h, growth medium was replaced with phenol red-free RPMI 1640 containing 5% charcoal dextran-treated (stripped) FBS. The cells were grown for an additional 24 h before treatment with either vehicle, 100 nm Dex, 10 nm E2, or Dex and E2 for 1 h. The cells were fixed in 1% formaldehyde and harvested in lysis buffer containing protease inhibitors. The nuclear contents were then sonicated using a Branson Sonifier 150 at setting 4 with 10-sec pulses, four times on ice. Sheared chromatin was precleared with rabbit IgG and protein A agarose/salmon sperm DNA and then immunoprecipitated overnight with 10 μl of polyclonal GR antibody (18), 10 μl of monoclonal ER antibody (Santa Cruz Biotechnology, Inc.), 2 μl of IgG (Millipore, Billerica, MA), and 10 μl of antibody to the phosphoserine 5 version of polymerase II (Pol II) (Covance, Princeton, NJ). After elution of protein:DNA complexes and DNA purification, PCR analysis was performed on immunoprecipitated and input DNA. PCR analysis of the glucocorticoid response elements (GREs), transcriptional start site (TSS), and estrogen response element (ERE) of trefoil factor 1 (TFF1) used the following primers: GRE 1919-1794 primer 1, 5′-GCTCAGAGACTTTGTGCGTATTTGG-3′ and primer 2, 5′-AGGCTTGATCAGAGAGGTTTG-3′; TSS primer 1, 5′AAAGCCCGGTACAGGACTCCATTTG-3′ and primer 2, 5′-ACCTCGTATGTCACAAACTCCACG-3′; and TFF1 ERE, 5′-TGGGCTTCATGAGCTCCTTCCCTT-3′ and primer 2, 5′-GATTCATAGTGAGAGATGGCCGGA-3′.
Statistical analysis
Data are presented as means ± sem. Statistical significance was determined by ANOVA with Tukey's post hoc analysis.
Results
Glucocorticoid and ER are expressed in the human uterine epithelial cell line ECC1
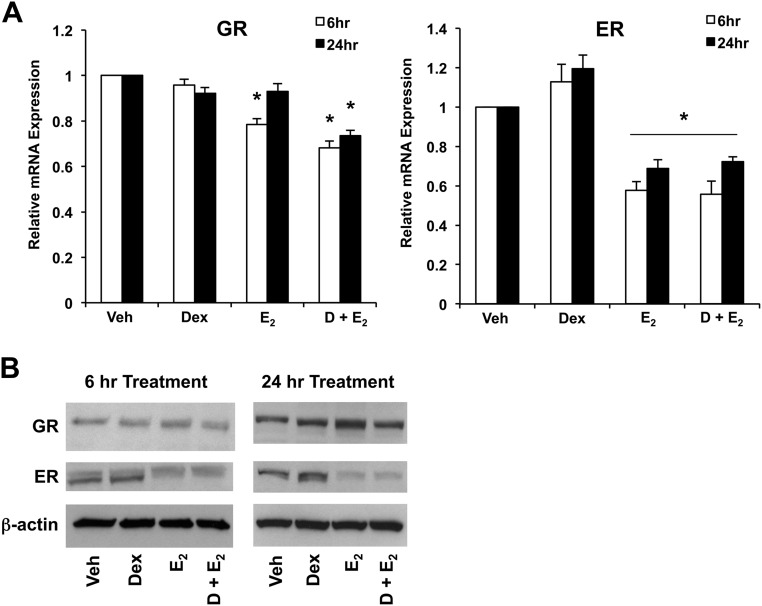
We and others have demonstrated that GR is expressed in the uterus, as well as uterine cell lines (14, 19). To assess whether GR and ER are present and sensitive to hormone treatment in ECC1 cells, we determined expression of GR and ER by QRT-PCR and Western blot analysis after 6 and 24 h of treatment of vehicle, 100 nm Dex, 10 nm E2, or Dex and E2. Expression of both human GR and ER mRNA was observed in ECC1 cells, and we found that E2 treatment in the presence and absence of Dex significantly reduced levels of ER at 6 and 24 h, as has been previously reported in other cell lines (Fig. 1A) (20). E2 down-regulation of ER protein levels was evident by Western blotting as early as 6 h and more apparent by 24 h (Fig. 1B). Treatment with E2 results in statistically significant changes in human GR mRNA levels at 6 h. However, mRNA expression is not statistically different at 24 h. Expression of GR protein expression is not statistically significant after treatment with Dex, E2, or Dex + E2 at either 6 or 24 h.
Fig. 1.
GR and ERα expression in response to Dex and E2 treatment in ECC1 cells. A, Expression of GR and ERα was measured by QRT-PCR. ECC1 cells treated with 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 were normalized to the housekeeping gene Cyclophilin B and compared with vehicle-treated cells. Bar graphs show mean ± sem. *, P < 0.05 as determined by ANOVA. B, GR and ERα protein levels were measured by Western blotting in whole-cell lysates from ECC1 cells treated for 6 and 24 h with vehicle (Veh), 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 (D + E2). The housekeeping gene β-actin was used as a control. The figure is representative of three experiments.
E2 antagonizes a subset of glucocorticoid-induced genes in uterine epithelial cells
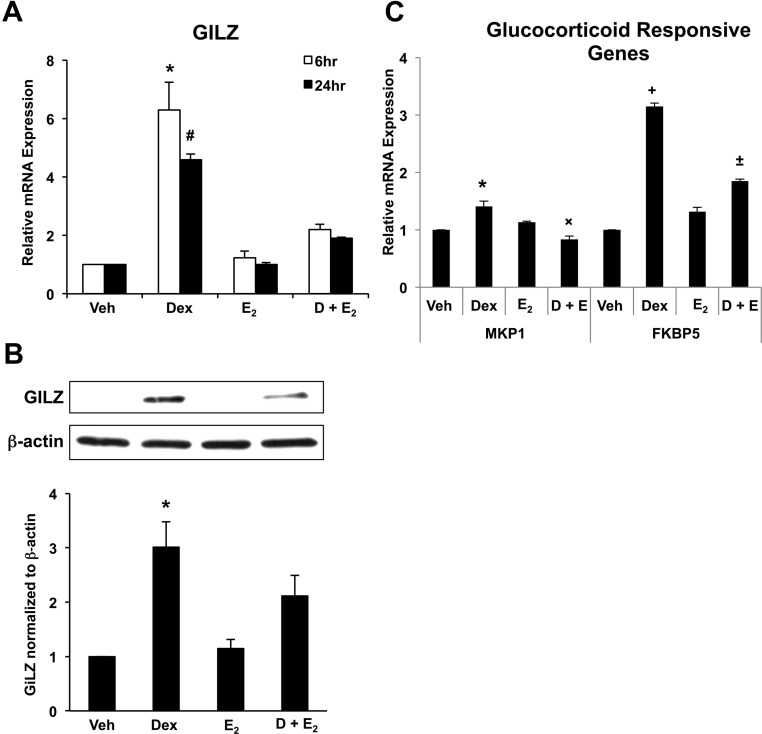
To identify the response of the uterine epithelium to glucocorticoids in vitro, ECC1 cells were treated with vehicle, 100 nm Dex, 10 nm E2, or Dex and E2 for either 6 or 24 h, and expression of the glucocorticoid-responsive gene GILZ was examined. Dex treatment rapidly induced expression of GILZ mRNA, whereas E2 treatment alone had no effect on GILZ expression (Fig. 2A). Interestingly, in cells treated concomitantly with Dex and E2, E2 was able to significantly repress GILZ induction by Dex, suggesting an antagonism of GILZ regulation by glucocorticoids and E2. To determine whether alterations in mRNA levels translated to alterations in protein expression, GILZ expression was analyzed by Western blotting (Fig. 2B). Dex significantly increased GILZ expression, and in cells treated with both Dex and E2, that induction was diminished. To determine whether the antagonism of glucocorticoid-induced gene expression by E2 is unique to GILZ, two additional glucocorticoid-responsive genes, MAPK phosphatase 1 (MKP1) and FK506-binding protein 5 (FKBP5), were also examined in response to hormone treatment. Both MKP1 and FKBP5 are antagonistically regulated by E2 in the presence of Dex, as is evident for GILZ, suggesting that this antagonism is not unique to the GILZ gene (Fig. 2C).
