Abstract
The clinical difference between brain tumors in adults and children is striking. Compared with adults, pediatric tumor types (mostly glial and neuronal) are more sensitive to adjuvant irradiation and chemotherapy. Pediatric tumors more often require craniospinal irradiation based on their propensity to disseminate within the neuraxis. The spectrum of side effects is broader in the child based on age and extent of treatment: radiation therapy brings increased risk of severe long-term sequelae affecting neurologic, endocrine, and cognitive function. In this review of glioma, ependymoma and medulloblastoma, we highlight the differences between adults and children including the higher incidence of spinal cord ependymoma and supratentorial high-grade glioma in the adult and a higher incidence of medulloblastoma in the child. With the exception of completely resected low-grade glioma, radiation therapy remains a standard of care for most patients. In some settings, the radiation oncologist should suggest further surgery or additional adjuvant therapy in an effort to optimize local tumor control. An effort is underway to better characterize adult and pediatric brain tumors biologically with an emphasis on improving our understanding of tumor genesis, malignant transformation, and some of the similarities and differences between tumor types and their response to conventional therapy.
INTRODUCTION
Brain tumors are uncommon and the etiology is generally unknown. The diagnosis cannot be anticipated and is shocking. Therapy is largely focused on local tumor control, combining the efforts of neurosurgery and radiation oncology. The use of chemotherapy is supplemental for most tumor types and considered critical for some, especially when it can be used to delay irradiation in the very young, lower the total dose required to achieve disease control, or work synergistically to improve outcome. Brain tumors are considered incurable by lay people who are often confused by the differences between primary and metastatic disease. Adults are typically diagnosed with high-grade brain tumors after middle age when acceptance of a poor prognosis by patient and caregiver may dampen pursuit of aggressive treatment. Brain tumors diagnosed in children provoke emotion but are fortunately cured with a high rate of success: side effects and not tumor control rates drive clinical trial designs in these patients.
Our understanding of brain tumor biology, prognosis after standard therapy, and the late effects of treatment is increasing. This information has been incorporated into clinical trials and frontline management for many but not all patients: the small number of cases and large variety of tumor types makes expert care inaccessible for most patients with primary brain tumors. The goal of this article is to explore the similarities and differences between the types of brain tumors diagnosed in children, adolescents, and adults focusing on medulloblastoma, ependymoma, and low-grade glioma. The focus will be to compare and contrast these tumors and their treatments across the ages and discuss areas of uncertainty to help the radiation oncologist who may not treat many of these patients understand and appreciate the nuances in this special aspect of our field. As the field of molecular tumor biology continues to expand, our appreciation of the similarities and differences between adults and children with the same histological diagnosis will be enhanced.
Epidemiology
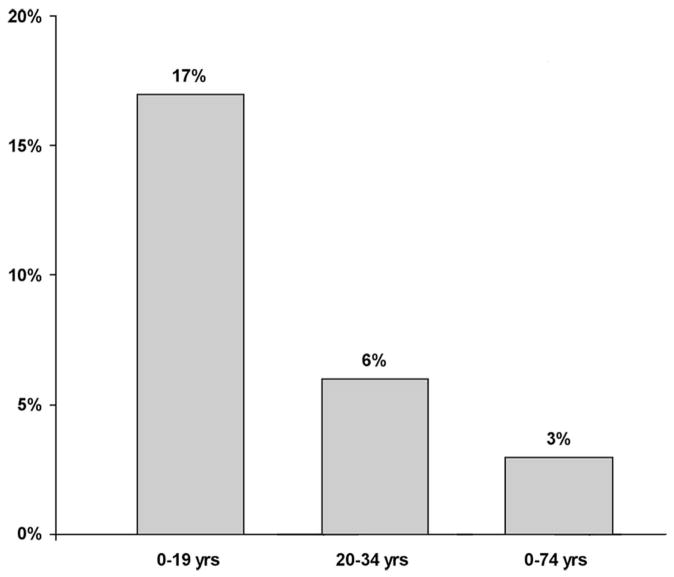
The distribution of tumors and tumor locations by age is a fascinating aspect of brain tumor epidemiology.1 For certain tumor types, such as medulloblastoma, ependymoma, and pilocytic astrocytoma, the incidence decreases with age (Fig. 1). Although the differences by age in the proportion of patients with ependymoma is much less than observed for medulloblastoma and low-grade glioma, it is important to note that posterior fossa ependymoma, supratentorial ependymoma, and spinal cord ependymoma are the most common in the very young, the adolescent, and in the adult, respectively. The most common site in young children is the infratentorial brain, and the proportion of tumors arising in the posterior fossa decreases with age (Fig. 2). In order of increasing age, the most common tumors (second most common in parenthesis) are the embryonal tumors, including medulloblastoma (pilocytic astrocytoma) in children ages 0–4 years; pilocytic astrocytoma (embryonal tumors) ages 5–9 years; malignant glioma ages 10–14 years; pituitary tumors which includes craniopharyngioma and germ cell tumors in ages 5–19 years; pituitary tumors (meningioma) ages 20–34 years, and meningioma (glioblastoma multiforme) ages 34–74 years. There are an estimated 20,500 primary brain tumors diagnosed each year in the US: 3750 cases occur in individuals age <19 years and 2870 cases in those age <15 years. It is important to recognize that the incidence of metastatic disease far exceeds the frequency of primary brain tumors in adults, whereas metastatic disease is comparatively uncommon in the pediatric age group.
