Abstract
Fibroblast growth factor-19 (FGF19) and its rodent ortholog, FGF15, are hormones produced in the distal small intestine and secreted into the circulation after a meal. In addition to controlling the enterohepatic circulation of bile acids, FGF15/19 also regulates systemic lipid and glucose metabolism. In these experiments we investigated the hypothesis that, like other gut-derived postprandial hormones, FGF15/19 can act in the central nervous system to elicit its metabolic effects. We found that FGF-receptors 1 and 4 are present in rat hypothalamus, and that their expression was reduced by up to 60% in high-fat fed rats relative to lean controls. Consistent with a potential role for brain FGF15/19 signaling to regulate energy and glucose homeostasis, and with a previous report that intracerebroventricular (i.c.v.) administration of FGF19 increases energy expenditure, we report that acute i.c.v. FGF19 reduces 24-h food intake and body weight, and acutely improves glucose tolerance. Conversely, i.c.v. administration of an FGF-receptor inhibitor increases food intake and impairs glucose tolerance, suggesting a physiological role for brain FGF receptor signaling. Together, these findings identify the central nervous system as a potentially important target for the beneficial effects of FGF19 in the treatment of obesity and diabetes.
Fibroblast growth factor-19 (FGF19) is an intestinal hormone secreted from ileal enterocytes in response to binding of bile acids at the nuclear receptor, farnesoid-X receptor. Because bile acids are resorbed in the distal small intestine after aiding in intraluminal digestion, activation of farnesoid-X receptor and secretion of FGF19 are thought to represent a late postprandial signal indicating a transition between the fed and fasting states (1). After its secretion into hepatic portal circulation, FGF19 acts in the liver to reduce the expression and activity of CYP7A1, the rate-limiting enzyme responsible for the conversion of cholesterol to bile acids (2). Moreover, FGF19 acts in the gallbladder to induce relaxation and refilling with bile (3). All of this is consistent with FGF19's well-established role in bile acid metabolism (4, 5).
FGF19 also regulates energy and glucose homeostasis in rodents. The rodent ortholog to human FGF19 is named FGF15. Although the two proteins are only about 50% homologous, FGF19 is biologically active in rodents. Recombinant FGF19 is also commercially available in a stable protein form, making it more suitable for pharmacological experiments. Chronic iv infusion of recombinant FGF19 alters bile acid homeostasis and results in substantial weight loss and improved glucose tolerance in both leptin-deficient and diet-induced obese (DIO) mice (6). Transgenic overexpression of FGF19 likewise renders mice resistant to high-fat diet (HFD)-induced obesity and glucose intolerance (7).
The physiological effects of FGF15/19 have been thought to occur primarily through signaling at FGF-receptor 4 (FGFR4), which is highly expressed in liver (8). In addition to its role in cholesterol and bile acid homeostasis, FGFR4 also contributes to regulation of systemic lipid and glucose metabolism. FGFR4-knockout mice on a normal chow diet exhibit features of the metabolic syndrome including increased adiposity and insulin resistance (9). Intriguingly, reexpressing FGFR4 in liver was not sufficient to rescue these defects, implying an additional role for FGFR4 signaling at other tissues.
Consistent with this possibility, a recent study identifying a role for FGF15/19 and FGFR4 to repress hepatic gluconeogenesis, trichoroacetic acid-cycle flux, and fatty acid oxidation concluded that the metabolic actions of FGF15/19 involve effects on additional tissues (1). The brain, and particularly the hypothalamus, is one possibility because it is important in the regulation of systemic lipid and glucose metabolism (10, 11). Indeed, one previous study reported that intracerebroventricular (i.c.v.) administration of FGF19 increases energy expenditure in mice (6), further suggesting a potential role for the brain. In this study, we directly assessed the actions of FGF15/19 in the central nervous system (CNS) to inhibit food intake and improve glucose tolerance.
Materials and Methods
Animals
The University of Cincinnati IACUC approved all animal protocols. Male Long-Evans rats from Harlan Labs (Indianapolis, IN) were singly housed in an AAALAC-approved facility with a 12-h light, 12-h dark cycle, and allowed ad libitum access to food and water unless otherwise noted.
Diets
The rats were maintained on pelleted rodent chow (Harlan Teklad, Indianapolis, IN), a purified 40% fat diet, or a purified 60% fat diet (Research Diets, New Brunswick, NJ).
