Abstract
Mutations in the gene encoding glucocerebrosidase (GBA), the enzyme deficient in the lysosomal storage disorder Gaucher disease, are associated with the development of Parkinson disease and other Lewy body disorders. In fact, GBA variants are currently the most common genetic risk factor associated with parkinsonism, and identified subjects with Parkinson disease are more than five times more likely to carry mutations in GBA. The mechanisms underlying this association are not known, but proposed theories include enhanced protein aggregation, alterations in lipid levels, and autophagy-lysosomal dysfunction promoting the retention of undegraded proteins. We review the genetic studies linking GBA to parkinsonism, as well as several of the mechanisms postulated to explain the association of GBA mutations and the synucleinopathies, which demonstrate how studies of a rare mendelian disease may provide insights into our understanding of a common complex disorder.
Keywords: Parkinson disease, Gaucher disease, Alpha synuclein, Glucoceerebrosidase, Lew body disorders
Introduction
Of the many genes now associated with parkinsonism, mutations in the gene encoding for glucocerebrosidase (GBA) are one of the most frequent genetic determinants known in Parkinson disease (PD). The surprising discovery of a link between a rare Mendelian disorder, Gaucher disease (GD), and the common complex disorders PD and Lewy body dementia (LBD) provides new opportunities to evaluate different pathways and mechanisms relevant to the pathogenesis of both disorders.
Glucocerebrosidase is a lysosomal enzyme that catalyzes the hydrolysis of glucocerebroside, a membrane glycolipid, to ceramide and glucose [1]. The gene for glucocerebrosidase (GBA, Online Mendelian Inheritance in Man [OMIM] #606463) was mapped to 1q21-22, cloned and sequenced more than two decades ago [2–4]. It is located in a very gene-rich area, where seven genes and two pseudogenes are found within 85 kb of sequence [5]. The GBA gene encompasses 7.6 kb of sequence and includes 11 exons and ten introns [3]. The glucocerebrosidase pseudogene (GBAP) is a highly homologous 5.7 kb sequence located 16 kb downstream, with the same organization of exons and introns as GBA [3, 6].
Mutations in GBA result in defective glucocerebrosidase, the enzyme implicated in the most common lipidosis, GD (type 1, OMIM#230800; type 2, OMIM#230900; type 3, OMIM#2301000). First described in 1882, GD presents with hepatosplenomegaly, anemia, thrombocytopenia, bone involvement, and neurologic symptoms (types 2 and 3) [7]. Mutations in GBA include point mutations, insertions, deletions, frameshift changes, splice site alterations, and recombinant alleles reported in GD patients of different ethnicities [8•]. Patients with identical GBA genotypes may exhibit considerable clinical heterogeneity. To date, there are approximately 300 mutations and polymorphisms in the GBA gene that have been identified in patients with varying presentations of GD [8•]. Consequently, it is still unclear how identical mutations can present such vast clinical variability [9, 10].
Among the many phenotypes associated with GD are patients presenting with parkinsonian symptoms. During past decades, several case reports and case studies describing such patients appeared in the literature [11–13]. Later, it was noted that some family members of patients with GD who carry GBA mutations had an increased susceptibility for developing PD [14, 15]. LBD also was reported to occur with increased frequency in GD patients and carriers [16]. In recent years, multiple independent studies from around the world have supported the original work identifying an association between GBA mutations and development of Lewy body disorders.
Studies in Patients with Gaucher Disease and Parkinsonism
In one of the first attempts to explore GBA-associated parkinsonism, different molecular approaches were used to evaluate a 48-year-old patient presenting with GD and atypical parkinsonism, including direct sequencing of GBA; a nearby gene, metaxin 1; and other known Parkinson genes (α-synuclein and parkin) using multiple techniques [17]. Gene sequencing and Southern blotting were used to evaluate the GBA locus and demonstrated that the patient had genotype L444P/D409H and also carried a duplication encompassing the GBA pseudogene and metaxin 1. Northern and Western blotting performed to assess GBA expression and protein levels revealed low expression and trace levels of protein. The authors then assembled a series of 17 patients with GD who developed parkinsonism [18]. Molecular analysis of these cases revealed 12 different GBA genotypes, although N370S was the most frequent GBA mutation found. No mutations were identified in the exonic regions of parkin and α-synuclein genes.
A larger study of 57 subjects was instrumental in establishing the association between the two disorders. The study searched for GBA mutations in brain samples from patients diagnosed with PD. Based on sequencing analyses, GBA alterations were detected in 12 samples (21%), including those from eight individuals with mutations (N370S, L444P, K198T, R329C) and four with alterations (T369M, E326K) [19] that occur with similar frequency in patients and controls and are considered GBA polymorphisms [20]. A group from northern Israel then explored the association between GBA mutations and PD by screening 99 Ashkenazi patients with idiopathic PD and 1,543 healthy Ashkenazi Jews for six GBA mutations (N370S, L444P, c.84dupG, IVS+1A>G, V394L, and R496H) commonly found among Ashkenazi Jews. The researchers identified these mutations in 31.3% of patients with PD versus 6.2% of healthy controls (P<0.001) [21].
