Abstract
Lipoprotein receptors are evolutionarily ancient proteins that are expressed on the surface of many cell types. Beginning with the appearance of the first primitive multicellular organisms several structurally and functionally distinct families of lipoprotein receptors evolved. Originally, these cell surface proteins were thought to merely mediate the traffic of lipids and nutrients between cells and in some cases, by functioning as scavenger receptors, remove other kinds of macromolecules, such as proteases and protease inhibitors from the extracellular space and the cell surface. Over the last decade, this picture has greatly expanded. We now appreciate that many of these receptors are not mere cargo transporters; they are deeply embedded in the machinery by which cells communicate with each other. By physically interacting and coevolving with fundamental signaling pathways, lipoprotein receptors have occupied essential and surprisingly diverse functions that are indispensable for integrating the complex web of cellular signal input during development and in differentiated tissues. Furthermore, lipoprotein receptors modulate cellular trafficking and localization of the amyloid precursor protein (APP) and the β-amyloid peptide (Aβ), suggesting a role in the pathogenesis of Alzheimer’s disease. Moreover, compelling evidence shows that low density lipoprotein receptor family members are involved in tumor development and progression.
Introduction
Lipid transport through the circulation, the extracellular space and across the plasma membrane involves the concerted action of a wide range of cell surface receptors, lipid carrier and transfer proteins, enzymes and cellular transporters. As an evolutionarily ancient process, it probably arose to distribute essential nutritional or endogenously synthesized lipids and hormones, but also lipid modified signaling proteins and other associated macromolecules between increasingly metabolically specialized tissues. Lipoprotein receptors are amongst the oldest components of this complex biochemical system. These cell surface receptors fall into two major groups: endocytic receptors that bind their cargo in the form of lipid carrying lipoproteins and mediate their internalization and eventually lysosomal delivery and a second group which promotes lipid exchange at the plasma membrane without cellular uptake of the protein component of the particle. The latter encompasses for example the scavenger type B receptors SR-B1, SR-B2 and CD36; while well known members of the first group include for instance the low-density lipoprotein (LDL) receptor and LDL receptor related proteins and the scavenger type A receptors (SRAs). In addition to their specialized functions as mediators of cellular lipid uptake, several of these proteins have - over the last few years - also been recognized for often unrelated roles as cellular signal transducers or signal modulators. In this review, we will restrict ourselves to only one particularly versatile subgroup – the LDL receptor related proteins. After a short overview of the evolution of the family, we will briefly address the traditional and more restricted role of the LDL receptor gene family (Figure 1) in ‘classic’ lipoprotein transport and the metabolism of other macromolecules, and then move on to focus mainly on the larger and rapidly expanding role of the LDL receptor gene family e.g. activation and modulation of tyrosine kinases, its role in cellular growth regulation and cancer, and regulation and integration of fundamental cellular signaling pathways in the central nervous system and during development.
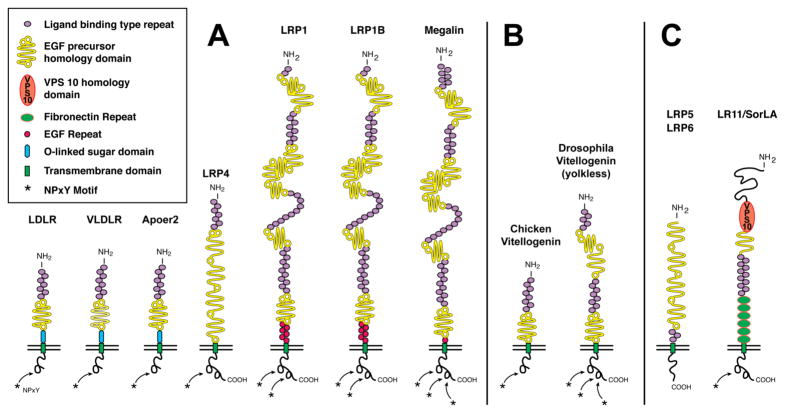
Figure 1. The LDL receptor gene family.
Panel A illustrates the core LDL receptor gene family as it exists in mammalian species. Panel B displays equivalent receptors that are structurally and functionally distinct family members in non-mammalian species. Panel C represents a subgroup of functionally important, but more distantly related family members that share some, but not all, of the structural requirements of the ‘core members’. In addition, they may also contain domains e.g. vacuolar protein sorting (VPS) domain, which are not present in the core family. These family members are characterized by one or more ligand binding domains, epidermal growth factor (EGF) – homology domains consisting of EGF repeats and YWTD propeller (β-propeller) domains involved in pH dependent release of ligands in the endosomes, a single transmembrane domain and a cytoplasmic tail containing at least one NPxY motifs. The latter represents both the endocytosis signal as well as a binding site for adaptor proteins linking the receptor to intracellular signaling pathways. Furthermore, LDLR, VLDLR, and Apoer2 carry an O-linked sugar domain.
Evolution of the LDL receptor gene family
Remarkably, the LDL receptor gene family seems to have appeared in an evolutionary burst coinciding with the appearance of the first multicellular organisms, rather than evolving gradually over time from the smaller and simpler to the larger and more complex family members. The worm Caenorhabditis elegans, one of the most primitive multicellular organisms that is separated from humans by an estimated 109 years of divergent evolution, contains in its genome several large and small family members that are not only structurally almost indistinguishable from their human counterparts, but the exon/intron structure is also virtually identical. This suggests that this ancient gene family arose rapidly by repeated duplication and concatenation of exon/intron blocks from a single primordial precursor gene. The gene family can be divided into a core of seven members (in mammals), which is defined by them sharing the same structural arrangement of four basic building blocks, and a diverse group of more distantly related proteins that share some, but not all structural features with the core members (reviewed in (Herz et al. 2002; Krieger et al. 1994; Nykjaer et al. 2002; Willnow et al. 1999)). Similar exon/intron structures and other inherent conserved sequence elements suggest that most core family members arose from a primordial gene containing one ligand binding domain (symbolized by the red ovals in Figure 1) followed by at least one YWTD-repeat containing propeller domain by duplication and exon shuffling (Springer 1998). This central minimal unit is present in all the core members of the gene family. In some, such as the LDL receptor, it accounts for essentially the entire extracellular portion.
Although C. elegans does not possess a systemic circulatory system, LDL receptor family members appear to have taken on roles related to lipid metabolism and nutrient transport as well as intercellular signaling already in such simple multicellular organisms. For instance, Ashrafi and colleagues found a role for a C. elegans homologue of the very-low-density lipoprotein (VLDL) receptor (wormbase gene T13C2.6) in intestinal lipid accumulation using a genome-wide RNAi approach in the worm (Ashrafi et al. 2003) and personal communications), while Kamikura and Cooper (Kamikura et al. 2003) showed a role for the C. elegans LRP1 and LRP2 genes in the export of the EGL-17/FGF signaling protein, which controls sex myoblast migration in the worm. In other early work, Yochem and colleagues (Yochem et al. 1993; Yochem et al. 1999) reported an essential role for LRP2 in the molting process. In neither case are the cellular mechanisms and functions of the receptors that lead to the described consequences in the mutant worms well understood.
Nutrient transport, in the form of lipids, vitellogenin or other macromolecules appears to be a function that has evolved early during a time when the first primitive multicellular organisms arose. It likely became indispensable when increasing specialization and differentiation of dedicated cells led to ‘outsourcing’ to satisfy essential cellular needs for the sake of greater efficiency of the organism. This is impressively illustrated by genetic defects in different LDL receptor family members in flies (Schonbaum et al. 1995) and in the chicken (Barber et al. 1991; Schneider 1996; Schneider et al. 2003) that result in infertility due to lack of yolk deposition. In C. elegans, the LDL receptor family member rme-2 is required for yolk endocytosis and hatching (Grant et al. 1999).
On the other hand, the broad functions the LDL receptor-related proteins (LRPs) have acquired as direct signal transducers (e.g. VLDLR and Apoer2, discussed later) or modulators and integrators of a diverse range of fundamental cellular signaling pathways (e.g. tyrosine kinases, Wnt, TGFβ, BMP) in higher organisms have so far not been identified or sufficiently investigated in C. elegans. For instance, no clear orthologue for the Wnt co-receptor LRP5 (arrow in the fly) (Pinson et al. 2000; Tamai et al. 2000; Wehrli et al. 2000) has as yet been found in the worm (Eisenmann 2005). This absence suggests that the gene encoding LRP5/arrow arose later, potentially from either LRP1 or LRP2, as both receptors contain the entire extracellular portion of LRP5/arrow in a single contiguous sequence block (Figure 1). It appears that increasingly sophisticated mechanisms of signal modulation and fine tuning by LRPs arose through progressive integration of the expanding gene family into these newly developing elementary primordial signaling complexes.
Lipoprotein Metabolism, Energy Homeostasis and Cellular Uptake of Nutrients and Hormones
Lipoprotein Metabolism
The LDL receptor (LDLR) was the first cellular lipoprotein receptor to be purified using traditional biochemical approaches and subsequently cloned in the 1970’s and early 1980s (Goldstein et al. 1995). Much of our fundamental understanding of cell biology, in particularly intracellular trafficking, endocytosis and vesicle recycling was elucidated with LDLR serving as a model. Arguably, it is also the most widely known lipoprotein receptor, because it is to a large extent responsible for controlling the concentration of plasma LDL, the lipoprotein that stands synonymous for ‘bad cholesterol’. LDLR is one of the most intensively investigated proteins, for basic scientific reasons as well as for its clinical importance. At the time of this writing, >15,000 references are listed in ‘Medline’ for the LDL receptor. Loss of LDLR function is the cause for familial hypercholesterolemia (FH, OMIM#606945), a genetic syndrome in which the risk for coronary artery disease and atherosclerosis is already greatly increased in heterozygous carriers. Homozygotes lacking all LDL receptor function develop plasma LDL levels approaching 1000 mg/dl, which cause a particularly rapid onset of atherosclerosis and can even lead to myocardial infarction already during early childhood.
Like all core members of the gene family, LDLR binds Apolipoprotein E (ApoE), a plasma protein of approximately 34 kDa that predominantly associates with triglyceride carrying chylomicrons and very low-density lipoproteins (VLDLs). Upon lipolysis by lipoprotein lipase in peripheral capillaries the ApoE content of chylomicrons increases significantly. These ApoE-enriched chylomicron remnants are then avidly taken up by the liver (Mahley et al. 1989; Mahley et al. 1991; Mahley et al. 1989; Mahley et al. 1995). By contrast, as VLDLs shed their triglycerides they are eventually converted to cholesterol-rich LDL which contains no ApoE, but only a single ApoB100 moiety as its sole apoprotein, which mediates its binding to LDLR. Yet, in FH patients, as well as in LDL receptor-deficient rabbits (Havel et al. 1982; Kita et al. 1982; Kita et al. 1982) and mice (Ishibashi et al. 1993), only LDL accumulates while chylomicron remnants continue to be cleared efficiently into the liver. This observation implied the existence of another ApoE-, but not ApoB100-specific lipoprotein receptor that works in concert with LDLR to remove chylomicron remnants from the circulation.
LRP1 (Herz et al. 1988) presented itself as a likely candidate for this job. It had not only one, but 4 potential ligand binding domains, i.e. strings of negatively charged cysteine-rich Ca2+-chelating repeat modules of the kind that were shown in LDLR to interact with ApoB as well as ApoE. Indeed, LRP1 could bind ApoE-containing lipoproteins in vitro, especially when the particles had undergone ApoE-enrichment (Kowal et al. 1990), as it occurs during the conversion of chylomicrons to remnants (Mahley et al. 1989). Moreover, LRP1 is highly expressed in the liver (Herz et al. 1988), the central clearing house for this kind of lipoproteins. Although LRP1 had all the right properties, it turned out to be difficult to conclusively prove its identity with the elusive chylomicron remnant receptor by biochemical means alone. In contrast to LDLR, LRP1-deficient knockout animals die early during embryonic development (Herz et al. 1992), precluding this straightforward genetic approach to solve the question. Ultimately, conditional liver-specific gene targeting of LRP1, performed on the background of conventional LDLR knockout mice, settled the issue by demonstrating unequivocally how LRP1 and LDLR together mediate the removal of chylomicron remnants by the liver in a concerted and mutually compensatory manner (Rohlmann et al. 1998). Furthermore, LRP1 knockin mice carrying inactivating alanine residues in the proximal NPTY motif of the cytoplasmic tail cause a late destruction of the liver and perinatal death. This indicates a critical endocytosis-independent signaling function of LRP1 in liver development and possibly also in transient hematopoietic microenvironment in this tissue (Roebroek et al. 2006).
LRP1 and VLDLR participate in lipoprotein metabolism through another mechanism, which involves binding of lipoprotein lipase (LPL) (Chappell et al. 1992; Nykjaer et al. 1993) and hepatic lipase (HL) (Kounnas et al. 1995; Verges et al. 2004). The extent to which lipase binding to either receptor affects lipid metabolism in humans is not entirely clear. Experiments in the mouse have revealed a role for VLDLR in LPL turnover, with mild fasting hypertriglyceridemia and decreased LPL activity in plasma (Yagyu et al. 2002). This is consistent with the observed reduction in adipose tissue mass of VLDLR knockout mice (Frykman et al. 1995). VLDLR is expressed on the surface of endothelial cells (Wyne et al. 1996), where particularly in muscle and adipose tissue it may be involved to some degree in regulating the transcytosis and presentation of LPL to circulating VLDL and chylomicrons. Significant hypertriglyceridemia has not been reported in VLDLR-deficient humans ((Boycott et al. 2005; Ozcelik et al. 2008; Turkmen et al. 2008), OMIM#192977).
LPL and HL are cleared by LRP1 in hepatocytes (Herz et al. 1995; Kounnas et al. 1995; van Vlijmen et al. 1999; Verges et al. 2004), which would serve to keep circulating lipase levels low by clearing the lipase into the liver. LRP2 can also bind LPL (Kounnas et al. 1993), but the physiological significance of this is less clear.
Energy Homeostasis
Interestingly, chylomicron remnant uptake by the liver is not the only example for lipoprotein transport in which LRP1 is involved. Descamps et al. (Descamps et al. 1993) found that insulin stimulated the rapid translocation of LRP1 to the surface of adipocytes within seconds of exposure to the hormone. Recently now, Hofmann et al. (Hofmann et al. 2007) reported that LRP1 expression in the adipocyte is involved in the control of systemic energy homeostasis. Mice lacking LRP1 in their adipocytes had increased energy expenditure, but decreased thermogenesis. Intriguingly, they also exhibited transient postprandial hypertriglyceridemia, in parallel with impaired cholesteryl ester uptake into adipose tissues, suggesting that LRP1 does indeed mediate to some degree postprandial chylomicron remnant uptake into adipocytes that is stimulated by insulin. A similar insulin-mediated increase of LRP1 activity has been reported by Heeren and colleagues (Laatsch et al. 2008) in the liver, further confirming the active role LRP1 takes in the regulation of energy homeostasis and metabolism.