Hypothalamic gene expression
Rats maintained on either chow or 60% HFD for 6 wk were killed in the fed condition or after a 48-h fast. cDNA from whole hypothalamus was prepared as in described elsewhere (12). Quantitative RT-PCR was performed using a TaqMan 7900 sequence detection system and TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA). Relative mRNA expression for FGFR1 (NM_024146.1) and FGFR4 (NM_001109904.1) was calculated relative to the housekeeping gene 18S (NM_213557.1), using the ΔΔCT method. We have previously reported the expression of other obesity-associated genes in these samples (12).
Surgical procedures
Rats were outfitted with stainless-steel cannulas in the third-cerebral ventricle (i3vt) as in described previously (13). Correct placement was confirmed by verifying that i3vt injection of 10 ng angiotensin II (American Peptides, Sunnyvale, CA) provoked an intake of at least 5 ml of water within 1 h, and/or by injecting dye into the cannula just before death and confirming its presence in the ventricles upon dissection.
Drugs
Recombinant FGF19 from ProSpecBio (Ness-Ziona, Israel) was dissolved in water at a concentration of 1 mg/ml and delivered in a volume of 3 μl. The FGFR inhibitor PD173074 was obtained from Tocris Bioscience (Minneapolis, MN), dissolved in dimethyl-sulfoxide at a concentration of 50 mg/ml, and delivered in a volume of 3 μl.
Feeding experiments
Rats were weighed 2 h before the onset of dark, and food hoppers were removed. Just before the onset of dark, weight-matched groups received an i3vt infusion of FGF19, PD173074, or vehicle, and hoppers were returned. Animals and hoppers were weighed again at the indicated intervals.
Glucose tolerance testing
Just before the experiment, fasted animals were weighed and separated into weight-matched groups for i3vt infusion of FGF19, PD173074, or vehicle. Rats were fasted for 12 h, beginning at the onset of the light phase. At 30 min after an i3vt infusion, rats received an ip injection of 2 mg/kg dextrose (FGF19 experiments) or 1 mg/kg dextrose (PD173074 experiment). Blood glucose was measured at baseline and at regular intervals thereafter using Accu-Check glucose meters and strips (Roche, Indianapolis, IN). Blood was collected from the tail vein at the indicated time points for measurement of plasma insulin (ELISA, Crystal Chem, Downers Grove, IL).
Statistical analyses
Data are presented as mean ± ses. Data were analyzed using ANOVA or repeated measures ANOVA (RM ANOVA), followed by Tukey's post hoc tests, or by t test as indicated. Data were analyzed using Prism (GraphPad Software, Inc., San Diego CA) or Sigma Stat (SYSTAT, San Jose CA) software with the critical value, α, set at P < 0.05.
Results
FGFR expression in hypothalamus
Because FGF15/19 is known to bind to and activate both FGFR1 and FGFR4 (14), we asked whether FGFR1 and/or FGFR4 are expressed in rat hypothalamus, and if fasting or high-fat feeding alters this expression. In agreement with the literature (15–17), FGFR1 mRNA was abundant in hypothalamus. We found that FGFR1 expression was reduced by up to 60% among animals maintained on a HFD compared with chow-fed controls (Fig 1A). There was no significant effect of fasting. Consistent with a previous report (see Supplemental Table 2 in Ref. 15), we also observed more moderate levels of FGFR4 mRNA in hypothalamus. Like FGFR1, FGFR4 expression was significantly lower in DIO rats, and this was more apparent in the fed condition (Fig 1B). It has also been reported that β-Klotho, a transmembrane glycoprotein that stabilizes the association between endocrine FGFs and their receptors, is present in mouse hypothalamus (see supplemental Table 3 in Ref. 15). However, because rat β-Klotho has not yet been well characterized, we were unable to measure changes in its expression.
Fig. 1.
FGFR mRNA expression in hypothalamus of lean and DIO rats. Both FGFR1 (panel A) and FGFR4 (panel B) mRNAs were expressed in whole rat hypothalamus. A, The expression of FGFR1 was lower in the hypothalamus of high-fat diet-fed rats relative to lean controls (two-way ANOVA, P < 0.001; Tukey), regardless of whether the rats were killed in the fed state or after a 48-h fast. B, The expression of FGFR4 was lower in the hypothalamus of high-fat diet-fed rats relative to lean controls, and this was more apparent among rats killed in the fed state (two-way ANOVA, P < 0.001; Tukey) (n = 6-8 per group; *, P < 0.05; **, P < 0.01).