Subsequently, various cohorts of patients with PD have been screened for common GBA mutations (most often N370S and L444P) or by sequencing the entire GBA gene [21–28, 29•, 30, 31•, 32, 33, 34•, 35, 36••]. These studies reported a higher frequency of GBA mutations among both Ashkenazi Jewish and non-Jewish populations with PD than in matched controls. Among different research centers, the frequency of heterozygous GBA mutations varied between 10.7% and 31.3% among Ashkenazi Jewish cases with PD and between 2.3% and 9.4% in non-Ashkenazi Jewish patients (Table 1). Some studies reported a lower frequency of GBA mutations [25], whereas others had statistically insignificant results [23, 28]. This discrepancy may be attributed to the specific mutations screened for in the respective studies, because it is now known that the mutation frequency differs greatly among different ethnic groups [37]. For example, among Ashkenazi Jews, the carrier frequency for GBA mutations is between 1 in 14 and 1 in 18, and the N370S variant accounts for 70% of the mutant alleles [1]. On the other hand, GBA mutations are found in less than 1% of the population in other ethnic groups, in which a greater number of different mutations are found. Consequently, screening for the GBA mutations common in Ashkenazi Jews is not a reliable strategy for other ethnic cohorts. Moreover, the N370S mutation may not be present in the Asian population, as it has not been identified in patients with Gaucher disease of East Asian ancestry [34•, 38].
Table 1.
Frequency of GBA mutation carriers in individual Parkinson cohorts
| Study | Population | Screened mutations | Sample size, n |
Carrier frequency, % |
P value | Most common variant(s) | ||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||||
| Lwin et al. 2004 [19] | Mixed | Whole GBA scanning | 57 | – | 21 | – | – | N370S |
| Aharon-Peretz et al. 2004 [21] | Ashkenazi Jews | N370S, L444P, c.84dupG, IVS2 +1A>G, V394L, R496H | 99 | 1543 | 31.3 | 6.2 | <0.0001 | N370S |
| Clark et al. 2005 [79] | Ashkenazi Jews | N370S | 160 | 92 | 10.7 | 4.3 | 0.2 | N370S |
| Sato et al. 2005 [22] | Caucasians (Canadian origin) | N370S, L444P, IVS2+1A>G, K198T, R329C, c.84dupG, RecNcil | 88 | 122 | 5.68 | 0.8 | 0.48 | RecNcil |
| Toft et al. 2006 [23] | Norwegian | N370S, L444P | 311 | 474 | 2.3 | 1.7 | 0.58 | N370S |
| Eblan et al. 2006 [24] | Mixed (no Jewish) | Whole GBA scanning | 33 | 31 | 12 | 3.2 | – | RecNcil, L444P |
| Tan et al. 2007 [25] | Chinese | L444P, N370S | 331 | 347 | 2.4 | 0 | 0.06 | L444P |
| Ziegler et al. 2007 [26] | Chinese | Whole GBA scanning | 92 | 92 | 4.3 | 1.1 | – | L444P |
| Wu et al. 2007 [28] | Taiwanese | L444P, RecNcil, R120W | 518 | 339 | 3.1 | 1.2 | 0.07 | L444P, RecNcil |
| Clark et al. 2007 [27] | Mixed (64% Jewish) | Whole GBA scanning | 278 (178) | 179 | 13.7 | 4.5 | – | N370S, c.84dupG |
| De Marco et al. 2008 [80] | Italian | N370S, L444P | 395 | 483 | 2.8 | 0.2 | 0.0018 | L444P |
| Spitz et al. 2008 [30] | Brazilian | N370S, L444P, G377S | 65 | 267 | 3 | 0 | 0.037 | L444P |
| Mata et al. 2008 [41] | Mixed | N370S, L444P | 721 | 554 | 2.9 | 0.4 | <0.001 | N370S, L444P |
| Gan-Or et al. 2008 [29•] | Ashkenazi Jews | N370S, R496H, L444P, c.84dupG, IVS2+1, V394L, D409H, RecTL | 420 | 333 | 17.9 | 4.2 | <0.0001 | N370S |
| Bras et al. 2009 [35] | Portuguese | Whole GBA scanning | 230 | 430 | 6.1 | 0.7 | – | N370S, N396T |
| Kalinderi et al. 2009 [33] | Greek | Whole GBA scanning | 172 | 132 | 4.7 | 0.8 | 0.048 | H255Q, L444P |
| Nichols et al. 2009 [32] | Mixed (<10% Jewish) | N370S, T369M, L444P, IVS6, IVS10 E326K, K303K, R262H, RecNcil | 1325 | 359 | 12.6 | 5.3 | – | E326K, T369M, N370S, L444P |
| Neumann et al. 2009 [31•] | British | Whole GBA scanning | 790 | 257 | 4.18 | 1.17 | 0.01 | L444P, N370S |
| Mitsui et al. 2009 [34•] | Japanese | Whole GBA scanning | 534 | 544 | 9.4 | 0.037 | 6.9 × 10–14 | R120W, RecNcil |
Most of the published studies specifically investigated sporadic PD. Recently, Nichols et al. [32] studied GBA alterations in familial PD. First, they screened all GBA exons in one proband from 96 selected families with cases of PD. Nine GBA alterations were found in 21 cases, including four novel alleles (21.8%). The selection of these patients was based on the LOD (logarithmic odds) scores for microsatellite markers close to the GBA locus. The authors then checked 1,325 familial PD cases from 566 families and 359 controls for the variations identified in the first 96 families, and detected 161 carriers (12.2%) in these patients versus 5.3% of controls. Although this study is important because it demonstrates the increased frequency of GBA mutations in familial PD cases, the authors also included subjects with E326K and T369M as mutation carriers. After removing subjects carrying these polymorphisms, the mutation rate for the remaining screened mutant alleles was 4.1% in cases versus 1.1% in controls, consistent with other studies screening for only a limited group of GBA mutations. However, in another recent study investigating familial Parkinson cases from Japan, GBA mutations were found in eight of 34 complex families and in five of 34 probands (14.7%), and all affected family members had concordant variants. This study showed GBA variants to be associated with familial PD cases as well as sporadic disease [34•].