Fig. 2.
Glucocorticoid-responsive GILZ expression in ECC1. A, Expression of GILZ was measured by QRT-PCR in ECC1 cells treated for 6 or 24 h with 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 (D + E2). Values were normalized to the housekeeping gene Cyclophilin B and compared with vehicle-treated (Veh) cells. Bar graphs show mean ± sem. The symbols represent values that are statistically different at P < 0.05 from one another as determined by Tukey's post hoc analysis after ANOVA. Treatment groups without symbols are not statistically different from each other. B, Whole-cell lysates were subjected to Western blot analysis for GILZ. The housekeeping gene β-actin was used as a control and for normalization. The bar graph represents the quantification of four experiments. C, Expression of MKP1 and FKBP5 was measured by QRT-PCR in ECC1 cells treated for 6 h with 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2. Values were normalized to the housekeeping gene Cyclophilin B and compared with vehicle-treated cells. Bar graphs show mean ± sem. The symbols represent values that are statistically different at P < 0.05 from one another as determined by Tukey's post hoc analysis after ANOVA.
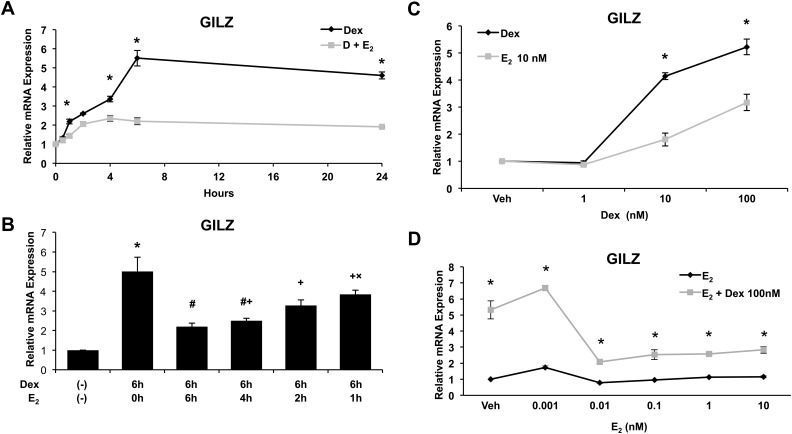
To further elucidate the kinetics of E2-mediated GILZ antagonism, GILZ expression was measured by QRT-PCR after a time course of hormone treatment. The repression of Dex-induced GILZ expression is rapid, occurring at significant level by 1 h after treatment (Fig. 3A). Dex pretreatment has no effect on the ability of E2 to antagonize glucocorticoid-induced GILZ expression (Fig. 3B). ECC1 cells treated with Dex for 6 h and E2 added at times coincident with or subsequent to Dex also demonstrated repression of GILZ expression by E2. Together, these results demonstrate that E2 antagonism of Dex-induced GILZ expression is rapid and suggests a direct mechanism.
Fig. 3.
Kinetics of GILZ regulation in response to Dex and E2 treatment. A, Expression of GILZ was measured by QRT-PCR in ECC1 cells treated for 0, 0.5, 1, 2, 4, 6, and 24 h with 100 nm Dex or 100 nm Dex and 10 nm E2 (D + E2). Graph shows mean ± sem. *, P < 0.05 as determined by ANOVA. B, Expression of GILZ was measured by QRT-PCR in ECC1 cells treated with vehicle (Veh) or 100 nm Dex for 6 h with varying lengths of time with 10 nm E2 (0, 6, 4, 2, and 1 h). Values were normalized to the housekeeping gene Cyclophilin B. Bar graphs show mean ± sem. The symbols represent values that are statistically different at P < 0.05 from one another as determined by Tukey's post hoc analysis after ANOVA. C, Expression of GILZ was measured by QRT-PCR in ECC1 cells treated with vehicle, Dex at varying concentrations, or Dex at varying concentrations with 10 nm E2. Graph shows mean ± sem. *, P < 0.05 as determined by ANOVA. D, Expression of GILZ was measured by QRT-PCR in ECC1 cells treated with vehicle, E2 at varying concentrations, or 100 nm Dex with varying concentrations of E2. Graph shows mean ± sem. *, P < 0.05 as determined by ANOVA.
We then evaluated the range of concentrations of Dex and E2 under which E2 antagonizes Dex-induced GILZ expression. ECC1 cells were treated with Dex concentrations from 1 to 100 nm and GILZ gene expression assessed. As shown in Fig. 3C, treatment with increasing doses of Dex results in dose-dependent increases in GILZ expression up to a saturating dose of 100 nm. Concomitant treatment with E2 causes significant repression up to the saturating dose. GILZ expression after a concentration range of E2 from 0.001 to 10 nm was assessed. Lower concentrations of E2 are equally capable of repressing Dex-induced GILZ expression, up to and including 0.01 nm E2. These data suggest that repression of GR signaling by E2 can occur at physiologically relevant doses (Fig. 3D). It is interesting that ER, at very low concentrations of E2, requires only partial occupancy by E2 to antagonize glucocorticoid-induced gene expression. At this time, however, we do not understand the basic mechanism underlying this observation.
