Abstract
Acetylcholine (ACh) has been established as a paracrine factor in the anterior pituitary gland, but the receptors mediating ACh action and the cell types bearing these receptors have not been identified. Our results showed that the expression of the nicotinic subunits mRNAs followed the order β2 > β1 = α9 > α4 in cultured rat pituitary cells. The expression of the subunits in immortalized LβT2 mouse gonadotrophs followed the order β2 > α4 = α1. M4 > M3 muscarinic receptor mRNA were also identified in pituitary and LβT2 cells. The treatment of cultured pituitary cells with GnRH down-regulated the expression of α9 and α4 mRNAs, without affecting the expression of M3 and M4 receptor mRNAs, and ACh did not alter the expression of GnRH receptor mRNA. We also performed double immunostaining to show the expression of β2-subunit and M4 receptor proteins in gonadotrophs. Functional nicotinic channels capable of generating an inward current, facilitation of electrical activity, and Ca2+ influx were identified in single gonadotrophs and LβT2 cells. In both cell types, the M3 receptor-mediated, phospholipase C-dependent Ca2+ mobilization activated an outward apamin-sensitive K+ current and caused hyperpolarization. The activation of M4 receptors by ACh inhibited cAMP production and GnRH-induced LH release in a pertussis toxin-sensitive manner. We concluded that multiple cholinergic receptors are expressed in gonadotrophs and that the main secretory action of ACh is inhibitory through M4 receptor-mediated down-regulation of cAMP production. The expression of nicotinic receptors in vitro compensates for the lack of regular GnRH stimulation of gonadotrophs.
Acetylcholine (ACh) is an agonist of the muscarinic ACh membrane receptor (mAChR) and nicotinic ACh membrane receptor channel (nAChR). mAChRs belong to the superfamily of G protein-coupled receptors. There are five subtypes of these receptors, termed M1-M5. The M1, M3, and M5 receptors signal predominantly through the Gq/11 pathway. This pathway activates phospholipase C, which catalyzes the production of inositol trisphosphate and diacylglycerol, intracellular messengers that release Ca2+ from intracellular stores and activate protein kinase C, respectively. In contrast, M2 and M4 receptors are coupled to the Gi/o signaling pathway. This pathway inhibits adenylyl cyclase activity and exhibits βγ dimmer-dependent effects on channel gating (1). nAChRs are members of the comparatively diverse Cys-loop family of ligand-gated channels. Seventeen subunits of this receptor have been identified and shown to assemble into a variety of receptor subtypes. The binding of (-)-nicotine ditartrate (nicotine), ACh, or other agonists to nAChRs stimulates cation influx through a channel and generally results in membrane depolarization. The pores of the activated channels are permeable to Na+ and K+ and in some neuronal subtypes to Ca2+ as well (2, 3).
ACh has also been established as an autocrine and a paracrine factor in the pituitary gland (4). Functional nAChRs have been described in the porcine intermediate pituitary cells at both the whole-cell and single-channel levels (5). These channels are depolarizing, and their activation facilitates Ca2+ influx directly by allowing flow through the pore of the channel and indirectly by activating voltage-gated Ca2+ channels (6). ACh released from frog melanotrophs also activates M1 receptors (7) and stimulates electrical activity and α-melanocyte-stimulating hormone release (8, 9). Moreover, mAChRs are present in rat (10) and sheep (11) anterior pituitary tissues, cultured rat anterior pituitary cells (12), and the mouse AtT-20 pituitary tumor cell line (13). Functional studies have also indicated the expression of these receptors in rat folliculo-stellate cells (14) and immortalized rat GH3 pituitary cells (15). Studies with anterior pituitary cells have also revealed that ACh regulates prolactin and GH secretion (16–19). However, the mAChR subtypes present in the subpopulations of endocrine anterior pituitary cells have not been identified. Furthermore, the composition, biophysical and electrophysiological properties, and effects on Ca2+ signaling of the nAChRs have not been studied in anterior pituitary cells.
Here, we investigated the expression and signaling functions of the nAChRs and mAChRs in gonadotrophs, cells that are critical for the control of reproduction (20). Our experiments were performed on cultured rat gonadotrophs and immortalized mouse LβT2 gonadotrophs. We identified three types of ACh receptors in these cells. The activation of these receptors by a common agonist inhibits cAMP production through M4 receptors, facilitates Ca2+ mobilization through M3 receptors, and causes depolarization and stimulation of Ca2+ influx through β2-containing nicotinic channels.
Materials and Methods
Chemicals
ACh, 11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one (AF-DX 116), apamin, atropine, bicuculline methiodide (BMI), 8-methyl-8-azabicyclo-3-endo[3.2.1]oct-3-yl-1,4-dihydro-2-oxo-3(2H)-quinazolinecarboxylic acid ester hydrochloride (DAU 5884), (-)-cytisine, (+)-tubocurarine chloride (dTC), iberiotoxin (IBTX), pirenzepine dihydrochloride (pirenzepine), N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-4-chlorobenzamide (PNU 282987), pertussis toxin (PTX), 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl-1H-imidazole hydrochloride, 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM 34), and 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride (Xe991) were purchased from Tocris Bioscience (Ellisville, MO). GnRH was purchased from Bachem (Torrance, CA), [d-pGlu1,d-Phe2,d-Trp3,6]GnRH (DpGlu) from Penninsula Laboratories (San Carlos, CA), and fura 2-AM from Invitrogen (Carlsbad, CA). Amphotericin B, α-bungarotoxin (αBTX), forskolin, HEPES, IBTX, ivermectin, nicotine, oxotremorine, 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U73122), 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5-pyrro-lidinedione, and 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5-pyrrolidinidione (U73343) were from Sigma (St. Louis, MO).
Cell cultures
Experiments were performed on anterior pituitary cells from normal postpubertal female Sprague Dawley rats obtained from Taconic Farms (Germantown, NY). Euthanasia was performed by asphyxiation with CO2, and the anterior pituitary glands from 20-50 rats in different stages of estrous cycle were removed after decapitation. The experiments were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. Anterior pituitary tissue was cut into 1 × 1-mm pieces using a tissue Chopper (Brinkmann Instruments, Westbury, NY). Tissue was than washed in PBS containing 0.3% BSA, 2 mm glutamine, and MEM vitamins (Invitrogen) and treated with 0.4% trypsin for 15 min at 37 C. The cells were mechanically dispersed using transfer pipette and filtered trough a 40-μm nylon mesh to remove tissue fragments. Cells were harvested by centrifugation at 300 × g for 10 min, and cell pellet was resuspended in medium 199 containing Earle's salts, sodium bicarbonate, 10% heat-inactivated horse serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). For electrophysiological and calcium measurements, cells were plated at low density (0.7 million per 25-mm glass coverslip coated with poly-lysine). For total RNA isolation, cells were plated on poly-lysine coated six-well plates, 4 million per well. For perifusion experiments, 10 million cells were incubated with preswollen cytodex-1 beads in 60-mm Petri dishes. All experiments were performed 24-48 h after dispersion. Immortalized mouse gonadotroph LβT2 cells were grown in DMEM supplemented with 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin in a humidified 5% CO2 atmosphere at 37 C.
RT-PCR analysis
Total RNA from the primary pituitary cells and LβT2 gonadotrophs was extracted using the RNeasy Mini kit (QIAGEN, Valencia, CA). Subsequently, 1 μg of total RNA was treated with deoxyribonuclease I (Invitrogen) and reverse transcribed with SuperScript III First Strand Synthesis SuperMix for quantitative RT-PCR (qRT-PCR) (Invitrogen). qRT-PCR was performed using predesigned TaqMan Gene Expression Assays for rat and mouse (Applied Biosystems, Foster City, CA) using the LightCycler TaqMan Master Mix and the LightCycler 2.0 Real-Time PCR system (Roche Applied Science, Indianapolis, IN). The target gene expression levels were determined by the comparative 2−δδC(T) quantification method using glyceraldehyde-3-phosphate dehydrogenase as a reference gene. The results were expressed as relative to the β2 nicotinic, M4 muscarinic, or GnRH receptor gene expression (set to 100%).
Intracellular calcium measurements
Calcium studies were performed on single isolated cells bathed in Krebs Ringer buffer with 2 μm fura 2-AM at room temperature for 60 min. Coverslips with cells were then mounted on the stage of an Observer-D1 microscope (Carl Zeiss, Oberkochen, Germany) attached to an ORCA-ER camera (Hamamatsu Photonics, Hamamatsu City, Japan) and a λ DG-4 wavelength switcher (Sutter, Novato, CA). Hardware control and image analysis was performed using Metafluor software (Molecular Devices, Downingtown, PA). Cells were examined under an oil immersion objective during exposure to alternating 340- and 380-nm light beams, and the intensity of light emission at 520 nm was measured.