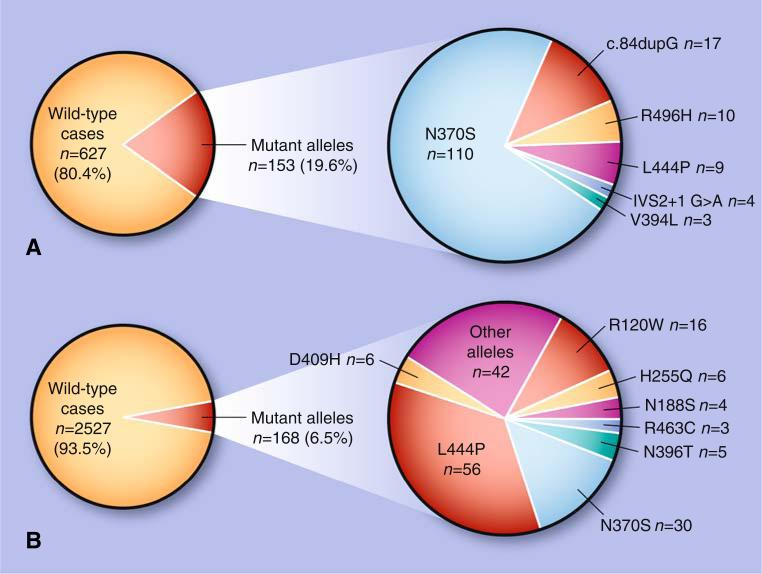
Although these initial cohort studies suggested that GBA mutations are a risk factor for parkinsonism, there were deficiencies in study design because of the lack of appropriate controls, mixed ethnicity of samples, screening for only a limited number of GBA mutations, and inaccurate definitions of GBA mutant alleles. To address these issues, a large multicenter cohort was assembled including patients from 16 different research centers, totaling 5,691 patients with PD (780 Ashkenazi Jews) and 4,898 controls (387 Ashkenazi Jews) [36]. Among Ashkenazi Jews, N370S and L444P were detected in 15% of patients and 3% of controls, whereas in patients with PD from other ethnic groups, the combined frequency of one of these two mutations was 3%. When full GBA sequencing was performed, 7% of non-Ashkenazi Jewish patients with PD were found to be mutation carriers. Overall, the odds ratio for carrying a GBA mutation in subjects with PD was 5.43 (95% CI, 3.89–7.57), rendering mutations in this gene a common risk factor for PD (Fig. 1). However, it is unknown whether any specific GBA mutations have a higher degree of association with development of PD, as the frequency of each mutated allele in different populations appeared to be a reflection of the carrier frequency in that specific population.
Fig. 1.
Frequency of GBA mutation carriers among patients with Parkinson disease and the distribution of different mutations in Ashkenazi Jews and non-Ashkenazi ethnic populations. A Twenty percent of Ashkenazi Jewish patients were found to carry a GBA mutation. B Among non-Ashkenazi Jewish patients for whom full sequencing was performed, 6.5% carried GBA mutations (from a meta-analysis by Sidransky et al. [36••]). The data in panel B are summarized based on whole GBA sequencing in three studies (Sidransky et al., 2009 [36••]; Neumann et al., 2009 [31•]; and Kalinderi et al., 2009 [33]). “Other alleles” include K(-27)R, K7E, R32H, R39H, R44C, R131C, R131S, D140H, R163Q, R163X, G193E, G193W, K198T, F213I, F216Y, R239C, R257Q, R262H, L268L, S271G, T323I, E326K, R329C, L336P, G344S, N370K, L371I, D380N, D380A, D443N, V460M, T482K, R496C, Q497R, c.1263-1317 del55bp, RecA456P, and RecNcil
GBA Mutations in Other Lewy Body Disorders
Because of the diversity of PD phenotypes encountered in these studies, investigators expanded their studies to determine whether GBA mutations are associated with other Lewy body disorders. The synucleinopathies include PD, LBD (encompassing dementia with Lewy bodies, Lewy body variant Alzheimer disease, and multiple system atrophy [MSA]). They are characterized by the deposition of inclusion bodies consisting primarily of fibrillated α-synuclein in the brainstem or cortical (limbic or neocortical) regions, as previously described [39, 40].
Initially, Goker-Alpan et al. [16] examined all GBA exons in DNA from brain samples of 75 autopsy cases with pathologically confirmed Lewy body disorders (28 PD, 35 LBD, and 12 MSA) and found GBA mutations in 23% of 35 cases with LBD, 4% of cases with PD, and none with MSA. Subsequently, screening for just N370S and L444P, Mata et al. [41] detected GBA alterations in 2 (3.5%) of 57 patients with dementia with Lewy bodies (P=0.045) compared with control subjects (0.4%). Farrer et al. [42] reported mutations in GBA in 6% of 50 brain samples from subjects with pathologically confirmed diffuse LBD. In a fourth study, Clark et al. [43] sequenced GBA in brain samples from 187 cases, including 95 individuals with LBD, 60 patients with Alzheimer disease, and 35 pathologically normal controls. They detected GBA mutations in 28% of cases with LBD, 10% of cases with Alzheimer disease, and 3% of control cases (P<0.001). Although GBA mutations were not found exclusively in cases with Lewy bodies, they showed that GBA carriers are significantly more likely to have Lewy bodies as a pathologic finding. Whereas the first study by Goker-Alpan et al. [16] had only 12 cases of MSA, a recent study from the United Kingdom screened all GBA exons from 108 pathologically confirmed cases of MSA and 257 controls. As with the first study, the authors failed to show any significant difference between cases and controls (P=0.66) [44]. Moreover, in a study from Poland of 66 patients with MSA, screening for mutations L444P and N370S yielded no mutation carriers [45].