Antagonism of glucocorticoid-induced GILZ expression by E2 is mediated at the transcriptional level and requires ER
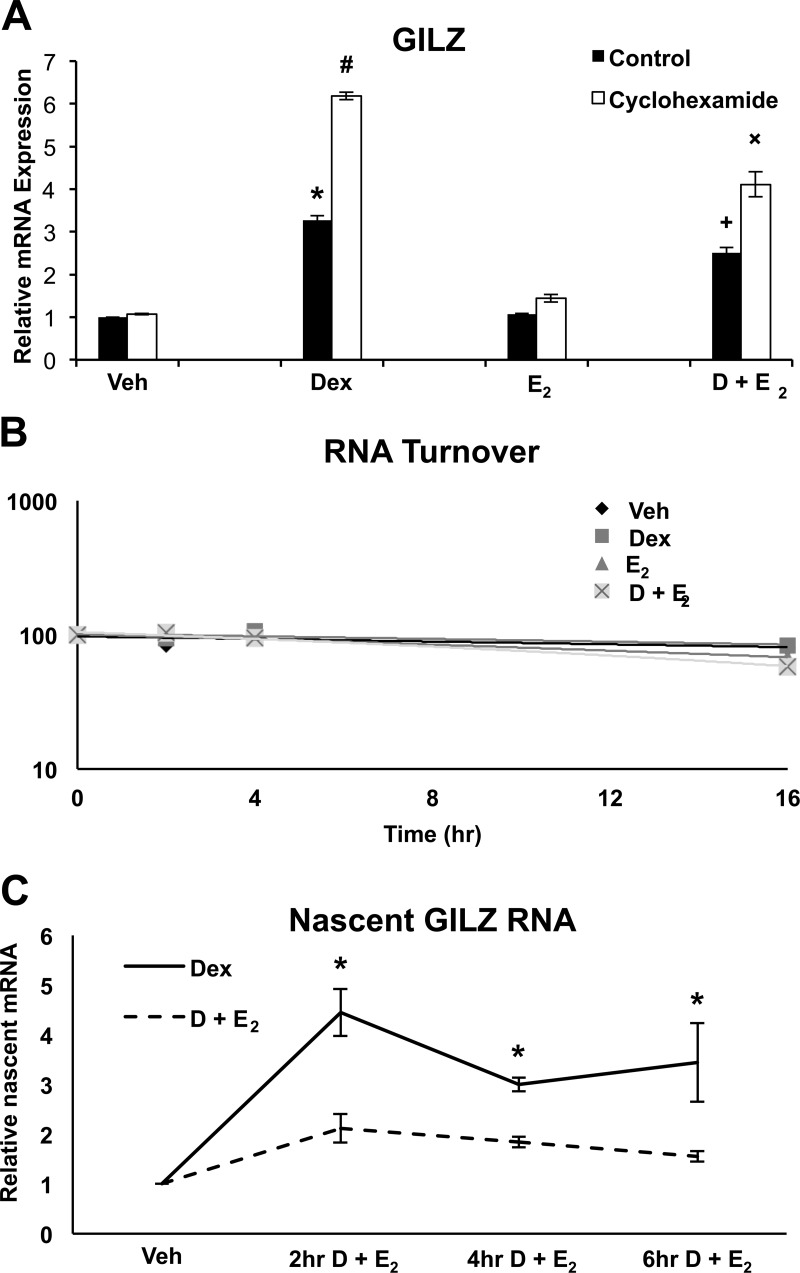
To understand the mechanism by which E2 antagonizes glucocorticoid-induced GILZ expression, ECC1 cells were evaluated for evidence of indirect or direct regulation by E2. The relative contribution of new protein synthesis and RNA turnover to the mechanism by which E2 disrupts glucocorticoid-induced GILZ gene expression was next examined. Cells were administered cycloheximide 1 h prior to hormone treatment to prevent new protein synthesis, and GILZ mRNA levels were QRT-PCR 6 h after treatment (Fig. 4A). Cycloheximide did not alter repression by E2 of Dex-induced GILZ expression. RNA turnover was also evaluated as a potential mechanism by which E2 mediates GILZ expression (Fig. 4B). ECC1 cells were first treated with hormone for 4 h to induce gene expression followed by administration of Actinomyocin D. Levels of GILZ mRNA were quantified at 0, 2, 4, and 16 h after the addition of Actinomyocin D. The slope of the best-fit line provides quantification of RNA decay. Although decay is slightly enhanced in Dex + E2 cells, levels are not statistically significant and most likely do not represent the primary mechanism by which E2 antagonizes glucocorticoid-induced gene expression. Nascent GILZ RNA expression in response to Dex and Dex + E2 treatment was examined to determine whether E2 antagonism of glucocorticoid-induced gene expression is a function of impeding transcriptional initiation (Fig. 4C). Cotreatment with Dex and E2 represses nascent RNA levels to a significant level as early as 2 h. Together, these data suggest that E2 directly regulates glucocorticoid-induced GILZ expression.
Fig. 4.
Transcriptional regulation is a likely mechanism of E2-mediated GILZ antagonism. A, ECC1 cells were treated with 10 μm cycloheximide for 1 h before hormone treatment. Cells were then administered 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 (D + E2) for 6 h and GILZ mRNA evaluated by QRT-PCR was compared to vehicle (Veh). Values were normalized to the housekeeping gene Cyclophilin B. Bar graphs show mean ± sem. The symbols represent values that are statistically different at P < 0.05 from one another as determined by Tukey's post hoc analysis after ANOVA. B, RNA decay was measured by treating ECC1 cells with vehicle, 100 nm Dex, or 100 nm Dex and 10 nm E2 for 4 h to induce gene expression followed by the addition of 5 μg/μl Actinomycin D. GILZ mRNA was measured by QRT-PCR at time points 0, 2, 4, and 16 h after the addition of Actinomycin D. Amount of mRNA at time 0 was set to 100%, and subsequent time points are reported as a percentage of 100. C, Relative levels of nascent RNA was measured by QRT-PCR in ECC1 cells after treatment with vehicle, 100 nm Dex, or 100 nm Dex and 10 nm E2 for 0, 2, 4, and 6 h. Values were normalized to nascent RNA for the housekeeping gene Cyclophilin B. Graph shows mean ± sem. *, P < 0.05 as determined by ANOVA.