Nutrient, Vitamin and Hormone Uptake
In addition to lipid uptake and the recently emerging roles in energy homeostasis, the LDL receptor gene family also participates in the transport of other cellular nutrients and vitamins. The role of lipoproteins, in particular the role of ApoB, LDL and VLDL in the transport of the fat-soluble vitamins A and E has long been recognized. Elegant work from Thomas Willnow’s lab, however, revealed that at least one member of the family, LRP2 (a.k.a. megalin) has carved out its own essential niche in vitamin metabolism that is independent of lipoprotein transport (Nykjaer et al. 1999). The kidney had been known for its role in converting circulating mono-hydroxylated vitamin D3 to its fully active dihydroxylated form, i.e. 1,25-[OH]2-cholecalciferol. It was also known that this conversion occurs in the proximal tubule, and that this is preceded by the secretion of vitamin D binding protein (DPB) into the primary glomerular filtrate and reuptake by the villous membranes of the proximal tubule cells (Willnow et al. 2002). This reuptake turned out to be mediated by LRP2. As a consequence, conditional knockout animals lacking LRP2 in their kidneys are severely vitamin D deficient, with all the clinical signs of rickets (Leheste et al. 2003). Conventional LRP2 knockout mice are more severely affected, with a considerable degree of strain-background dependent variation of perinatal lethality resulting from extensive developmental craniofacial malformations that include midline defects such as cleft palate, exencephaly and holoprosencephaly (Willnow et al. 1996). LRP2-deficiency in humans is the cause of Donnai-Barrow syndrome (OMIM#222448), which partially phenocopies the mouse model (Kantarci et al. 2007). The differences between LRP2-deficient mice and humans could also be due in part to another potential role of LRP2 in the cellular uptake and transport of steroid hormones (Hammes et al. 2005), which may manifest itself differently in both species.
Burk and colleagues recently reported an unexpected role for Apoer2 (a.k.a. LRP8) as well as for LRP2 in the transport of yet another micronutrient, the trace element selenium (Burk et al. 2007; Olson et al. 2008; Olson et al. 2007). Selenium is present in the active center of glutathione peroxidase, thioredoxin reductase, as well as deiodinases and is thus essential for cellular functions. Selenium is specifically incorporated into the amino acid selenocysteine and transported to target tissues in the form of the selenocysteine-enriched selenoprotein P1 (Sepp1) (Burk et al. 2005). The two organs that are primarily affected by Sepp1 deficiency are the testis and the brain (Burk et al. 2007; Olson et al. 2007), resulting in male infertility caused by defects in sperm morphology and motility, neurological defects (Peters et al. 2006) and rapid onset of neurodegeneration when the animals were maintained on a selenium deficient diet (Burk et al. 2007). Male Apoer2 knockout mice show the same sperm abnormalities (Andersen et al. 2003) and they are also highly susceptible to neurodegeneration under selenium deficient feeding conditions (Burk et al. 2007). The testis is particularly susceptible to Sepp1 (Hill et al. 2003; Schomburg et al. 2003) or Apoer2 deficiency, with abnormal spermatogenesis occurring even when normal selenium levels are present in the diet. Interestingly, Apoer2 knockout male mice not only showed reduced expression of selenoprotein phospholipid hydroperoxide glutathione peroxidase (PHGPx), which is required for sperm maturation, but also increased levels of clusterin (a.k.a. ApoJ), thereby revealing another previously unrecognized ligand for Apoer2 (Andersen et al. 2003). LRP2-mutant mice also display urinary selenium loss, which correlates with Sepp1 excretion in their urine. In addition, selenium and glutathione peroxidase activity in the brain are significantly reduced, suggesting that LRP2 prevents urinary Sepp1 loss and participates in brain selenium and Sepp1 uptake (Chiu-Ugalde et al. 2010).
Protease Metabolism
A second large group of ligands that bind to LDL receptor family members are broad spectrum proteases and protease inhibitors (reviewed in (Herz et al. 2001; Strickland et al. 1995; Strickland et al. 2003)). The first indication that LRP1 has a role independent of lipoprotein metabolism arose from the discovery that it was identical to the extensively studied α2-macroglobulin receptor (Kristensen et al. 1990; Strickland et al. 1990). α2-Macroglobulin (α2M) is a proteinase inhibitor with a broad spectrum of specificity (Travis et al. 1983; Van Leuven et al. 1994). It is present in high concentrations in plasma. Native α2-macroglobulin is not recognized by any receptor. Upon attack of a bait region by a proteinase, however, it undergoes a rapid conformational change, trapping the proteinase and exposing a receptor binding site that mediates its rapid removal from the circulation by LRP1 in the liver. In addition to protease trapping and removal, activated α2M can also bind signaling proteins, notably platelet-derived growth factor BB (PDGF-BB) and transforming growth factor β1 (TGFβ1) (Crookston et al. 1993; LaMarre et al. 1991). As will be discussed in more detail later, both proteins also bind directly to LRP1, which further physically associates with their cognate signaling receptors (Boucher et al. 2003; Boucher et al. 2002; Huang et al. 2003; Loukinova et al. 2002; Takayama et al. 2005). Taken together these findings illustrate the tightly interwoven functions of LRP1 as a signal integrator of tissue remodeling processes that involve a variety of signaling proteins, as well as proteases and their inhibitors.
Other proteases and inhibitors that bind to LRP1 and some of the other family members include plasminogen activators and their inhibitor PAI-1 (Bu et al. 1992; Herz et al. 1992; Nykjaer et al. 1992; Orth et al. 1992), coagulation factors and metalloproteinases (Bovenschen et al. 2003; Herz et al. 2001; Lillis et al. 2008; Narita et al. 1995; Strickland et al. 2006; Strickland et al. 2003). A complete account is beyond the scope of the present review, but the unprecedented number and diversity of ligands for these multifunctional receptors impressively document their complex role in vivo. The deep integration into such a wide array of biological processes also explains why it is often proving difficult to isolate and unambiguously demonstrate a single specific function, especially for the most versatile member of the family, LRP1, in vivo. Disruption of LRP1 function, even in a specific cell type or tissue, often results in a complex composite phenotype caused by the simultaneous loss of more than one function at a time.
There is one protease of considerable clinical importance, however, which is a direct ligand for LRP1: tissue-type plasminogen activator (tPA) (Bu et al. 1992). Its primary clinical use as a thrombolytic agent is to restore blood flow after an acute coronary artery occlusion or thromboembolism. A serious side effect of tPA treatment is an increased incidence of stroke due to an increase in cerebrovascular permeability and bleeding, which may occur through a LRP1-mediated cell signaling event and not through generalized degradation of the vascular basement membrane by tPA (Yepes et al. 2003). Conditional LRP1 knockout experiments in macrophages have further shown that LRP1 controls macrophage migration in concert with tPA and PAI-1 through the adhesion molecule Mac-1 (Cao et al. 2006).
Regulation of Cellular Signal Transduction
Over the course of the last decade it has become abundantly clear that modulation of cellular signaling by the LDL receptor gene family is not merely an indirect phenomenon (May et al. 2003; Schneider et al. 2003; Strickland et al. 2003; Willnow et al. 2007). In fact most family members are directly, i.e. physically involved in either transmitting a signal across the plasma membrane by themselves or in modulating such signals by increasing or decreasing the activity of other fundamental signaling pathways. The latter include for instance membrane receptor tyrosine kinases, Wnt, TGF-β, Bmp, Shh, and others. In the following we summarize the main principles and mechanisms by which the different members of the family control a wide variety of diverse biological responses in the embryo and in the adult.
Signaling through regulated proteolysis
LRP1 cannot only remove active proteinases from the cell surface and extracellular space, it is also itself cleaved by cell surface metalloproteinases, resulting in the shedding of the extracellular domain (Hahn-Dantona et al. 2001; Liu et al. 2009; May et al. 2003; May et al. 2002; Rozanov et al. 2004; Strickland et al. 1995). Apoer2 can be cleaved in a similar manner, which is likely to occur physiologically in response to signals that activate typical or atypical forms protein kinase C (Herrlich et al. 1998; Herrlich et al. 2008; May et al. 2003). In both cases, this cleavage is regulated by the glycosylation state of the receptor (May et al. 2003). Shedding of the extracellular domain may affect cellular signals on the short- as well as long-range, by sequestration of regulators of cell proliferation, e.g. PDGF or TGFβ in the case of LRP1 (Huang et al. 2003; Loukinova et al. 2002), or of the neuronal signaling protein Reelin in the case of Apoer2 (Koch et al. 2002). Moreover, release of the extracellular domain leaves behind a short extracellular stub, the membrane spanning segment and the intracellular domain (May et al. 2002). Further processing of the truncated receptor occurs constitutively through the action of γ-secretase, the same intramembraneous aspartyl protease that mediates the processing of the amyloid precursor protein (APP) involved in Alzheimer’s disease (Wolfe et al. 1999). This second cleavage results in the release of the intracellular domain, which can translocate to the nucleus where it could, in principle, modulate transcriptional activity of specific, but currently as yet unidentified target genes. In contrast to the nuclear translocation of the carboxyl-terminal fragment of LRP1 and possibly Apoer2 (Zurhove et al. 2008), the potential for proteolytic processing of the LRP1B and LRP4 extracellular domains provides for an additional, independent level of signal modulation by LDL receptor family members (Dietrich et al. 2010). Mutational analysis of both receptors in the mouse revealed an anchorage-independent function of the extracellular domains that was sufficient to ensure normal development (LRP1B) or allow for a mitigated phenotype in the LRP4 mutants, respectively (Johnson et al. 2005; Marschang et al. 2004). In contrast, a complete knockout of either receptor is lethal (Dietrich et al. 2010; Weatherbee et al. 2006). The metalloproteinase-mediated cleavage of the LRP1B and LRP4 extracellular domains suggest a physiological role, however, the significance of this cleavage event will have to be verified and evaluated in vivo (Dietrich et al. 2010). This suggests that the extracellular domains of LRP1B and LRP4, in the absence of proper membrane integration, may serve as decoy receptors in the extracellular space, by binding ligands and thereby modulating intracellular signaling events that are critical to normal growth and development.
Transmembrane Signaling - Activation of Tyrosine Kinases
The first definitive indication that LDL receptor family members can directly transmit signals across the plasma membrane by activating cytoplasmic kinase cascades came from the analysis of mice lacking VLDLR and Apoer2 (Trommsdorff et al. 1999). Both receptors bind the extracellular signaling protein Reelin (D’Arcangelo et al. 1999; Hiesberger et al. 1999). Oligomeric Reelin (Utsunomiya-Tate et al. 2000) induces clustering of the receptors (Strasser et al. 2004), thereby facilitating transphosphorylation of non-receptor Src family tyrosine kinases (SFKs) (Arnaud et al. 2003; Ballif et al. 2003; Bock et al. 2003; Howell et al. 1999) which are recruited to the cytoplasmic tail of the receptors through interactions with the adaptor protein Disabled-1 (Dab1) (Howell et al. 1997). This adaptor protein itself binds to ‘NPxY’ motifs in the receptor tails (Howell et al. 1999; Trommsdorff et al. 1998), and also to phosphoinositide-rich regions of the plasma membrane (Stolt et al. 2003). Both interactions are necessary for Reelin signal transduction, which increases signal specificity through stringent compartmentalization (Stolt et al. 2005; Xu et al. 2005). Activation of SFKs is the master switch that turns on a broader and diverging signaling cascade (Arnaud et al. 2003; Arnaud et al. 2003; Assadi et al. 2003; Ballif et al. 2004; Ballif et al. 2003; Beffert et al. 2002; Bock et al. 2003; Bock et al. 2003; Bock et al. 2004; Feng et al. 2007; Hiesberger et al. 1999; Howell et al. 1999; Mayer et al. 2006; Zhang et al. 2007), which acts on multiple targets, most notably cytoskeletal components such as actin and microtubules (Assadi et al. 2003; Brich et al. 2003; Jossin 2004; Jossin et al. 2004; Kawauchi et al. 2008; Lambert de Rouvroit et al. 2001; Pramatarova et al. 2003; Stockinger et al. 2000; Winder 2004), molecular motors (Beffert et al. 2002; Horiuchi et al. 2007; Pramatarova et al. 2006; Verhey et al. 2001) and ion channels, specifically the NMDA receptor (Figure 5 (Beffert et al. 2005; Chen et al. 2005; D’Arcangelo 2005; Groc et al. 2007; Isosaka et al. 2006; May et al. 2004; Qiu et al. 2007; Qiu et al. 2006; Sinagra et al. 2005)). The pathways that are activated by Reelin are essential in the embryo for brain development, i.e. the ordered formation of cortical layers in the neocortex and in the cerebellum, but also in the adult where they regulate synaptic transmission and synaptic plasticity (Beffert et al. 2006; Beffert et al. 2005; Beffert et al. 2004; May et al. 2004; Qiu et al. 2007; Qiu et al. 2006; Sinagra et al. 2005; Weeber et al. 2002), dendrite and dendritic spine formation (D’Arcangelo 2006; Jossin et al. 2007; Matsuki et al. 2008; Niu et al. 2004; Niu et al. 2008; Trommsdorff et al. 1999) and neuronal survival (Beffert et al. 2006). The central role that particularly Apoer2 plays in the synapse could explain a part of the still enigmatic mechanisms that underlie Alzheimer’s disease (reviewed in (Herz et al. 2000; Herz et al. 2006)). A second tyrosine kinase pathway in the brain is controlled by LRP1. TrkC, a tyrosine kinase receptor with essential functions in brain development and neurite outgrowth, is transactivated by ligand binding to LRP1 (Shi et al. 2009). The binding of either α2M or tPA to LRP1 results in activation of Src family kinases which in turn activate downstream targets including Akt and MEK/Erk. Gene silencing of LRP1 led to a functional abrogation of TrkC activation, even in the presence of activating ligand NGF-β. The finding that LRP1 is necessary for this process is underscored by overexpression experiments with RAP, a molecular chaperone that antagonizes ligand binding to LRP1, which also blocks TrkC kinase signaling.
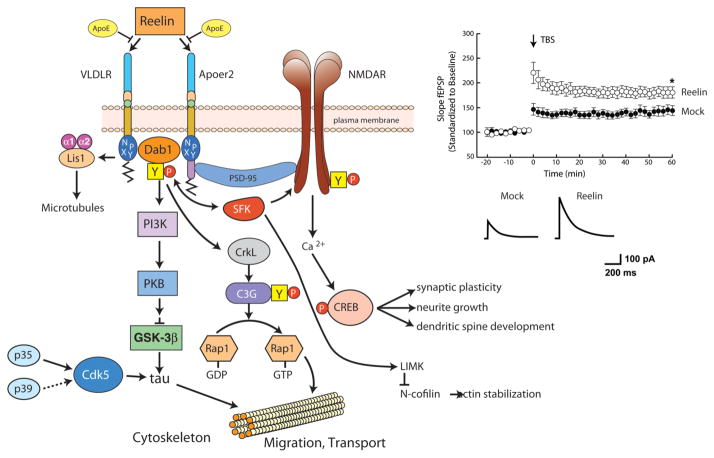
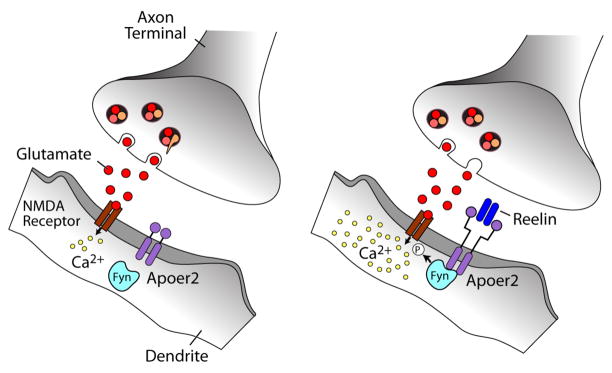
Figure 5. Reelin signaling through VLDLR and Apoer2 in neurons (modified from (Herz et al. 2006)).