Histopathologic Findings in GBA Mutation Carriers
Analyses of postmortem brain tissue from patients with GD who developed parkinsonism demonstrated classic PD pathology as well as Lewy bodies in hippocampal regions CA2 through CA4, which are areas affected in GD [18, 46, 47]. Kono et al. [48] used positron emission tomography to investigate underlying dopaminergic dysfunction in parkinsonism associated with GBA mutations and demonstrated presynaptic dopaminergic dysfunction characteristic of patients with PD. Neumann et al. [31•] showed that in addition to classic PD pathology, in PD samples carrying GBA mutations, α-synuclein inclusions were detectable in cortical areas corresponding to Braak stages 5 to 6. These findings are similar to LBD, suggesting that GBA carriers have more advanced neuropathologic disease. This result is similar to the study by Clark et al. [43] reporting GBA as a marker for cortical Lewy body pathologic findings, independent of histopathologic findings typical of Alzheimer disease.
Clinical Findings in GBA Mutation Carriers
Parallel to studies focusing on the mutation analysis of patients with parkinsonism, several groups focused on the associated clinical manifestations in GBA mutation carriers, including age of onset, motor symptoms, cognitive deficits, and response to l-dopa treatment. Although the earliest studies in patients with GD and PD reported early onset and treatment-refractory parkinsonism with prevalent cognitive decline [11, 18], subsequent publications show there is a broad spectrum of parkinsonian phenotypes among GBA mutation carriers, with variations in both age of onset and treatment response.
The first patients reported with GD and PD developed parkinsonian features in their 40s and 50s, an age earlier than that of most patients with sporadic Parkinson cases [14, 18]. In subsequent studies, the age of onset of motor impairment was reported to be 1.7–6 years earlier in GBA mutation carriers than in patients without GBA mutations [21, 25, 27, 28, 31•, 32, 33]. GBA mutations also have been associated with earlier-onset (<50 years) PD, in which the frequency of GBA mutations was found to be twice that of late-onset cases [27, 28, 32]. Even among cohorts in which PD developed before the age of 50 years, GBA mutation carriers had an earlier age of onset of clinical symptoms [27, 29•].
There also are conflicting reports with respect to the efficacy of l-dopa treatment in carriers of GBA mutations. While some reports described parkinsonian symptoms in patients with GD to be poorly responsive to l-dopa treatment [18], several others reported good or excellent response to treatment among GBA carriers [26, 28, 31•, 41, 49].
Overall, most studies could not detect any significant difference in clinical manifestations and disease progression between GBA carriers and controls applying the unified PD rating scale, Mini Mental State Examination, and Hoehn and Yahr clinical evaluation scale. Parkinsonism associated with GBA mutations appears to be phenotypically indistinguishable from sporadic PD. However, some studies found a higher frequency of cognitive decline [31•, 49], bradykinesia [50], and olfactory dysfunction [49] and a lower frequency of rigidity [27, 29•] to be associated with GBA mutations.
Generally it has been difficult to ascertain whether a correlation exists between the GBA genotype and the severity of parkinsonian manifestations. Although most studies reported N370S as the most common mutation among patients with PD, it has been suggested that more severe GBA mutations confer an increased risk of developing parkinsonism [29•]. Mutations such as R120W and L444P also were reported to confer an increased risk in cases of familial PD [34].
Mechanisms for the Association of GBA Mutations and the Synucleinopathies
The relationship between GBA mutations and the pathogenesis of PD and other Lewy body disorders is not clear. Most autosomal recessively inherited forms of PD, as in the case of parkin, DJ-1, PINK1, and ATP13A2, are considered to be the result of loss-of-function mutations and present with early-onset disease. On the other hand, autosomal dominant forms of PD generally are attributed to gain-of-function mutations, as seen with the genes for α-synuclein and LRRK2 [51]. In parkinsonism associated with GBA mutations, both gain- and loss-of-function theories have been postulated. The neuropathologic findings in patients with both GD and PD, as well as GBA carriers, appear to be typical of other synucleinopathies, suggesting that glucocerebrosidase may contribute to aggregation of α-synuclein through enhanced protein aggregation or as a consequence of glucocerebrosidase deficiency.
Evidence Implicating Lipid Alterations as a Loss of GBA Function
Glucocerebrosidase degrades glucocerebroside to ceramide. It previously was postulated that alterations in ceramide metabolism are associated with Lewy body formation. By reviewing the literature, Bras et al. [52] identified a group of genes involved in ceramide metabolism associated with Lewy body pathology. The role of lipid homeostasis and lysosomal function is an expanding field of research. Conradi et al. [53] identified the accumulation of lipofuscin spheroids in the cortical regions of brains from patients with neuropathic GD. These lipofuscin deposits, proposed to be ganglioside in origin, are undigested lysosomal material and indicate inefficient lysosomal degradation in GD.