I3vt FGF19 and food intake
Weight-matched groups of rats maintained on chow received FGF19 or vehicle i3vt. I3vt FGF19 reduced 24-h chow intake by approximately 25% and induced significant weight loss relative to controls (Fig 2, A–C). In light of the changes in FGFR expression in hypothalamus of HFD rats, we hypothesized that the central anorexic effects of FGF19 would be blunted under HF-feeding conditions. Weight-matched groups, on a 40% HFD for 6 wk, received FGF19 i3vt or vehicle as before. I3vt FGF19 elicited a 20% reduction in 24-h food intake accompanied by a significant reduction in body weight (Fig 2, D and E), indicating no obvious impairment of CNS FGF19 action in DIO.
Fig. 2.
Infusion of FGF19 directly into the brain reduces food intake and body weight. In two independent experiments conducted in different cohorts of chow-fed rats (A–C[b]), infusion of FGF19 directly into the i3vt elicited a reduction in food intake over 24 h (RM ANOVA, P < 0.05; Tukey), accompanied by a reduction in body weight (t test). D, The same dose of FGF19, given i3vt, was also sufficient to reduce food intake over 24 h in rats maintained on a high-fat diet for 6 wk (RM ANOVA, P < 0.01; Tukey) and to E, significantly reduce body weight, relative to vehicle (VEH)-treated controls (t test, P < 0.05) (n = 5-9 per group; **, P < 0.01; *, P < 0.05).
I3vt FGFR inhibition and food intake
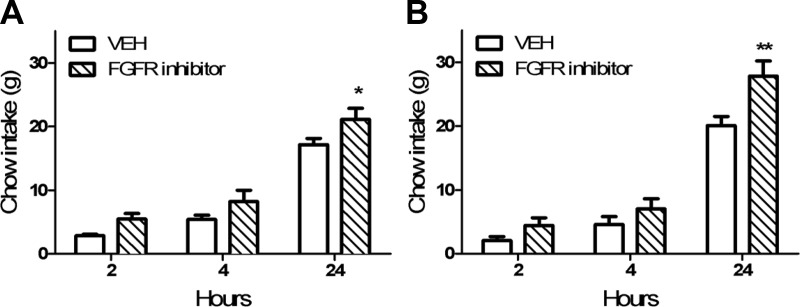
To test the hypothesis that endogenous CNS FGFR signaling is important in the regulation of energy balance, we inhibited FGFRs in the CNS with an acute i3vt infusion of the compound PD173074 and monitored food intake over 24 h. PD173074 induced a 38% increase in 24-h food intake (Fig 3, A and B), implying that endogenous CNS FGFR signaling is anorexigenic.
Fig. 3.
Infusion of an FGFR inhibitor directly into the brain increases food intake. In two independent experiments conducted in different cohorts of chow-fed rats, infusion of the FGFR inhibitor PD173074 increased 24-h food intake (RM ANOVA, P < 0.05; Tukey) (n = 4-7 per group; *, P < 0.05). VEH, Vehicle. **, P < 0.01.
I3vt FGF19 and glucose tolerance
We next asked whether FGF19 acts in the brain to regulate glucose tolerance. Weight-matched rats were treated with i3vt FGF19 or vehicle 30 min before an ip glucose challenge. FGF19 improved glucose tolerance (Fig 4, A–D). Plasma insulin did not differ between the groups at any time point (Fig 4E).
Fig. 4.
CNS FGFR signaling regulates glucose tolerance. In two independent experiments conducted in different cohorts of weight-matched chow-fed rats (A–E), infusion of FGF19 directly into the i3vt improved glucose tolerance, as measured by the time course (RM ANOVA, P < 0.05; Tukey) (A and C) or by the area under the curve (AUC, t tests) (B and D). E, This was not associated with differences in plasma insulin (RM ANOVA, P > 0.05). F, i3vt infusion of an FGFR inhibitor impaired glucose tolerance, as measured by the time course (RM ANOVA, P < 0.05; Tukey), G, Area under the curve [t test, nonsignificant (ns) (n = 3-7 rats per group; ***, P < 0.001; **, P < 0.01; *, P < 0.05].VEH, Vehicle.
i3vt FGFR inhibition and glucose tolerance
Inhibiting FGFRs locally in the CNS with i3vt PD173074 significantly impaired glucose tolerance (Fig 4, F and G), supporting a role for endogenous CNS FGFR signaling in the regulation of glucose homeostasis.