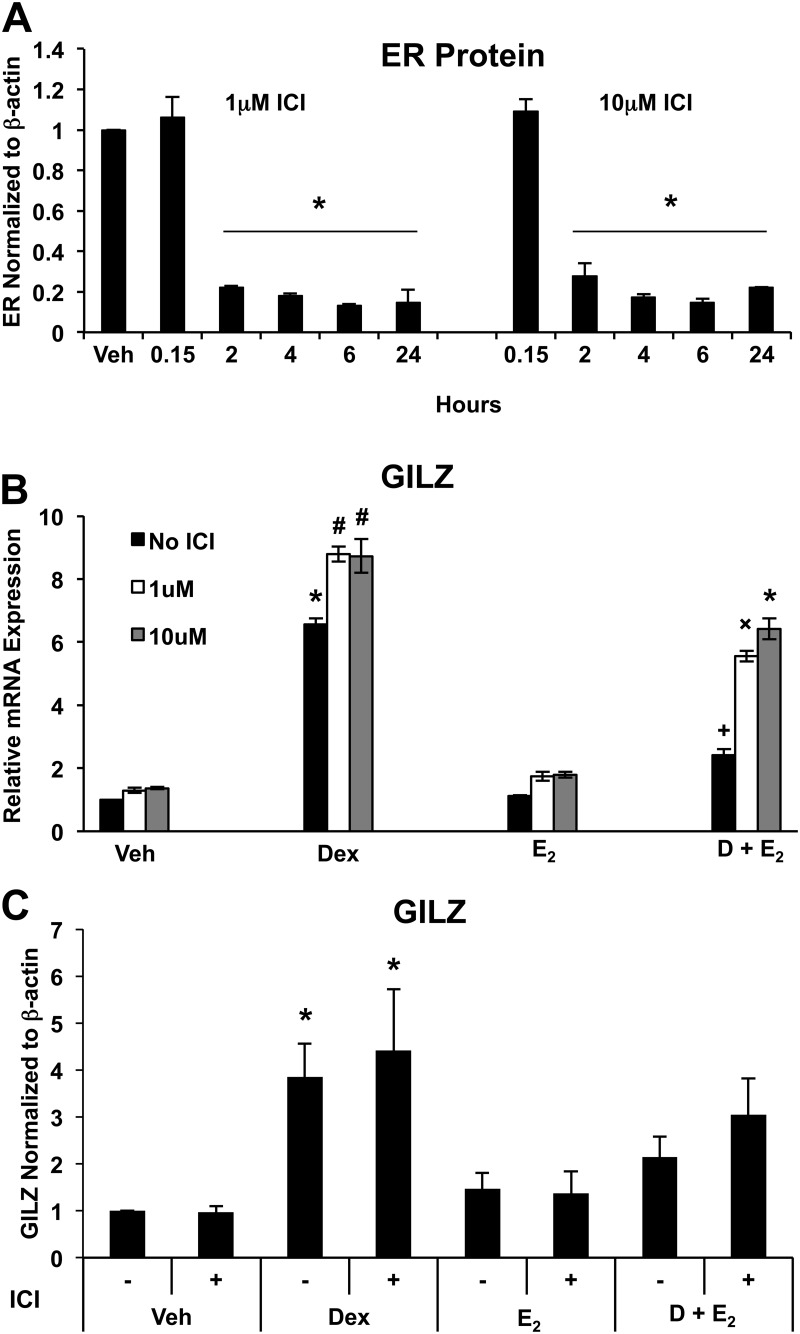
To investigate the role of ER in the regulation of GILZ expression in ECC1 cells, we used ICI 182,780, a steroidal ER antagonist that competitively binds, down-regulates, and degrades both ERα and ERβ. GILZ mRNA and protein expression were then evaluated after treatment with ICI 182,780 and vehicle, 100 nm Dex, 10 nm E2, or Dex and E2. ICI 182,780 significantly repressed ER expression 2 h after treatment at both 1 and 10 μm concentrations (Fig. 5A). Decreasing the intracellular levels of ER attenuated the ability of E2 to inhibit GILZ induction by Dex. At 24 h, ECC1 cells treated with Dex and E2 in the absence of ICI 182,780 have a 63% reduction in GILZ expression compared with Dex alone, whereas addition of 1 and 10 μm ICI 182,780 diminished the level of repression to 37 and 26%, respectively (Fig. 5B). Interestingly, ICI 182,780 also increased Dex induction of GILZ expression. Reduction in ER-mediated repression of Dex-induced GILZ expression by ICI 182,780 cotreatment was also evident by Western blotting, although not to significant levels at 24 h (Fig. 5C).
Fig. 5.
ER depletion by ICI 182,780 inhibits E2-mediated repression of GILZ expression. A, Whole-cell lysates from ECC1 cells treated over a time course with 1 or 10 μm ICI 182,780 (ICI) were subjected to Western blot analysis for ERα. The housekeeping gene β-actin was used as a control and for normalization. The bar graph shows mean ± sem and represents the quantification of four experiments. *, P < 0.05 as determined by ANOVA. B, GILZ expression was determined by QRT-PCR in ECC1 cells cotreated with vehicle (Veh), 1 μm, or 10 μm ICI and 24 h of vehicle, 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 (D + E2) for 24 h. Values were normalized to the housekeeping gene Cyclophilin B. Bar graphs show mean ± sem. Symbols represent groups with means statistically different at P < 0.05 as determined by ANOVA. C, Whole-cell lysates from ECC1 cells treated with or without 1 μm ICI and vehicle, 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 for 24 h were subjected to Western blot analysis for GILZ. The housekeeping gene β-actin was used as a control and for normalization. The bar graph shows mean ± sem and represents the quantification of four experiments. Symbols represent groups with means statistically different at P < 0.05 as determined by ANOVA.
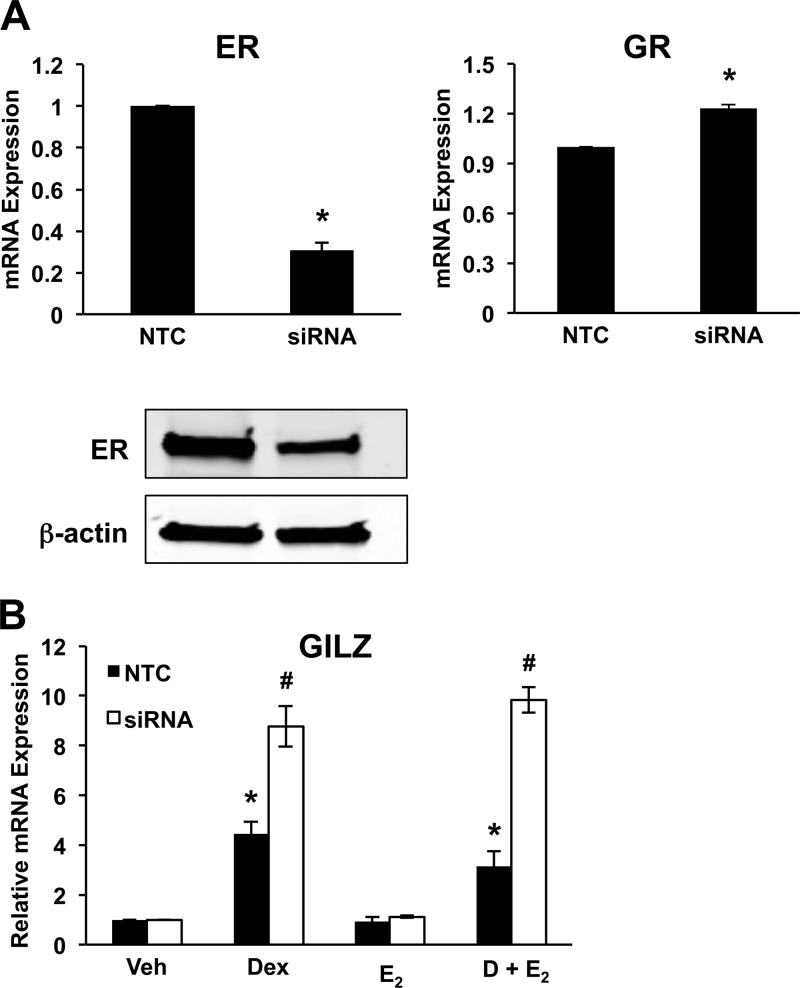
Analysis of GILZ expression after exposure of cells to the ER inhibitor ICI 182,780 showed that antagonizing ER expression and activity mitigated the ability of E2 to repress glucocorticoid-induced gene expression. To determine the role of ERα in mediating E2 antagonism of glucocorticoid-induced GILZ expression, cells were transfected with siRNA against ERα before hormone treatment. As shown in Fig. 6A, the transfection of ERα siRNA into ECC1 cells significantly reduced the expression of ERα, whereas the expression of the housekeeping gene β-actin was not affected. Transfection with nontargeting siRNA (NTC) did not change the expression of ERα or GR. RNA isolated from cells transfected with either NTC or ERα siRNA and treated for 6 h with vehicle, 100 nm Dex, 10 nm E2, or Dex and E2 was examined for GILZ expression by QRT-PCR (Fig. 6B). Knockdown of ERα expression by siRNA abrogated the ability of E2 to repress Dex-induced GILZ gene expression, confirming the essential role of ERα in E2 antagonism. Interestingly, induction of GILZ RNA by Dex increased in cells transfected with ERα siRNA or treated with ER antagonist ICI 182,780 (Fig. 5B).