Fig. 1.
Distribution of tumor types by age. Medulloblastoma includes all types of embryonal tumors, ependymoma includes differentiated and anaplastic subtypes, grade I glioma denotes pilocytic astrocytoma and grades I–IV glioma include patients with pilocytic astrocytoma, glioblastoma multiforme, oligodendroglioma and all other types of glioma except ependymoma.
Fig. 2.
Proportion of tumors involving the cerebellum by age group.
Pathobiology
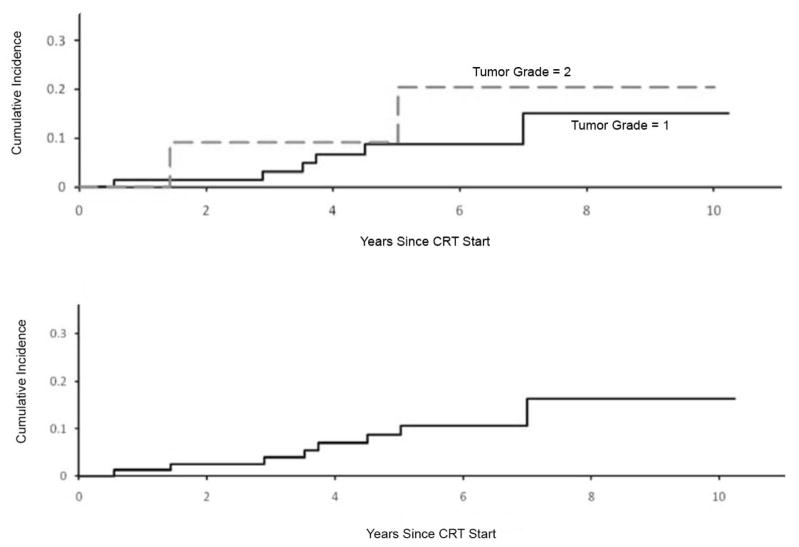
Medulloblastoma and low-grade gliomas are among the tumors featured in the recent revision of the WHO classification of brain tumors.2 An expanded view of medulloblastoma now includes large-cell medulloblastoma, anaplastic medulloblastoma, medulloblastoma with extensive nodularity, and desmoplastic/nodular medulloblastoma in order of decreasing risk of treatment failure using standard therapy. There is increased agreement that these subtypes will be used in the stratification for treatment, although controversies remain on how this will be accomplished at the institutional level. More controversial may be the reclassification of ganglioglioma as a WHO grade I tumor and the relatively new entity of pilomyxoid astrocytoma as WHO grade II. The latter is based on the interpretation of tumor aggressiveness in surgical neuropathology series where local tumor progression was not independent of optochiasmatic tumor location and subtotal resection.3 There are no reports that pilomyxoid astrocytoma has an increased rate of progression after definitive irradiation. Our recent report on disease control in pediatric low-grade glioma after definitive irradiation showed no difference in local failure by tumor grade using the 2007 criteria; however, on the basis of the previous classification system WHO grade II tumors had a statistically higher local failure rate after 54 Gy (Fig. 3).4 There has been no change in the classification of the most commonly encountered variants of ependymoma which continue to be controversial in their grading or agreement amongst neuropathologists.5 Clear cut differences in tumor control have been observed in large series of patients treated with definitive therapy that show anaplastic ependymoma to have a poor prognosis compared to its differentiated counterpart.6,7 Most will agree that clearly anaplastic and differentiated tumors may be graded with a high level of agreement. Unclear is the spectrum of tumors in between and the prognostic significance of focal anaplasia encountered in a largely differentiated ependymoma and a tumor with a high level of mitotic activity but lacking vascular proliferation or vice versa. For clinical trial purposes, a four-tiered system with obviously differentiated ependymoma, obviously anaplastic ependymoma, and two levels in between may be a solution with the lowest grade group considered for observation or adjuvant radiation therapy without chemotherapy.
Fig. 3.
Local tumor progression after 54Gy for pediatric low-grade glioma based on WHO 2003 (upper) and WHO 2007 (lower) classification criteria.
Similar to the challenges in classifying ependymomas in the pediatric setting, there is often discordance between pathologists in the classification of common adult tumors, such as meningiomas and malignant gliomas. Based on the WHO 2007 revisions, meningioma will continue to be classified as benign, atypical, and anaplastic. The distinction between atypical and anaplastic is often treacherous for pathologists. To improve the classification, the revised criteria now include brain invasion as a diagnostic feature of grade II meningioma. The data support brain invasion as a poor prognostic factor but with a lower mortality rate than those classified as anaplastic. Although the classification of malignant gliomas has been refined significantly during the last two decades, with clear-cut guidelines for distinguishing grade III lesions, such as anaplastic astrocytoma, from grade IV lesions, such as glioblastoma multiforme, recent molecular studies have demonstrated that these histological groups may actually encompass multiple entities with similar morphological features, but distinct pathways of tumorigenesis. It remains uncertain whether such factors influence the optimal therapeutic approach, and most likely will not alter the central role that radiotherapy plays in management. However, these molecular aspects may eventually play a major role in determining the optimal chemotherapeutic approach that should be coupled with irradiation.