Left Panel, The oligomeric signaling protein Reelin binds to VLDLR and Apoer2 with high affinity at the cell surface and induces receptor clustering and transactivation of Src family tyrosine kinases (SFKs), resulting in phosphorylation and thereby activation of disabled-1 (Dab1), an adaptor protein that interacts with NPxY motifs in both receptor tails. Phosphorylated Dab1 activates phosphatidylinositol 3-kinase (PI3K) and subsequently protein kinase B (PKB, Akt), which in turn inhibits glycogen synthase kinase-3β (GSK3β) and reduces phosphorylation of the microtubule stabilizing protein, tau. Tyrosine-phosphorylated Dab1 also recruit Crk and Crk-like (CrkL), which regulate actin cytoskeleton rearrangement through the phosphorylation of the guanine nucleotide exchange factor, C3G, and Rap1-GTP. Actin depolymerisation is also controlled by LIM kinase (LIMK), which inhibits N-cofilin by serine phosphorylation. Lis1 binds tyrosine-phosphorylated Dab1 and participates in the formation of a Pafah1b complex, which regulates microtubule functions. Cyclin-dependent kinase 5 (Cdk5) and its activators p35 and p39 are assumed to function in parallel with Reelin. In the synapse, Apoer2 associates with the post-synaptic scaffolding protein PSD95 through a 59 amino acid sequence encoded by the alternatively spliced exon 19, thus functionally coupling the Reelin signaling complex to the NMDA receptor (NMDAR). Reelin-activated SFKs phosphorylate the NMDAR on tyrosines in the NR2 subunits, thereby potentiating NMDAR-mediated Ca2+ influx. Elevated intracellular Ca2+ levels activate the transcription and survival factor cAMP-response element binding protein (CREB), which initiates the expression of genes that are important for synaptic plasticity, neurite growth and dendritic spine development. Right panel, Reelin potentiates long-term potentiation (LTP) (reproduced from (Weeber et al. 2002)) and enhances NMDAR-mediated whole cell current in wild type CA1 pyramidal neurons.
Modulation of Tyrosine Kinase Receptor Signaling
Although none of the LDL receptor family members harbor a kinase domain in their short cytoplasmic tails, clustering of Apoer2 and VLDLR can directly activate tyrosine kinases through recruitment of SFKs into the complex (Arnaud et al. 2003; Ballif et al. 2003; Beffert et al. 2006; Bock et al. 2003; Strasser et al. 2004). LRP1 employs another mechanism to modulate the activity of a membrane tyrosine kinase receptor, by binding PDGF-BB and by forming a complex with the PDGF receptor β (Figure 2A (Boucher et al. 2002; Loukinova et al. 2002)). Complex formation with the PDGF receptor alters its subcellular trafficking (Takayama et al. 2005). In the absence of LRP1 PDGFRβ is rapidly endocytosed in response to PDGF exposure. Endocytosis of the PDGFβ receptor has been shown to be required for activation (Muratoglu et al.). This coincides with an increased association of PDGFRβ with the E3 ubiquitin ligase c-Cbl and a concomitant increase in ubiquitination (Figure 2B (Takayama et al. 2005)). In the absence of LRP1, basal activity of PDGFRβ is increased, possibly due to ligand-independent dimerization or increased sensitivity to autocrine stimulation, whereas this threshold is higher in the presence of LRP1. This increased basal activity leads to continuous mitogenic signaling, which readily explains the increased vascular smooth muscle cell proliferation and migration in smooth muscle cell-specific LRP1 knockout mice (Boucher et al. 2003; Takayama et al. 2005; Zhou et al. 2009). In response to PDGF receptor activation, LRP1 is phosphorylated on a tyrosine residue in the second NPxY within its cytoplasmic tail (Barnes et al. 2003; Barnes et al. 2001; Loukinova et al. 2002). Intriguingly, this phosphorylation event is prevented by the binding of ApoE-containing lipoproteins to LRP1 (Boucher et al. 2002), providing an attractive mechanism that could explain the powerful effect of ApoE on preventing PDGF-induced smooth muscle cell migration in vitro (Figure 2A (Herz et al. 2004; Ishigami et al. 1998; Ishigami et al. 2000; Swertfeger et al. 2002; Zhu et al. 2003) ). It may also be relevant for explaining the atheroprotective effect of locally, i.e. macrophage produced ApoE in the vascular wall (Fazio et al. 1997; Linton et al. 1995; Shimano et al. 1995; Yu et al. 2006).
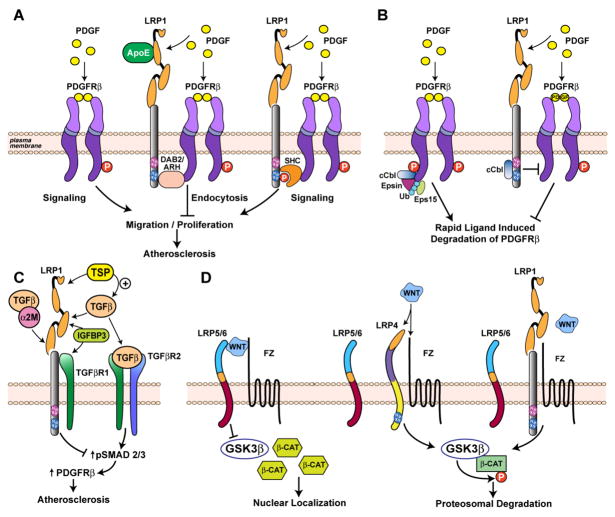
Figure 2. Modulation of PDGF, TGFβ, and Wnt signaling activity by the LDL receptor family.
Panel A, Regulation of PDGF signaling and suppression of atherosclerosis by LRP1. In the absence of LRP1, PDGF binds PDGF receptor β, induces its tyrosine phosphorylation, and activates PDGF-dependent migratory and proliferative signals. In the presence of LRP1, PDGF binds to both LRP1 and PDGF receptor β. Binding of ApoE-containing lipoproteins to LRP1 block LRP1 tail phosphorylation. In its unphosphorylated state, LRP1 interacts with adaptor proteins that are involved in the regulation of endocytosis (e.g. Dab2), reduces extracellular PDGF by endocytosis and degradation and prevents PDGF-dependent vascular smooth muscle cell migration and proliferation. In the absence of ApoE, LRP1 undergoes tyrosine phosphorylation in response to PDGF, which leads to recruitment of the Shc adaptor protein and activates proliferative and migratory signaling. Panel B, In the absence of LRP1 enhanced binding of cCbl to PDGFRβ results in increased monoubiquitination of PDGFRβ. This increases the affinity of the receptor for the ubiquitin interacting motifs in adaptor molecules such as Epsin/Eps15, and direct the receptor to coated pit endocytosis. LRP1 can interact with cCbl independently, which may competitively or sterically interfere with the association of cCbl with PDGFRβ, thereby decreasing the rate at which the receptor is ubiquitinated and removed from the cell surface by endocytosis. Panel C, Suppression of TGFβ and PDGF signaling and protection of vascular wall integrity by LRP1. Lack of LRP1 leads to enhanced complex formation of TGFβR1 and TGFβR2 and activation of TGFβ signaling resulting in phosphorylated SMAD2/3 (pSMAD2/3) accumulation, and increased PDGF receptor β expression and activation in smooth muscle cells. Moreover, LRP1 binds TGFβ directly or indirectly through α2M, internalizes insulin-like growth factor binding protein (IGFBP3) and the TGFβ activator thrombospondin (TSP), and sequesters TGFβ receptor. Panel D, Suppression of canonical Wnt signaling by LRP1 and LRP4. The ternary complex of Frizzled-1, co-receptor LRP5/6, and Wnt ligand at the cell surface leads to inhibition of GSK-3β and subsequent accumulation of β-catenin which translocates to the nucleus and mediates gene transcription. LRP1 and LRP4 suppress canonical Wnt signaling, probably by competing for LRP5/6 in the Wnt/Fz signaling complex at the membrane. In addition, LRP4 decreases Wnt signaling through its ligand binding domain by binding, sequestering or internalizing Wnt and BMP ligands and modulators. NPxY motif one
 and two
and two
 .
.
LDL receptor-deficient mice that are also lacking LRP1 in the smooth muscle cells of their aortas spontaneously develop striking atherosclerotic lesions and abdominal aneurysms, even when plasma cholesterol levels are low (Boucher et al. 2003). Moreover, they are extremely susceptible to cholesterol-feeding, resulting in the development of rampant atherosclerosis and death from abdominal and mesenteric vessel occlusion at cholesterol levels equal to those present in mice lacking only their LDL receptors (Boucher et al. 2003). Blockade of PDGFR signaling with the tyrosine kinase inhibitor Gleevec prevented atherosclerosis progression upon cholesterol-feeding in the LRP1-deficient cohort, suggesting that PDGFR signals are responsible for the variations in atherogenicity at a given cholesterol level (Boucher et al. 2003). Intriguingly, ApoE deficient mice are also more susceptible to lesion development than LDL receptor knockouts (Ishibashi et al. 1994; Plump et al. 1992; Zhang et al. 1992), raising the possibility that this difference could be caused by the abolished suppression of PDGF-induced LRP1 tyrosine phosphorylation by ApoE.
Modulation of TGF-β Signaling
Huang and colleagues identified a large TGFβ1 binding protein on the cell surface which they called TGFβ receptor V (O’Grady et al. 1991). They later identified this protein as LRP1 and showed, that LRP1 also binds insulin growth factor binding protein 3 (IGFBP3) and that it was required for growth inhibition by IGFBP3 and TGFβ1 (Huang et al. 2003). Signaling by TGFβ1 involves the ligand-induced heterodimerization of TGFβR-I and II (Luo et al. 1996). Huang and colleagues showed that LRP1 can form a complex with TGFβR-I (Huang et al. 2003) and proposed that the relative ratio of TGFβR-I and II is critical for partitioning of receptor-bound TGFβ between the clathrin/signaling and a caveolae/degradation pathway (Huang et al. 2005). This model is consistent with the presence of LRP1 in caveolae (Boucher et al. 2002), where it can sequester TGFβR-I and prevent its association with TGFβR-II. In the absence of LRP1, TGFβ signal repression is abolished, resulting in a massive increase of pSmad2 accumulation in the nucleus of LRP1 deficient smooth muscle cells (Figure 2C (Boucher et al. 2007)).
Thrombospondin is an activator of TGFβ and itself a ligand for LRP (Godyna et al. 1995; Mikhailenko et al. 1995; Mikhailenko et al. 1997). IGFBP3 binding to LRP1 and TGFβR-I has also been proposed to activate a growth inhibitory cascade (Huang et al. 2005). Thus, LRP1 can in principle suppress the activation of TGFβ receptor-dependent signaling pathways in at least five distinct ways: direct binding of TGFβ1, possibly indirectly by removing TGFβ1 bound to α2M, endocytosis of thrombospondin, sequestration of TGFβR-1, and endocytosis of IGFBP3 (Figure 2C (Boucher et al. 2007)). The existence of multiple independent mechanisms impressively confirms the central role LRP1 has in the regulation of TGFβ function. VLDLR is also a high affinity receptor for thrombospondins that mediate growth suppression (Blake et al. 2008). This appears to involve, however, a pathway that activates Akt and Erk, rather than Smads (Oganesian et al. 2008).
Conditional knockout mice lacking LRP1 only in their smooth muscle cells present with medial thickening of the aorta, which is accompanied by disruption of elastic layers and increased fibrosis (Boucher et al. 2003). This Marfan-like syndrome is consistent with the simultaneous activation of proliferative PDGFRβ-dependent and fibrosis- and elastolysis-inducing TGFβ-dependent signaling pathways. This regulation of vascular wall maintenance, potentially also involving ApoE as a modulator in vivo, is an excellent example for the integration of two fundamentally distinct signaling pathways by LRP1. Even though these pathways are distinct, they overlap at the Smad transcriptional level. Activation of Smad4 transcription leads to massive upregulation of both receptor and ligand of the PDGF pathway (Gotzmann et al. 2006). Therefore, LRP1 inhibits a cascade of proliferation/inflammation at two subsequent steps.
Modulation of Wnt, Bmp and Shh signaling
Recent studies in vitro and in vivo have provided evidence that some LDL receptor family members are also involved in the regulation of at least two other morphogenetic signaling pathways, those involving Wnt and Bmp proteins (Johnson et al. 2005; Spoelgen et al. 2005; Zilberberg et al. 2004). LRP1 has been reported to interact with human Frizzled-1, which mediates Wnt signaling together with another co-receptor, LRP5/6 (the orthologue of Drosophila arrow) and disrupts the receptor/co-receptor complex formation, leading to the repression of canonical Wnt signaling (Pinson et al. 2000; Tamai et al. 2000; Wehrli et al. 2000; Zilberberg et al. 2004). LRP4 suppresses Wnt signaling, probably by competing for LRP5/6 in the Wnt/Fz signaling complex at the membrane level and through its ligand binding domain by altering the effect of extracellular ligands (Figure 2D (Dietrich et al. 2010; Johnson et al. 2005; Weatherbee et al. 2006)). LRP4 knockout mice present with several remarkable phenotypes, including defects of kidney development (Karner et al. 2010; Li et al. 2010; Weatherbee et al. 2006), limb development (Drogemuller et al. 2007; Duchesne et al. 2006; Johnson et al. 2005; Simon-Chazottes et al. 2006; Weatherbee et al. 2006), tooth development (Johnson et al. 2005) and failure to form neuromuscular junctions (Weatherbee et al. 2006). Not all of these defects can be explained by deregulation of Wnt signaling alone, suggesting that LRP4 is also involved in the execution or modulation of other signaling pathways. This has now been confirmed by the recent discovery that LRP4 forms a complex with the muscle-specific tyrosine kinase MUSK, which is required for the formation of neuromuscular junctions ((Kim et al. 2008; Zhang et al. 2008) and discussed in detail below). The hypomorphic phenotype caused by expression of a truncated, and thus apparently partially functional, LRP4 provides further mechanistic insights (Dietrich et al. 2010; Johnson et al. 2005; Johnson et al. 2006). In principle, two distinct mechanisms by which a soluble LRP4 ectodomain may modulate signaling events can be postulated. First, soluble LRP4 may serve as a scavenger receptor for extracellular signaling ligands (Dietrich et al. 2010) and second, the non-membrane anchored LRP4 through interaction with MuSK retains a partial ability to induce AChR clustering (Kim et al. 2008; Zhang et al. 2008).
Another member of the gene family, LRP2, participates in forebrain development by removing Bmp4 from the extracellular space (Spoelgen et al. 2005). In the absence of LRP2, Bmp4 expression is increased leading to a subsequent loss of sonic hedgehog (Shh) expression in the ventral forebrain. LRP2 can also bind and thereby remove Shh from the extracellular space (McCarthy et al. 2002). By thus regulating the levels of an upstream (Bmp4) as well as a downstream (Shh) morphogen in the developing brain, LRP2 participates in the integration and feed-back regulation of both signaling pathways.