It has been shown that α-synuclein binds to lipids in the plasma membrane and synaptic vesicles [54]. Although α-synuclein normally is an unstructured soluble protein, it can aggregate to form insoluble amyloid fibrils in pathologic conditions. The binding of lipids may be able to prevent the formation of the fibrillar forms of α-synuclein and the aggregation of this protein. It has been postulated that accumulation of polyunsaturated lipids that might accumulate as a result of deficient glucocerebrosidase activity may alter the sphingolipid composition of membranes and disrupt membrane binding of α-synuclein, thereby enhancing its accumulation in the cytoplasm [55–57].
Evidence of lysosomal dysfunction in another lysosomal lipid storage disease, Niemann-Pick type C, may represent an additional facet of the effect of unbalanced lipid homeostasis [56]. In this case, the dysregulation of lipid and cholesterol trafficking leads to a buildup in the lysosome and disrupts proteolysis, which may increase levels of undegraded proteins. In addition, Martinez et al. [57] showed that GM1 ganglioside lipid rafts interact with α-synuclein, and both Martinez et al. [57] and Sharon et al. [58] showed that fatty acids also may contribute to the oligomerization of α-synuclein.
To explore whether alterations in glucocerebrosidase affect α-synuclein metabolism, Manning-Boğ et al. [59] analyzed in vitro and in vivo effects of exposure to conduritol-β-epoxide (CBE), an established inhibitor of glucocerebrosidase, and demonstrated that exposure to CBE could increase α-synuclein accumulation as detected by increased immunoreactivity in cultured cells and in the substantia nigra of a mouse model. They also observed that exposure to CBE induced astrocyte activation and aggregation of α-synuclein within these cells. This might correspond to the previous observation of astrogliosis in postmortem brains of patients with GD who developed PD [47].
However, there are some shortcomings in the theory that GBA mutations in subjects with parkinsonism result in lipid alterations due to a loss of functional glucocerebrosidase activity. Ceramide levels in the cell are tightly regulated, and ceramide can be derived from many different degradative and synthetic pathways. The amount of enzymatic activity in Gaucher heterozygotes should be sufficient to prevent substrate accumulation, and there is no evidence that ceramide is deficient in patients with GD, even those severely affected.
Gain-of-Function Role for GBA Mutations
There are other observations that favor a gain-of-function role for GBA mutations. Recent studies demonstrated that some mutations in GBA result in a misfolded protein [60]. Misfolded glucocerebrosidase might contribute to parkinsonism by leading to lysosomal insufficiency, by impairing autophagic pathways necessary for preventing the synucleinopathies, or by overwhelming the ubiquitin–proteasome pathway.
Lysosomal Alteration and the Synucleinopathies
One potential mode of action is that glucocerebrosidase dysfunction might lead to lysosomal insufficiency, thereby reducing α-synuclein degradation. Lysosomes acquire proteins via fusion with vesicles or via specific receptor-mediated incorporation. de Duve [61] and Essner and Novikoff [62] canonically described the lysosome as the major cellular compartment for protein degradation. The lysosome is a specific target of investigation for studies of the synucleinopathies because multiple lines of evidence, including identification of genetic mutations and familial forms of PD, implicate impaired lysosomal function. Mutations in ATP13A2, a lysosomal-resident P-type ATPase, have been associated with the onset of parkinsonism with dementia [63], juvenile parkinsonism, and young-onset PD [64]. Cuervo et al. [65] originally demonstrated that α-synuclein was degraded via chaperone-mediated autophagy, a lysosomal receptor-based pathway that degrades proteins containing the KFERQ peptide sequence (approximately 40% of all known proteins). Moreover, this pathway may be blocked by the A53T and A30P mutant variants of α-synuclein, as well as posttranslationally dopamine-modified variants of α-synuclein [66]. One speculation is that disturbances in the lysosome contribute to reduced α-synuclein degradation and consequently promote its aggregation.
Autophagic Dysfunction
An alternate theory is that GBA mutations may cause autophagic dysfunction, interfering with a process required for neuronal survival. Macroautophagy is a ubiquitous biochemical process common to all eukaryotic organisms. For autophagy to occur, induction via the mTOR or beclin 1/vps34 (class III PI3K) pathway initiates the production and involvement of various autophagy (Atg) proteins, including Atg5 and Atg7, and the posttranslational addition of a phosphatidylethanolamine to MAP-1A LC3 (also known as Atg8), which is a constituent and reliable biomarker of the double-membraned autophagic vacuole [67, 68]. The newly formed double-membraned structure envelops the cytoplasmic material, including protein aggregates and defective mitochondria, for delivery and fusion to acidic compartments such as lysosomes and late endosomes, where their contents ultimately are degraded.
In addition to the lysosome, the autophagic vacuole also has been determined to be a compartment requisite for α-synuclein clearance [69, 70] through the engulfment of the cytoplasmic or vesicular form of α-synuclein and its delivery to the lysosome for final degradation. Moreover, gene replacement of beclin 1 [71], which upregulates autophagy, ameliorates the deleterious effects of α-synuclein overexpression in animal models of PD and LBD. Reduced autophagy also can promote α-synuclein oligomerization [70]. Furthermore, disruption of autophagy, by ablation of the Atg5 or Atg7 genes, leads to the promotion of neuronal deposition of lipofuscin and polyubiquitinated aggregates [72, 73]. Finally, disruption of the autophagic pathway can reduce lipid metabolism, as Singh et al. [74] reported that lipids are transported to the lysosome via autophagy for degradation.