Disease Presentation
Radiation oncologists are important gatekeepers in the treatment of patients with brain tumors. Critical evaluation of diagnostic information and the ability to choose from a variety of radiation delivery modalities places the radiation oncologist in a position to affect outcome. In the treatment of brain tumors, assessing the extent of resection prior to radiation therapy serves as a critical example of the radiation oncologist in this pivotal position. Because radiation therapy is generally accepted as a one-time treatment, following the standard of care in the evaluation of patients for radiation therapy and looking for opportunities to optimize disease control will ultimately save lives. Over the practice experience of any physician, one will find a patient where further surgery will lead to cure without irradiation or improve the ability of radiation therapy to achieve local tumor control. Accepting only timely and high-quality diagnostic information prior to treatment may improve the opportunity for cure when the results affect targeting (treatment volume) and the prescribed dose. A recent study for standard-risk (non-metastatic and limited residual disease) medulloblastoma showed the importance of quality in imaging and its impact on outcome.8 Children with inadequate neuraxis imaging prior to craniospinal irradiation had a lower event-free and overall survival than those who had proper imaging. The major pitfall for these patients was motion-susceptible spinal imaging but also included under-appreciation of residual or metastatic disease. Because patients with non-diagnosed metastatic or high-risk medulloblastoma were treated with low-dose craniospinal irradiation, they experienced relapse when higher doses of radiation therapy would have increased the probability of cure. This level of performance should be unacceptable to investigators in future trials.
The extent of resection prior to radiation therapy is a prognostic factor for most brain tumors. Radical resection should be considered for the potential advantage it may provide in many histological subgroups. Radiation oncologists should challenge their surgical colleagues and focus on a team approach to optimize the patient for radiation therapy. Second surgery prior to radiation therapy may often be accomplished with limited morbidity because of the elective nature of the procedure, capitalizing on the brain relaxation achieved by the initial debulking. Because the most technically demanding aspects of tumor resection are often undertaken at the end of a long operative procedure in a patient debilitated from the events leading to diagnosis, in the absence of a firm histological diagnosis, and in the setting of an edematous brain, it is sometimes preferable to leave residual disease, rather than “pushing” the initial resection. The decision regarding whether to attempt a second resection is influenced by the histology and the potential resectability of the residual disease. For certain tumors, a separate operative trajectory may help to achieve gross-total resection if the configuration of the residual tumor following the initial resection suggests that an alternate approach may improve accessibility.
In view of the importance of post-resection imaging to subsequent treatment planning, MR imaging is performed within 72 hours of surgery to acutely assess hemostasis, differentiate between ischemia and retraction injury, evaluate residual tumor, and (if it is amenable) further resection and improved later interpretation of residual enhancement from hemostatic products placed in the operative bed. Imaging immediately prior to treatment planning can be of great value, for it not only identifies patients for whom further resection might be appropriate, but it also improves the definition of the tumor bed and gross tumor volume.
The availability of sophisticated radiation therapy modalities does not substitute for critical case review and a team approach when considering local tumor control. For example, a clinical trial is not required to prove that a patient with incompletely resected ependymoma is likely to fare worse after highly sophisticated radiation therapy than the same patient who is referred for second surgery and achieves gross total resection prior to a more simple treatment. For uncommon tumors and in the setting of limited experience, case review by experts to assist in the decision making process should be undertaken prior to treatment. This is especially true for low-grade brain and spinal cord tumors when radiation therapy may not be indicated if complete tumor resection is achieved. Another critical example is found in the treatment of recurrent disease after definitive therapy. It may seem facile to perform radiosurgery rather than to resect residual or metastatic disease; however, this determination needs to be made on a case-by-case basis, depending on the characteristics of the lesion. Because the tumor and normal tissues may acutely react to such treatment and require prolonged and debilitating steroid treatment, plus repetitive imaging evaluation to differentiate between recurrence and reaction, a brief operation may prove to be an easier and safer path to follow in many instances.