Shh is palmitoylated at its amino terminus (Buglino et al. 2008; Maity et al. 2005) and also modified by a cholesterol-moiety at its carboxyl-terminus (Cooper et al. 1998). Likewise, Wnts are also palmitoylated (Willert et al. 2003; Zhai et al. 2004). These lipid modifications promote the association of the proteins with lipoprotein particles and this is important for regulating their short-range and long-range signals in insects (Eaton 2008; Panakova et al. 2005). To what extent lipoprotein particles regulate Shh or Wnt signaling in higher organisms such as mammals is not clearly understood.
Roles in Cellular Growth Regulation and Cancer
The functions of LRP1 as a powerful negative modulator of cellular proliferation in smooth muscle cell proliferation in atherosclerosis and its role in the regulation of numerous growth factors with well-documented roles in cancer biology (tyrosine kinase receptors, Wnt, Shh, TGFβ) suggest that LDL receptor family members might themselves be involved in tumor development or progression. Evidence for this was indeed found several years ago, when Lisitsyn and colleagues first demonstrated that LRP1B, the closest relative to LRP1, was frequently deleted in non-small cell lung cancer cell lines (Liu et al. 2000). In a series of follow-up studies, mutations of LRP1B in urothelial malignancies (Langbein et al. 2002), esophageal squamous cell carcinomas (Sonoda et al. 2004), gliomas (Roversi et al. 2006), head and neck cancers (Nakagawa et al. 2006), cervical adenocarcinomas, B-cell lymphomas (Rahmatpanah et al. 2006), and leukemias (Taylor et al. 2007) have been reported. These findings were recently confirmed in a mutation screen in primary pulmonary adenocarcinomas (Ding et al. 2008). In this study, LRP1B was the fourth most commonly mutated gene, only surpassed in frequency by p53, k-ras, and EGFR tyrosine kinase mutations. Initial evidence for a potential involvement of the chromsome 2q21 gene locus, where LRP1B maps, was derived from chromosomal analysis in which mutations had been linked to decreased overall survival and reduced response to chemotherapy (Shiseki et al. 1994).
Mechanistically, LRP1B has been implicated in the trafficking of the PDGF receptor β and the urokinase receptor (Seki et al. 2005; Tanaga et al. 2004). However, the functions of these proteins are also regulated by LRP1, which is expressed normally and not mutated in the aforementioned cancers. This functional overlap is suggestive of a distinct mechanistic and non-redundant role of LRP1B in the control of cell cycle and proliferation in the context of cancer. An investigational obstacle to understanding this presumed tumor suppressor is the extremely low expression in mouse and human lung tissues for LRP1B (Li et al. 2005). In the mouse, expression is mainly limited to the brain and testis. In human, the distribution pattern is more ubiquitous with relatively low expression levels in the lung and other tissues where cancer occurrence has been linked to LRP1B (Li et al. 2005). Yet, several studies have also shown an increased frequency of LRP1B mutations to a higher grade and advanced staging of several tumor types, suggesting a role as a tumor suppressor (Herrmann et al. 1991). One could therefore speculate that an as yet unidentified microenvironmental factor, e.g. local inflammatory processes or hypoxia, might trigger a protective upregulation of LRP1B, which needs to be abolished for the cancer to progress. Studies into the role of specific genes in cancers with known environmental causes have proved difficult. In the hypomorphic mutant LRP1B mouse model we created in our laboratory, no spontaneous tumor development was observed (Marschang et al. 2004). Taken together with the gene analysis data in humans this suggests that LRP1B mutations are frequent and important secondary tumor-promoting, rather than primary tumor-inducing events. These findings suggest that LRP1B probably functions in the lung in a manner analogous to that shown for LRP1 in the vascular wall, i.e. as a signal integrator of as yet undefined growth regulating signaling pathways. So far, the molecular identification of these pathways has been hampered by the extremely low expression of LRP1B in the normal lung in mice and in humans (Marschang et al. 2004).
While LRP1B is the only member of the LDL gene family known to harbor frequent genetic and epigenetic alterations in malignancies, other family members have also been implicated in playing a role in cancer. Initially, LRP1 gene polymorphisms were suspected to pose an increased risk for the development of breast cancer, but this was not confirmed in follow-up studies (Benes et al. 2003; Jakubowska et al. 2009). LRP1 is involved in a wide variety of physiological processes, some of which may play a role in cancer. The regulation of angiogenesis through negative regulation of the PDGF receptor β on vascular endothelial cells, and the effect on anti-angiogenic activity of thrombospondin-2 would suggest a role in the control of local tumor growth (Boucher et al. 2003; Zhou et al. 2009), on the other hand, LRP1-mediated activation of metalloproteases 2 and 9 was shown to promote cancer cell migration and invasion (Song et al. 2009). The lack of naturally occurring LRP1 mutations suggests that these functions are an indispensible part of the physiological repertoire of the receptor, which in themselves are neither tumor promoting or suppressing.
In addition, LRP1 and LRP4 can also function as inhibitors of the Wnt signaling pathway (Ohazama et al. 2008). Wnt signaling and its main downstream target β-catenin regulate a variety of mechanism that are pertinent to cancer development and progression. Unbridled Wnt signaling leads to cellular hyperproliferation, rearrangement of cellular architecture, and increased resistance to medical treatment.
Roles of the Gene Family in the Nervous System
For several reasons, the expanding roles of the gene family in the nervous system deserve particular attention. First, mounting evidence is emerging that several members of the family are directly or indirectly involved in neuronal survival and degeneration, specifically in the incompletely understood mechanisms that underlie the pathogenesis of Alzheimer’s disease (Figure 3). Second, a wealth of insights into the molecular mechanisms has accumulated over the last few years that have provided us with novel unique examples for the molecular mechanisms by which LDL receptor family members participate in the control of the most complex human organ.
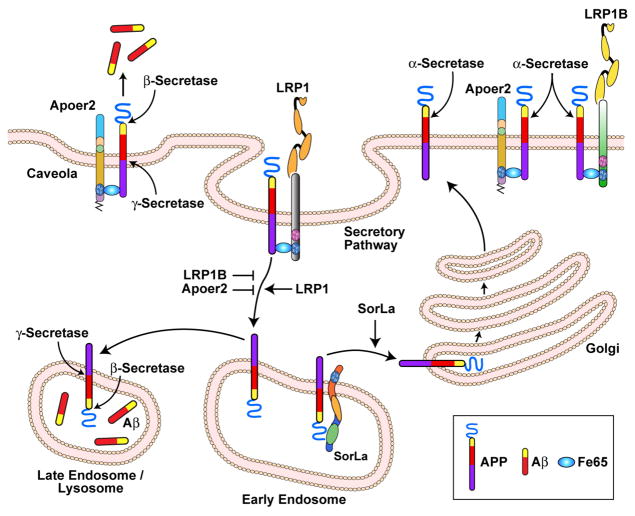
Figure 3. Regulatory influence of LDLR family members on APP processing and Aβ production.
APP bound to the cell surface is primarily processed by the α-secretase favoring the non-amyloidogenic pathway. Endocytosis of APP to the endosomal/lysosomal compartments results in increased β-secretase mediated cleavage and enhanced Aβ-production. The fast internalization rate of LRP1 facilitates APP endocytosis and its processing to Aβ. By contrast, LRP1B and Apoer2 endocytose slowly, which results in a longer residence time of APP at the cell surface promoting non-amyloidogenic processing. Apoer2 is associated with caveolae shifting APP from membrane non-raft to raft domains and thereby facilitating processing by β-secretase. SorLa prevents β-secretase access to APP and subsequent Aβ production by intracellularly shuttling APP to the Golgi compartment.
Aβ and Alzheimer’s Disease
Alzheimer’s Disease (AD) is the most prevalent form of late-onset dementia in humans - currently affecting 20–30 million individuals worldwide (Selkoe 2001). Progressive dysfunction of neurons in the limbic system and the associated cortex, accumulation of extracellular aggregates of the APP-derived 42-residue amyloid-β protein, and intraneuronal aggregates of the microtubule-associated phosphoprotein tau in the form of neurofibrillary tangles are key histopathological hallmarks of AD (Hardy et al. 2002). However, recent evidence has shown that Aβ42 oligomers inhibit hippocampal LTP, enhance long-term depression (LTD), and reduce dendritic spine density in normal rodent hippocampus. These low molecular weight aggregates are now believed to be the primary toxic species of the Aβ peptide in AD (Shankar et al. 2008).
Aβ ranges in size from 37 to 43 amino acids and is a cleavage product of APP that is processed through two alternative pathways. In the non-amyloidogenic pathway, APP is first cleaved by α-secretases (TACE and ADAM9/10) within the amyloid peptide sequence which prevents Aβ production, giving rise to the socalled a-carboxyl-terminal fragment (α-CTF). This cleavage event predominately takes place at or near the cell surface (Deuss et al. 2008). In the amyloidogenic pathway, APP is first cleaved by β-secretase (BACE-1) to form the amino-terminus of what is known as the β-carboxyl-terminal fragment (β-CTFβ). Maximal BACE1 activity requires acidic pH, as is present not at the plasma membrane, but within the subcellular compartments of the secretory pathway, including the trans-Golgi network and endosomes (Cole et al. 2007). Both α- and β-CTFs are further processed by the γ-secretase complex (consisting of presenilin 1/2, nicastrin, Aph1 and Pen2) (Tolia et al. 2009) within the transmembrane domain, resulting in the release of Aβ peptides of variable length for the amyloidogenic, and the shorter p3 peptide for the non-amyloidogenic pathway (Zheng et al. 2006). In both pathways, the γ-secretase cleavage releases the APP intracellular domain (AICD) fragment which subsequently can translocate to the nucleus where it interacts, together with Fe65 and Tip60, with the LRP1 promotor and suppresses its transcription (Liu et al. 2007).
LRP1 facilitates APP internalisation and enhances Aβ production
LRP1 binds APP and modulates its processing to Aβ. Soluble Kunitz protease inhibitor containing forms of APP (sAPP751/770) as well as their corresponding full length membrane-bound isoforms bind directly to LRP1 (Knauer et al. 1996; Kounnas et al. 1995). This interaction is sensitive to the LRP1 inhibitor receptor-associated protein (RAP) suggesting a direct interaction between extracellular domains of APP751/770 and LRP1 (Knauer et al. 1996). However, for the neuronal APP isoform (APP695), which lacks the KPI domain, an intracellular interaction with LRP1 bridged by the cytoplasmic adaptor protein Fe65 could be shown (Kinoshita et al. 2001; Trommsdorff et al. 1998). In the absence of LRP1, Aβ production, APP secretion, and APP internalization are affected, independent of the APP isoform (Pietrzik et al. 2002). A characteristic property of LRP1 is its short half life at the cell surface (t1/2 < 0.5min) (Li et al. 2000). This rapid endocytosis rate facilitates APP internalization and favors its subsequent intracellular processing through BACE1 and therefore enhances Aβ production (Cam et al. 2005) (Figure 3). Intriguingly, mice overexpressing functional LRP1 mini receptors on neurons have increased levels of oligomeric Aβ in brain (Zerbinatti et al. 2004).
LRP1 mediates Aβ transport across the blood brain barrier (BBB)
Another important mechanism to maintain Aβ homeostasis in the brain is the elimination of Aβ from the brain across the BBB which is mediated mainly by the cell surface LRP1; localized predominantly on the abluminal side of the cerebral endothelium (Deane et al. 2009; Deane et al. 2008; Deane et al. 2004; Shibata et al. 2000). Furthermore, brain endothelial expression of LRP1 at the BBB is reduced in AD mouse models and AD patients thus making it unfavorable for Aβ clearance from the brain (Deane et al. 2004; Shibata et al. 2000). Besides the direct binding of Aβ to LRP1, it also binds to various chaperone molecules e.g. ApoJ (Bell et al. 2007), α2-macroglobulin (Qiu et al. 1999) and lactoferrin (Narita et al. 1997), which thus also affect this clearance mechanism, whereas ApoE changes the rapid LRP1 dependent clearance in an isoform specific manner (ApoE4>ApoE3>ApoE2) (Deane et al. 2008). Once Aβ is transported to the blood side, soluble LRP1 (sLRP1) acts as a major peripheral Aβ binding protein that normally sequesters ~70–90% of circulating Aβ - suggesting a “peripheral sink” for Aβ that regulates Aβ removal from the brain (Figure 4 (Sagare et al. 2007)).
Figure 4. LRP1 mediates transcytosis of Aβ through the BBB.
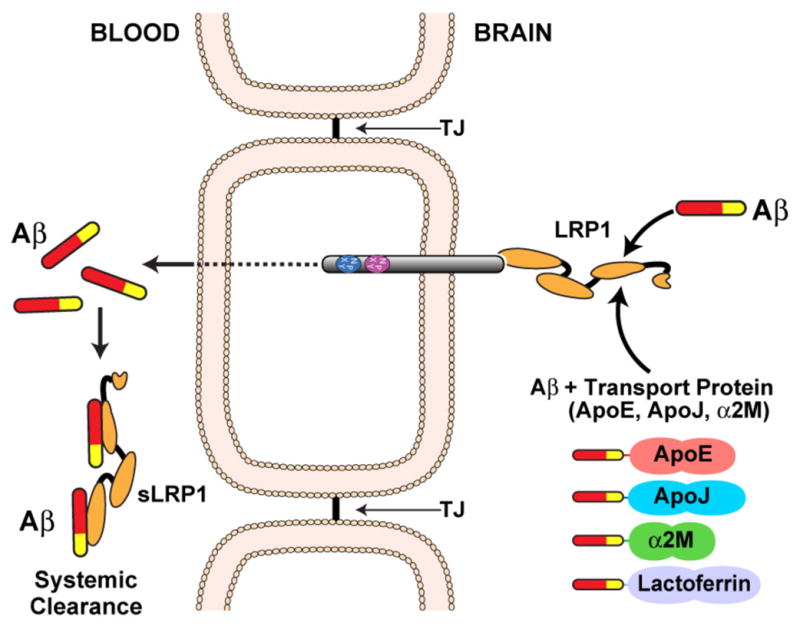
Cell surface LRP1 at the abluminal membrane binds Aβ and initiates its transcytosis across the BBB followed by its excretion into the blood stream. In addition, Aβ binding to LRP1 is influenced by Aβ transport proteins (ApoE, ApoJ, α2M, and lactoferrin). In the circulation, the soluble form of LRP1 binds >70% of Aβ and promotes its degradation.
LRP1B decreases APP internalization and reduces Aβ production
LRP1B has high homology (59%) to the amino acid sequence of LRP1 and shares an almost identical structural organization. However, LRP1B contains two additional exons, one encoding an additional ligand binding repeat found in ligand binding domain IV and the other an additional 33-amino acid sequence within its cytoplasmic tail (Liu et al. 2001). As a result of this high degree of homology to LRP1, minireceptors of LRP1B – with only the fourth binding domain (mLRP1B4) – also bind APP inside and outside of the cellular membrane (Cam et al. 2004). In contrast to LRP1, overexpression of mLRP1B4 extensively accumulates APP at the surface, which is likely based on its slow endocytosis rate (t1/2 > 10min) (Cam et al. 2004; Liu et al. 2001). The APP retention at the surface significantly decreases Aβ production (Figure 3), which is accompanied by an enhanced sAPPα secretion (Cam et al. 2004). The divergent endocytosis properties of LRP1B and LRP1 suggest that these receptors antagonize their respective function by competing for binding of common ligands (Marzolo et al. 2009).