Fig. 6.
siRNA-mediated knockdown of ERα inhibits E2-mediated repression of GILZ expression. A, ECC1 cells, transfected with ERα siRNA or NTC, were assessed for the extent of knockdown compared with NTC by QRT-PCR and Western blotting. GR expression levels were evaluated by QRT-PCR under in ERα knockdown ECC1 cells. Values were normalized to the housekeeping genes Cyclophilin B (QRT-PCR) or β-actin (Western blotting). Bar graphs show mean ± sem. *, P < 0.05 as determined by ANOVA. B, ECC1 cells transfected with NTC or ERα siRNA were treated for 6 h with vehicle (Veh), 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 (D + E2). Expression of GILZ was evaluated by QRT-PCR. Values were normalized to the housekeeping gene Cyclophilin B and compared with vehicle-treated cells. Bar graphs show mean ± sem. The symbols represent values that are statistically different at P < 0.05 from one another as determined by Tukey's post hoc analysis after ANOVA.
Because the ER antagonist ICI 182,780 disrupts signaling by both ERα and ERβ, we assessed the relative contribution of ERβ to E2-mediated antagonism of glucocorticoid-induced GILZ expression, by treating ECC1 cells with either the ERβ agonist Liq or the ERβ antagonist PHTPP (21, 22). Liq alone does not alter GILZ mRNA expression (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). However, similar to E2, the ERβ agonist is able to antagonize GILZ expression by Dex. Treating ECC1 cells with PHTPP, an ERβ antagonist, and vehicle, 100 nm Dex, 10 nm E2, or Dex and E2 does not block the ability of E2 to repress glucocorticoid-induced GILZ expression (Supplemental Fig. 1B). These data suggest that although ERβ may also antagonize glucocorticoid-regulated GILZ expression, it is a mechanism distinct from E2 and ERα.
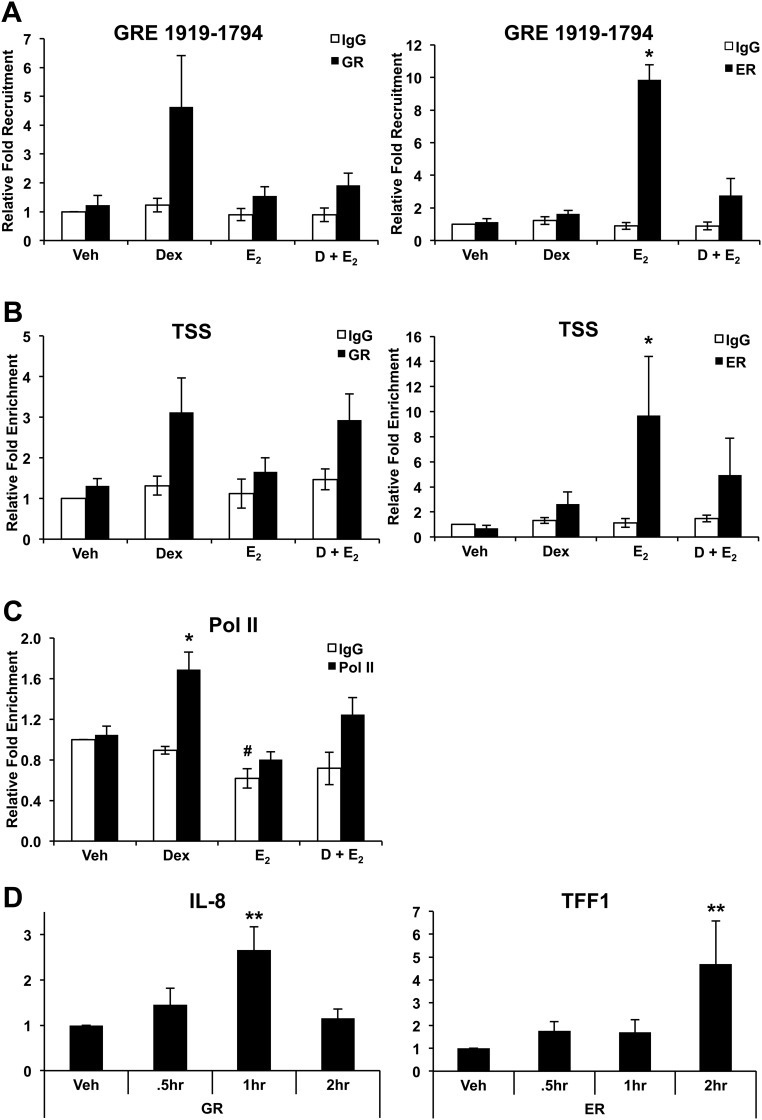
Given that ERα is required for E2-mediated antagonism of GILZ induction by Dex, ChIP assays were performed on human genomic DNA from ECC1 cells treated with vehicle, 100 nm Dex, 10 nm E2, or Dex and E2 for 1 h to examine the recruitment of ERα to the GILZ promoter in vivo. Occupancy of GR or ERα at GREs at the previously identified position −1919 to −1794 and the TSS was monitored by QRT-PCR amplification using specific primers (23). Interestingly, both GR and ERα are recruited to this GRE in the GILZ promoter in a hormone dependent manner, although not to the GRE located at −2220 to −2133 (data not shown). In the presence of Dex and E2, the levels of GR associated with the chromatin are reduced at GRE −1919 to −1794, potentially the mechanism by which GILZ expression is reduced in the presence of E2 and glucocorticoids. Recruitment of ERα to the promoter of GILZ by E2 suggests transcriptional regulation by both steroid hormones and is consistent with the finding that GILZ expression increases in the absence of ER (Fig. 7A). GR regulates many genes through binding near to their TSS (24). To determine whether receptor also occupies the TSS, we evaluated the recruitment of GR and ERα to the TSS (Fig. 7B). GR is recruited to this region within 1 h. The TSS region evaluated overlaps with the region determined by Tynan et al. (25) to be important for ERα regulation of GILZ. ERα is also recruited to the TSS after E2 treatment, although in the presence of both Dex and E2, occupancy is not statistically different from vehicle. In light of decreased nascent GILZ RNA in the presence of Dex and E2 (Fig. 4C), activated Pol II occupancy at the TSS was assessed after treatment with vehicle, 100 nm Dex, 10 nm E2, or Dex and E2 for 1 h. As expected, the TSS of ECC1 cells treated with Dex show increased activated Pol II occupancy, and this occupancy is decreased upon Dex and E2 cotreatment (Fig. 7C). Recruitment of GR to a nuclear factor κB (NFκB) site in IL-8 and ERα to an ERE in TFF1 were also analyzed as a control, as have been previously described (Fig. 7D) (26, 27). Under the same concentrations of Dex that induced GR occupancy of the GILZ promoter, GR occupancy of an NFκB site in the promoter of IL-8 is significantly increased by 1 h after treatment. ERα occupancy of the evaluated ERE within the promoter of TFF1 is significantly enhanced 2 h after treatment with E2 at the same concentration used to induced occupancy of the GILZ promoter. ChIP results in the presence of Dex and E2 suggest interplay between GR and ERα in the occupancy of the GILZ promoter.