Discussion
The present observations clearly demonstrate that i.c.v. administration of the ileal hormone FGF19 suppresses food intake over 24 h and induces weight loss in both lean and DIO rats (Fig 2). This is consistent with growing evidence that FGF15/19 acts in an insulin-like manner (1, 8), whereas other gut-derived hormones have faster and more transient effects on feeding behavior (18). Moreover, our data are consistent with previous reports that i.c.v. FGF19 increases energy expenditure, and that its chronic peripheral administration or transgenic overexpression is sufficient to reduce body weight and increase energy expenditure in DIO mice (6–7, 19). The literature regarding effects of chronic peripheral administration of FGF19 on food intake, however, is equivocal. Some studies report decreased food intake (19), others report no effect (6, 19), and its transgenic overexpression increases food intake when expressed relative to body weight (7). These disparate findings may result from a tension between the direct actions of FGF19 on anorexigenic circuits and an increased drive to eat that is secondary to increased energy expenditure. Alternatively they may reflect competing effects of FGF15/19 in the brain vs. the periphery. Insulin, for example, has catabolic central effects and anabolic peripheral effects. Additional studies will be necessary to further address these interesting possibilities.
Importantly, the present data indicate that i.c.v. FGF19 acutely improves glucose tolerance (Fig 4). Consistent with this, chronic peripheral administration or transgenic overexpression of FGF19 is sufficient to improve glucose tolerance in DIO or leptin-deficient mice (6, 7). Because our i.c.v. dose was roughly 100 times smaller than the optimal peripheral dose [1 mg/kg·body weight (1, 6, 8)], we think it is unlikely the hormone is escaping the brain to elicit its effects in the periphery. Intriguingly, acute peripheral administration of FGF15/19 induces insulin-independent increases in hepatic glycogen synthesis (8) and inhibits hepatic gluconeogenesis (1), but those studies did not explicitly assess the extent to which these outcomes rely on FGFR signaling in the liver. The current data raise the possibility that CNS FGFR signaling contributes to the regulation of hepatic glycogen synthesis and/or gluconeogenesis, and that the observed improvements in glucose tolerance reflect an insulin-like, but insulin-independent effect of brain FGF19 signaling. Additional studies investigating effects on hepatic lipid and carbohydrate metabolism and related signaling pathways are necessary to address this.
Consistent with the finding that i3vt infusion of FGF19 alters energy and glucose homeostasis, mRNAs for FGFRs 1 and 4 were found in rat hypothalamus, and their expression was significantly reduced in DIO rats relative to their lean controls (Fig 1). These data are in agreement with several previous reports that FGFR1 is present in hypothalamus (16–17), at qualitatively comparable levels to its expression in adipose tissue (15). Specifically, FGFR1 is expressed in the arcuate and lateral hypothalamus (16), key regions important for the control of glucose and lipid metabolism. The expression of FGFR4 and β-klotho in hypothalamus is at least 1 order of magnitude lower compared with liver (15). Importantly, however, the hypothalamus is a heterogeneous structure. As such, the expression of FGFR4 and/or β-klotho in a small group of hypothalamic cells can have disproportionately large systemic effects. A more thorough understanding of the functional neuroanatomy of brain FGFRs and effects of FGFR signaling on downstream signaling pathways and gene expression is a priority for future research.
We found that inhibiting the endogenous activity of CNS FGFRs with PD173074 increased food intake (Fig 3) and impaired glucose tolerance (Fig 4). Our data agree with previous reports that endogenous CNS FGFR signaling alters energy balance (20–21). However, because PD173074 inhibits both FGFR1 and FGFR4 (22, 23), we cannot discriminate the relative contribution of these two receptors to these effects. Intriguingly, like many other postprandial gut hormones, FGF15/19 may also be expressed in restricted regions of the adult brain (http://mouse.brain-map.org) (Supplemental Table 2 in Ref. 15, 24), although the extent of its neuroanatomical distribution has not yet been fully explored. Additional studies will be necessary to parse the relative contributions of brain and gut FGF15/19 to the regulation of metabolism.