Apoer2 decreases APP internalization and enhances or reduces Aβ production
Interestingly, Apoer2 is expressed in multiple splice variants on the cell surface, which may increase their potential for functional diversity (Sun et al. 1999). However, the influence of Apoer2 on APP processing is poorly understood, as Apoer2 is capable of both reducing as well as enhancing Aβ production. Similar to LRP1 and LRP1B, Apoer2 shows extracellular as well as intracellular interaction with APP. The extracellular interaction is enhanced by the matrix molecule F-spondin (Hoe et al. 2005), whereas the intracellular interaction is stabilized by Fe65 (Hoe et al. 2006). In the presence of both fusion proteins, the slow internalization rate of Apoer2 results in complex retention at the surface and reduces APP endocytic trafficking and subsequent production of Aβ (Figure 3 (Hoe et al. 2005)). In this context, binding of ApoE to Apoer2 enhances APP endocytosis and Aβ production in an isoform-specific manner (He et al. 2007). Intriguingly, Apoer2 is present in caveolae (Riddell et al. 2001) and recent studies showed that relocation of APP to raft membrane domains promotes BACE1 cleavage and subsequent Aβ production (Figure 3 (Fuentealba et al. 2007)).
SorLA modulates intracellular APP trafficking and reduces Aβ production
SorLA/LR11 is a more distant member of the lipoprotein receptor family that shares some, but not all, structural properties of this family. In addition, it contains a domain with homology to the yeast sorting protein (Vps10p) family of intracellular sorting receptors that transports proteins from the Golgi network into vacuoles (Spoelgen et al. 2006). SorLA/LR11 expression is significantly decreased in AD brains (Andersen et al. 2005) and cerebrospinal fluid (Ma et al. 2009). Binding studies demonstrate a direct extracellular interaction between SorLA/LR11 and APP that is independent of APPs KPI domain (Andersen et al. 2005). In contrast to the above mentioned lipoprotein receptors, SorLA/LR11 does not alter APP endocytosis (Spoelgen et al. 2006). Overexpression of SorLA/LR11 in neurons causes redistribution of APP to the Golgi network and endosomes and decreased processing to Aβ by BACE1 (Figure 3 (Andersen et al. 2005)). Furthermore, SorLA/LR11 binds BACE1 and competes for the interaction with APP (Spoelgen et al. 2006). These findings are confirmed by the disruption of SorLA/LR11 expression in knockout mice, which results in increased levels of Aβ. Moreover, genetic studies have shown a correlation between SorLA/LR11 gene variants with an enhanced risk of sporadic AD, providing direct genetic evidence for a role of this receptor in AD pathogenesis (Rogaeva et al. 2007).
Together, these findings emphasize the complex involvement of ApoE receptors in Aβ production and clearance and their potential contribution to AD.
ApoE receptor signaling in neurons - Apoer2 and VLDLR
The analysis of VLDLR and Apoer2 knockout mice provided the first clear insight into a linear signaling pathway in which LDL receptor family members play a pivotal role as direct activators of an intraneuronal signaling cascade. This pathway is initiated by Reelin-binding to Apoer2 and VLDLR, which activates intracellular kinases that control migration of neurons and the lamination of the cortex during development of the embryonic brain (Figure 5 (D’Arcangelo et al. 1999; Hiesberger et al. 1999; Trommsdorff et al. 1999)). Mice lacking both receptors exhibit a phenotype identical to that of the reeler mouse mutant, and of another strain of mice carrying mutations in the cytoplasmic adaptor protein Disabled-1 (Dab1) (D’Arcangelo 2005; D’Arcangelo 2005; Herz et al. 2006; Levenson et al. 2008; May et al. 2005; Tissir et al. 2003; Tueting et al. 2006).
Reelin-binding induces receptor clustering and subsequent activation of SFKs through proximity-triggered transphosphorylation of Dab1 bound to the NPxY motif of both receptor tails (Arnaud et al. 2003; Bock et al. 2003). The tyrosine phosphorylated form of Dab1 enhances the activity of Src family tyrosine kinases, primarily Fyn, which in turn activates a cascade that results in the inhibition of glycogen synthase kinase-3β (GSK3β). This enzyme is the major kinase for the microtubule stabilizing protein t in the neurons and activation of the Reelin pathway thus leads to reduced phosphorylation of this protein (Beffert et al. 2002; Hiesberger et al. 1999). Activation of the same branch of this pathway stabilizes the actin cytoskeleton of neuronal processes by phosphorylation of the actin depolymerizing protein, N-cofilin (Chai et al. 2009). Recently, it was also shown that thrombospondin-1 binds to Apoer2 and VLDLR in the brain where it participates in the chain migration of postnatal neurons along the rostral stream (Blake et al. 2008). A detailed schematic summary of the signaling cascade that is activated by Apoer2 and VLDLR is shown in Figure 5 (Herz et al. 2006).
After the end of the neuronal migration period Reelin expression shifts from its former predominant site in layer I Cajal-Retzius neurons to a subpopulation of inhibitory interneurons that are dispersed throughout the cortex and hippocampus (Herz et al. 2006; Tissir et al. 2003). Reelin secretion from these neurons regulates synaptic plasticity (Palop et al. 2006; Palop et al. 2007), neuronal survival (Beffert et al. 2006) and protects from hyperexcitation as it occurs in epilepsy (Haas et al. 2002; Heinrich et al. 2006). An important role of Reelin in the adult brain is the Apoer2-mediated induction of NMDA receptor subunit (NR2) tyrosine-phosphorylation. This phosphorylation step regulates surface distribution and activity of the NMDA receptor (Figure 6 (Herz et al. 2006; Lau et al. 2007)). For the functional coupling of the Reelin/Apoer2 receptor complex to NMDAR the presence of an alternatively spliced exon - containing a 59 amino acid sequence in the cytoplasmic tail - is required (Beffert et al. 2005). This sequence includes binding sites for several adaptor proteins including post synaptic density protein (PSD95) (Beffert et al. 2005; Hoe et al. 2006) and cJun-N-terminal kinase interacting proteins (JIPs) (Gotthardt et al. 2000; Stockinger et al. 2000) that are essential for the activation of Src family kinases and the following phosphorylation of the NMDA receptor subunits (Kennedy et al. 2007). Mice lacking this additional 59 amino acid sequence (exon 19) show deficits in learning, prominent fear conditioning defects (Beffert et al. 2005) and enhanced neuronal loss during aging (Beffert et al. 2006).
Figure 6. Reelin signaling through Apoer2 potentiates NMDAR-mediated Ca2+ influx at excitatory synapses.
Ligation of Apoer2 by Reelin in the dendrite activates the SFK Fyn, which phosphorylates the NMDAR on NR2 subunits. Increased NMDAR phosphorylation stabilizes NMDAR at the plasma membrane (Snyder et al. 2005), and can also increase Ca2+ conductance. In this manner, the Reelin signaling pathway participates directly in the mechanisms that regulate synaptic plasticity, memory and learning.
The mechanisms by which Apoer2 and Vldlr regulate synaptic functions may be directly relevant to the pathogenesis of late-onset Alzheimer’s disease, in particular to a form of the disease that is driven by ApoE4 through a poorly understood mechanism (Corder et al. 1993; Schmechel et al. 1993). Recent work from our laboratory showed that activation of Apoer2 and Vldlr by Reelin in cultured neurons (Chen et al. 2005) and in hippocampal slices of mice (Weeber et al. 2002) can prevent the synaptic suppression caused by synthetic and native amyloid-β oligomers (Chen et al. 2010; Durakoglugil et al. 2009).
ApoE receptor signaling in neurons - LRP1
In addition to its role as a signal transducer/modulator in the vascular wall, LRP1 also is involved in signal modulation in neurons (Bacskai et al. 2000; Bu et al. 2006; Hayashi et al. 2007; Lillis et al. 2008; Liu et al. 2007; May et al. 2005; May et al. 2004; McLoughlin et al. 2008; Schneider et al. 2003; Zhuo et al. 2000), although the underlying mechanisms there are less well understood. For instance, neuron-specific knockouts of LRP1 using a Cre recombinase driven by the Synapsin-I promoter (May et al. 2004) develop severe behavioral and motor abnormalities, including hyperactivity, tremor, and dystonia that might be related to a role of LRP1 in the regulation of postsynaptic NMDA responses (May et al. 2004). This NMDA receptor modulation may involve a tissue-type plasminogen activator-dependent mechanism that requires the second NPxY motif in LRP1 (Martin et al. 2008).
ApoE receptor signaling in neurons - LRP4
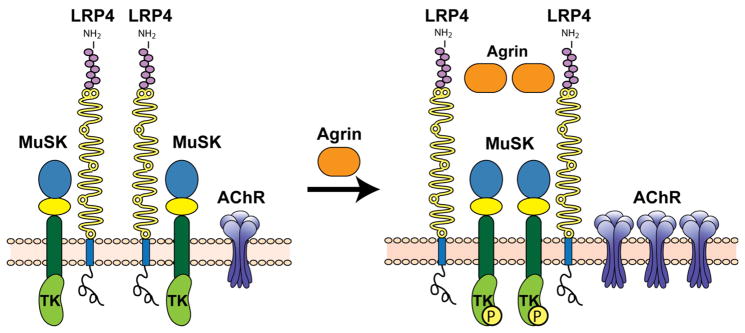
LRP4 is unique inasmuch as its size falls between the smaller members of the family (LDLR, Apoer2, and VLDLR) and the large receptors (LRP1, LRP1B, and LRP2). It plays a crucial role in neuromuscular synapse function and development (Kim et al. 2008; Weatherbee et al. 2006; Zhang et al. 2008). LRP4 knockout mice develop severe neuromuscular junction defects, resulting in perinatal lethality due to postpartum asphyxia (Weatherbee et al. 2006). Mechanistically, LRP4 serves as the obligate co-receptor for the muscle-specific tyrosine kinase MuSK and is required for the activation of MuSK by the signaling protein Agrin (Figure 7 (Kim et al. 2008; Zhang et al. 2008)). Interestingly, mice expressing an extracellularly truncated allele is viable (Dietrich et al. 2010; Johnson et al. 2005; Johnson et al. 2006). We hypothesize that release of the ectodomain into the extracellular space results in a soluble LRP4 that can still bind and present agrin to MuSK and thereby induce its activation. This mechanism combines two of the previously discussed distinct functional features that characterize the role of LRPs in cellular signal transduction, i.e. their absolute requirement as unique receptors for certain signaling proteins, and their ability to directly or indirectly control the activation of other primary cellular signaling mechanisms, such as tyrosine or serine/threonine kinases in response to ligand binding.
Figure 7. LRP4 serves as a receptor for agrin and as a coreceptor for the tyrosine kinase MUSK in muscle.
(Kim et al. 2008; Zhang et al. 2008). The membrane tyrosine kinase, MUSK, is required for the induction of acetylcholine receptor (AChR) clustering during the formation of neuromuscular junctions (endplates). MUSK does not bind the signaling protein agrin directly, but must form a complex with LRP4, a coreceptor for agrin. Agrin binding to LRP4 enhances complex formation of MUSK with LRP4 and induces transphosphorylation of MUSK followed by acetylcholine receptor (AChR) clustering.
In conclusion, in this review we have attempted to summarize and highlight a representative cross-section of some of the most prominent principles by which one particular class of lipoprotein receptors, the LDL receptor gene family, functions in the control of an expanding range of fundamental metabolic and morphogenetic pathways that in many cases are not, or only remotely so, related to lipid metabolism. Knockout models of these multifunctional receptors have confirmed their crucial roles, for instance in morphogenetic signaling pathways. On the other hand, the wealth of these emerging findings now draws a new picture of this ancient gene family that is very different from the traditional one in which these receptors were solely viewed as transporters for lipoproteins and other macromolecules, following in the footsteps of the namesake of the family, the LDL receptor. It now appears that the need for integrating traditional linear signaling cascades, and to coordinate or modulate their appropriate cellular response in relation to other extracellular or environmental conditions, arose with the appearance of the first primitive multicellular organisms, in which neighboring cells needed to develop a more sophisticated network to communicate with each other than the more primitive unicellular organisms require. Complete comprehension of LDL receptor family members in complex mammalian organisms, particularly in the brain, will require increasingly sophisticated genetic approaches. We may not have seen nothin’ yet.
Acknowledgments
We are indebted to all present of former members of our own laboratory and of the Department of Molecular Genetics whose work and dedication has contributed to the scientific findings we have had the privilege to review and to Nancy Heard for artwork. We acknowledge the many colleagues in the field whose work we were not able to include or discuss in the depth it clearly deserves. We also acknowledge support from the National Institutes of Health, the Alexander-von-Humboldt Foundation, the American Heart Association, the American Health Assistance Foundation, the Consortium for Frontotemporal Dementia Research, and the University of Texas Southwestern Medical Center. M.D. and M.F.D. are supported by fellowships from the Boehringer Ingelheim Foundation, Germany.