Electrophysiological measurements
Membrane potential and whole-cell currents were measured in single isolated pituitary cells using the amphotericin-perforated patch-clamp technique at room temperature. Briefly, cells were continuously perfused with an extracellular solution containing: 150 mm NaCl, 3 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, and 10 mm glucose (pH was adjusted to 7.3 with NaOH). In some experiments, bath CaCl2 was omitted and 5 mm EGTA added to bath medium. The pipette solution contained: 70 mm K-aspartate, 70 mm KCl, 3 mm MgCl2, and 10 mm HEPES (pH was adjusted to 7.2 with KOH). Before measurement, amphotericin B was added to the pipette solution from a stock solution (20 mg/ml in dimethyl sulfoxide, always freshly prepared) to obtain a final concentration of 200 μg/ml. The holding potential for ACh-induced current recording was at −60 mV (pituitary cells in culture), or −40 mV (pituitary cell lines), if not otherwise stated. Recordings were performed using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Data were captured and stored using the pClamp 10 software packages in conjunction with the Digidata 1322A A/D converter (Axon Instruments). All data have been corrected for liquid junction potential (9.7 mV, positive). Solutions were delivered to the recording chamber by a gravity-driven microperfusion system (ALA Scientific Instruments, Westbury, NY).
Immunohistochemistry
Adult rat pituitaries were fixed in Bouin's fix solution for 48 h. Next, they were dehydrated in a series of alcohol washes, immersed in xylene, and embedded in paraffin. Five-micrometer coronal sections were cut on a microtome and mounted on gelatin-coated slides. The sections were then deparaffinized with xylene and rehydrated, and the antigen was retrieved in 0.5 m Tris-HCl buffer (pH 9.5) by heating in microwave oven. The β2-subunit immunofluorescence staining was performed using the tyramide signal amplification kit with horseradish peroxidase-goat antirabbit IgG and Alexa Fluor 488 tyramide according to the manufacturer's instructions (Invitrogen). The sections were incubated with polyclonal rabbit antinicotinic ACh receptor, β2-subunit (0.65 μg/ml; GeneTex, Irvine, CA), for 1 h at room temperature. After immunostaining for the β2-subunit, we used microwave treatment to avoid false colocalization between the two rabbit antisera (21). The sections were immersed in citrate buffer at pH 6.0, brought to the boiling point in the microwave oven at maximum power, and then microwaved again for 5 min at half power. After cooling down, the sections were stained for LH (1:200 dilution) overnight at 4 C. The sections were then incubated with donkey antirabbit Alexa Fluor 350 (1:200 dilution) for 2 h at room temperature. After permeabilization with 0.3% Triton X-100 in PBS with 1% BSA for 30 min, a mouse antibody complementary to the M4 subunit of the muscarinic receptor was applied overnight at 4 C (10 μg/ml; Millipore, Bedford, MA). The sections were then incubated with donkey antimouse Alexa Fluor 555 (1:200 dilution) for 2 h at room temperature. Finally, the sections were mounted in Mowiol (Calbiochem, San Diego, CA) and examined under the Zeiss Axiovert optical fluorescent microscope with an EC Plan-Neofluar ×63 objective. The images were collected using the Apotome system for optical sectioning. Triple labeling procedures were performed with special attention paid to the possible secondary antibody cointeractions and false colocalizations. In separate experiments, it was determined that secondary antibodies did not react with other primary or secondary antibodies applied. Normal rabbit and normal mouse IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used as negative controls at dilutions used for the primary antibodies. No specific labeling was detected after this treatment or after the omission of primary antibodies. Furthermore, all of the primary antibodies used were routinely probed in Western blotting. In the anterior pituitary tissue, each of them recognized a band with the size that corresponded to the size of investigated protein (data not shown).
cAMP and LH measurement
cAMP and LH secretion was monitored using cell column perifusion experiments. Briefly, cells attached on cytodex-1 beads were transferred from Petri dishes to 0.5-ml chambers and perifused with Hanks' M199 containing 25 mm HEPES, 0.1% BSA, and penicillin (100 U/ml)/streptomycin (100 μg/ml) for 2.5 h at a flow rate of 0.5 ml/min and at 37 C to establish stable basal secretion. Fractions were collected in 1-min intervals and later assayed for cAMP contents and LH secretion, using our specific cAMP antiserum, 125I-cAMP from PerkinElmer Life Sciences (Boston, MA), and rat LH ELISA kit was from Shibayagi (Shibukawa, Japan).
Data analysis
All numerical values in the text are reported as mean ± sem. Significant differences between means were determined by ANOVA accompanied with a post hoc statistical test; P values of less than 0.05 were considered significant.
Results
Expression of AChR mRNA and protein transcripts in the anterior pituitary gland
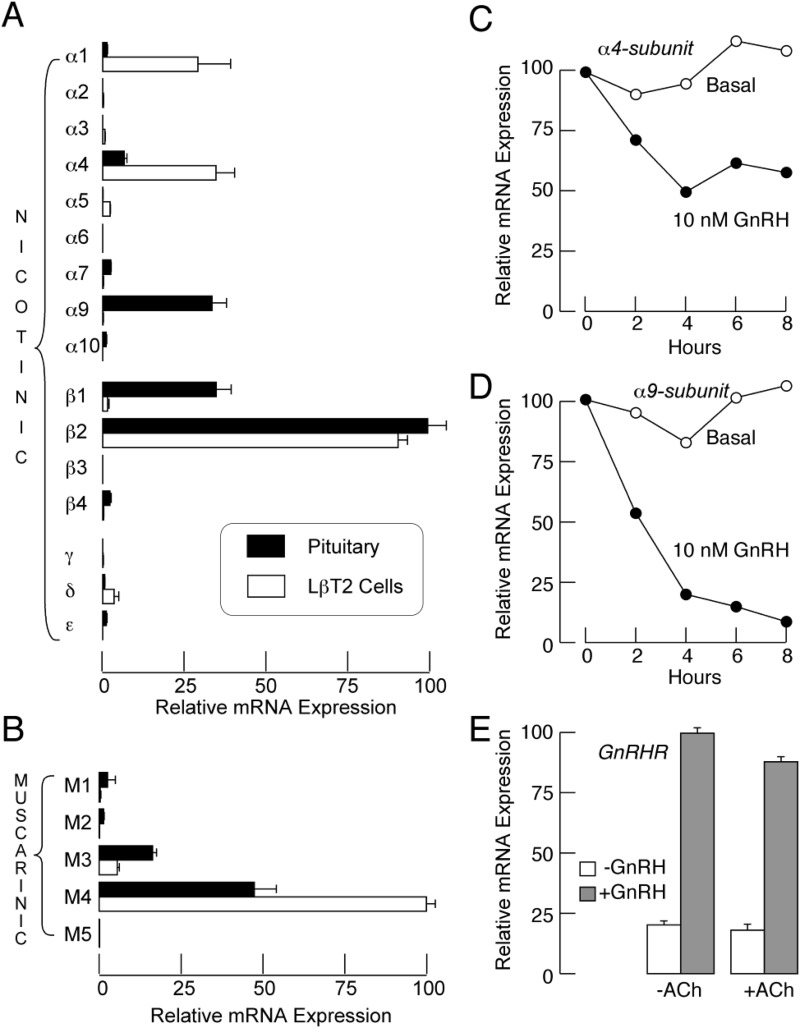
qRT-PCR analysis was performed on mRNA extracted from cultured rat anterior pituitary cells. We looked for expression of the mRNAs of the nicotinic-α1-, -α2-, -α3-, -α4-, -α5-, -α6-, -α7-, -α9-, -α10-, -β1-, -β2-, -β3-, -β4-, -γ-, -δ-, and -ϵ-subunits and the muscarinic M1, M2, M3, M4, and M5 receptors. The pituitary cells expressed several nicotinic subunits. The quantities of the subunits followed the following order: β2 > β1 = α9 > α4 > α7 = β4, with > reflecting significant difference and = the lack of significant difference among pairs (ANOVA). mRNA transcripts for mAChRs were also identified in the pituitary cells. The quantities of the isoforms followed the following order: M4 > M3 > M1 = M2. In immortalized LβT2 gonadotrophs, the order was: β2 > α4 = α1 > δ = α5 = β1 subunits (Fig. 1A) and M4 > M3 (Fig. 1B).
Fig. 1.