Fig. 7.
Association of GR and ERα with the promoter of GILZ. A, Recruitment of GR and ERα to GRE at position −1919 to −1794 was assessed by ChIP assay after treatment with vehicle (Veh), 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 (D + E2) for 1 h in ECC1 cells. Enrichment of the promoter region measured by QRT-PCR. Bar graphs show mean ± sem. *, P < 0.05 as determined by ANOVA. B, Recruitment of GR and ERα to the GILZ TSS was examined by ChIP after treatment with vehicle, 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2. Enrichment measured by primers directed to the TSS and quantified by QRT-PCR. Bar graphs show mean ± sem. *, P < 0.05 as determined by ANOVA. C, Recruitment of activated Pol II to the TSS of GILZ determined by ChIP after treatment of ECC1 cells with vehicle, 100 nm Dex, 10 nm E2, or 100 nm Dex and 10 nm E2 for 1 h. Enrichment measured by QRT-PCR. Bar graphs show mean ± sem. The symbols represent values that are statistically different at P < 0.01 from one another as determined by Tukey's post hoc analysis after ANOVA. D, Recruitment of GR to a NFκB site in IL-8 and ERα to an ERE in TFF1 were analyzed by ChIP and quantified by QRT-PCR for control. Bar graphs show mean ± sem. **, P < 0.01 as determined by ANOVA.
E2 antagonizes GILZ induction by glucocorticoids in the mouse whole uterus
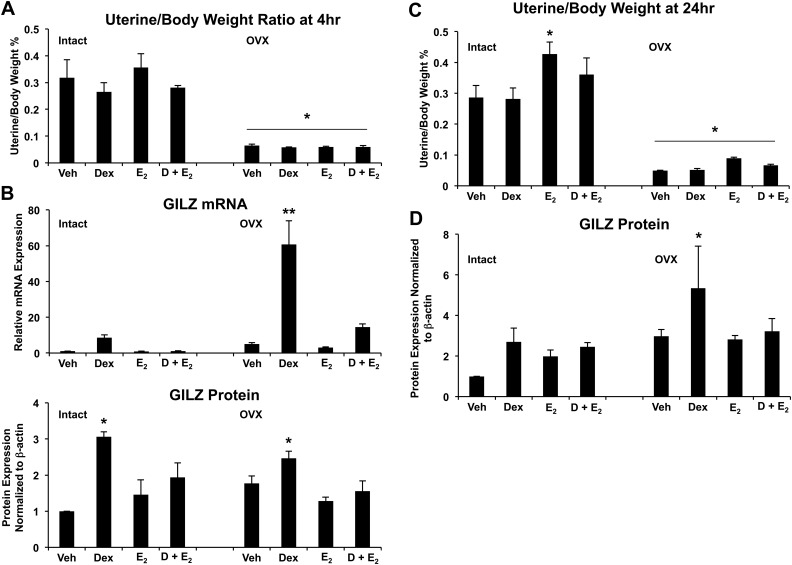
To determine whether E2-mediated antagonism of glucocorticoid-induced GILZ expression was specific to the uterine epithelium or common to the entire uterus, female C57Bl/6 mice were given a single ip injection of 1 mg/kg of Dex, 10 μg/kg of E2, or 1 mg/kg of Dex and 10 μg/kg and uterus harvested for uterine weight, RNA, and protein. Our lab has previously reported that glucocorticoids can block the biological effects of E2 in the rat uterus, including edema in the stroma and myometrium, measured by uterine weight (13). A single injection of Dex in the mouse is able to antagonize the E2-mediated increase in uterine weight at a statistically significant level by 24 h (Fig. 8C), although the trend appears as early as 4 h (Fig. 8A). When examining GILZ mRNA levels, vehicle-treated OVX mice display a 5-fold increase in GILZ expression at 4 h compared with their intact counterparts, which may be a reflection of unopposed GILZ expression in the absence of E2 in these mice (Fig. 8B). Levels of GILZ mRNA were strongly induced by Dex injection in both intact and OVX mice, although to a greater extent in OVX mice, which lack endogenous E2. The higher levels of GILZ expression in vehicle-treated OVX mice, as well as the exaggerated response to Dex (60-fold increase of GILZ expression), suggest that there is a basal level of repression on GILZ expression by estrogen that is relieved by the absence of endogenous estrogens in OVX mice. GILZ protein was quantified at 24 h. GILZ protein is significantly up-regulated by Dex alone and up-regulation by Dex is inhibited at 24 h with the addition of E2 (Fig. 8D). Subsequently, protein levels of GILZ are greater in OVX mice than intact mice, again representing their lack of endogenous estrogens.
Fig. 8.
Global E2-mediated repression of GILZ expression in the mouse uterus. A, Proportion uterine weight relative to body weight was determined in intact and OVX mice (n = 7 per group) after 4 h of treatment with 1 mg/kg of Dex, 10 μg/kg of E2, or 1 mg/kg of Dex and 10 μg/kg of E2. Bar graphs show mean ± sem. *, P < 0.05 as determined by ANOVA. B, GILZ expression (evaluated by QRT-PCR and Western blotting) was determined in intact and OVX mice when stimulated with vehicle (Veh), 1 mg/kg of Dex, 10 μg/kg of E2, or 1 mg/kg of Dex and 10 μg/kg of E2 (D + E2). mRNA was normalized to Cyclophillin B, and protein was normalized to β-actin. Bar graphs show mean ± sem. **, P < 0.01 as determined by ANOVA. C, Proportion uterine weight relative to body weight was determined in mice (n = 7 per group) after 24 h of treatment with 1 mg/kg of Dex, 10 μg/kg of E2, or 1 mg/kg of Dex and 10 μg/kg of E2. Bar graphs show mean ± sem. The symbols represent values that are statistically different at P < 0.05 from one another as determined by Tukey's post hoc analysis after ANOVA. D, Western blot analysis determined GILZ expression in whole uterus from intact and OVX mice. Values were normalized to the house keeping protein β-actin. Bar graphs show mean ± sem. *, P < 0.05 as determined by ANOVA.
Discussion
The inflammatory response plays a critical role in both implantation during pregnancy and host defense in the uterus. Although this response is thought to be under the primary control of the female sex steroid hormones, progesterone and E2, evidence for glucocorticoids' role in the uterus continues to increase. Here, we describe a model in which cells from the human uterine epithelium can be examined in terms of responsiveness to both E2 and glucocorticoids. The ECC1 cell line expresses both ER and GR and is responsive to their ligand's. We previously reported that glucocorticoids can block the biological effects of E2 in the rat uterus (14). This study provides the first evidence that Dex and E2 act antagonistically to regulate gene expression in uterine epithelial cells. Examining glucocorticoid-induced gene expression, we identified GILZ, an antiinflammatory glucocorticoid-induced gene, as one of the targets of this antagonism and focused on determining the mechanism of regulation.