Treatment and Outcome
Medulloblastoma
Medulloblastoma is first among CNS embryonal tumors and malignant brain tumors in children when it comes to understanding the efficacy of available therapy, tumor biology, and the acute and late effects of radiation therapy. After decades of successful treatment using craniospinal irradiation, significant late effects observed in long-term survivors led to trials hoping to reduce or eliminate radiation therapy. Chemotherapy has been successfully used to reduce radiation dose and volume for most patients.8 In selected low-risk patients, radiation therapy has been altogether eliminated. The implementation of risk-adapted therapy has relied on neuraxis staging using MR imaging and cytological analysis of cerebrospinal fluid. The future promises to increase the specificity of risk-adapted therapy by relying on histopathologic and molecular analyses that retrospectively have demonstrated the potential to identify low and high-risk populations while supplementing current staging procedures. Because salvage options are few for these patients, reducing therapy will require strict adherence to protocol guidelines and age will continue to be an overriding factor in the selection of therapy. Available data suggest that older standard-risk patients may benefit less than younger children from craniospinal dose reductions from current dosage levels, which are already reduced from historical levels. Accordingly, emphasis in recent studies has been placed on reducing the primary site target volume, which is the location for the most insidious and feared side effects of treatment by clinicians (necrosis and secondary malignancy) and parents (cognitive dysfunction and hearing loss). Future challenges will continue to be the treatment of the youngest children with high-stage disease who suffer tremendously from the only effective therapy: high-dose craniospinal irradiation. While children age <3 years with localized disease have benefited from the administration of multiagent chemotherapy as a way to avoid or delay craniospinal irradiation,9,10 and the approach of administering early focal irradiation to the primary site,11 the overall survival results remain below those achieved in older children. In part, this reflects that chemotherapy alone has had suboptimal results for eradicating sub-clinical or overt neuraxis disease spread. At present, the choice of optimal regimens for chemotherapy remains controversial, and even with intensive therapy a significant percentage of patients with unfavorable biological factors or obvious metastatic disease succumb to disease progression. In contrast, the prognosis is substantially better for patients with desmoplastic tumor histology.
The most common question asked by radiation oncologists is whether full posterior fossa irradiation remains the standard of care. The answer is that in the setting of post-irradiation cyclophosphamide-based dose-intensive chemotherapy, full posterior fossa irradiation is not required.12 The question remains unanswered for patients treated with the non-intensive chemotherapy regimen employed by most centers. The second most common question is lowering the craniospinal dose from 23.4 Gy to 18 Gy in the non-protocol setting. The answer is that a sufficient number of patients have not been treated using 18 Gy CSI and that the risk of neuraxis failure after 23.4 Gy remains an impediment to cure. Accordingly, this is a question that remains to be addressed in a phase III randomized study, as is currently being conducted within the Children’s Oncology Group.
When comparing and contrasting pediatric and adult medulloblastoma, the question often arises: what is standard therapy for the adult with medulloblastoma? Adult medulloblastoma represents <1% of adult intracranial tumors. The data in the literature are inconclusive concerning the differences in prognostic and treatment factors compared to children because of the small sample size and patient ascertainment biases in the adult series. It does appear that adults have a higher probability of having desmoplastic histology and a lateralized lesion compared to children, which would infer a better prognosis. A recent Surveillance, Epidemiology, and End Results (SEER) database analysis was recently reported that assessed survival rates and prognostic factors in adult medulloblastoma.13 Four-hundred fifty-four patients with adult medulloblastoma were diagnosed from 1973–2004 in the 17 regions covered by SEER. The overall median survival was 10.6 years and has improved during each of the last three decades. In a multivariate analysis, diagnosis at age <20 years, diagnosis after the 1980’s, gross total resection, and the use of craniospinal irradiation were favorable prognostic factors, whereas large cell histology was associated with poor survival. The extent of resection and full-dose craniospinal irradiation has been associated with improved survival in other smaller series.14,15 Adults appear to have a higher rate of “late” relapse and perhaps a higher rate of extracranial dissemination compared to their younger counterparts. While the role of pre-irradiation, concurrent, or post-irradiation chemotherapy has not been proven, a prospective trial in 36 patients suggest the treatment is well-tolerated and might be associated with an improvement in survival particularly in high-risk patients.16
Clinical and molecular staging of medulloblastoma should be realized for the next series of institutional and cooperative group trials based on a variety of markers that have undergone statistical validation. In a recent report, the amplification of MYC/MYCN and gains or losses in 6q or 17q was prognostic for overall survival in pediatric patients.17 Comparing adults to children, molecular markers divided the two types on the basis of 17q gain, MYC/MYCN amplification, and CDK6. For adults, 17q gains were less frequent but had similar prognostic value, in addition to minimal MYC/MYCN amplification but more amplification of CDK6. When not amplified, the latter two carried a better prognosis. Although 10q deletions appear to be similar in adults and children, they have not been prognostic for children but appear to carry a poor prognosis in adults.18,19
Ependymoma
Intracranial ependymoma is most often diagnosed in very young children. Investigators were keen to test the promise of conformal radiation therapy when it became available after decades of unsuccessful radiotherapy avoidance using surgery and conventional chemotherapy. The advent of conformal radiation therapy coincided with improved neuroimaging, neurosurgical navigation, and the systematic use of second surgery prior to irradiation for patients initially treated with incomplete resection. More effective surgery combined with high-dose (59.4 Gy) post-operative radiation therapy increased local tumor control rates and event-free and overall survival with evidence that the pattern of failure has now shifted from predominantly local to nearly evenly divided between local and distant failure.7,20 Although ependymoma is a tumor prone to neuraxis dissemination, a benefit to prophylactic craniospinal irradiation for patients with initially localized disease remains uncertain. The role of craniospinal irradiation has long been obscured by local tumor progression.21 These results raise the question about the potential usefulness of craniospinal irradiation in age-appropriate high-risk patients and the value of adjuvant chemotherapy in the setting of optimal local tumor control measures. Early and now longer-term results suggest that high-dose focal irradiation can be safely administered through systematic targeting, and that cognitive function is preserved. As a result, investigators have moved to include conformal therapy in the frontline management of children regardless of age. Concerns about the use of high-dose irradiation in these patients have been answered with late effects research showing the absence of severe negative sequelae.22–24 Future studies using smaller target volume margins and newer treatment modalities should further address these concerns.