Expression of ACh receptor subunits in pituitary cells. A and B, qRT-PCR analysis of expression of mRNAs for nAChR subunits (A) and mAChRs (B) in pituitary cells (closed bars) and LβT2 cells (open bars). Data shown are means ± sem values from four (A) and five (B) experiments. C and D, Time course of down-regulation of α4 (C) and α9 (D) mRNAs expression by GnRH in pituitary cells. E, The lack of effect of ACh on GnRH receptor mRNA expression in pituitary cells.
To test whether the expression patterns of these receptors in vitro depend on GnRH, we stimulated cultured pituitary cells with 10 nm GnRH for 6 h. This treatment resulted in a 50% reduction of α4-mRNA expression (Fig. 1C) and a 95% reduction of α9-mRNA expression (Fig. 1D). In contrast, the expression of other nicotinic subunits and M3- and M4-mAChR mRNAs was not affected by GnRH (data not shown). Further, the similar ACh treatment had no effect on the expression of GnRH receptor-mRNA and did not affect GnRH-induced up-regulation of this transcript (Fig. 1E).
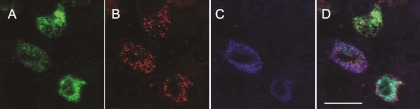
By analyzing the pituitary tissue sections using triple immunofluorescence, we further confirmed that the LH-containing cells (gonadotrophs) expressed both β2-subunit and M4 subunit proteins (Fig. 2). A large number of cells throughout the anterior pituitary was intensely stained for both the β2-subunit and for LH. The staining for the M4 subunit in LH-positive cells was less pronounced and appeared to follow a punctuate pattern. Some of the cells that were positively stained for the β2, M4 or both subunits were negative for LH. The immunohistochemical analysis of the other subunits and receptors was inconclusive.
Fig. 2.
Triple immunofluorescence labeling of β2-subunit of nAChR, M4 subtype of mAChR, and LH in anterior pituitary sections. A, β2-subunit (green fluorescence). B, M4 subunit (red fluorescence). C, LH-containing cells (blue fluorescence). D, Overlay, both β2-subunit and M4 subunit were observed in LH-containing cells. Nuclei were devoid of staining in all cases. Scale bar, 20 μm.
Taken together, these results indicate that both rat and mouse gonadotrophs express M4 and M3 types of muscarinic receptors as well as different subunits of nicotinic receptors. However, the ultimate structure of the receptors may differ between the two cell types, and the receptor composition is influenced by GnRH.
Characterization of Ca2+-mobilizing mAChRs in gonadotrophs
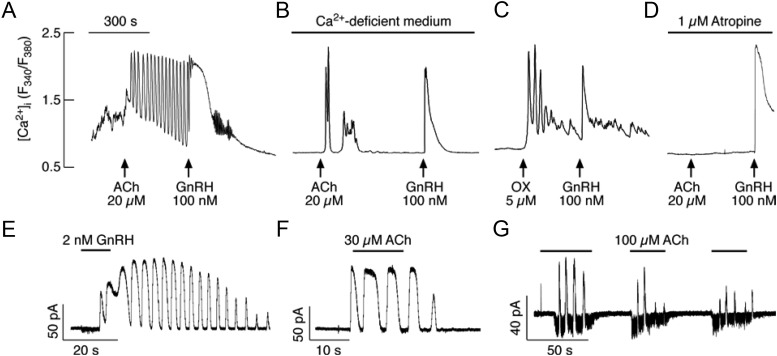
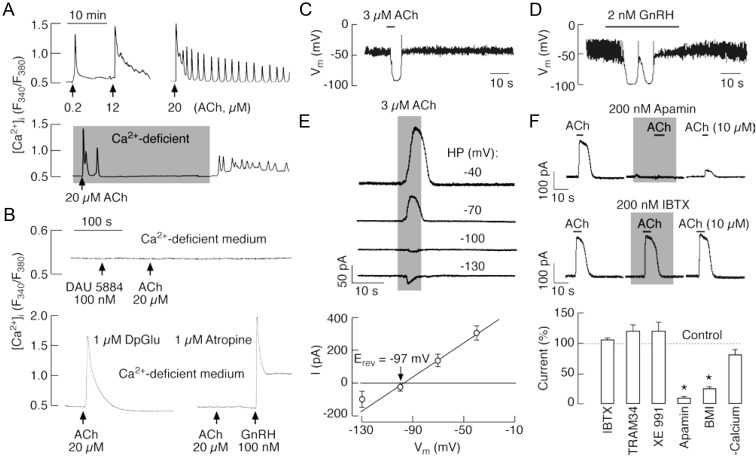
Gonadotrophs were initially identified by their cell type-specific morphology and, subsequent to experimentation, by addition of GnRH, a highly specific Ca2+-mobilizing agonist for these cells, which stimulates high-amplitude [Ca2+]i oscillations only in gonadotrophs (22). In 20 of 50 gonadotrophs, 20 μm ACh also induced high-amplitude [Ca2+]i transients when bathed in Ca2+-containing medium (Fig. 3A). The ACh- and GnRH-induced [Ca2+]i responses were also observed in Ca2+-deficient medium (Fig. 3B). This indicated that the increase in [Ca2+]i was a result of Ca2+ release from intracellular stores. Consistent with the role of Ca2+-mobilizing mAChR in this response, in cells bathed in Ca2+-deficient medium, the ACh-induced [Ca2+]i oscillations were mimicked by the application of 5 μm oxotremorine (Fig. 3C), a nonselective mAChR agonist (26), and inhibited by the application of 1 μm atropine (Fig. 3D), a nonselective mAChR antagonist.
Fig. 3.
Agonist-induced oscillatory calcium and current responses in identified gonadotrophs. A and B, Examples of Ca2+ responses to application of ACh and GnRH in gonadotrophs bathed in Ca2+-containing (A) and Ca2+-deficient (B) medium. C and D, Oxotremorine (OX) stimulated Ca2+ oscillations (C) and atropine inhibited effects of ACh on [Ca2+]i response (D) in GnRH-responsive cells bathed in Ca2+-deficient medium. In this and following figures, arrows indicate the start of agonist application. E and F, Voltage clamp amphotericin-perforated whole-cell recording of Ca2+ oscillations monitored as an outward Ca2+-activated current in gonadotrophs stimulated with GnRH (E) and ACh (F). G, In a fraction of gonadotrophs, ACh stimulated inward and outward currents. All cells were voltage clamped at −60 mV. Horizontal bars indicate duration of agonist application.
Furthermore, we characterized the electrophysiological properties of these receptors through amphotericin-perforated whole-cell recordings by monitoring [Ca2+]i oscillations as apamin-sensitive small conductance (SK) Ca2+-activated potassium (KCa) channels (SK channels) (23–25). Similar to GnRH (Fig. 3E), ACh also induced an oscillatory membrane current in nine of 12 gonadotrophs, with peak amplitude of 37 ± 17 pA (Fig. 3F). In the majority of amphotericin-perforated cells, 100 μm ACh simultaneously induced outward and inward currents (Fig. 3G). The inward current was due to activation of nAChRs (see Fig. 7 below).
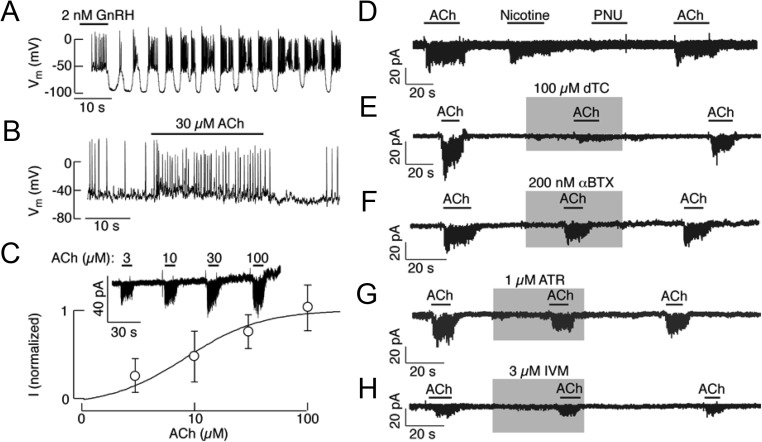
Fig. 7.