Nonetheless it is apparent that the FGF19/FGFR4 signaling axis has an important physiological role in the regulation of metabolism. First, plasma levels of FGF19 are negatively associated with indices of metabolic disease in humans (25). Consistent with this, both the FGF15-knockout mouse and the FGFR4-knockout mouse are glucose intolerant relative to littermate controls. This glucose intolerance can be rescued by exogenous administration of FGF19 to the FGF15-knockout mouse (8), but reexpressing a constitutively active form of FGFR4 in the liver of FGFR4 knockout mice was not sufficient to rescue the phenotype (9). This finding implicates FGFR4 signaling in other tissues, including, perhaps, hypothalamus, in the physiological regulation of metabolism.
The therapeutic potential of FGF19 in the treatment of metabolic disorders has received considerable recent attention (26–28). At pharmacological doses, FGF19 elicits comparable improvements on metabolic endpoints as another endocrine FGF, FGF21 (19); chronic FGF21 treatment reduces body weight and improves glucose tolerance in rodent and primate models of obesity and diabetes (29, 30). The similarities in the actions of these two endocrine FGFs have led to speculation that the underlying mechanisms are shared (19). FGF21 binds preferentially to FGFR1 in complex with the coreceptor β-Klotho (14). FGFR1 is only sparsely expressed in liver, but is highly expressed in hypothalamus and in white adipose tissue (15). Like FGF19, FGF21 acts in the CNS to regulate systemic energy and glucose metabolism (31). Recent evidence, however, suggests that the mechanisms by which FGF15/19 and FGF21 elicit improvements in metabolism are at least partially dissociated. Whereas fat-specific FGFR1-knockout mice were resistant to several metabolic effects of FGF21, both acute and chronic peripheral FGF19 was effective in this model (32).
In the present study we confirm previous reports that FGFRs 1 and 4 are present in rat hypothalamus and further demonstrate that high-fat feeding significantly reduces their expression (Fig 1). In addition, we find that small doses of FGF19 delivered acutely into the brain's ventricular system can elicit significant changes in peripheral metabolism. Specifically, i3vt FGF19 reduces food intake and body weight in both lean and DIO rats (Fig 2) and acutely improves glucose tolerance (Fig 4). Finally, inhibiting FGFRs acutely in the CNS increases food intake and impairs glucose tolerance (Figs. 3 and 4), suggesting a physiological role for CNS FGFR-signaling in the regulation of peripheral metabolism. Together these data indicate that FGF15/19 can be added to the list of gut-derived hormones capable of acting in the CNS to regulate systemic metabolism.
Acknowledgments
We thank Adam Chambers and Bernadette Grayson (University of Cincinnati, Cincinnati, OH) for technical assistance.
This work was supported by National Institutes of Health Grants DK082173 and HL111319 to (K.K.R.), DK084310 (to R.K.), DK017844 (to S.C.W.), and DK093848 (to R.J.S.).
Disclosure Summary: R.J.S. receives research support from Ablaris, Johnson and Johnson, Novo Nordisk, and Pfizer, is a paid speaker for Johnson and Johnson, Merck, Novo Nordisk, and Pfizer, serves as a consultant for Angiogen, Eli Lilly, Johnson and Johnson, Novartis, Novo Nordisk, Takeda, and Zafgen, and has equity in Zafgen. R.K. receives research support from Johnson and Johnson. K.K.R., R.G.A., S.G., and S.C.W. have no disclosures.
Footnotes
- CNS
- Central nervous system
- DIO
- diet-induced obese
- FGF
- fibroblast growth factor
- FGFR
- FGF receptor
- HFD
- high-fat diet
- i3vt
- third-cerebral ventricle
- RM ANOVA
- repeated measures ANOVA.