GILZ has been well characterized in terms of its regulation by glucocorticoids (28–30). It was originally discovered as part of a systematic study of genes transcriptionally induced by glucocorticoids and responsible for glucocorticoid-induced apoptosis in T-lymphocytes but has subsequently been found to be under the control of glucocorticoids in several other immune cell types (28, 31, 32). Transcriptional regulation of GILZ by glucocorticoids involves GR promoter binding. The GILZ promoter has been shown to have both GREs and an ERE (23, 25). Scanning by ChIP and mutational analysis has demonstrated three bona fide GREs within the promoter of GILZ (23). In ECC1 cells, one of these confirmed sites, the GRE located at −1919 to −1794, appears to be important for regulation of GILZ by GR and ER. Regulation of GILZ expression can also occur outside GR binding to GREs, and vasopressin, aldosterone, and E2 have also been shown to stimulate GILZ expression (25, 33, 34). E2 regulates GILZ in a cell type-specific manner, and although an ERE was identified in the GILZ promoter, it is not required for regulation by E2 (25). The region of the GILZ promoter between −104 and −69, containing a functional octamer-binding site (Oct-1) and a cAMP response element-binding protein binding site, mediates E2-dependent down-regulation in MCF-7 cells and up-regulation in HeLa and HEK293 cells. Our studies show that alone E2 does not regulate GILZ expression in ECC1 cells. ChIP assays in this study suggest there is recruitment of ERα to both the GRE located at −1919 to −1794 and the TSS. GR bound to these regions is diminished in the presence of Dex and E2, suggesting mechanism by which the presence of both hormones prevents promoter binding. It is interesting to consider the recruitment of ERα to the GRE and TSS in light of the data from the ER knockdown experiments. Reduction of ERα gene expression, targeted degradation of ER protein, and the absence of estrogen in OVX mice permit greater GILZ induction by Dex. Compared with intact mice, mice lacking circulating E2 have higher basal levels of GILZ expression, which is further exaggerated by Dex treatment. This is true for both gene expression after 4 h of treatment and protein expression after 24 h of treatment. Recruitment of ERα to the promoter of GILZ by E2 suggests that although E2 alone does not change the expression of GILZ, the expression of GILZ is nonetheless modulated by ERα. Regulation of GILZ expression by glucocorticoids in uterine epithelial cells appears to be regulated by GR recruitment to more than one location on the promoter. In addition to the GRE, the TSS, which includes base pairs from −94 to +45, demonstrates some GR recruitment along with significant ERα recruitment. The TSS contains several putative transcription factor binding sites, including cAMP response element-binding protein, activator protein 1, and Forkhead box A1, which have all been shown to facilitate DNA binding by GR (35, 36). The TSS also encompasses some of the region shown to be important for E2 regulation of GILZ expression (25). Results from this study, as well as those from Tynan et al. (25), provide evidence of interplay between GR and ER for direct gene regulation of GILZ, where gene transcription requires receptor binding not only on classic GREs but also to other transcription factor elements. Identifying GILZ as a target of gene regulation by glucocorticoids and E2 in the uterine epithelium represents one facet of their coordinated effect on the local immune system.
Defining the key regulators of uterine function established the female sex steroid hormones as important mediators. However, new signaling pathways are now being recognized. Glucocorticoids appear to balance functions during implantation and pregnancy, mediating both pro- and antiinflammatory actions, including suppression of natural killer cells, promotion of trophoblast growth and invasion vs. induction of apoptosis, and restriction of cytotrophoblast growth and invasion (37–44). Although we have focused on the antagonism of GILZ, an antiinflammatory protein, by glucocorticoids and E2, the induction of MKP1 and FKBP5 gene expression by glucocorticoids is also antagonized by E2, both of which are also mediators of immune function (45, 46). GILZ represents one target gene that, although classically identified as a glucocorticoid-regulated gene, is under the direct control of glucocorticoids and E2. GILZ regulation may be representative of a cohort of immune regulatory genes controlled by both glucocorticoids and endogenous estrogens in vivo responsible for the integration of immune regulation and female reproductive function.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- Dex
- dexamethasone
- E2
- estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- FBS
- fetal bovine serum
- FKBP5
- FK506-binding protein 5
- GILZ
- glucocorticoid-induced leucine zipper
- GR
- glucocorticoid receptor
- GRE
- glucocorticoid response element
- Liq
- Liquiritigenin
- MKP1
- MAPK phosphatase 1
- NFκB
- nuclear factor κB
- NTC
- nontarget control
- OVX
- ovariectomized
- PHTPP
- 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- Pol II
- polymerase II
- QRT-PCR
- quantitative real-time PCR
- siRNA
- small interfering RNA
- TFF1
- trefoil factor 1
- TSS
- transcriptional start site.
References
- 1. Ozturk S, Demir R. 2010. Particular functions of estrogen and progesterone in establishment of uterine receptivity and embryo implantation. Histol Histopathol 25:1215-1228 [DOI] [PubMed] [Google Scholar]
- 2. Norwitz ER, Schust DJ, Fisher SJ. 2001. Implantation and the survival of early pregnancy. N Engl J Med 345:1400-1408 [DOI] [PubMed] [Google Scholar]
- 3. Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. 2000. Embryo implantation. Dev Biol 223:217-237 [DOI] [PubMed] [Google Scholar]
- 4. Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. 2010. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod 16:135-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fahey JV, Schaefer TM, Wira CR. 2006. Sex hormone modulation of human uterine epithelial cell immune responses. Integr Comp Biol 46:1082-1087 [DOI] [PubMed] [Google Scholar]
- 6. Bigsby RM, Young PC. 1993. Progesterone and dexamethasone inhibition of uterine epithelial cell proliferation: studies with antiprogesterone compounds in the neonatal mouse. J Steroid Biochem Mol Biol 46:253-257 [DOI] [PubMed] [Google Scholar]
- 7. Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. 1995. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9:1608-1621 [DOI] [PubMed] [Google Scholar]
- 8. Reichardt HM, Umland T, Bauer A, Kretz O, Schütz G. 2000. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol 20:9009-9017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zanchi NE, Filho MA, Felitti V, Nicastro H, Lorenzeti FM, Lancha AH., Jr 2010. Glucocorticoids: extensive physiological actions modulated through multiple mechanisms of gene regulation. J Cell Physiol 224:311-315 [DOI] [PubMed] [Google Scholar]
- 10. Yudt MR, Cidlowski JA. 2002. The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Mol Endocrinol 16:1719-1726 [DOI] [PubMed] [Google Scholar]
- 11. Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. 1985. Primary structure and expression of a functional human glucocorticoid receptor cdna. Nature 318:635-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou J, Cidlowski JA. 2005. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids 70:407-417 [DOI] [PubMed] [Google Scholar]