Intracranial ependymoma represents less than 2% of primary tumors of adulthood. The largest review of prognostic factors and outcomes was published by a combined group of 24 French neurosurgical centers involving 152 patients.25 Pathology and radiographic studies were centrally reviewed in this report. The 5- and 10-year overall survival rates were 84.8 and 76.5%, respectively. On multivariate analysis, overall survival rates were associated with tumor grade (p<0.001), extent of resection (p=0.006), patient age (p=0.004) and performance status (p=0.03). The impact of localized radiotherapy had a positive impact on incompletely resected low-grade tumors. No retrospective review of adults with ependymoma has shown a benefit of the addition of chemotherapy, though there are no studies comparing combined radiation and temodar with radiation alone.26 From the available data, adults appear to have the same prognostic and treatment related factors as their pediatric counterparts. Complete gross resection and high-dose (≥54 Gy) localized radiotherapy offer the best chance for prolonged disease-free survival.27,28
Genetic differences in ependymoma are considered to be the key to successful risk classification for future clinical trials and highlight many of the differences between pediatric and adult ependymoma. Chromosomal gains of 1q are commonly associated with ependymoma in children, particularly the intracranial and anaplastic tumor types.29 Others have shown that genetic imbalances are predictive of tumor site, histologic subtype, and the age of the patient, suggesting further differences between adult and pediatric ependymoma.30
Low-Grade Glioma
Resection is the preferred approach for pediatric low-grade glioma followed by observation, unless adjuvant therapy is indicated based on symptoms or risk of further progression. Chemotherapy is the preferred approach for young children with unresectable residual tumors, based on concerns about the long-term effects of radiation therapy among patients where survivorship is high. Although most patients will eventually require radiation therapy, the goal of chemotherapy is to delay irradiation in the hope of minimizing the cognitive and endocrine sequelae. The indications for radiation therapy are likely to increase if research can identify those likely to experience progression during or after chemotherapy, or if the side effects of radiation therapy can be predicted based on clinical and biologic factors or mitigated using improved targeting approaches. The current concern amongst radiation oncologists who treat these patients is the risk of observation after chemotherapy in patients with central or optic pathway tumors: these patients risk loss of function when symptomatic tumor progression occurs in the absence of changes in neuroimaging and because the response to radiation therapy can be measured in months to years for some patients.
An effort is underway to better characterize adult and pediatric gliomas with an emphasis on improving our understanding of malignant transformations, similarities, and differences between low and high-grade gliomas in adults and children.31–33 This information is necessary to develop in vitro and pre-clinical models of low-grade glioma. There is limited information on the genetic abnormalities associated with low-grade glioma. As noted in a recent report, the genetic abnormalites observed in adult are rarely observed in children.34 Clinically, gliomas in children are distinct from their adult counterparts and these differences are supported by molecular pathobiology.35–37
Combined modality therapy
Despite their biological differences, the treatment of adults and children with high-grade or so-called malignant brain tumors appears to be converging. Combined modality therapy will soon be the standard for nearly all WHO grade III and IV tumors in adults and children. This applies to the most common high-grade or malignant CNS tumors in children, medulloblastoma and ependymoma, and the less common but deadly forms of glioma: anaplastic astrocytoma and glioblastoma multiforme.
For medulloblastoma, it is conceivable that the standard therapy for adults and children should be the same considering the risks of treatment effects in older children to be similar to their adult counterparts. For high-grade glioma, radiation therapy and temozolomide appear to be a standard of care for both adults38 and children despite less promising results in children in at least one trial.39 However, given the suboptimal survival results with this combination, a host of studies in both children and adults are examining molecularly targeted agents in conjunction with irradiation and conventional chemotherapy.
For ependymoma, investigators are eager to test the role of newer chemotherapy (anti-angiogenic) agents considering that conventional chemotherapy has to date shown limited independent benefit on survival. Although the potential role of conventional agents continues to be tested in the cooperative group setting for patients with newly diagnosed disease, the role of molecularly targeted therapeutic approaches is increasingly being examined in patients with progressive disease. For example, the Collaborative Ependymoma Research Network has recently launched separate trials for adults and children with recurrent ependymoma, both employing bevacizumab. The adult trial also includes temozolomide and the pediatric trial includes the tyrosine kinase inhibitor lapatinib.