References
- 1. Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. 2011. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab 13:729-738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2:217-225 [DOI] [PubMed] [Google Scholar]
- 3. Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA. 2006. Identification of a hormonal basis for gallbladder filling. Nat Med 12:1253-1255 [DOI] [PubMed] [Google Scholar]
- 4. Jones S. 2008. Mini-review: endocrine actions of fibroblast growth factor 19. Mol Pharmacol 5:42-48 [DOI] [PubMed] [Google Scholar]
- 5. Itoh N. 2010. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res 342:1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. 2004. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145:2594-2603 [DOI] [PubMed] [Google Scholar]
- 7. Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. 2002. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143:1741-1747 [DOI] [PubMed] [Google Scholar]
- 8. Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. 2011. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331:1621-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. 2007. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes 56:2501-2510 [DOI] [PubMed] [Google Scholar]
- 10. Sandoval DA, Obici S, Seeley RJ. 2009. Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discov 8:386-398 [DOI] [PubMed] [Google Scholar]
- 11. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature 404:661-671 [DOI] [PubMed] [Google Scholar]
- 12. Gutierrez-Aguilar R, Kim DH, Woods SC, Seeley RJ. 2012. Expression of new loci associated with obesity in diet-induced obese rats: from genetics to physiology. Obesity 20:306-312 [DOI] [PubMed] [Google Scholar]
- 13. Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D'Alessio D, Tso P, Seeley RJ, Woods SC. 2011. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav 103:10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. 2007. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282:26687-26695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. 2010. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 24:2050-2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuo A, Tooyama I, Isobe S, Oomura Y, Akiguchi I, Hanai K, Kimura J, Kimura H. 1994. Immunohistochemical localization in the rat brain of an epitope corresponding to the fibroblast growth factor receptor-1. Neuroscience 60:49-66 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. 1995. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res 701:201-226 [DOI] [PubMed] [Google Scholar]
- 18. Ryan KK, Woods SC, Seeley RJ. 2012. Central nervous system mechanisms linking the consumption of palatable high fat diets to the defense of greater adiposity. Cell Metab 15:137-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adams AC, Coskun T, Rovira AR, Schneider MA, Raches DW, Micanovic R, Bina HA, Dunbar JD, Kharitonenkov 2012. A Fundamentals of FGF19, FGF21 action in vitro and in vivo. PloS One 7:e38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sasaki K, Oomura Y, Suzuki K, Muto T, Hanai K, Tooyama I, Kimura H, Yanaihara N. 1991. Effects of fibroblast growth factors and platelet-derived growth factor on food intake in rats. Brain Res Bull 27:327-332 [DOI] [PubMed] [Google Scholar]
- 21. Li AJ, Oomura Y, Hori T, Aou S, Sasaki K, Kimura H, Tooyama I. 1996. Fibroblast growth factor receptor-1 in the lateral hypothalamic area regulates food intake. Exp Neurol 137:318-323 [DOI] [PubMed] [Google Scholar]
- 22. Taylor JG, VI, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, Yu Y, Chen QR, Shah K, Youngblood V, Fang J, Kim SY, Yeung C, Helman LJ, Mendoza A, Ngo V, Staudt LM, Wei JS, Khanna C, Catchpoole D, Qualman SJ, Hewitt SM, Merlino G, Chanock SJ, Khan J. 2009. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest 119:3395-3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pardo OE, Latigo J, Jeffery RE, Nye E, Poulsom R, Spencer-Dene B, Lemoine NR, Stamp GW, Aboagye EO, Seckl MJ. 2009. The fibroblast growth factor receptor inhibitor PD173074 blocks small cell lung cancer growth in vitro and in vivo. Cancer Res 69:8645-8651 [DOI] [PubMed] [Google Scholar]
- 24. The gene expression nervous system altas (GENSAT) Project. NINDS Contracts 1INS02331 and HHSN271200723701C to the Rockefeller University, New York, NY [Google Scholar]
- 25. Barutcuoglu B, Basol G, Cakir Y, Cetinkalp S, Parildar Z, Kabaroglu C, Ozmen D, Mutaf I, Bayindir O. 2011. Fibroblast growth factor-19 levels in type 2 diabetic patients with metabolic syndrome. Ann Clin Lab Sci 41:390-396 [PubMed] [Google Scholar]
- 26. Strack AM, Myers RW. 2004. Modulation of metabolic syndrome by fibroblast growth factor 19 (FGF19)? Endocrinology 145:2591-2593 [DOI] [PubMed] [Google Scholar]
- 27. Wu X, Li Y. 2011. Therapeutic utilities of fibroblast growth factor 19. Expert Opin Ther Targets 15:1307-1316 [DOI] [PubMed] [Google Scholar]
- 28. Long YC, Kharitonenkov A. 2011. Hormone-like fibroblast growth factors and metabolic regulation. Biochim Biophys Acta 1812:791-795 [DOI] [PubMed] [Google Scholar]
- 29. Véniant MM, Komorowski R, Chen P, Stanislaus S, Winters K, Hager T, Zhou L, Wada R, Hecht R, Xu J. 2012. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology 153:4192-4203 [DOI] [PubMed] [Google Scholar]
- 30. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. 2008. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149:6018-6027 [DOI] [PubMed] [Google Scholar]
- 31. Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. 2010. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59:1817-1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. 27 August 2012. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab 10.1016/j.molmet.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]