Electrophysiological characterization of nAChR in pituitary gonadotrophs. A and B, Current-clamp traces of GnRH (A) and ACh (B) induced electrical activity. Notice that GnRH-induced increase in the frequency of action potentials was periodically interrupted with transient hyperpolarization, whereas ACh application caused a sustained depolarization in this particular gonadotroph. C, Concentration-dependent effect of ACh on the amplitude of inward current in identified gonadotrophs. Top, Representative traces. Bottom, Mean ± sem values, with the estimated EC50 of 8.6 μm (n = 5). D, Stimulation of inward current by 100 μm ACh, 30 μm nicotine, but not by 1 μm PNU 282987. E, Inhibition of ACh-induced inward current by dTC. F–H, The lack of effect of αBTX, atropine (ATR), and ivermectin (IVM) on ACh-induced inward current in identified gonadotrophs. All traces were obtained using standard whole-cell patch-clamp recording. Gray areas and horizontal bars indicate duration of drug application.
In immortalized LβT2 gonadotrophs, ACh induced low frequency [Ca2+]i oscillations (0.49 ± 0.04 spikes per minute) or a nonoscillatory response when the cells were bathed in Ca2+-containing medium (Fig. 4A, top). In the oscillatory cells, spike frequency did not obviously increase with increased ACh concentration. Twenty-two of 40 cells bathed in a Ca2+-deficient medium were also responsive to ACh. Several initial spikes were observed in Ca2+-deficient medium, but these spikes were abolished after prolonged receptor activation. The administration of Ca2+ to the bath medium was accompanied by a secondary increase in [Ca2+]i (Fig. 4A, bottom). Thus, Ca2+-mobilizing mAChRs are also operative in LβT2 gonadotrophs.
Fig. 4.
ACh-induced Ca2+ mobilization and activation of SK-type potassium channels in LβT2 gonadotrophs. A, Representative records of oscillatory and nonoscillatory [Ca2+]i responses induced by variable ACh concentrations (top) and extracellular calcium dependence of sustained but not early spiking (bottom). Gray area indicates period during which cells were exposed to Ca2+-deficient medium. B, Pharmacological identification of receptors responsible for ACh-induced Ca2+ mobilization. Top, Inhibition of Ca2+-mobilizing action of 20 μm ACh in cells bathed in Ca2+-deficient medium and treated with 100 nm DAU 5884, a M3-mAChR-specific inhibitor. Bottom, Insensitivity of ACh-induced Ca2+ response to DpGlu, a specific antagonist of GnRH receptor (left), and inhibition by atropine, a general mAChR blocker (right), in cells bathed in Ca2+-deficient medium. C and D, Current-clamp traces of ACh (C)- and GnRH (D)-induced transient hyperpolarization. E and F, Activation of KCa channels by ACh in LβT2 gonadotrophs. E, Effect of the holding potential (HP) on the amplitude of ACh-induced outward current: representative traces (top) and mean ± sem values (bottom). The reversal potential (Erev = −97 ± 5 mV, n = 3) was near to the calculated reversal potential for K+ ions. F, Sensitivity of ACh-induced current to KCa channel blockers. Top, Inhibition of ACh-induced current by apamin (200 nm), a small conductance KCa channel blocker, but not IBTX (200 nm), a highly specific blocker for KCa1.1 channels. Bottom, Summary histogram showing effects of removal of bath Ca2+ and addition of K+ channel blockers: apamin (200 nm), IBTX (100 nm), BMI (30 μm), TRAM 34 (1 μm), and Xe991 (1 μm), in cell bathed in Ca2+-containing medium. *, P < 0.01. LβT2 cells were voltage clamped at −40 mV using amphotericin-perforated whole-cell recording.
Ca2+-mobilizing action of ACh in gonadotrophs could be mediated by βγ dimmer of M4 type of Gi/o-coupled receptors and/or the M3 type of Gq/11-coupled receptors, because three other subtypes of G protein-coupled mAChRs are poorly expressed in both cell types (Fig. 1). Pituitary gonadotrophs treated overnight with PTX remained responsive to ACh (data not shown), excluding the role of Gi/o-coupled M4 subtype of mAChR in calcium mobilization. On the other hand, ACh did not evoke Ca2+ responses in 39 of 39 cells that were bathed in a Ca2+-deficient medium in the presence of 100 nm DAU 5884, a M3-mAChR-specific antagonist (Fig. 4B, top), ACh also stimulated Ca2+ mobilization in gonadotrophs with inhibited GnRH receptors by DpGlu (Fig. 4B, bottom). However, ACh did not evoke a response in cells bathed in Ca2+-deficient medium containing 1 μm atropine (Fig. 4B, bottom). These data clearly indicate that the Ca2+-mobilizing action of ACh is mediated by the M3 type of mAChRs.
Activation of apamin-sensitive KCa channels by M3-mAChRs in LβΤ2 cells
In further experiments, we examined effects of ACh on electrophysiological properties of LβT2 cells. In amphotericin-perforated cells, both ACh (3-10 μm) and GnRH induced transient hyperpolarization (Fig. 4, C and D). In cells voltage clamped at −40 mV, ACh induced an outward current with a maximum peak amplitude of 122 ± 15 pA (n = 42) (Fig. 4, E and F), suggesting that KCa channels may play a role in hyperpolarization of the plasma membrane. The amplitude of the ACh-induced current was dependent on the membrane potential and decreased at holding potentials less than −40 mV (Fig. 4E, top). The estimated reversal potential for this current was −97 ± 5 mV, close to K+ equilibrium potential (Fig. 4E, bottom).
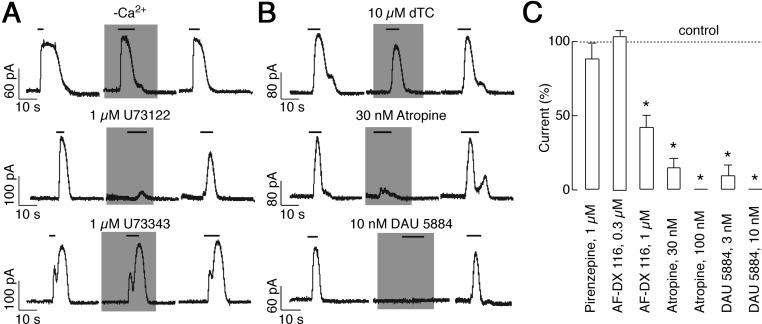
The nature of the channel involved in ACh-mediated hyperpolarization was examined using selective KCa channel blockers under voltage-clamp conditions. At a holding potential of −40 mV, the KCa2 blockers apamin (200 nm) and BMI (30 μm) (27) rapidly and reversibly reduced the ACh-evoked outward current to 9.6 ± 6 and 26 ± 2%, respectively (Fig. 4F, top and bottom). The current was insensitive to IBTX (100 nm) (Fig. 4F, middle), a specific inhibitor of KCa1.1 channels (27). The current was also insensitive to 1 μm TRAM 34, a blocker of KCa1 and KCa3.1 channels (28). It also did not respond to 1 μm Xe991, a Kv7/KCNQ channel inhibitor in the submicromolar concentration range (Fig. 4F, bottom) (29). These results indicated that mAChR-mediated Ca2+ signaling in LβT2 cells was coupled to the SK type of KCa channels.
Consistent with this conclusion, ACh-induced outward current was also observed in Ca2+-deficient medium (Fig. 4F, bottom, and Fig. 5A, top). The peak amplitude of this current was 80 ± 9% of the current amplitude observed in the presence of Ca2+. The outward current was completely inhibited by 1-min pretreatment with 1 μm U73122, a specific phospholipase C inhibitor, whereas 1 μm U73343, the inactive analog, did not inhibit the outward current (Fig. 5A, middle and bottom). The ACh-induced SK current was also reduced by 30 nm atropine, a nonselective mAChR antagonist (Fig. 5B, middle). The current was reduced to 14 ± 6%. However, the current was not affected by 10 μm dTC, a nonselective nAChR antagonist (Fig. 5B, top). AF-DX 116, a M2-mAChR-specific antagonist in the nanomolar-concentration range (30), was also ineffective at 300 nm and 1 μm AF-DX 116 reduced outward current to 41 ± 8%. In addition, 1 μm pirenzepine, an antagonist highly specific to M1 (31), had no effect (Fig. 5C). Of the tested muscarinic blockers, the M3 inhibitor DAU 5884 was the most effective when considering concentration-dependent effects. DAU 5884 at a concentration of 3 nm reduced ACh-induced outward current to 9 ± 7% (Fig. 5, B and C). These results indicate that the Ca2+-mobilizing action of ACh mediated by the M3 type of mAChRs is coupled to activation of SK current, leading to hyperpolarization of the cell membrane.
Fig. 5.