To complicate matters, ceramides are known inducers of autophagy [75], although their effect in GD or in GBA mutation carriers has not been characterized. Although these degradative pathways are intricately linked, it would appear that either mutated glucocerebrosidase or accumulated glucocerebroside may disrupt cellular pathways necessary for autophagic–lysosomal degradation. Moreover, as these pathways have significant crosstalk, it is conceivable that disturbances in one metabolic system, such as glucocerebrosidase function, would lead to proteolytic failure, ultimately resulting in α-synuclein aggregation, Lewy body formation, and neural degeneration.
Endoplasmic Reticulum Stress and Interruptions to the Ubiquitin–Proteasome Pathway
Another theory is that mutant glucocerebrosidase might overwhelm the ubiquitin–proteasome pathway, causing a delay in the degradation of accumulated proteins, including α-synuclein [76]. The proteasomal pathway degrades mutant glucocerebrosidase, and it has been shown that some mutant variants are not trafficked from the endoplasmic reticulum to the lysosome, where they attain their mature functional conformation, but rather are transported to the proteasome for degradation. Ron and Horowitz [77] showed that glucocerebrosidase undergoes ubiquitination and is degraded. This degradative process involves the heat shock protein chaperones and, consequently, is sensitive to alterations in levels of molecular chaperones. The same authors proposed that one deleterious mechanism of mutant GBA is that the protein undergoes parkin-mediated ubiquitination, creating an imbalance in protein degradation resulting in secondary toxicity [78].
One observation that conflicts with a gain-of-function mechanism relates to the spectrum of GBA mutations encountered in subjects with PD. Among the many different mutations encountered are c.84dupG, IVS2+1G>A, and recombinant alleles that would be considered null alleles. These mutations, albeit rare, make it hard to support a gain-of-function mechanism when no protein is being made. However, the very truncated forms of the mutant protein still might induce endoplasmic reticulum stress.
Conclusions
The association of glucocerebrosidase with parkinsonism was discovered not through genomic techniques, but rather through careful clinical observation and investigations. This unanticipated finding has opened new avenues for research on the synucleinopathies. Although the full pathogenesis of these disorders remains elusive, probing the contribution of mutations in GBA to the disease process very likely will prove fruitful, as this finding suggests that glucocerebrosidase and α-synuclein are implicated in a common cellular pathway. Whether the route is related to protein accumulation or to lipid dysregulation, the metabolic pathways, protein structure, protein interactions, and other properties relevant to this enzyme merit close evaluation. This story also illustrates how studies of rare disorders can provide insights into mechanisms and pathways relevant to common diseases.
Acknowledgments
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health. The authors thank Dr. Nahid Tayebi, Dr. Grisel Lopez, Dr. Ehud Goldin, Sarah Klontz, and Jae H. Choi for their critical reading of the manuscript.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Arash Velayati, Section on Neurogenetics, Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health, Building 35, Room 1A213, 35 Convent Drive, MSC 3708, Bethesda, MD 20892, USA.
W. Haung Yu, Taub Institute for Alzheimer's Disease Research, Department of Pathology, Columbia University, New York, NY, USA.
Ellen Sidransky, Section on Neurogenetics, Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health, Building 35, Room 1A213, 35 Convent Drive, MSC 3708, Bethesda, MD 20892, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Beutler E. Gaucher disease: new molecular approaches to diagnosis and treatment. Science. 1992;256:794–799. doi: 10.1126/science.1589760. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, Wilder S, Horowitz Z, et al. The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics. 1989;4:87–96. doi: 10.1016/0888-7543(89)90319-4. [DOI] [PubMed] [Google Scholar]
- 3.Sorge J, West C, Westwood B, Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Nat Acad Sci U S A. 1985;82:7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barneveld RA, Keijzer W, Tegelaers FP, et al. Assignment of the gene coding for human beta-glucocerebrosidase to the region q21–q31 of chromosome 1 using monoclonal antibodies. Hum Genet. 1993;64:227–231. doi: 10.1007/BF00279398. [DOI] [PubMed] [Google Scholar]
- 5.Winfield SL, Tayebi N, Martin BM, et al. Identification of three additional genes contiguous to the glucocerebrosidase locus on chromosome 1q21: implications for Gaucher disease. Genome Res. 1997;7:1020–1026. doi: 10.1101/gr.7.10.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorge J, Gross E, West C, Beutler E. High level transcription of the glucocerebrosidase pseudogene in normal subjects and patients with Gaucher disease. J Clin Invest. 1990;86:1137–1141. doi: 10.1172/JCI114818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaucher PCE. Thesis, Doctor of Medicine. Octave Doin; Paris: 1882. On primary epithelioma of the spleen: idiopathic hypertrophy of the spleen without leukemia. [Google Scholar]
- 8•.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat. 2008;29:567–583. doi: 10.1002/humu.20676. [This work provides a comprehensive review of the structure of GBA and the mutations identified.] [DOI] [PubMed] [Google Scholar]