References
- 1.Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–6. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, et al. Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem. 2003;278:23989–95. doi: 10.1074/jbc.M302157200. [DOI] [PubMed] [Google Scholar]
- 3.Arnaud L, Ballif BA, Cooper JA. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol Cell Biol. 2003;23:9293–302. doi: 10.1128/MCB.23.24.9293-9302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–72. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 6.Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, et al. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–6. doi: 10.1038/ng1257. [DOI] [PubMed] [Google Scholar]
- 7.Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2000;97:11551–6. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, et al. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol. 2004;14:606–10. doi: 10.1016/j.cub.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Ballif BA, Arnaud L, Cooper JA. Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res Mol Brain Res. 2003;117:152–9. doi: 10.1016/s0169-328x(03)00295-x. [DOI] [PubMed] [Google Scholar]
- 10.Barber DL, Sanders EJ, Aebersold R, Schneider WJ. The receptor for yolk lipoprotein deposition in the chicken oocyte. J Biol Chem. 1991;266:18761–70. [PubMed] [Google Scholar]
- 11.Barnes H, Ackermann EJ, van der Geer P. v-Src induces Shc binding to tyrosine 63 in the cytoplasmic domain of the LDL receptor-related protein 1. Oncogene. 2003;22:3589–97. doi: 10.1038/sj.onc.1206504. [DOI] [PubMed] [Google Scholar]
- 12.Barnes H, Larsen B, Tyers M, van Der Geer P. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J Biol Chem. 2001;276:19119–25. doi: 10.1074/jbc.M011437200. [DOI] [PubMed] [Google Scholar]
- 13.Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, et al. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J Neurosci. 2006;26:2041–52. doi: 10.1523/JNEUROSCI.4566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, et al. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–64. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- 15.Beffert U, Nematollah Farsian F, Masiulis I, Hammer RE, Yoon SO, et al. ApoE receptor 2 controls neuronal survival in the adult brain. Curr Biol. 2006;16:2446–52. doi: 10.1016/j.cub.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–79. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Beffert U, Weeber EJ, Morfini G, Ko J, Brady ST, et al. Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission. J Neurosci. 2004;24:1897–906. doi: 10.1523/JNEUROSCI.4084-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–18. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benes P, Jurajda M, Zaloudik J, Izakovicova-Holla L, Vacha J. C766T low-density lipoprotein receptor-related protein 1 (LRP1) gene polymorphism and susceptibility to breast cancer. Breast Cancer Res. 2003;5:R77–81. doi: 10.1186/bcr591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blake SM, Strasser V, Andrade N, Duit S, Hofbauer R, et al. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 2008 doi: 10.1038/emboj.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- 22.Bock HH, Jossin Y, Liu P, Forster E, May P, et al. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem. 2003;278:38772–9. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- 23.Bock HH, Jossin Y, May P, Bergner O, Herz J. Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J Biol Chem. 2004;279:33471–9. doi: 10.1074/jbc.M401770200. [DOI] [PubMed] [Google Scholar]
- 24.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–32. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 25.Boucher P, Li WP, Matz RL, Takayama Y, Auwerx J, et al. LRP1 functions as an atheroprotective integrator of TGFbeta and PDFG signals in the vascular wall: implications for Marfan syndrome. PLoS One. 2007;2:e448. doi: 10.1371/journal.pone.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, et al. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low Density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–13. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- 27.Bovenschen N, Herz J, Grimbergen JM, Lenting PJ, Havekes LM, et al. Elevated plasma factor VIII in a mouse model of low-density lipoprotein receptor-related protein deficiency. Blood. 2003;101:3933–9. doi: 10.1182/blood-2002-07-2081. [DOI] [PubMed] [Google Scholar]
- 28.Boycott KM, Flavelle S, Bureau A, Glass HC, Fujiwara TM, et al. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am J Hum Genet. 2005;77:477–83. doi: 10.1086/444400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brich J, Shie FS, Howell BW, Li R, Tus K, et al. Genetic modulation of tau phosphorylation in the mouse. J Neurosci. 2003;23:187–92. doi: 10.1523/JNEUROSCI.23-01-00187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bu G, Cam J, Zerbinatti C. LRP in amyloid-beta production and metabolism. Ann N Y Acad Sci. 2006;1086:35–53. doi: 10.1196/annals.1377.005. [DOI] [PubMed] [Google Scholar]
- 31.Bu G, Williams S, Strickland DK, Schwartz AL. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1992;89:7427–31. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J Biol Chem. 2008;283:22076–88. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005;25:215–35. doi: 10.1146/annurev.nutr.24.012003.132120. [DOI] [PubMed] [Google Scholar]
- 34.Burk RF, Hill KE, Olson GE, Weeber EJ, Motley AK, et al. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J Neurosci. 2007;27:6207–11. doi: 10.1523/JNEUROSCI.1153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cam JA, Zerbinatti CV, Knisely JM, Hecimovic S, Li Y, et al. The low density lipoprotein receptor-related protein 1B retains beta-amyloid precursor protein at the cell surface and reduces amyloid-beta peptide production. J Biol Chem. 2004;279:29639–46. doi: 10.1074/jbc.M313893200. [DOI] [PubMed] [Google Scholar]
- 36.Cam JA, Zerbinatti CV, Li Y, Bu G. Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the beta-amyloid precursor protein. J Biol Chem. 2005;280:15464–70. doi: 10.1074/jbc.M500613200. [DOI] [PubMed] [Google Scholar]
- 37.Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, et al. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25:1860–70. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci. 2009;29:288–99. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chappell DA, Fry GL, Waknitz MA, Iverius P-H, Williams SE, et al. The Low Density Lipoprotein Receptor-related Protein/α2-Macroglobulin Receptor Binds and Mediates Catabolism of Bovine Milk Lipoprotein Lipase. J Biol Chem. 1992;267:25764–25767. [PubMed] [Google Scholar]
- 40.Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–16. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A. 2010;107:12011–6. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiu-Ugalde J, Theilig F, Behrends T, Drebes J, Sieland C, et al. Mutation of megalin leads to urinary loss of selenoprotein P and selenium deficiency in serum, liver, kidneys and brain. Biochem J. 2010 doi: 10.1042/BJ20100779. [DOI] [PubMed] [Google Scholar]
- 43.Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–7. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 45.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 46.Crookston KP, Webb DJ, Lamarre J, Gonias SL. Binding of platelet-derived growth factor-BB and transforming growth factor-beta 1 to alpha 2-macroglobulin in vitro and in vivo: comparison of receptor-recognized and non-recognized alpha 2-macroglobulin conformations. Biochem J. 1993;293(Pt 2):443–50. doi: 10.1042/bj2930443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Arcangelo G. Apoer2: a reelin receptor to remember. Neuron. 2005;47:471–3. doi: 10.1016/j.neuron.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 48.D’Arcangelo G. The reeler mouse: anatomy of a mutant. Int Rev Neurobiol. 2005;71:383–417. doi: 10.1016/s0074-7742(05)71016-3. [DOI] [PubMed] [Google Scholar]
- 49.D’Arcangelo G. Reelin mouse mutants as models of cortical development disorders. Epilepsy Behav. 2006;8:81–90. doi: 10.1016/j.yebeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 50.D’Arcangelo G, Homayouni R, Keshvara L, Rice D, Sheldon M, et al. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 51.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deane R, Sagare A, Hamm K, Parisi M, Lane S, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–13. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deane R, Sagare A, Zlokovic BV. The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer’s disease. Curr Pharm Des. 2008;14:1601–5. doi: 10.2174/138161208784705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–44. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 55.Descamps O, Bilheimer D, Herz J. Insulin stimulates receptor-mediated uptake of apoE-enriched lipoproteins and activated alpha 2-macroglobulin in adipocytes. J Biol Chem. 1993;268:974–81. [PubMed] [Google Scholar]
- 56.Deuss M, Reiss K, Hartmann D. Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17. Curr Alzheimer Res. 2008;5:187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- 57.Dietrich MF, van der Weyden L, Prosser HM, Bradley A, Herz J, et al. Ectodomains of the LDL receptor-related proteins LRP1b and LRP4 have anchorage independent functions in vivo. PLoS One. 2010;5:e9960. doi: 10.1371/journal.pone.0009960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drogemuller C, Leeb T, Harlizius B, Tammen I, Distl O, et al. Congenital syndactyly in cattle: four novel mutations in the low density lipoprotein receptor-related protein 4 gene (LRP4) BMC Genet. 2007;8:5. doi: 10.1186/1471-2156-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duchesne A, Gautier M, Chadi S, Grohs C, Floriot S, et al. Identification of a doublet missense substitution in the bovine LRP4 gene as a candidate causal mutation for syndactyly in Holstein cattle. Genomics. 2006;88:610–21. doi: 10.1016/j.ygeno.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Durakoglugil MS, Chen Y, White CL, Kavalali ET, Herz J. Reelin signaling antagonizes beta-amyloid at the synapse. Proc Natl Acad Sci U S A. 2009;106:15938–43. doi: 10.1073/pnas.0908176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eaton S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat Rev Mol Cell Biol. 2008;9:437–45. doi: 10.1038/nrm2414. [DOI] [PubMed] [Google Scholar]
- 63.Eisenmann DM. Wnt signaling. WormBook. 2005:1–17. doi: 10.1895/wormbook.1.7.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, et al. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci U S A. 1997;94:4647–52. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng L, Allen NS, Simo S, Cooper JA. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 2007;21:2717–30. doi: 10.1101/gad.1604207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1995;92:8453–7. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuentealba RA, Barria MI, Lee J, Cam J, Araya C, et al. ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP) endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity. Mol Neurodegener. 2007;2:14. doi: 10.1186/1750-1326-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol. 1995;129:1403–10. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Bases of Inherited Disease. Vol. 62. New York: McGraw-Hill Publishing Company; 1995. pp. 1981–2030. [Google Scholar]
- 70.Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, et al. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–24. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 71.Gotzmann J, Fischer AN, Zojer M, Mikula M, Proell V, et al. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene. 2006;25:3170–85. doi: 10.1038/sj.onc.1209083. [DOI] [PubMed] [Google Scholar]
- 72.Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–26. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, et al. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–75. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, et al. Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci. 2002;22:5797–802. doi: 10.1523/JNEUROSCI.22-14-05797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276:15498–503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- 76.Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–62. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 77.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 78.Havel RJ, Kita T, Kotite L, Kane JP, Hamilton RL, et al. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis. 1982;2:467–74. doi: 10.1161/01.atv.2.6.467. [DOI] [PubMed] [Google Scholar]
- 79.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–41. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He X, Cooley K, Chung CH, Dashti N, Tang J. Apolipoprotein receptor 2 and X11 alpha/beta mediate apolipoprotein E-induced endocytosis of amyloid-beta precursor protein and beta-secretase, leading to amyloid-beta production. J Neurosci. 2007;27:4052–60. doi: 10.1523/JNEUROSCI.3993-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heinrich C, Nitta N, Flubacher A, Muller M, Fahrner A, et al. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci. 2006;26:4701–13. doi: 10.1523/JNEUROSCI.5516-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herrlich A, Daub H, Knebel A, Herrlich P, Ullrich A, et al. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc Natl Acad Sci U S A. 1998;95:8985–90. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herrlich A, Klinman E, Fu J, Sadegh C, Lodish H. Ectodomain cleavage of the EGF ligands HB-EGF, neuregulin1-{beta}, and TGF-{alpha} is specifically triggered by different stimuli and involves different PKC isoenzymes. FASEB J. 2008 doi: 10.1096/fj.08-113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herrmann MA, Hay ID, Bartelt DH, Jr, Ritland SR, Dahl RJ, et al. Cytogenetic and molecular genetic studies of follicular and papillary thyroid cancers. J Clin Invest. 1991;88:1596–604. doi: 10.1172/JCI115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herz J, Beffert U. Apolipoprotein E receptors: linking brain development and Alzheimer’s disease. Nat Rev Neurosci. 2000;1:51–8. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- 86.Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–34. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 87.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–9. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 88.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–21. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 89.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, et al. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–27. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herz J, Hui DY. Lipoprotein receptors in the vascular wall. Curr Opin Lipidol. 2004;15:175–81. doi: 10.1097/00041433-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 91.Herz J, Qiu SQ, Oesterle A, DeSilva HV, Shafi S, et al. Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1995;92:4611–5. doi: 10.1073/pnas.92.10.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–84. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–9. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 94.Hill KE, Zhou J, McMahan WJ, Motley AK, Atkins JF, et al. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem. 2003;278:13640–6. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 95.Hoe HS, Magill LA, Guenette S, Fu Z, Vicini S, et al. FE65 interaction with the ApoE receptor ApoEr2. J Biol Chem. 2006;281:24521–30. doi: 10.1074/jbc.M600728200. [DOI] [PubMed] [Google Scholar]
- 96.Hoe HS, Pocivavsek A, Chakraborty G, Fu Z, Vicini S, et al. Apolipoprotein E receptor 2 interactions with the N-methyl-D-aspartate receptor. J Biol Chem. 2006;281:3425–31. doi: 10.1074/jbc.M509380200. [DOI] [PubMed] [Google Scholar]
- 97.Hoe HS, Wessner D, Beffert U, Becker AG, Matsuoka Y, et al. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol Cell Biol. 2005;25:9259–68. doi: 10.1128/MCB.25.21.9259-9268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hofmann SM, Zhou L, Perez-Tilve D, Greer T, Grant E, et al. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J Clin Invest. 2007;117:3271–82. doi: 10.1172/JCI31929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horiuchi D, Collins CA, Bhat P, Barkus RV, Diantonio A, et al. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17:1313–7. doi: 10.1016/j.cub.2007.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 1997;16:121–32. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine [corrected] phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–8. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999;19:5179–88. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang SS, Huang JS. TGF-beta control of cell proliferation. J Cell Biochem. 2005;96:447–62. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- 104.Huang SS, Ling TY, Tseng WF, Huang YH, Tang FM, et al. Cellular growth inhibition by IGFBP-3 and TGF-beta1 requires LRP-1. FASEB J. 2003;17:2068–81. doi: 10.1096/fj.03-0256com. [DOI] [PubMed] [Google Scholar]
- 105.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–93. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: Tests of the hypothesis in knockout mice lacking the LDL receptor, apoE, and both proteins. Proc Natl Acad Sci USA. 1994;91:4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishigami M, Swertfeger DK, Granholm NA, Hui DY. Apolipoprotein E inhibits platelet-derived growth factor-induced vascular smooth muscle cell migration and proliferation by suppressing signal transduction and preventing cell entry to G1 phase. J Biol Chem. 1998;273:20156–61. doi: 10.1074/jbc.273.32.20156. [DOI] [PubMed] [Google Scholar]