Characterization of mAChR expressed in LβT2 gonadotrophs. A, Dependence of ACh-induced Ca2+ mobilization on phospholipase C signaling pathway. ACh-induced outward current from LβT2 cells persisted in Ca2+-deficient medium (top), was completely inhibited by 1 min of pretreatment with U73122, a specific phospholipase C inhibitor (middle), and was not affected by pretreatment with inactive analog U73343 (bottom). B, ACh-induced outward current was insensitive to dTC (top) but was abolished by atropine (middle) and DAU 5884 (bottom). C, Summary histogram showing the effect of atropine, pirenzepine, DAU 5884, and AF-DX 116 on the amplitude of ACh-induced currents. Voltage clamp amphotericin-perforated whole-cell recording from LβT2 cell voltage clamped at −40 mV are shown. Cells were stimulated with 10 μm ACh. *, P < 0.01.
Characterization of nAChRs in gonadotrophs
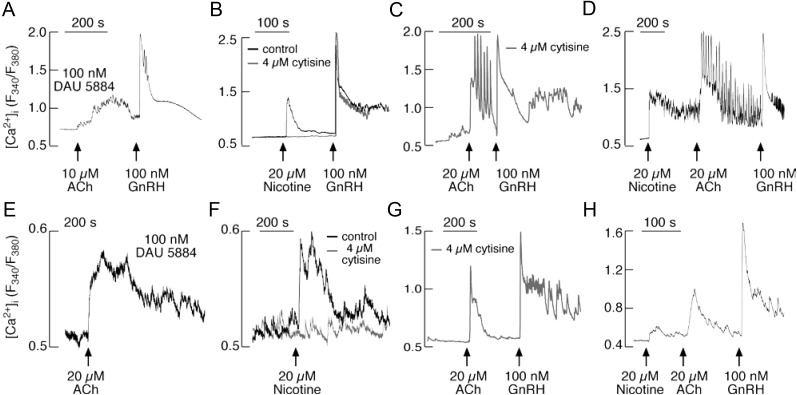
To clarify whether gonadotrophs express functional nAChRs and to examine their influence on calcium influx, we bathed primary cultures of pituitary cells and LβT2 cells in Ca2+-containing medium and examined the effects of ACh and nicotine, a nAChR-specific agonist, on Ca2+ signaling in individual cells. To exclude contribution of M3-mAChRs on calcium signaling (32), in initial experiments, 100 nm DAU 5884 was applied. The application of 20 μm ACh induced small amplitude nonoscillatory [Ca2+]i signals (Fig. 6A) in 50% of the gonadotrophs (25 of 50). Nicotine (20 μm) also induced an increase of the [Ca2+]i in 14 of 18 GnRH-positive cells (Fig. 6, B and D). When pituitary cells were bathed in Ca2+-deficient medium, nicotine did not elevate [Ca2+]i (data not shown). In nine cells bathed in medium containing calcium and 4 μm cytisine, a specific blocker of β2-containing nAChRs (33), nicotine was also ineffective at raising [Ca2+]i (Fig. 6B). However, in a fraction of cells bathed in medium containing 4 μm cytisine, ACh induced high-amplitude [Ca2+]i response (Fig. 6C). Similarly, in approximately 50% of nicotine-responsive cells, ACh induced an additional high-amplitude oscillatory Ca2+ response (Fig. 6D). These results indicated that the majority of rat gonadotrophs express nAChRs containing the β2-subunit, in addition to M3-mAChRs.
Fig. 6.
Agonist-induced Ca2+ influx in single pituitary cells and LβT2 gonadotrophs. Top, Characterization of Ca2+ influx pathway in pituitary gonadotrophs. Effects of ACh (A) and nicotine (B) on [Ca2+]i in gonadotrophs bathed in Ca2+-containing medium. Nicotine was ineffective in cells bathed in medium containing cytisine, a specific blocker of β2-subunit-containing nAChR (B). In most of the cells, ACh induced additional rise in [Ca2+]i in the presence of cytisine (C) or nicotine (D). GnRH was added at the end of recording to identify gonadotrophs. Bottom, Characterization of Ca2+ influx pathway in LβT2 cells. Nicotine induced a rise in [Ca2+]i in cells with blocked M3-mAChR by DAU 5884 (E). Stimulatory effects of nicotine on Ca2+ influx was abolished in cells bathed with 4 μm cytisine (F). In a fraction of cells, ACh induced additional rise in [Ca2+]i in the presence of cytisine (G) or nicotine (H).
In further experiments, we examined whether immortalized LβT2 cells express functional nAChRs. A smaller percentage of these cells (24 of 72) showed an ACh-induced increase in [Ca2+]i when bathed in medium containing 100 nm DAU 5884, a M3-mAChR-specific antagonist (Fig. 6E). Nicotine also induced a smaller fraction of LβT2 cells (27 of 86) to increase Ca2+ influx (Fig. 6F). The cells bathed in medium containing 4 μm cytisine, however, did not exhibit the nicotine-induced rise in [Ca2+]i (37 of 39 cells) (Fig. 6F), but a fraction of cells (six of eight) responded to ACh (Fig. 6G). Furthermore, in the most of the cells (four of five) responding to 20 μm nicotine, the administration of 20 μm ACh further elevated [Ca2+]i (Fig. 6H). In contrast, none of cells (n = 16) responded to the application of nicotine when bathed in a medium containing 20 μm ACh and 100 nm DAU 5884 (data not shown). These results indicate that the β2-containing nAChRs are also functional in a fraction of immortalized gonadotrophs.
The nAChRs expressed in rat gonadotrophs were further characterized using standard whole-cell patch-clamp recordings. The gonadotrophs were identified at the beginning of experiment (before the cytosolic molecules necessary for the response were washed out) using GnRH. In current-clamped cells, GnRH caused oscillations in the membrane potential, resulting in the bursting type of electrical activity (Fig. 7A). In contrast, ACh induced sustained depolarization and increased action potential frequency in a fraction of gonadotrophs (Fig. 7B). In 28 of the 39 voltage-clamped gonadotrophs, ACh induced an inward depolarizing current in a concentration-dependent manner (Fig. 7C, top). The maximum amplitude of inward current induced by 100 μm ACh was 18 ± 4 pA, and the estimated EC50 was 8.6 ± 4.7 μm (Fig. 7C, bottom). If the single channel conductance of β2-containing nAChRs is about 30 pS as in other tissues, the estimated number of channels per cell was 10.
Figure 7, D–H, summarizes the pharmacological characteristics of ACh-induced inward current. Nicotine evoked an inward current similar to that invoked by ACh, whereas PNU 282987, a selective agonist of α7-nAChRs (34), had no effect on the current (Fig. 7D). dTC, an antagonist of several subtypes of the nAChR, including the α4β2 subtype (35), inhibited the ACh-induced inward current (Fig. 7E). Similarly, 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl-1H-imidazole hydrochloride, a general inhibitor of receptor-mediated calcium channels (known as SKF 96365), inhibited ACh-induced current (data not shown). In contrast, αBTX had no effect (Fig. 7F). αBTX is a potent inhibitor of the α7, α8, and α9 homomers as well as neuromuscular nAChRs but has no effect on heteromeric neuronal or peripheral nAChRs (36). Furthermore, the ACh-induced inward current was not inhibited by atropine (Fig. 7G), a nonselective competitive antagonist of the mAChR (1). Ivermectin, a positive allosteric modulator of α7-nAChRs (37), was also ineffective (Fig. 7H). These results indicate that pituitary gonadotrophs express functional heteromeric nAChRs.