Treatment Guidelines
Three-dimensional radiation therapy planning and image registration software permits the evaluation of local treatment failure relative to the targeted and irradiated volume. This type of analysis in cooperative group trials and for individual patients treated with non-protocol therapy increasingly shows that complex treatment guidelines with sequential volume reductions or non-specific normal tissue constraints may lead to marginal miss and treatment failure when the areas containing the greatest tumor burden do not receive the prescription dose. As we continue to test the safety of smaller clinical target volume margins and move to use smaller planning target volume margins with image-guidance, it is critical that (1) trials be designed for simplicity in targeting and dose prescription, (2) normal tissue tolerances be volumetrically defined and supported by peer reviewed data and (3) that total dose and uniformity be more consistently applied. Total dose and target volume margins will always be research questions in clinical trials for brain tumors; however, the standard of care should be clear. Relevant questions: what are the standard doses and target volumes for medulloblastoma, ependymoma, and low-grade glioma outside of a clinical trial?
Late Effects
Understanding where the risks of normal tissue irradiation are high or low can be used effectively in the planning process to minimize the risk or impact of side effects. Patients with brain tumors are vulnerable to wide ranging side effects from radiation therapy that are magnified in young children and enhanced by tumor and treatments preceding radiation therapy. Side effects range from treatable deficits that rarely impact long-term function to severe and debilitating side effects that result in the loss of functional independence. For most brain tumors that require radiation therapy, the competing risk of treatment failure exceeds the risk of the most severe side effects. Should radiation normal tissue tolerance guidelines be observed when there is little chance for cure?
Neurovascular effects of irradiation may results in motor, sensory, coordination, hearing or visual deficits and are most often attributable to parenchymal necrosis or infarction within the high-dose volume. The primary covariates are time after irradiation, radiation dose and patient age (Fig. 4).
Fig. 4.
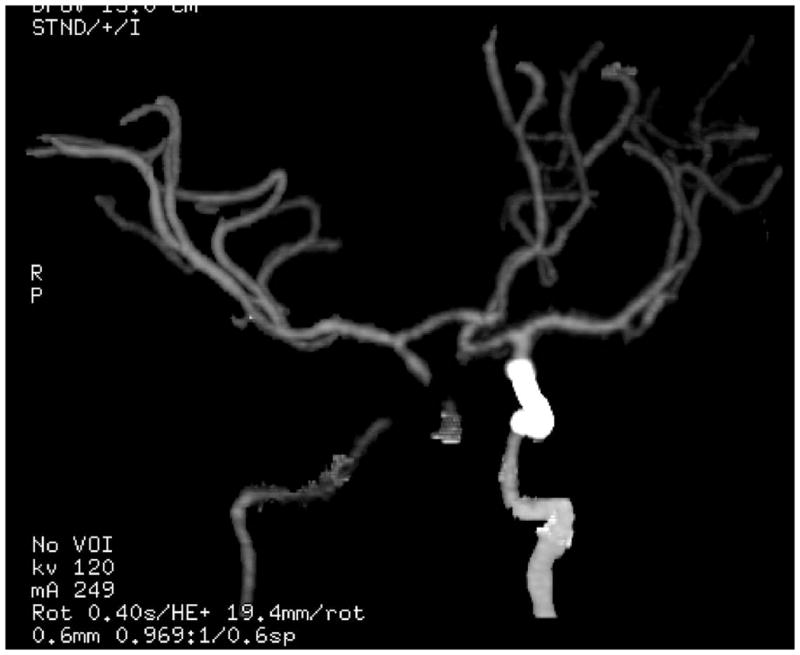
CT angiogram demonstrating right internal carotid stenosis and left internal carotid stent in a patient with craniopharyngioma after two separate courses of suprasellar external beam irradiation.
Endocrinopathies are strongly influenced by radiation dose to the hypothalamus, which is the organ most at risk for pituitary function. In the absence of pre-existing deficits and for a given dose to the hypothalamus, the order of incidence is growth hormone, thyroid hormone, glucocorticoid, and gonadotropin deficiency. Although some might consider hypothalamic obesity to be a radiation-related endocrinopathy, comparable control groups have yet to be identified. Finally, diabetes insipidus does not directly result from hypothalamic-pituitary axis (HPA) irradiation at the doses used in human clinical trials. When it occurs after irradiation, it often results from an acquired structural deficit of the HPA due to tumor expansion or brain shift. The major research question is no longer “which dose does an endocrine deficiency occur or as a surrogate?” but “why hormone replacement is required?” or rather, “why for a given high dose of radiation not all patients will develop an endocrine deficiency?” Fig. 5 shows the incidence of hormone replacement in a carefully followed cohort of children with centrally located low-grade glioma treated with doses >40 Gy. The graph is meant to show the incidence of hormone deficiencies after high-dose irradiation and that some patients do not develop deficiencies or require hormone replacement >5 years after treatment.40
Fig. 5.
Incidence of endocrinopathy after >40Gy irradiation of the hypothalamus.