- 108.Ishigami M, Swertfeger DK, Hui MS, Granholm NA, Hui DY. Apolipoprotein E inhibition of vascular smooth muscle cell proliferation but not the inhibition of migration is mediated through activation of inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2000;20:1020–6. doi: 10.1161/01.atv.20.4.1020. [DOI] [PubMed] [Google Scholar]
- 109.Isosaka T, Hattori K, Yagi T. NMDA-receptor proteins are upregulated in the hippocampus of postnatal heterozygous reeler mice. Brain Res. 2006;1073–1074:11–9. doi: 10.1016/j.brainres.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 110.Jakubowska A, Gronwald J, Menkiszak J, Gorski B, Huzarski T, et al. BRCA1-associated breast and ovarian cancer risks in Poland: no association with commonly studied polymorphisms. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0390-5. [DOI] [PubMed] [Google Scholar]
- 111.Johnson EB, Hammer RE, Herz J. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum Mol Genet. 2005;14:3523–38. doi: 10.1093/hmg/ddi381. [DOI] [PubMed] [Google Scholar]
- 112.Johnson EB, Steffen DJ, Lynch KW, Herz J. Defective splicing of Megf7/Lrp4, a regulator of distal limb development, in autosomal recessive mulefoot disease. Genomics. 2006;88:600–9. doi: 10.1016/j.ygeno.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 113.Jossin Y. Neuronal migration and the role of reelin during early development of the cerebral cortex. Mol Neurobiol. 2004;30:225–51. doi: 10.1385/MN:30:3:225. [DOI] [PubMed] [Google Scholar]
- 114.Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol. 2007;27:7113–24. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, et al. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci. 2004;24:514–21. doi: 10.1523/JNEUROSCI.3408-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kamikura DM, Cooper JA. Lipoprotein receptors and a disabled family cytoplasmic adaptor protein regulate EGL-17/FGF export in C. elegans. Genes Dev. 2003;17:2798–811. doi: 10.1101/gad.1136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet. 2007;39:957–9. doi: 10.1038/ng2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karner CM, Dietrich MF, Johnson EB, Kappesser N, Tennert C, et al. Lrp4 regulates initiation of ureteric budding and is crucial for kidney formation--a mouse model for Cenani-Lenz syndrome. PLoS One. 2010;5:e10418. doi: 10.1371/journal.pone.0010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawauchi T, Hoshino M. Molecular pathways regulating cytoskeletal organization and morphological changes in migrating neurons. Dev Neurosci. 2008;30:36–46. doi: 10.1159/000109850. [DOI] [PubMed] [Google Scholar]
- 120.Kennedy NJ, Martin G, Ehrhardt AG, Cavanagh-Kyros J, Kuan CY, et al. Requirement of JIP scaffold proteins for NMDA-mediated signal transduction. Genes Dev. 2007;21:2336–46. doi: 10.1101/gad.1563107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–42. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, et al. Lrp4 Is a Receptor for Agrin and Forms a Complex with MuSK. Cell. 2008 doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kinoshita A, Whelan CM, Smith CJ, Mikhailenko I, Rebeck GW, et al. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J Neurosci. 2001;21:8354–61. doi: 10.1523/JNEUROSCI.21-21-08354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kita T, Brown MS, Bilheimer DW, Goldstein JL. Delayed clearance of very low density and intermediate density lipoproteins with enhanced conversion to low density lipoprotein in WHHL rabbits. Proc Natl Acad Sci U S A. 1982;79:5693–7. doi: 10.1073/pnas.79.18.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kita T, Goldstein JL, Brown MS, Watanabe Y, Hornick CA, et al. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1982;79:3623–7. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Knauer MF, Orlando RA, Glabe CG. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP) Brain Res. 1996;740:6–14. doi: 10.1016/s0006-8993(96)00711-1. [DOI] [PubMed] [Google Scholar]
- 127.Koch S, Strasser V, Hauser C, Fasching D, Brandes C, et al. A secreted soluble form of ApoE receptor 2 acts as a dominant-negative receptor and inhibits Reelin signaling. EMBO J. 2002;21:5996–6004. doi: 10.1093/emboj/cdf599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kounnas MZ, Chappell DA, Strickland DK, Argraves WS. Glycoprotein 330, a member of the low density lipoprotein receptor family, binds lipoprotein lipase in vitro. J Biol Chem. 1993;268:14176–81. [PubMed] [Google Scholar]
- 129.Kounnas MZ, Chappell DA, Wong H, Argraves WS, Strickland DK. The cellular internalization and degradation of hepatic lipase is mediated by low density lipoprotein receptor-related protein and requires cell surface proteoglycans. J Biol Chem. 1995;270:9307–12. doi: 10.1074/jbc.270.16.9307. [DOI] [PubMed] [Google Scholar]
- 130.Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–40. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 131.Kowal RC, Herz J, Weisgraber KH, Mahley RW, Brown MS, et al. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J Biol Chem. 1990;265:10771–9. [PubMed] [Google Scholar]
- 132.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–37. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 133.Kristensen T, Moestrup SK, Gliemann J, Bendtsen L, Sand O, et al. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the alpha 2-macroglobulin receptor. FEBS Lett. 1990;276:151–5. doi: 10.1016/0014-5793(90)80530-v. [DOI] [PubMed] [Google Scholar]
- 134.Laatsch A, Merkel M, Talmud PJ, Grewal T, Beisiegel U, et al. Insulin stimulates hepatic low density lipoprotein receptor-related protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 135.LaMarre J, Wollenberg GK, Gonias SL, Hayes MA. Cytokine binding and clearance properties of proteinase-activated alpha 2-macroglobulins. Lab Invest. 1991;65:3–14. [PubMed] [Google Scholar]
- 136.Lambert de Rouvroit C, Goffinet AM. Neuronal migration. Mech Dev. 2001;105:47–56. doi: 10.1016/s0925-4773(01)00396-3. [DOI] [PubMed] [Google Scholar]
- 137.Langbein S, Szakacs O, Wilhelm M, Sukosd F, Weber S, et al. Alteration of the LRP1B gene region is associated with high grade of urothelial cancer. Lab Invest. 2002;82:639–43. doi: 10.1038/labinvest.3780458. [DOI] [PubMed] [Google Scholar]
- 138.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–26. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 139.Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, et al. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003;17:247–9. doi: 10.1096/fj.02-0578fje. [DOI] [PubMed] [Google Scholar]
- 140.Levenson JM, Qiu S, Weeber EJ. The role of reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene. Biochim Biophys Acta. 2008;1779:422–31. doi: 10.1016/j.bbagrm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 141.Li Y, Lu W, Bu G. Striking differences of LDL receptor-related protein 1B expression in mouse and human. Biochem Biophys Res Commun. 2005;333:868–73. doi: 10.1016/j.bbrc.2005.05.170. [DOI] [PubMed] [Google Scholar]
- 142.Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275:17187–94. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 143.Li Y, Pawlik B, Elcioglu N, Aglan M, Kayserili H, et al. LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani-Lenz syndrome. Am J Hum Genet. 2010;86:696–706. doi: 10.1016/j.ajhg.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–7. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 146.Liu CX, Li Y, Obermoeller-McCormick LM, Schwartz AL, Bu G. The putative tumor suppressor LRP1B, a novel member of the low density lipoprotein (LDL) receptor family, exhibits both overlapping and distinct properties with the LDL receptor-related protein. J Biol Chem. 2001;276:28889–96. doi: 10.1074/jbc.M102727200. [DOI] [PubMed] [Google Scholar]
- 147.Liu CX, Musco S, Lisitsina NM, Forgacs E, Minna JD, et al. LRP-DIT, a putative endocytic receptor gene, is frequently inactivated in non-small cell lung cancer cell lines. Cancer Res. 2000;60:1961–7. [PubMed] [Google Scholar]
- 148.Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Q, Zhang J, Tran H, Verbeek MM, Reiss K, et al. LRP1 shedding in human brain: roles of ADAM10 and ADAM17. Mol Neurodegener. 2009;4:17. doi: 10.1186/1750-1326-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Loukinova E, Ranganathan S, Kuznetsov S, Gorlatova N, Migliorini M, et al. PDGF-induced tyrosine phosphorylation of the LDL receptor-related protein (LRP): Evidence for integrated interaction between LRP and the PDGF receptor. J Bio Chem. 2002 doi: 10.1074/jbc.M200427200. submitted. [DOI] [PubMed] [Google Scholar]
- 151.Luo K, Lodish HF. Signaling by chimeric erythropoietin-TGF-beta receptors: homodimerization of the cytoplasmic domain of the type I TGF-beta receptor and heterodimerization with the type II receptor are both required for intracellular signal transduction. EMBO J. 1996;15:4485–96. [PMC free article] [PubMed] [Google Scholar]
- 152.Ma QL, Galasko DR, Ringman JM, Vinters HV, Edland SD, et al. Reduction of SorLA/LR11, a sorting protein limiting beta-amyloid production, in Alzheimer disease cerebrospinal fluid. Arch Neurol. 2009;66:448–57. doi: 10.1001/archneurol.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mahley RW, Hui DY, Innerarity TL, Beisiegel U. Chylomicron remnant metabolism. Role of hepatic lipoprotein receptors in mediating uptake. Arteriosclerosis. 1989;9:I14–8. [PubMed] [Google Scholar]
- 154.Mahley RW, Hussain MM. Chylomicron and chylomicron remnant catabolism. Current Opinion in Lipidology. 1991;2:170–176. [Google Scholar]
- 155.Mahley RW, Hussain MM, Greenman B, Fisher M, Vogel T, et al. Intravenous infusion of apolipoprotein E accelerates clearance of plasma lipoprotins in rabbits. J Clin Invest. 1989;83:2125–2130. doi: 10.1172/JCI114126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mahley RW, Rall SC. In: Type III Hyperlipoproteinemia (Dysbetalipoproteinemia): The Role of Apolipoprotein E in Normal and Abnormal Lipoprotein Metabolism. Metabolic and Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Vol. 61. New York: McGraw-Hill Book Co; 1995. pp. 1953–1980. [Google Scholar]
- 157.Maity T, Fuse N, Beachy PA. Molecular mechanisms of Sonic hedgehog mutant effects in holoprosencephaly. Proc Natl Acad Sci U S A. 2005;102:17026–31. doi: 10.1073/pnas.0507848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marschang P, Brich J, Weeber EJ, Sweatt JD, Shelton JM, et al. Normal development and fertility of knockout mice lacking the tumor suppressor gene LRP1b suggest functional compensation by LRP1. Mol Cell Biol. 2004;24:3782–93. doi: 10.1128/MCB.24.9.3782-3793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martin AM, Kuhlmann C, Trossbach S, Jaeger S, Waldron E, et al. The functional role of the second NPXY motif of the LRP1 beta-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors. J Biol Chem. 2008;283:12004–13. doi: 10.1074/jbc.M707607200. [DOI] [PubMed] [Google Scholar]
- 160.Marzolo MP, Bu G. Lipoprotein receptors and cholesterol in APP trafficking and proteolytic processing, implications for Alzheimer’s disease. Semin Cell Dev Biol. 2009;20:191–200. doi: 10.1016/j.semcdb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Matsuki T, Pramatarova A, Howell BW. Reduction of Crk and CrkL expression blocks reelin-induced dendritogenesis. J Cell Sci. 2008;121:1869–75. doi: 10.1242/jcs.027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.May P, Bock HH, Herz J. Integration of endocytosis and signal transduction by lipoprotein receptors. Sci STKE. 2003;2003:PE12. doi: 10.1126/stke.2003.176.pe12. [DOI] [PubMed] [Google Scholar]
- 163.May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J Biol Chem. 2003;278:37386–92. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- 164.May P, Herz J, Bock HH. Molecular mechanisms of lipoprotein receptor signalling. Cell Mol Life Sci. 2005;62:2325–38. doi: 10.1007/s00018-005-5231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–43. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 166.May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24:8872–83. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mayer H, Duit S, Hauser C, Schneider WJ, Nimpf J. Reconstitution of the Reelin signaling pathway in fibroblasts demonstrates that Dab1 phosphorylation is independent of receptor localization in lipid rafts. Mol Cell Biol. 2006;26:19–27. doi: 10.1128/MCB.26.1.19-27.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McCarthy RA, Barth JL, Chintalapudi MR, Knaak C, Argraves WS. Megalin functions as an endocytic sonic hedgehog receptor. J Biol Chem. 2002;277:25660–7. doi: 10.1074/jbc.M201933200. [DOI] [PubMed] [Google Scholar]
- 169.McLoughlin DM, Miller CC. The FE65 proteins and Alzheimer’s disease. J Neurosci Res. 2008;86:744–54. doi: 10.1002/jnr.21532. [DOI] [PubMed] [Google Scholar]
- 170.Mikhailenko I, Kounnas MZ, Strickland DK. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor mediates the cellular internalization and degradation of thrombospondin. A process facilitated by cell-surface proteoglycans. J Biol Chem. 1995;270:9543–9. doi: 10.1074/jbc.270.16.9543. [DOI] [PubMed] [Google Scholar]
- 171.Mikhailenko I, Krylov D, Argraves KM, Roberts DD, Liau G, et al. Cellular internalization and degradation of thrombospondin-1 is mediated by the amino-terminal heparin binding domain (HBD). High affinity interaction of dimeric HBD with the low density lipoprotein receptor-related protein. J Biol Chem. 1997;272:6784–91. doi: 10.1074/jbc.272.10.6784. [DOI] [PubMed] [Google Scholar]
- 172.Muratoglu SC, Mikhailenko I, Newton C, Migliorini M, Strickland DK. The LDL receptor-related protein 1 (LRP1) forms a signaling complex with the PDGF receptor-{beta} in endosomes and regulates activation of the mapk pathway. J Biol Chem. doi: 10.1074/jbc.M109.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nakagawa T, Pimkhaokham A, Suzuki E, Omura K, Inazawa J, et al. Genetic or epigenetic silencing of low density lipoprotein receptor-related protein 1B expression in oral squamous cell carcinoma. Cancer Sci. 2006;97:1070–4. doi: 10.1111/j.1349-7006.2006.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Narita M, Bu G, Olins GM, Higuchi DA, Herz J, et al. Two receptor systems are involved in the plasma clearance of tissue factor pathway inhibitor in vivo. J Biol Chem. 1995;270:24800–4. doi: 10.1074/jbc.270.42.24800. [DOI] [PubMed] [Google Scholar]
- 175.Narita M, Holtzman DM, Schwartz AL, Bu G. Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem. 1997;69:1904–11. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- 176.Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- 177.Niu S, Yabut O, D’Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci. 2008;28:10339–48. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nykjaer A, Bengtsson-Olivecrona G, Lookene A, Moestrup SK, Petersen CM, et al. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds lipoprotein lipase and beta-migrating very low density lipoprotein associated with the lipase. J Biol Chem. 1993;268:15048–55. [PubMed] [Google Scholar]
- 179.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–15. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 180.Nykjaer A, Petersen CM, Moller B, Jensen PH, Moestrup SK, et al. Purified alpha 2-macroglobulin receptor/LDL receptor-related protein binds urokinase.plasminogen activator inhibitor type-1 complex. Evidence that the alpha 2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J Dairy Res. 1992;59:273–85. [PubMed] [Google Scholar]
- 181.Nykjaer A, Willnow TE. The low-density lipoprotein receptor gene family: a cellular Swiss army knife? Trends Cell Biol. 2002;12:273–80. doi: 10.1016/s0962-8924(02)02282-1. [DOI] [PubMed] [Google Scholar]
- 182.O’Grady P, Kuo MD, Baldassare JJ, Huang SS, Huang JS. Purification of a new type high molecular weight receptor (type V receptor) of transforming growth factor beta (TGF-beta) from bovine liver. Identification of the type V TGF-beta receptor in cultured cells. J Biol Chem. 1991;266:8583–9. [PubMed] [Google Scholar]
- 183.Oganesian A, Armstrong LC, Migliorini MM, Strickland DK, Bornstein P. Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells. Mol Biol Cell. 2008;19:563–71. doi: 10.1091/mbc.E07-07-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ohazama A, Johnson EB, Ota MS, Choi HY, Porntaveetus T, et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One. 2008;3:e4092. doi: 10.1371/journal.pone.0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Olson GE, Winfrey VP, Hill KE, Burk RF. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J Biol Chem. 2008;283:6854–60. doi: 10.1074/jbc.M709945200. [DOI] [PubMed] [Google Scholar]
- 186.Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J Biol Chem. 2007;282:12290–7. doi: 10.1074/jbc.M611403200. [DOI] [PubMed] [Google Scholar]