Inhibition of cAMP and LH release by M4-mAChRs in gonadotrophs
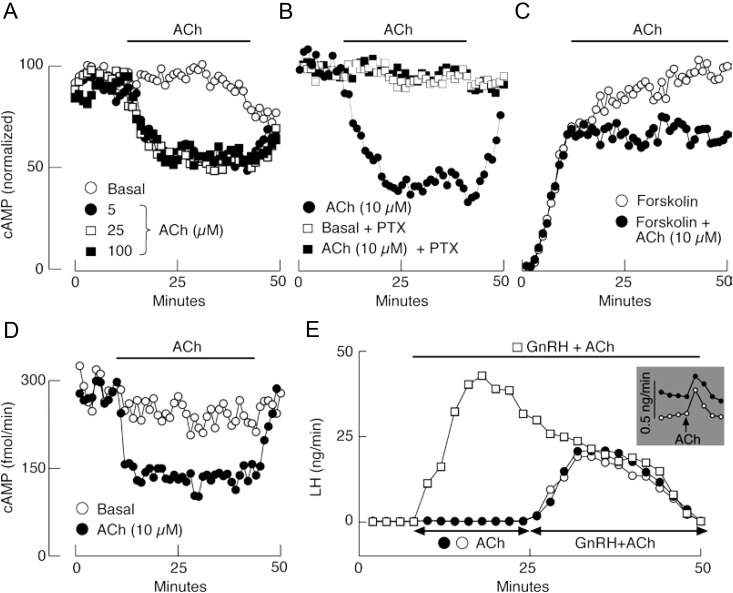
As mentioned above, pituitary gonadotrophs also express M4-mAChR mRNA and protein (Figs. 1 and 2). Because these receptors are coupled to Gi/o signaling pathway, leading to inhibition of cAMP production, we used cAMP measurements to determine whether they are functional. ACh rapidly inhibited basal cAMP release in perifused pituitary cells (Fig. 8A). This inhibitory action was lost in cells treated overnight with 250 ng/ml PTX, an inhibitor of the Gi/o signaling pathway (Fig. 8B). Forskolin (1 μm), an activator of adenylyl cyclase, induced cAMP release that was also inhibited by 10 μm ACh (Fig. 8C). ACh also reversibly inhibited cAMP release in perifused LβT2 cells (Fig. 8D), indicating that gonadotrophs probably contributed to ACh-mediated inhibition of cAMP production in mixed pituitary cells. Furthermore, we observed that pretreatment with ACh for 15 min, time sufficient to reach the plateau level in inhibition of cAMP production (Fig. 8B), inhibited GnRH-induced LH release (Fig. 8E). This inhibition was lost in cells treated with PTX (data not shown). ACh alone was very poor stimulator of LH release (Fig. 8E, inset). These results indicated that the coactivation of M4 receptors limits the secretory potency of GnRH.
Fig. 8.
ACh-induced inhibition of adenylyl cyclase and LH release in perifused pituitary cells. A–C, Effects of ACh on cAMP release in cultured pituitary cells. A, Inhibition of basal cAMP release by ACh. B, PTX sensitivity of 10 μm ACh-induced inhibition of cAMP release. C, ACh (10 μm)-induced inhibition of forskolin (1 μm) stimulated cAMP release. D, Effect of ACh on cAMP release in LβT2 cells. Horizontal bars indicate duration of ACh treatment. E, Effects of ACh on GnRH induced LH release. Inhibitory effect on GnRH-stimulated LH release was observed after pretreatment with ACh (10 μm) for 15 min. Inset shows a small increase in LH release triggered by ACh alone.
Discussion
The functional role of ACh in gonadotropin signaling was hypothesized as early as the 1940s. It was during this period that a rapid atropine-sensitive cholinergic component was found to be involved in gonadotropin release (38). McCann's group expanded upon this in vivo work in the early 1970s. They showed that the intraventricular application of atropine blocked the release of gonadotropins (39). Additional direct evidence implicating ACh in GnRH-controlled gonadotropin release was obtained in other in vitro experiments (40, 41). Subsequently, studies revealed ACh-mediated stimulation and inhibition of GnRH release through action at the mAChRs and nAChRs (42–48). More recently, functional nAChRs and M1- and M2-mAChRs were found in immortalized mouse GnRH neurons (49). Cholinergic afferents to rat GnRH neurons in the preoptic area were also identified (50). However, many aspects of cholinergic regulations of gonadotropin release remain unclear (51).
Here, we showed that nAChR and mAChR are also expressed in gonadotrophs, indicating that ACh may also influence gonadotroph function directly. nAChRs were expressed in 50-70% of identified rat gonadotrophs and in approximately 30% of LβT2 cells using calcium imaging. The expression of these channels in cultured pituitary cells was further documented in electrophysiological experiments, showing that ACh and nicotine generated an inward current capable of facilitating action potential firing. In addition, we showed through imaging analysis that ACh promotes Ca2+ influx. Several lines of evidence support the conclusion that the nAChR pentamers may be heteromeric α4β2 receptors (52, 53). Among the nicotinic receptor subunits, the β2 mRNA is most highly expressed in both mixed anterior pituitary cells and immortalized LβT2 gonadotrophs. The α4 mRNA was present in both cell types. The presence of the β2 protein was also present in the gonadotrophs as shown by immunohistochemistry. The ability of cytisine to block ACh-induced [Ca2+]i increase in both cell types is consistent with the presence of β2-containing nAChRs in gonadotrophs (33). dTC, an antagonist of several nAChR subtypes, including the α4β2 subtype (35), also inhibited the ACh-induced inward current. In contrast, αBTX did not inhibit the ACh-induced current. αBTX is a potent inhibitor of α7, α8, and α9 homomers and neuromuscular nAChRs, but it does not inhibit heteromeric neuronal and peripheral nAChRs (36). PNU 282987, a selective agonist of α7-nAChRs (34), failed to induce the inward current in pituitary gonadotrophs. Furthermore, the ACh-induced inward current was not facilitated by ivermectin, a positive allosteric modulator of α7-nAChRs (37).
Our qRT-PCR showed that α1 mRNA was significantly expressed in mouse LβT2 cells, whereas α9 mRNA was most highly expressed in mixed rat anterior pituitary cells cultured for 2 d. The finding that α9 mRNA expression was reduced by 95% 6 h after GnRH treatment further suggested that the α9-subunit has a limited role in forming functional nAChRs in cycling female rats. The α9-subunit is expressed in cochlear hair cells (54), breast cancer cells (55), and the skeletal muscle of the tongue and epithelium (56). Mice lacking the α9 gene fail to develop mature synaptic connections and cochlear outer hair cell efferent innervations (57, 58). Thus, homomeric α9 receptors may be important during development and neural differentiation (59). Together, these data suggest that the nAChRs of the two cell types differ in their α-subunit composition.
The mRNA transcripts for calcium-mobilizing M3-type mAChRs were also expressed in both cell types, independent of GnRH. Functional receptors were identified in approximately 70% of cells, as demonstrated by the ability of ACh to raise [Ca2+]i in cells bathed in calcium-deficient medium. In normal gonadotrophs, ACh always triggered baseline calcium oscillations, whereas in immortalized LβT2 cells, both oscillatory and nonoscillatory patterns of Ca2+ signaling were observed. The difference in response may be related to the ratio of inositol 1,4,5-trisphosphate and [Ca2+]i that is needed for the proper bidirectional effects of calcium on inositol 1,4,5-trisphosphate receptor channels to generate calcium oscillations (60, 61). No response was elicited in cells bathed in Ca2+-deficient medium and DAU 5884, a M3-mAChR-specific antagonist (32), indicating that this receptor subtype accounts for the Ca2+-mobilizing actions of ACh.
In LβT2 cells, the ACh-induced rise in [Ca2+]i activated a large outward current and was sufficient to hyperpolarize the cell membrane. Biophysical and pharmacological investigations have revealed that apamin-sensitive SK channels are responsible for the outward current and transient hyperpolarizations of the LβT2 cells. These channels are also expressed in normal gonadotrophs and are activated by GnRH (23–25). Like the Ca2+ signals, the ACh-induced SK current was abolished in cells bathed in DAU 5884-containing medium. This result confirmed that functional M3-mAChRs are expressed in these cells. The coupling of these receptors to the phospholipase C signaling pathway was shown through experiments applying U73122, an inhibitor of this enzyme (62), and with U73343, an inactive analog. Others have also observed that ACh-stimulated GH secretion is dependent on phosphatidyl inositol in the bovine anterior pituitary. However, the receptor subtype has not been identified (63). The nonsecretory folliculo-stellate cells of the rat anterior pituitary also express Ca2+-mobilizing mAChRs, which are most likely of the M1 subtype (14).
The ability of ACh to inhibit basal and vasoactive intestinal peptide-stimulated adenylyl cyclase activity and prolactin release in immortalized GH3 lacto-somatotrophs suggests that M2- or M4-mAChRs are also expressed in anterior pituitary cells (64). Here, we showed that M4-mAChR mRNA was robustly expressed in primary pituitary cells and LβT2 cells as well as that M4-mAChR protein was present in the identified gonadotrophs. We further showed that ACh inhibits the basal and forskolin-stimulated cAMP production and GnRH-induced LH secretion in perifused pituitary cells. This inhibition was sensitive to the administration of PTX toxin, indicating that the receptors were coupled the Gi/o signaling pathway. The same receptor coupling was also found in LβT2 cells.