- 13. Rhen T, Grissom S, Afshari C, Cidlowski JA. 2003. Dexamethasone blocks the rapid biological effects of 17β-estradiol in the rat uterus without antagonizing its global genomic actions. FASEB J 17:1849-1870 [DOI] [PubMed] [Google Scholar]
- 14. Whirledge S, Dixon D, Cidlowski JA. 2012. Glucocorticoids regulate gene expression and repress cellular proliferation in human uterine leiomyoma cells. Horm Cancer 3:79-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rabin DS, Johnson EO, Brandon DD, Liapi C, Chrousos GP. 1990. Glucocorticoids inhibit estradiol-mediated uterine growth: possible role of the uterine estradiol receptor. Biol Reprod 42:74-80 [DOI] [PubMed] [Google Scholar]
- 16. Rhen T, Cidlowski JA. 2006. Estrogens and glucocorticoids have opposing effects on the amount and latent activity of complement proteins in the rat uterus. Biol Reprod 74:265-274 [DOI] [PubMed] [Google Scholar]
- 17. Pakrasi PL, Cheng HC, Dey SK. 1983. Prostaglandins in the uterus: modulation by steroid hormones. Prostaglandins 26:991-1009 [DOI] [PubMed] [Google Scholar]
- 18. Cidlowski JA, Bellingham DL, Powell-Oliver FE, Lubahn DB, Sar M. 1990. Novel antipeptide antibodies to the human glucocorticoid receptor: recognition of multiple receptor forms in vitro and distinct localization of cytoplasmic and nuclear receptors. Mol Endocrinol 4:1427-1437 [DOI] [PubMed] [Google Scholar]
- 19. Ho CK, Tetsuka M, Hillier SG. 1999. Regulation of 11β-hydroxysteroid dehydrogenase isoforms and glucocorticoid receptor gene expression in the rat uterus. J Endocrinol 163:425-431 [DOI] [PubMed] [Google Scholar]
- 20. Saceda M, Lippman ME, Chambon P, Lindsey RL, Ponglikitmongkol M, Puente M, Martin MB. 1988. Regulation of the estrogen receptor in mcf-7 cells by estradiol. Mol Endocrinol 2:1157-1162 [DOI] [PubMed] [Google Scholar]
- 21. Compton DR, Sheng S, Carlson KE, Rebacz NA, Lee IY, Katzenellenbogen BS, Katzenellenbogen JA. 2004. Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen receptor β antagonist activity. J Med Chem 47:5872-5893 [DOI] [PubMed] [Google Scholar]
- 22. Mersereau JE, Levy N, Staub RE, Baggett S, Zogovic T, Zogric T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC. 2008. Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Mol Cell Endocrinol 283:49-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Haqq C, Yamamoto KR. 2004. Chromatin immunoprecipitation (chip) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci USA 101:15603-15608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, Myers RM. 2009. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res 19:2163-2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tynan SH, Lundeen SG, Allan GF. 2004. Cell type-specific bidirectional regulation of the glucocorticoid-induced leucine zipper (gilz) gene by estrogen. J Steroid Biochem Mol Biol 91:225-239 [DOI] [PubMed] [Google Scholar]
- 26. Nissen RM, Yamamoto KR. 2000. The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev 14:2314-2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289-1297 [DOI] [PubMed] [Google Scholar]
- 28. D'Adamio F, Zollo O, Moraca R, Ayroldi E, Bruscoli S, Bartoli A, Cannarile L, Migliorati G, Riccardi C. 1997. A new dexamethasone-induced gene of the leucine zipper family protects t lymphocytes from tcr/cd3-activated cell death. Immunity 7:803-812 [DOI] [PubMed] [Google Scholar]
- 29. Ayroldi E, Riccardi C. 2009. Glucocorticoid-induced leucine zipper (gilz): a new important mediator of glucocorticoid action. FASEB J 23:3649-3658 [DOI] [PubMed] [Google Scholar]
- 30. Fan H, Morand EF. 2012. Targeting the side effects of steroid therapy in autoimmune diseases: the role of gilz. Discov Med 13:123-133 [PubMed] [Google Scholar]
- 31. Berrebi D, Bruscoli S, Cohen N, Foussat A, Migliorati G, Bouchet-Delbos L, Maillot MC, Portier A, Couderc J, Galanaud P, Peuchmaur M, Riccardi C, Emilie D. 2003. Synthesis of glucocorticoid-induced leucine zipper (gilz) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and il-10. Blood 101:729-738 [DOI] [PubMed] [Google Scholar]
- 32. Godot V, Garcia G, Capel F, Arock M, Durand-Gasselin I, Asselin-Labat ML, Emilie D, Humbert M. 2006. Dexamethasone and il-10 stimulate glucocorticoid-induced leucine zipper synthesis by human mast cells. Allergy 61:886-890 [DOI] [PubMed] [Google Scholar]
- 33. Gomez M, Raju SV, Viswanathan A, Painter RG, Bonvillain R, Byrne P, Nguyen DH, Bagby GJ, Kolls JK, Nelson S, Wang G. 2010. Ethanol upregulates glucocorticoid-induced leucine zipper expression and modulates cellular inflammatory responses in lung epithelial cells. J Immunol 184:5715-5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. 2005. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 280:39970-39981 [DOI] [PubMed] [Google Scholar]
- 35. Herrlich P. 2001. Cross-talk between glucocorticoid receptor and ap-1. Oncogene 20:2465-2475 [DOI] [PubMed] [Google Scholar]
- 36. Belikov S, Astrand C, Wrange O. 2009. Foxa1 binding directs chromatin structure and the functional response of a glucocorticoid receptor-regulated promoter. Mol Cell Biol 29:5413-5425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boomsma CM, Keay SD, Macklon NS. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst Rev 2007:CD005996. [DOI] [PubMed] [Google Scholar]
- 38. Ogasawara M, Aoki K. 2000. Successful uterine steroid therapy in a case with a history of ten miscarriages. Am J Reprod Immunol 44:253-255 [DOI] [PubMed] [Google Scholar]
- 39. Quenby S, Farquharson R, Young M, Vince G. 2003. Successful pregnancy outcome following 19 consecutive miscarriages: case report. Hum Reprod 18:2562-2564 [DOI] [PubMed] [Google Scholar]
- 40. Quenby S, Kalumbi C, Bates M, Farquharson R, Vince G. 2005. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil Steril 84:980-984 [DOI] [PubMed] [Google Scholar]
- 41. Mandl M, Ghaffari-Tabrizi N, Haas J, Nöhammer G, Desoye G. 2006. Differential glucocorticoid effects on proliferation and invasion of human trophoblast cell lines. Reproduction 132:159-167 [DOI] [PubMed] [Google Scholar]
- 42. Librach CL, Feigenbaum SL, Bass KE, Cui TY, Verastas N, Sadovsky Y, Quigley JP, French DL, Fisher SJ. 1994. Interleukin-1β regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem 269:17125-17131 [PubMed] [Google Scholar]
- 43. Ma Y, Pan F, McNeil M. 2002. Formation of dtdp-rhamnose is essential for growth of mycobacteria. J Bacteriol 184:3392-3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crocker IP, Barratt S, Kaur M, Baker PN. 2001. The in-vitro characterization of induced apoptosis in placental cytotrophoblasts and syncytiotrophoblasts. Placenta 22:822-830 [DOI] [PubMed] [Google Scholar]
- 45. Nakamura N, Shimaoka Y, Tougan T, Onda H, Okuzaki D, Zhao H, Fujimori A, Yabuta N, Nagamori I, Tanigawa A, Sato J, Oda T, Hayashida K, Suzuki R, Yukioka M, Nojima H, Ochi T. 2006. Isolation and expression profiling of genes upregulated in bone marrow-derived mononuclear cells of rheumatoid arthritis patients. DNA Res 13:169-183 [DOI] [PubMed] [Google Scholar]
- 46. Vandevyver S, Dejager L, Van Bogaert T, Kleyman A, Liu Y, Tuckermann J, Libert C. 2012. Glucocorticoid receptor dimerization induces mkp1 to protect against tnf-induced inflammation. J Clin Invest 122:2130-2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.