- 187.Orth K, Madison EL, Gething MJ, Sambrook JF, Herz J. Complexes of tissue-type plasminogen activator and its serpin inhibitor plasminogen-activator inhibitor type 1 are internalized by means of the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Proc Natl Acad Sci U S A. 1992;89:7422–6. doi: 10.1073/pnas.89.16.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ozcelik T, Akarsu N, Uz E, Caglayan S, Gulsuner S, et al. Mutations in the very low-density lipoprotein receptor VLDLR cause cerebellar hypoplasia and quadrupedal locomotion in humans. Proc Natl Acad Sci U S A. 2008;105:4232–6. doi: 10.1073/pnas.0710010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–73. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- 190.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 192.Peters MM, Hill KE, Burk RF, Weeber EJ. Altered hippocampus synaptic function in selenoprotein P deficient mice. Mol Neurodegener. 2006;1:12. doi: 10.1186/1750-1326-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pietrzik CU, Busse T, Merriam DE, Weggen S, Koo EH. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 2002;21:5691–700. doi: 10.1093/emboj/cdf568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 195.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–53. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 196.Pramatarova A, Ochalski PG, Chen K, Gropman A, Myers S, et al. Nck beta interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol Cell Biol. 2003;23:7210–21. doi: 10.1128/MCB.23.20.7210-7221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pramatarova A, Ochalski PG, Lee CH, Howell BW. Mouse disabled 1 regulates the nuclear position of neurons in a Drosophila eye model. Mol Cell Biol. 2006;26:1510–7. doi: 10.1128/MCB.26.4.1510-1517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Qiu S, Weeber EJ. Reelin signaling facilitates maturation of CA1 glutamatergic synapses. J Neurophysiol. 2007;97:2312–21. doi: 10.1152/jn.00869.2006. [DOI] [PubMed] [Google Scholar]
- 199.Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26:12943–55. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Qiu Z, Strickland DK, Hyman BT, Rebeck GW. Alpha2-macroglobulin enhances the clearance of endogenous soluble beta-amyloid peptide via low-density lipoprotein receptor-related protein in cortical neurons. J Neurochem. 1999;73:1393–8. doi: 10.1046/j.1471-4159.1999.0731393.x. [DOI] [PubMed] [Google Scholar]
- 201.Rahmatpanah FB, Carstens S, Guo J, Sjahputera O, Taylor KH, et al. Differential DNA methylation patterns of small B-cell lymphoma subclasses with different clinical behavior. Leukemia. 2006;20:1855–62. doi: 10.1038/sj.leu.2404345. [DOI] [PubMed] [Google Scholar]
- 202.Riddell DR, Sun XM, Stannard AK, Soutar AK, Owen JS. Localization of apolipoprotein E receptor 2 to caveolae in the plasma membrane. J Lipid Res. 2001;42:998–1002. [PubMed] [Google Scholar]
- 203.Roebroek AJ, Reekmans S, Lauwers A, Feyaerts N, Smeijers L, et al. Mutant Lrp1 knock-in mice generated by recombinase-mediated cassette exchange reveal differential importance of the NPXY motifs in the intracellular domain of LRP1 for normal fetal development. Mol Cell Biol. 2006;26:605–16. doi: 10.1128/MCB.26.2.605-616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101:689–95. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Roversi G, Pfundt R, Moroni RF, Magnani I, van Reijmersdal S, et al. Identification of novel genomic markers related to progression to glioblastoma through genomic profiling of 25 primary glioma cell lines. Oncogene. 2006;25:1571–83. doi: 10.1038/sj.onc.1209177. [DOI] [PubMed] [Google Scholar]
- 207.Rozanov DV, Hahn-Dantona E, Strickland DK, Strongin AY. The low density lipoprotein receptor-related protein LRP is regulated by membrane type-1 matrix metalloproteinase (MT1-MMP) proteolysis in malignant cells. J Biol Chem. 2004;279:4260–8. doi: 10.1074/jbc.M311569200. [DOI] [PubMed] [Google Scholar]
- 208.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–31. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–53. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schneider WJ. Vitellogenin receptors: oocyte-specific members of the low-density lipoprotein receptor supergene family. Int Rev Cytol. 1996;166:103–37. doi: 10.1016/s0074-7696(08)62507-3. [DOI] [PubMed] [Google Scholar]
- 211.Schneider WJ, Nimpf J. LDL receptor relatives at the crossroad of endocytosis and signaling. Cell Mol Life Sci. 2003;60:892–903. doi: 10.1007/s00018-003-2183-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schomburg L, Schweizer U, Holtmann B, Flohe L, Sendtner M, et al. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J. 2003;370:397–402. doi: 10.1042/BJ20021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schonbaum CP, Lee S, Mahowald AP. The Drosophila yolkless gene encodes a vitellogenin receptor belonging to the low density lipoprotein receptor superfamily. Proc Natl Acad Sci U S A. 1995;92:1485–9. doi: 10.1073/pnas.92.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Seki N, Bujo H, Jiang M, Tanaga K, Takahashi K, et al. LRP1B is a negative modulator of increased migration activity of intimal smooth muscle cells from rabbit aortic plaques. Biochem Biophys Res Commun. 2005;331:964–70. doi: 10.1016/j.bbrc.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 215.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 216.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shi Y, Mantuano E, Inoue G, Campana WM, Gonias SL. Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci Signal. 2009;2:ra18. doi: 10.1126/scisignal.2000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, et al. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–99. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shimano H, Ohsuga J, Shimada M, Namba Y, Gotoda T, et al. Inhibition of diet-induced atheroma formation in transgenic mice expressing apolipoprotein E in the arterial wall. J Clin Invest. 1995;95:469–76. doi: 10.1172/JCI117687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shiseki M, Kohno T, Nishikawa R, Sameshima Y, Mizoguchi H, et al. Frequent allelic losses on chromosomes 2q, 18q, and 22q in advanced non-small cell lung carcinoma. Cancer Res. 1994;54:5643–8. [PubMed] [Google Scholar]
- 221.Simon-Chazottes D, Tutois S, Kuehn M, Evans M, Bourgade F, et al. Mutations in the gene encoding the low-density lipoprotein receptor LRP4 cause abnormal limb development in the mouse. Genomics. 2006;87:673–7. doi: 10.1016/j.ygeno.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 222.Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, et al. Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro. J Neurosci. 2005;25:6127–36. doi: 10.1523/JNEUROSCI.1757-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–8. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 224.Song H, Li Y, Lee J, Schwartz AL, Bu G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009;69:879–86. doi: 10.1158/0008-5472.CAN-08-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sonoda I, Imoto I, Inoue J, Shibata T, Shimada Y, et al. Frequent silencing of low density lipoprotein receptor-related protein 1B (LRP1B) expression by genetic and epigenetic mechanisms in esophageal squamous cell carcinoma. Cancer Res. 2004;64:3741–7. doi: 10.1158/0008-5472.CAN-04-0172. [DOI] [PubMed] [Google Scholar]
- 226.Spoelgen R, Hammes A, Anzenberger U, Zechner D, Andersen OM, et al. LRP2/megalin is required for patterning of the ventral telencephalon. Development. 2005;132:405–14. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- 227.Spoelgen R, von Arnim CA, Thomas AV, Peltan ID, Koker M, et al. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J Neurosci. 2006;26:418–28. doi: 10.1523/JNEUROSCI.3882-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Springer TA. An extracellular beta-propeller module predicted in lipoprotein and scavenger receptors, tyrosine kinases, epidermal growth factor precursor, and extracellular matrix components. J Mol Biol. 1998;283:837–62. doi: 10.1006/jmbi.1998.2115. [DOI] [PubMed] [Google Scholar]
- 229.Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, et al. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and −2. J Biol Chem. 2000;275:25625–32. doi: 10.1074/jbc.M004119200. [DOI] [PubMed] [Google Scholar]
- 230.Stolt PC, Chen Y, Liu P, Bock HH, Blacklow SC, et al. Phosphoinositide binding by the disabled-1 PTB domain is necessary for membrane localization and Reelin signal transduction. J Biol Chem. 2005;280:9671–7. doi: 10.1074/jbc.M413356200. [DOI] [PubMed] [Google Scholar]
- 231.Stolt PC, Jeon H, Song HK, Herz J, Eck MJ, et al. Origins of peptide selectivity and phosphoinositide binding revealed by structures of disabled-1 PTB domain complexes. Structure. 2003;11:569–79. doi: 10.1016/s0969-2126(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 232.Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, et al. Receptor clustering is involved in Reelin signaling. Mol Cell Biol. 2004;24:1378–86. doi: 10.1128/MCB.24.3.1378-1386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, et al. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–4. [PubMed] [Google Scholar]
- 234.Strickland DK, Kounnas MZ, Argraves WS. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995;9:890–8. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]
- 235.Strickland DK, Medved L. Low-density lipoprotein receptor-related protein (LRP)-mediated clearance of activated blood coagulation co-factors and proteases: clearance mechanism or regulation? J Thromb Haemost. 2006;4:1484–6. doi: 10.1111/j.1538-7836.2006.01987.x. [DOI] [PubMed] [Google Scholar]
- 236.Strickland DK, Ranganathan S. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J Thromb Haemost. 2003;1:1663–70. doi: 10.1046/j.1538-7836.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- 237.Sun XM, Soutar AK. Expression in vitro of alternatively spliced variants of the messenger RNA for human apolipoprotein E receptor-2 identified in human tissues by ribonuclease protection assays. Eur J Biochem. 1999;262:230–9. doi: 10.1046/j.1432-1327.1999.00394.x. [DOI] [PubMed] [Google Scholar]
- 238.Swertfeger DK, Bu G, Hui DY. Low density lipoprotein receptor-related protein mediates apolipoprotein E inhibition of smooth muscle cell migration. J Biol Chem. 2002;277:4141–6. doi: 10.1074/jbc.M109124200. [DOI] [PubMed] [Google Scholar]
- 239.Takayama Y, May P, Anderson RG, Herz J. Low density lipoprotein receptor-related protein 1 (LRP1) controls endocytosis and c-CBL-mediated ubiquitination of the platelet-derived growth factor receptor beta (PDGFR beta) J Biol Chem. 2005;280:18504–10. doi: 10.1074/jbc.M410265200. [DOI] [PubMed] [Google Scholar]
- 240.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 241.Tanaga K, Bujo H, Zhu Y, Kanaki T, Hirayama S, et al. LRP1B attenuates the migration of smooth muscle cells by reducing membrane localization of urokinase and PDGF receptors. Arterioscler Thromb Vasc Biol. 2004;24:1422–8. doi: 10.1161/01.ATV.0000133607.80554.09. [DOI] [PubMed] [Google Scholar]
- 242.Taylor KH, Pena-Hernandez KE, Davis JW, Arthur GL, Duff DJ, et al. Large-scale CpG methylation analysis identifies novel candidate genes and reveals methylation hotspots in acute lymphoblastic leukemia. Cancer Res. 2007;67:2617–25. doi: 10.1158/0008-5472.CAN-06-3993. [DOI] [PubMed] [Google Scholar]
- 243.Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- 244.Tolia A, De Strooper B. Structure and function of gamma-secretase. Semin Cell Dev Biol. 2009;20:211–8. doi: 10.1016/j.semcdb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 245.Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 246.Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–60. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 247.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 248.Tueting P, Doueiri MS, Guidotti A, Davis JM, Costa E. Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev. 2006;30:1065–77. doi: 10.1016/j.neubiorev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 249.Turkmen S, Hoffmann K, Demirhan O, Aruoba D, Humphrey N, et al. Cerebellar hypoplasia, with quadrupedal locomotion, caused by mutations in the very low-density lipoprotein receptor gene. Eur J Hum Genet. 2008;16:1070–4. doi: 10.1038/ejhg.2008.73. [DOI] [PubMed] [Google Scholar]
- 250.Utsunomiya-Tate N, Kubo K, Tate S, Kainosho M, Katayama E, et al. Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody. Proc Natl Acad Sci U S A. 2000;97:9729–34. doi: 10.1073/pnas.160272497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Van Leuven F, Umans L, Lorent K, Hilliker C, Serneels L, et al. Molecular analysis of the human and mouse alpha 2M family. Ann N Y Acad Sci. 1994;737:163–71. doi: 10.1111/j.1749-6632.1994.tb44310.x. [DOI] [PubMed] [Google Scholar]
- 252.van Vlijmen BJ, Rohlmann A, Page ST, Bensadoun A, Bos IS, et al. An extrahepatic receptor-associated protein-sensitive mechanism is involved in the metabolism of triglyceride-rich lipoproteins. J Biol Chem. 1999;274:35219–26. doi: 10.1074/jbc.274.49.35219. [DOI] [PubMed] [Google Scholar]
- 253.Verges M, Bensadoun A, Herz J, Belcher JD, Havel RJ. Endocytosis of hepatic lipase and lipoprotein lipase into rat liver hepatocytes in vivo is mediated by the low density lipoprotein receptor-related protein. J Biol Chem. 2004;279:9030–6. doi: 10.1074/jbc.M312908200. [DOI] [PubMed] [Google Scholar]
- 254.Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol. 2001;152:959–70. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- 256.Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–52. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 257.Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–30. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 258.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 259.Willnow TE, Hammes A, Eaton S. Lipoproteins and their receptors in embryonic development: more than cholesterol clearance. Development. 2007;134:3239–49. doi: 10.1242/dev.004408. [DOI] [PubMed] [Google Scholar]
- 260.Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, et al. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci U S A. 1996;93:8460–4. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Willnow TE, Nykjaer A. Pathways for kidney-specific uptake of the steroid hormone 25-hydroxyvitamin D3. Curr Opin Lipidol. 2002;13:255–60. doi: 10.1097/00041433-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 262.Willnow TE, Nykjaer A, Herz J. Lipoprotein receptors: new roles for ancient proteins. Nat Cell Biol. 1999;1:E157–62. doi: 10.1038/14109. [DOI] [PubMed] [Google Scholar]
- 263.Winder SJ. Filopodia formation and Disabled degradation downstream of Reelin. Biochem J. 2004;384:e1–2. doi: 10.1042/BJ20041588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–7. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 265.Wyne KL, Pathak K, Seabra MC, Hobbs HH. Expression of the VLDL receptor in endothelial cells. Arterioscler Thromb Vasc Biol. 1996;16:407–15. doi: 10.1161/01.atv.16.3.407. [DOI] [PubMed] [Google Scholar]
- 266.Xu M, Arnaud L, Cooper JA. Both the phosphoinositide and receptor binding activities of Dab1 are required for Reelin-stimulated Dab1 tyrosine phosphorylation. Brain Res Mol Brain Res. 2005;139:300–5. doi: 10.1016/j.molbrainres.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yagyu H, Lutz EP, Kako Y, Marks S, Hu Y, et al. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem. 2002;277:10037–43. doi: 10.1074/jbc.M109966200. [DOI] [PubMed] [Google Scholar]
- 268.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, et al. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–40. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yochem J, Greenwald I. A gene for a low density lipoprotein receptor-related protein in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:4572–6. doi: 10.1073/pnas.90.10.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yochem J, Tuck S, Greenwald I, Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126:597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- 271.Yu H, Zhang W, Yancey PG, Koury MJ, Zhang Y, et al. Macrophage apolipoprotein E reduces atherosclerosis and prevents premature death in apolipoprotein E and scavenger receptor-class BI double-knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:150–6. doi: 10.1161/01.ATV.0000194096.89476.73. [DOI] [PubMed] [Google Scholar]
- 272.Zerbinatti CV, Wozniak DF, Cirrito J, Cam JA, Osaka H, et al. Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A. 2004;101:1075–80. doi: 10.1073/pnas.0305803101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem. 2004;279:33220–7. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- 274.Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, et al. LRP4 Serves as a Coreceptor of Agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhang G, Assadi AH, McNeil RS, Beffert U, Wynshaw-Boris A, et al. The Pafah1b complex interacts with the Reelin receptor VLDLR. PLoS One. 2007;2:e252. doi: 10.1371/journal.pone.0000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 277.Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhou L, Takayama Y, Boucher P, Tallquist MD, Herz J. LRP1 regulates architecture of the vascular wall by controlling PDGFRbeta-dependent phosphatidylinositol 3-kinase activation. PLoS One. 2009;4:e6922. doi: 10.1371/journal.pone.0006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zhu Y, Hui DY. Apolipoprotein E binding to low density lipoprotein receptor-related protein-1 inhibits cell migration via activation of cAMP-dependent protein kinase A. J Biol Chem. 2003;278:36257–63. doi: 10.1074/jbc.M303171200. [DOI] [PubMed] [Google Scholar]
- 280.Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, et al. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci. 2000;20:542–9. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zilberberg A, Yaniv A, Gazit A. The low density lipoprotein receptor-1, LRP1, interacts with the human frizzled-1 (HFz1) and down-regulates the canonical Wnt signaling pathway. J Biol Chem. 2004;279:17535–42. doi: 10.1074/jbc.M311292200. [DOI] [PubMed] [Google Scholar]
- 282.Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008;1:ra15. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]