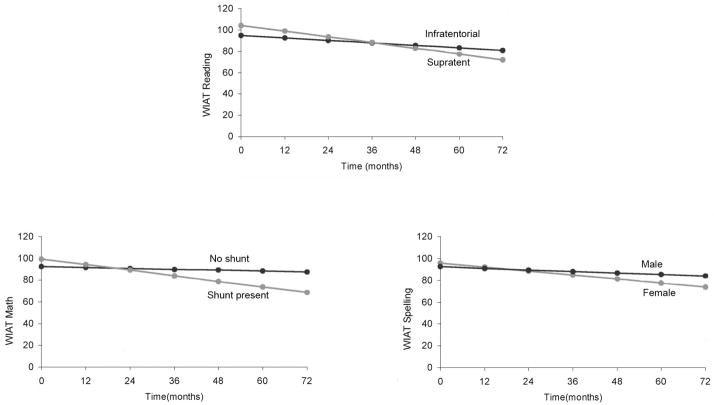
When evaluating adults or children for cognitive effects after irradiation, the consistent finding is that a significant proportion of patients have evidence of baseline deficits and that there are contributing factors other than radiation dose (Fig. 6). The late effects observed in children with medulloblastoma treated with radiation therapy are clearly attributable to radiation dose and volume. Effects are known to be exacerbated by age, but it is becoming clearer that pre-existing conditions, including surgical morbidity, are mitigating factors. Hydrocephalus and posterior fossa syndrome are among the most important factors. Children with medulloblastoma are prone to deficiencies in a broad range of domains. Whether radiotherapy volume reduction or dose reduction will improve outcome in these patients remains to be seen.
Fig. 6.
Reading, math, and spelling scores in children with medulloblastoma after craniospinal irradiation and by specific clinical factors of tumor location, CSF shunting, and sex.
CONCLUSION
Brain tumor is a well recognized diagnosis in pediatric oncology and these patients represent everything that is positive and negative about current treatment: increasing cure rates, the promise of new treatments, deleterious effects of therapy, and a lack of understanding about the impact of current treatment on long-term survivorship. The impact of long-term sequelae on functional outcome is also important in adults, particularly those with low-grade lesions, who may be expected to have long-term survival. Unlike low-grade gliomas in childhood, adult lesions commonly transition to higher histological grades years later while in the interim they have been capable of leading productive lives. In children, the value of terms such as benign and malignant may be blurred by histopathologic diagnosis, tumor location, or extent. Patients and caregivers need to be oriented to the fatal nature of the vast majority of tumors when untreated, and also the relative merits of all treatment options based on peer-reviewed data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CBTRUS 2008: Statistical Report: Primary Brain Tumors in the United States, 2000–2004. Published by the Central Brain Tumor Registry of the United States.
- 2.Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK. Surgical neuropathology update: a review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med. 2008;132:993–1007. doi: 10.5858/2008-132-993-SNUARO. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez C, Figarella-Branger D, Girard N. Pilocytic astrocytomas in children: prognostic factors—a retrospective study of 80 cases. Neurosurgery. 2003;53:544–553. doi: 10.1227/01.neu.0000079330.01541.6e. [DOI] [PubMed] [Google Scholar]
- 4.Merchant TE, Kun LE, Wu S. A phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. doi: 10.1200/JCO.2008.20.9494. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison D, Kocak M, Figarella-Branger D. Histopathological grading of intracranial pediatric ependymomas. Neuro Oncol. 2008;10:407. [Google Scholar]
- 6.Merchant TE, Jenkins JJ, Burger PC. Influence of tumor grade on time to progression after irradiation for localized ependymoma in children. Int J Radiat Oncol Biol Phys. 2002;53:52–57. doi: 10.1016/s0360-3016(01)02801-2. [DOI] [PubMed] [Google Scholar]
- 7.Merchant TE, Li C, Xiong X. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packer RJ, Gajjar A, Vezina G. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 9.Duffner PK, Horowitz ME, Krischer JP. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 10.Rutkowski S, Bode U, Deinlein F. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 11.Merchant TE, Kun LE, Krasin MJ. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2008;70:782–787. doi: 10.1016/j.ijrobp.2007.07.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashley D, Merchant TE, Zhou T. P9934: Systemic chemotherapy, second look surgery and conformal radiation therapy limited to the posterior fossa and primary site for children >8 months and <3 years with nonmetastatic medulloblastoma: A Children’s Oncology Group phase III study. Neuro Oncol. 2008;10:436–437. [Google Scholar]
- 13.Lai R. Survival of patients with adult medulloblastoma: a population-based study. Cancer. 2008;112:1568–1574. doi: 10.1002/cncr.23329. [DOI] [PubMed] [Google Scholar]
- 14.Chan AW, Tarbell NJ, Black PM. Adult medulloblastoma: prognostic factors and patterns of relapse. Neurosurgery. 2000;47:623–631. doi: 10.1097/00006123-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Abacioglu U, Uzel O, Sengoz M. Medulloblastoma in adults: treatment and prognostic factors. Int J Radiat Oncol Biol Phys. 2002;54:855–860. doi: 10.1016/s0360-3016(02)02986-3. [DOI] [PubMed] [Google Scholar]