In conclusion, we showed that multiple subtypes of functional AChRs are expressed in rat gonadotrophs and mouse LβT2 cells in vitro but not all of the cells express all of the receptors, suggesting that gonadotroph cells are functionally heterogeneous. Also the rat pituitary gonadotrophs exhibited nicotinic responses more often than the mouse-derived LβT2 cells, which could reflect the species difference. However, the physiological relevance of nAChRs is questionable, because GnRH down-regulated the expression of two subunits, suggesting that the expression of these receptors in vitro could reflect a lack of periodic GnRH receptor activation. It also appears that the secretory activity of both nAChR and mAChR is limited due to coactivation of M4 receptors. cAMP is known to contribute to control of the exocytotic pathway (65), which could indicate that the main physiological action of ACh in gonadotrophs is inhibitory rather than stimulatory. This does not argue against the potential role of ACh-stimulated Ca2+ influx and mobilization pathways in other cellular functions of gonadotrophs. Further studies are also needed to characterize the expression of nAChR and mAChR in males vs. females, different stages of estrous cycle and developmental stages, and to identify the physiological conditions that stimulate the local release of ACh by pituitary cells and that lead to the activation of these receptors in gonadotrophs.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development (to M.K., M.T., and S.S.S.), the Internal Grant Agency of Academy of Sciences IAA500110910 and Project Excellence P304/12/G069 (to H.Z.), and the Serbian Ministry of Education, Science, and Technological Development Project 41014 (to I.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACh
- Acetylcholine
- AF-DX 116
- 11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
- BMI
- bicuculline methiodide
- αBTX
- α-bungarotoxin
- DAU 5884
- 8-methyl-8-azabicyclo-3-endo[3.2.1]oct-3-yl-1,4-dihydro-2-oxo-3(2H)-quinazolinecarboxylic acid ester hydrochloride
- DpGlu
- [d-pGlu1,d-Phe2,d-Trp3,6]GnRH
- dTC
- (+)-tubocurarine chloride
- IBTX
- iberiotoxin
- KCa
- Ca2+-activated potassium
- mAChR
- muscarinic ACh membrane receptor
- nAChR
- nicotinic ACh membrane receptor
- nicotine
- (-)-nicotine ditartrate
- pirenzepine
- pirenzepine dihydrochloride
- PNU 282987
- N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-4-chlorobenzamide
- PTX
- pertussis toxin
- qRT-PCR
- quantitative RT-PCR
- SK
- small conductance
- TRAM 34
- 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- U73122
- 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione
- Xe991
- 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride
- U73343
- 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5-pyrrolidenedione.
References
- 1. Caulfield MP, Birdsall NJ. 1998. International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50:279-290 [PubMed] [Google Scholar]
- 2. Hogg RC, Raggenbass M, Bertrand D. 2003. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147:1-46 [DOI] [PubMed] [Google Scholar]
- 3. Zouridakis M, Zisimopoulou P, Poulas K, Tzartos SJ. 2009. Recent advances in understanding the structure of nicotinic acetylcholine receptors. IUBMB Life 61:407-423 [DOI] [PubMed] [Google Scholar]
- 4. Denef C. 2008. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol 20:1-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang ZW, Feltz P. 1990. Nicotinic acetylcholine receptors in porcine hypophyseal intermediate lobe cells. J Physiol 422:83-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poisbeau P, Trouslard J, Feltz P, Schlichter R. 1994. Calcium influx through neuronal-type nicotinic acetylcholine receptors present on the neuroendocrine cells of the porcine pars intermedia. Neuroendocrinology 60:378-388 [DOI] [PubMed] [Google Scholar]
- 7. Van Strien FJ, Roubos EW, Vaudry H, Jenks BG. 1996. Acetylcholine autoexcites the release of proopiomelanocortin-derived peptides from melanotrope cells of Xenopus laevis via an M1 muscarinic receptor. Endocrinology 137:4298-4307 [DOI] [PubMed] [Google Scholar]
- 8. Lamacz M, Tonon MC, Louiset E, Cazin L, Strosberg D, Vaudry H. 1989. Acetylcholine stimulates α-melanocyte-stimulating hormone release from frog pituitary melanotrophs through activation of muscarinic and nicotinic receptors. Endocrinology 125:707-714 [DOI] [PubMed] [Google Scholar]
- 9. Louiset E, Cazin L, Duval O, Lamacz M, Tonon MC, Vaudry H. 1990. Effect of acetylcholine on the electrical and secretory activities of frog pituitary melanotrophs. Brain Res 533:300-308 [DOI] [PubMed] [Google Scholar]
- 10. Schaeffer JM, Hsueh AJ. 1980. Acetylcholine receptors in the rat anterior pituitary gland. Endocrinology 106:1377-1381 [DOI] [PubMed] [Google Scholar]
- 11. Burt DR, Taylor RL. 1980. Muscarinic receptor binding in sheep anterior pituitary. Neuroendocrinology 30:344-349 [DOI] [PubMed] [Google Scholar]
- 12. Taylor RL, Burt DR. 1980. Pituitary cell cultures contain muscarinic receptors. Eur J Pharmacol 65:305-308 [DOI] [PubMed] [Google Scholar]
- 13. Heisler S, Larose L, Morisset J. 1983. Muscarinic cholinergic inhibition of cyclic AMP formation and adrenocorticotropin secretion in mouse pituitary tumor cells. Biochem Biophys Res Commun 114:289-295 [DOI] [PubMed] [Google Scholar]
- 14. Nakajima Y, Uchiyama M, Shirai Y, Sakuma Y, Kato M. 2001. Acetylcholine increases intracellular Ca2+ in the rat pituitary folliculostellate cells in primary culture. Am J Physiol Endocrinol Metab 280:E608–E615 [DOI] [PubMed] [Google Scholar]
- 15. Wojcikiewicz RJ, Dobson PR, Brown BL. 1984. Muscarinic acetylcholine receptor activation causes inhibition of cyclic AMP accumulation, prolactin and growth hormone secretion in GH3 rat anterior pituitary tumour cells. Biochim Biophys Acta 805:25-29 [DOI] [PubMed] [Google Scholar]
- 16. Carmeliet P, Baes M, Denef C. 1989. The glucocorticoid hormone dexamethasone reverses the growth hormone-releasing properties of the cholinomimetic carbachol. Endocrinology 124:2625-2634 [DOI] [PubMed] [Google Scholar]
- 17. Carmeliet P, Denef C. 1988. Immunocytochemical and pharmacological evidence for an intrinsic cholinomimetic system modulating prolactin and growth hormone release in rat pituitary. Endocrinology 123:1128-1139 [DOI] [PubMed] [Google Scholar]
- 18. Carmeliet P, Denef C. 1989. Synthesis and release of acetylcholine by normal and tumoral pituitary corticotrophs. Endocrinology 124:2218-2227 [DOI] [PubMed] [Google Scholar]
- 19. Carmeliet P, Maertens P, Denef C. 1989. Stimulation and inhibition of prolactin release from rat pituitary lactotrophs by the cholinomimetic carbachol in vitro. Influence of hormonal environment and intercellular contacts. Mol Cell Endocrinol 63:121-131 [DOI] [PubMed] [Google Scholar]
- 20. Sealfon SC, Weinstein H, Millar RP. 1997. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev 18:180-205 [DOI] [PubMed] [Google Scholar]
- 21. Tóth ZE, Mezey E. 2007. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem 55:545-554 [DOI] [PubMed] [Google Scholar]
- 22. Stojilkovic SS, Tabak J, Bertram R. 2010. Ion channels and signaling in the pituitary gland. Endocr Rev 31:845-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tse A, Hille B. 1992. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science 255:462-464 [DOI] [PubMed] [Google Scholar]
- 24. Kukuljan M, Stojilkovic SS, Rojas E, Catt KJ. 1992. Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Lett 301:19-22 [DOI] [PubMed] [Google Scholar]
- 25. Zemková H, Vancek J. 1997. Inhibitory effect of melatonin on gonadotropin-releasing hormone-induced Ca2+ oscillations in pituitary cells of newborn rats. Neuroendocrinology 65:276-283 [DOI] [PubMed] [Google Scholar]
- 26. Ishii M, Kurachi Y. 2006. Muscarinic acetylcholine receptors. Curr Pharm Des 12:3573-3581 [DOI] [PubMed] [Google Scholar]