- 9.Koprivica V, Stone DL, Park JK, et al. Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet. 2000;66:1777–1786. doi: 10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goker-Alpan O, Hruska KS, Orvisky E, et al. Divergent phenotypes in Gaucher disease implicate the role of modifiers. J Med Genet. 2005;42:e37. doi: 10.1136/jmg.2004.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neudorfer O, Giladi N, Elstein D, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996;89:691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 12.Machaczka M, Rucinska M, Skotnicki AB, Jurczak W. Parkinson's syndrome preceding clinical manifestation of Gaucher's disease. Am J Hematol. 1999;61:216–217. doi: 10.1002/(sici)1096-8652(199907)61:3<216::aid-ajh12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Várkonyi J, Rosenbaum H, Baumann N, et al. Gaucher disease associated with parkinsonism: four further case reports. Am J Med Genet A. 2003;116A:348–351. doi: 10.1002/ajmg.a.10028. [DOI] [PubMed] [Google Scholar]
- 14.Goker-Alpan O, Schiffmann R, LaMarca ME, et al. Parkinsonism among Gaucher disease carriers. J Med Genet. 2004;41:937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halperin A, Elstein D, Zimran A. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis. 2006;36:426–428. doi: 10.1016/j.bcmd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Goker-Alpan O, Giasson BI, Eblan MJ, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 17.Tayebi N, Callahan M, Madike V, et al. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab. 2001;73:313–321. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- 18.Tayebi N, Walker J, Stubblefield B, et al. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab. 2003;79:104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 19.Lwin A, Orvisky E, Goker-Alpan O, et al. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81(1):70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Park JK, Tayebi N, Stubblefield BK, et al. The E326K mutation and Gaucher disease: mutation or polymorphism? Clin Genet. 2002;61(1):32–34. doi: 10.1034/j.1399-0004.2002.610106.x. [DOI] [PubMed] [Google Scholar]
- 21.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2004;351(19):1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 22.Sato C, Morgan A, Lang AE, et al. Analysis of the glucocerebrosidase gene in Parkinson's disease. Mov Disord. 2005;20(3):367–370. doi: 10.1002/mds.20319. [DOI] [PubMed] [Google Scholar]
- 23.Toft M, Pielsticker L, Ross OA, et al. Glucocerebrosidase gene mutations and Parkinson disease in the Norwegian population. Neurology. 2006;66(3):415–417. doi: 10.1212/01.wnl.0000196492.80676.7c. [DOI] [PubMed] [Google Scholar]
- 24.Eblan MJ, Nguyen J, Ziegler SG, et al. Glucocerebrosidase mutations are also found in subjects with early-onset parkinsonism from Venezuela. Mov Disord. 2006;21(2):282–283. doi: 10.1002/mds.20766. [DOI] [PubMed] [Google Scholar]
- 25.Tan EK, Tong J, Fook-Chong S, et al. Glucocerebrosidase mutations and risk of Parkinson disease in Chinese patients. Arch Neurol. 2007;64(7):1056–1058. doi: 10.1001/archneur.64.7.1056. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler SG, Eblan MJ, Gutti U, et al. Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Mol Genet Metab. 2007;91(2):195–200. doi: 10.1016/j.ymgme.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark LN, Ross BM, Wang Y, et al. Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology. 2007;69(12):1270–1277. doi: 10.1212/01.wnl.0000276989.17578.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu YR, Chen CM, Chao CY, et al. Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol Neurosurg Psychiatry. 2007;78(9):977–979. doi: 10.1136/jnnp.2006.105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70(24):2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [This study included the largest cohort of Ashkenazi Jewish subjects with parkinsonism screened for GBA mutations.] [DOI] [PubMed] [Google Scholar]
- 30.Spitz M, Rozenberg R, Pereira Lda V. Reis Barbosa E: Association between Parkinson's disease and glucocerebrosidase mutations in Brazil. Parkinsonism Relat Disord. 2008;14(1):58–62. doi: 10.1016/j.parkreldis.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 31•.Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132(Pt 7):1783–1794. doi: 10.1093/brain/awp044. [The authors sequenced the GBA gene in a large series of brains from subjects with PD as well as controls. They identified mutations in 4.2% of patients versus 1.2% of controls.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols WC, Pankratz N, Marek DK, et al. Parkinson Study Group-PROGENI Investigators: Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72(4):310–316. doi: 10.1212/01.wnl.0000327823.81237.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci Lett. 2009;452(2):87–89. doi: 10.1016/j.neulet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 34•.Mitsui J, Mizuta I, Toyoda A, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch Neurol. 2009;66(5):571–576. doi: 10.1001/archneurol.2009.72. [This study, conducted in Japan, demonstrates that in this cohort, GBA mutations are encountered frequently in subjects with PD. The spectrum of mutations, however, differed from cohorts of other ethnicities.] [DOI] [PubMed] [Google Scholar]
- 35.Bras J, Paisan-Ruiz C, Guerreiro R, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiol Aging. 2009;30(9):1515–1517. doi: 10.1016/j.neurobiolaging.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [This multicenter international collaborative study combined the results from 16 different centers in four continents. Studying more than 5000 patients and an equal number of controls, the authors demonstrated that the odds ratio for carrying a GBA mutation in patients with PD was greater than 5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutti U, Fung HC, Hruska KS, et al. The need for appropriate genotyping strategies for glucocerebrosidase mutations in cohorts with Parkinson disease. Arch Neurol. 2008;65:850–851. doi: 10.1001/archneur.65.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan L, Hsu CM, Tsai CH, et al. Mutation analysis of Gaucher disease patients in Taiwan: high prevalence of the RecNciI and L444P mutations. Blood Cells Mol Dis. 2006;36:422–425. doi: 10.1016/j.bcmd.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 39.McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 40.Wenning GK, Jellinger KA. The role of alpha-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol. 2005;109:129–140. doi: 10.1007/s00401-004-0935-y. [DOI] [PubMed] [Google Scholar]