- 16.Brandes AA, Ermani M, Amista P. The treatment of adults with medulloblastoma: a prospective trial. Int J Radiat Oncol Biol Phys. 2003;57:755–761. doi: 10.1016/s0360-3016(03)00643-6. [DOI] [PubMed] [Google Scholar]
- 17.Pfister S, Remke M, Benner A. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27:1627–1636. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 18.Mendrzyk F, Radlwimmer B, Joos S. Genomic and protein expression profiling identifies CDK6 as novel independent prognostic marker in medulloblastoma. J Clin Oncol. 2005;23:8853–8862. doi: 10.1200/JCO.2005.02.8589. [DOI] [PubMed] [Google Scholar]
- 19.Perry A, Miller CR, Gujrati M. Malignant gliomas with primitive neuroectodermal tumor-like components: a clinicopathologic and genetic study of 53 cases. Brain Pathol. 2009;19:81–90. doi: 10.1111/j.1750-3639.2008.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merchant TE. Three-dimensional conformal radiation therapy for ependymoma. Childs Nerv Sys. doi: 10.1007/s00381-009-0892-9. in press. [DOI] [PubMed] [Google Scholar]
- 21.Timmermann B, Kortmann RD, Kuhl J. Combined postoperative irradiation and chemotherapy for anaplastic ependymomas in childhood: results of the German prospective trials HIT 88/89 and HIT 91. Int J Radiat Oncol Biol Phys. 2000;46:287–295. doi: 10.1016/s0360-3016(99)00414-9. [DOI] [PubMed] [Google Scholar]
- 22.Brannon Morris E, Li C, Khan RB. Evolution of neurological impairment in pediatric infratentorial ependymoma patients. J Neurooncol. doi: 10.1007/s11060-009-9866-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conklin HM, Li C, Xiong X, Ogg RJ. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26:3965–3970. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant TE, Chitti RM, Li C. Factors associated with neurological recovery of brainstem function following post-operative conformal radiation therapy in infratentorial ependymoma. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2009.01.079. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metellus P, Barrie M, Figarella-Branger Multicentric French study on adult intracranial ependymomas: prognostic factors analysis and therapeutic considerations from a cohort of 152 patients. Brain. 2007;130:1338–1349. doi: 10.1093/brain/awm046. [DOI] [PubMed] [Google Scholar]
- 26.Ruda R, Gilbert M, Soffietti R. Ependymomas of the adult: molecular biology and treatment. Curr Opin Neurol. 2008;21:754–761. doi: 10.1097/WCO.0b013e328317efe8. [DOI] [PubMed] [Google Scholar]
- 27.Metellus P, Barrie M, FigarellaBranger D. Intracranial ependymomas in adult patients: retrospective analysis of 121 cases from the multicentric French study. Neurochirurgie. 2007;53:66–75. doi: 10.1016/j.neuchi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Rogers L, Pueschel J, Spetzler R. Is gross-total resection sufficient treatment for posterior fossa ependymomas? J Neurosurg. 2005;102:629–636. doi: 10.3171/jns.2005.102.4.0629. [DOI] [PubMed] [Google Scholar]
- 29.Mendrzyk F, Korshunov A, Benner A. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res. 2006;12:2070–2079. doi: 10.1158/1078-0432.CCR-05-2363. [DOI] [PubMed] [Google Scholar]
- 30.Jeuken JW, Sprenger SH, Gilhuis J. Correlation between localization, age, and chromosomal imbalances in ependymal tumours as detected by CGH. J Pathol. 2002;197:238–244. doi: 10.1002/path.1086. [DOI] [PubMed] [Google Scholar]
- 31.Broniscer A, Baker SJ, West AN. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 32.Pfister S, Janzarik WG, Remke M. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forshew T, Tatevossian RG, Lawson AR. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y, Pang JC, Ng HK. Pilocytic astrocytomas do not show most of the genetic changes commonly seen in diffuse astrocytomas. Histopathology. 2000;37:437–444. doi: 10.1046/j.1365-2559.2000.01005.x. [DOI] [PubMed] [Google Scholar]
- 35.Hambardzumyan D, Amankulor NM, Helmy KY. Modeling adult gliomas using RCAS/t-va technology. Transl Oncol. 2009;2:89–95. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faury D, Nantel A, Dunn SE. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007;25:1196–1208. doi: 10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- 37.Eckert A, Kloor M, Giersch A. Microsatellite instability in pediatric and adult high-grade gliomas. Brain Pathol. 2007;17:146–150. doi: 10.1111/j.1750-3639.2007.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins JM, Marshall DT, Patel S. High-dose radiotherapy to 78 Gy with or without temozolomide for high grade gliomas. J Neurooncol. doi: 10.1007/s11060-008-9779-y. (in press) [DOI] [PubMed] [Google Scholar]
- 39.Broniscer A, Chintagumpala M, Fouladi M. Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. J Neurooncol. 2006;76:313–319. doi: 10.1007/s11060-005-7409-5. [DOI] [PubMed] [Google Scholar]
- 40.Merchant TE, Conklin HM, Wu S. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. doi: 10.1200/JCO.2008.21.2738. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]