- 27. Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. 2005. International union of pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57:463-472 [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Xu YQ, Liang YY, Gongora R, Warnock DG, Ma HP. 2007. An intermediate-conductance Ca(2+)-activated K (+) channel mediates B lymphoma cell cycle progression induced by serum. Pflugers Arch 454:945-956 [DOI] [PubMed] [Google Scholar]
- 29. Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. 1998. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282:1890-1893 [DOI] [PubMed] [Google Scholar]
- 30. Mansfield KJ, Liu L, Mitchelson FJ, Moore KH, Millard RJ, Burcher E. 2005. Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br J Pharmacol 144:1089-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uwada J, Anisuzzaman AS, Nishimune A, Yoshiki H, Muramatsu I. 2011. Intracellular distribution of functional M(1)-muscarinic acetylcholine receptors in N1E-115 neuroblastoma cells. J Neurochem 118:958-967 [DOI] [PubMed] [Google Scholar]
- 32. Gosens R, Bromhaar MM, Tonkes A, Schaafsma D, Zaagsma J, Nelemans SA, Meurs H. 2004. Muscarinic M(3) receptor-dependent regulation of airway smooth muscle contractile phenotype. Br J Pharmacol 141:943-950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Papke RL, Heinemann SF. 1994. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the β2 subunit. Mol Pharmacol 45:142-149 [PubMed] [Google Scholar]
- 34. Faghih R, Gopalakrishnan M, Briggs CA. 2008. Allosteric modulators of the α7 nicotinic acetylcholine receptor. J Med Chem 51:701-712 [DOI] [PubMed] [Google Scholar]
- 35. Astles PC, Baker SR, Boot JR, Broad LM, Dell CP, Keenan M. 2002. Recent progress in the development of subtype selective nicotinic acetylcholine receptor ligands. Curr Drug Targets CNS Neurol Disord 1:337-348 [DOI] [PubMed] [Google Scholar]
- 36. Dwoskin LP, Crooks PA. 2001. Competitive neuronal nicotinic receptor antagonists: a new direction for drug discovery. J Pharmacol Exp Ther 298:395-402 [PubMed] [Google Scholar]
- 37. Collins T, Millar NS. 2010. Nicotinic acetylcholine receptor transmembrane mutations convert ivermectin from a positive to a negative allosteric modulator. Mol Pharmacol 78:198-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Everett JW, Sawyer CH, Markee JE. 1949. A neurogenic timing factor in control of the ovulatory discharge of luteinizing hormone in the cyclic rat. Endocrinology 44:234-250 [DOI] [PubMed] [Google Scholar]
- 39. Libertun C, McCann SM. 1973. Blockade of the release of gonadotropins and prolactin by subcutaneous or intraventricular injection of atropine in male and female rats. Endocrinology 92:1714-1724 [DOI] [PubMed] [Google Scholar]
- 40. Simonovic I, Motta M, Martini L. 1974. Acetylcholine and the release of the follicle-stimulating hormone-releasing factor. Endocrinology 95:1373-1379 [DOI] [PubMed] [Google Scholar]
- 41. Fiorindo1 RP, Martini L. 1975. Evidence for a cholinergic component in the neuroendocrine control of luteinizing hormone (LH) secretion. Neuroendocrinology 18:322-332 [DOI] [PubMed] [Google Scholar]
- 42. Libertun C, McCann SM. 1976. Blockade of the postorchidectomy increase in gonadotropins by implants of atropine into the hypothalamus. Proc Soc Exp Biol Med 152:143-146 [DOI] [PubMed] [Google Scholar]
- 43. Billiar RB, Kalash J, Romita V, Tsuji K, Kosuge T. 1988. Neosurugatoxin: CNS acetylcholine receptors and luteinizing hormone secretion in ovariectomized rats. Brain Res Bull 20:315-322 [DOI] [PubMed] [Google Scholar]
- 44. Kalash J, Romita V, Billiar RB. 1989. Third ventricular injection of α-bungarotoxin decreases pulsatile luteinizing hormone secretion in the ovariectomized rat. Neuroendocrinology 49:462-470 [DOI] [PubMed] [Google Scholar]
- 45. Kalra SP, Kalra PS. 1983. Neural regulation of luteinizing hormone secretion in the rat. Endocr Rev 4:311-351 [DOI] [PubMed] [Google Scholar]
- 46. Richardson SB, Prasad JA, Hollander CS. 1982. Acetylcholine, melatonin, and potassium depolarization stimulate release of luteinizing hormone-releasing hormone from rat hypothalamus in vitro. Proc Natl Acad Sci USA 79:2686-2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Negro-Vilar A. 1982. The median eminence as a model to study presynaptic regulation of neural peptide release. Peptides 3:305-310 [DOI] [PubMed] [Google Scholar]
- 48. Koren D, Egozi Y, Sokolovsky M. 1992. Muscarinic involvement in the regulation of gonadotropin-releasing hormone in the cyclic rat. Mol Cell Endocrinol 90:87-93 [DOI] [PubMed] [Google Scholar]
- 49. Krsmanovic LZ, Mores N, Navarro CE, Saeed SA, Arora KK, Catt KJ. 1998. Muscarinic regulation of intracellular signaling and neurosecretion in gonadotropin-releasing hormone neurons. Endocrinology 139:4037-4043 [DOI] [PubMed] [Google Scholar]
- 50. Turi GF, Liposits Z, Hrabovszky E. 2008. Cholinergic afferents to gonadotropin-releasing hormone neurons of the rat. Neurochem Int 52:723-728 [DOI] [PubMed] [Google Scholar]
- 51. Samson WK. 1998. More pieces of the puzzle in place, even more discovered missing. Endocrinology 139:4035. [DOI] [PubMed] [Google Scholar]
- 52. Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. 1992. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41:31-37 [PubMed] [Google Scholar]
- 53. Puskar NL, Xiu X, Lester HA, Dougherty DA. 2011. Two neuronal nicotinic acetylcholine receptors, α4β4 and α7, show differential agonist binding modes. J Biol Chem 286:14618-14627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Drescher DG, Ramakrishnan NA, Drescher MJ, Chun W, Wang X, Myers SF, Green GE, Sadrazodi K, Karadaghy AA, Poopat N, Karpenko AN, Khan KM, Hatfield JS. 2004. Cloning and characterization of α9 subunits of the nicotinic acetylcholine receptor expressed by saccular hair cells of the rainbow trout (Oncorhynchus mykiss). Neuroscience 127:737-752 [DOI] [PubMed] [Google Scholar]
- 55. Wu CH, Lee CH, Ho YS. 2011. Nicotinic acetylcholine receptor-based blockade: applications of molecular targets for cancer therapy. Clin Cancer Res 17:3533-3541 [DOI] [PubMed] [Google Scholar]
- 56. Hollenhorst MI, Lips KS, Weitz A, Krasteva G, Kummer W, Fronius M. 2012. Evidence for functional atypical nicotinic receptors that activate K+-dependent Cl- secretion in mouse tracheal epithelium. Am J Respir Cell Mol Biol 46:106-114 [DOI] [PubMed] [Google Scholar]
- 57. Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. 1994. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79:705-715 [DOI] [PubMed] [Google Scholar]
- 58. Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. 1999. Role of α9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23:93-103 [DOI] [PubMed] [Google Scholar]
- 59. Murthy V, Taranda J, Elgoyhen AB, Vetter DE. 2009. Activity of nAChRs containing α9 subunits modulates synapse stabilization via bidirectional signaling programs. Dev Neurobiol 69:931-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Keizer J, Li YX, Stojilkovic S, Rinzel J. 1995. InsP3-induced Ca2+ excitability of the endoplasmic reticulum. Mol Biol Cell 6:945-951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li YX, Keizer J, Stojilkovic SS, Rinzel J. 1995. Ca2+ excitability of the ER membrane: an explanation for IP3-induced Ca2+ oscillations. Am J Physiol 269:C1079–C1092 [DOI] [PubMed] [Google Scholar]
- 62. Zheng L, Paik WY, Cesnjaj M, Balla T, Tomic M, Catt KJ, Stojilkovic SS. 1995. Effects of the phospholipase-C inhibitor, U73122, on signaling and secretion in pituitary gonadotrophs. Endocrinology 136:1079-1088 [DOI] [PubMed] [Google Scholar]
- 63. Young PW, Bicknell RJ, Schofield JG. 1979. Acetylcholine stimulates growth hormone secretion, phosphatidyl inositol labelling, 45Ca2+ efflux and cyclic GMP accumulation in bovine anterior pituitary glands. J Endocrinol 80:203-213 [DOI] [PubMed] [Google Scholar]
- 64. Onali P, Eva C, Olianas MC, Schwartz JP, Costa E. 1983. In GH3 pituitary cells, acetylcholine and vasoactive intestinal peptide antagonistically modulate adenylate cyclase, cyclic AMP content, and prolactin secretion. Mol Pharmacol 24:189-194 [PubMed] [Google Scholar]
- 65. Sikdar SK, Zorec R, Mason WT. 1990. cAMP directly facilitates Ca-induced exocytosis in bovine lactotrophs. FEBS Lett 273:150-154 [DOI] [PubMed] [Google Scholar]