- 41.Mata IF, Samii A, Schneer SH, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol. 2008;65:379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrer MJ, Williams LN, Algom AA, et al. Glucosidase-beta variations and Lewy body disorders. Parkinsonism Relat Disord. 2009;15:414–416. doi: 10.1016/j.parkreldis.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark LN, Kartsaklis LA, Wolf Gilbert R, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66:578–583. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segarane B, Li A, Paudel R, et al. Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology. 2009;72:1185–1186. doi: 10.1212/01.wnl.0000345356.40399.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamrozik Z, Lugowska A, Slawek J, Kwiecinski H. Glucocerebrosidase mutations p.L444P and p.N370S are not associated with multisystem atrophy, progressive supranuclear palsy and cortico-basal degeneration in Polish patients. J Neurol. 2009 Nov 1; doi: 10.1007/s00415-009-5363-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 46.Bembi B, Zambito Marsala S, Sidransky E, et al. Gaucher's disease with Parkinson's disease: clinical and pathological aspects. Neurology. 2003;61:99–101. doi: 10.1212/01.wnl.0000072482.70963.d7. [DOI] [PubMed] [Google Scholar]
- 47.Wong K, Sidransky E, Verma A, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Kono S, Shirakawa K, Ouchi Y, et al. Dopaminergic neuronal dysfunction associated with parkinsonism in both a Gaucher disease patient and a carrier. J Neurol Sci. 2007;252:181–184. doi: 10.1016/j.jns.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 49.Goker-Alpan O, Lopez G, Vithayathil J, et al. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65:1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gan-Or Z, Giladi N, Orr-Urtreger A. Differential phenotype in Parkinson's disease patients with severe versus mild GBA mutations. Brain. 2009;132:e125. doi: 10.1093/brain/awp161. [DOI] [PubMed] [Google Scholar]
- 51.Hardy J, Lewis P, Revesz T, et al. The genetics of Parkinson's syndromes: a critical review. Curr Opin Genet Dev. 2009;19:254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275:5767–5773. doi: 10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conradi NG, Sourander P, Nilsson O, et al. Neuropathology of the Norrbottnian type of Gaucher disease. Morphological and biochemical studies. Acta Neuropathol. 1984;65:99–109. doi: 10.1007/BF00690463. [DOI] [PubMed] [Google Scholar]
- 54.Jo E, McLaurin J, Yip CM, et al. alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 55.Schlossmacher MG, Cullen V, Müthing J. The glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2005;352:728–731. [PubMed] [Google Scholar]
- 56.Simons K, Gruenberg J. Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol. 2000;10:459–462. doi: 10.1016/s0962-8924(00)01847-x. [DOI] [PubMed] [Google Scholar]
- 57.Martinez Z, Zhu M, Han S, Fink AL. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- 58.Sharon R, Bar-Joseph I, Frosch MP, et al. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron. 2003;37:583–595. doi: 10.1016/s0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 59.Manning-Boğ AB, Schüle B, Langston JW. Alpha-synucleinglucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30(6):1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 60.Sawkar AR, Adamski-Werner SL, Cheng WC, et al. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem Biol. 2005;12:1235–1244. doi: 10.1016/j.chembiol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 61.de Duve C. From cytases to lysosomes. Fed Proc. 1964;23:1045–1049. [PubMed] [Google Scholar]
- 62.Essner E, Novikoff AB. Localization of acid phosphatase activity in hepatic lysosomes by means of electron microscopy. J Biophys Biochem Cytol. 1961;9:773–784. doi: 10.1083/jcb.9.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramirez A, Heimbach A, Grundemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 64.Di Fonzo A, Chien HF, Socal M, et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- 65.Cuervo AM, Stefanis L, Fredenburg R, et al. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 66.Martinez-Vicente M, Talloczy Z, Kaushik S, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sou YS, Tanida I, Komatsu M, et al. Phosphatidylserine in addition to phosphatidylethanolamine is an in vitro target of the mammalian Atg8 modifiers, LC3, GABARAP, and GATE-16. J Biol Chem. 2006;281:3017–3024. doi: 10.1074/jbc.M505888200. [DOI] [PubMed] [Google Scholar]
- 69.Webb JL, Ravikumar B, Atkins J, et al. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 70.Yu WH, Dorado B, Figueroa HY, et al. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am J Pathol. 2009;175:736–747. doi: 10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer B, Potkar R, Trejo M, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 73.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 74.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scarlatti F, Bauvy C, Ventruti A, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384–18391. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- 76.Dawson TM. Parkin and defective ubiquitination in Parkinson's disease. J Neural Transm Suppl. 2006;70:209–213. doi: 10.1007/978-3-211-45295-0_32. [DOI] [PubMed] [Google Scholar]
- 77.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14:2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 78.Horowitz M, Ron I. Interaction between parkin and glucocerebrosidase: a possible link between Parkinson disease and Gaucher disease. Mol Genet Metab. 2009;96:S27. doi: 10.1093/hmg/ddq292. [DOI] [PubMed] [Google Scholar]
- 79.Clark LN, Nicolai A, Afridi S, et al. Pilot association study of the beta-glucocerebrosidase N370S allele and Parkinson's disease in subjects of Jewish ethnicity. Mov Disord. 2005;20:100–103. doi: 10.1002/mds.20320. [DOI] [PubMed] [Google Scholar]
- 80.De Marco EV, Annesi G, Tarantino P, et al. Glucocerebrosidase gene mutations are associated with Parkinson's disease in southern Italy. Mov Disord. 2008;23:460–463. doi: 10.1002/mds.21892. [DOI] [PubMed] [Google Scholar]