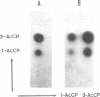
Abstract
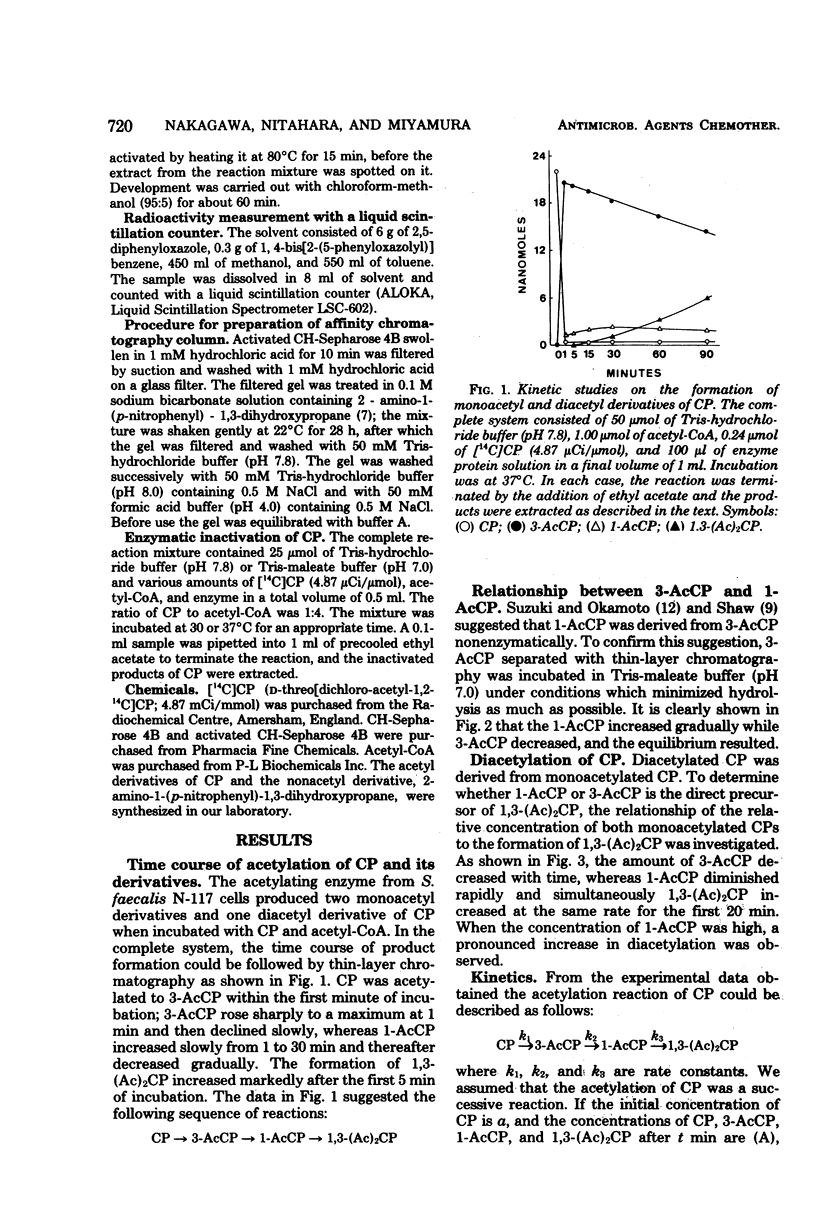
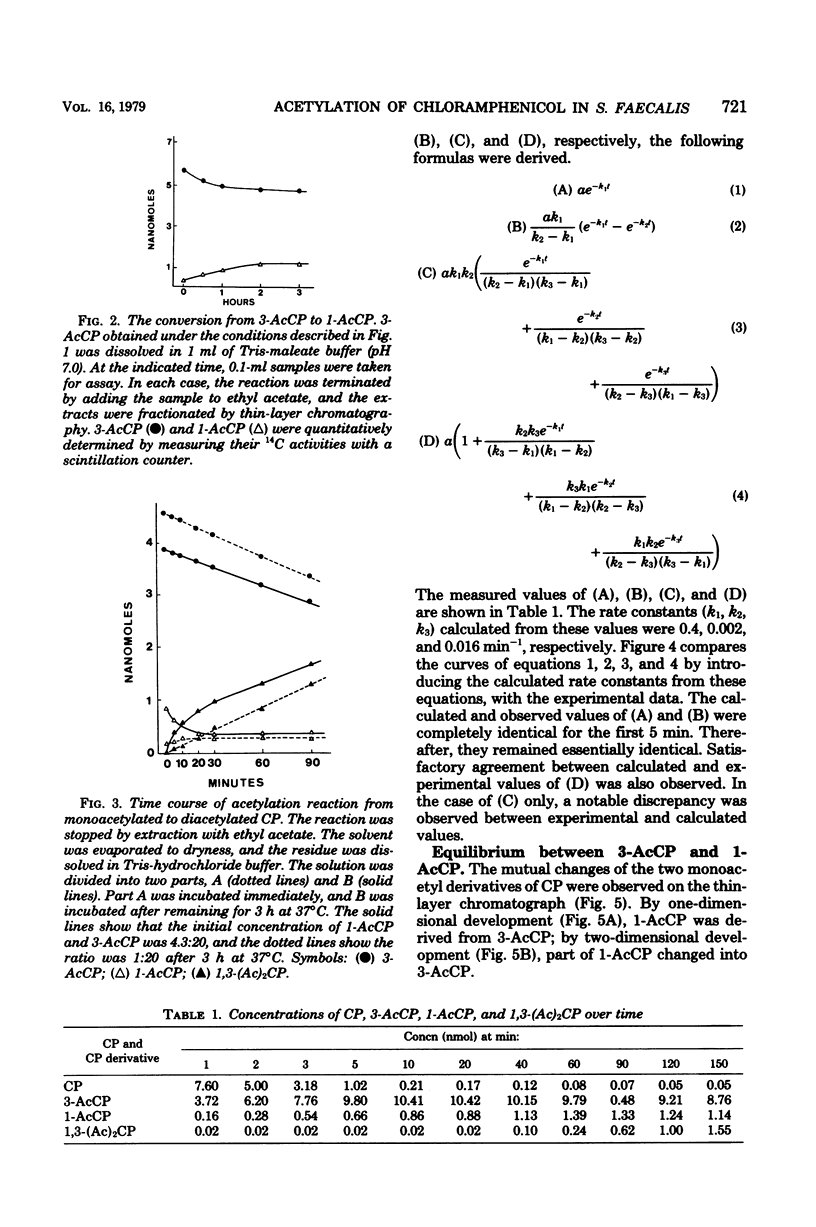
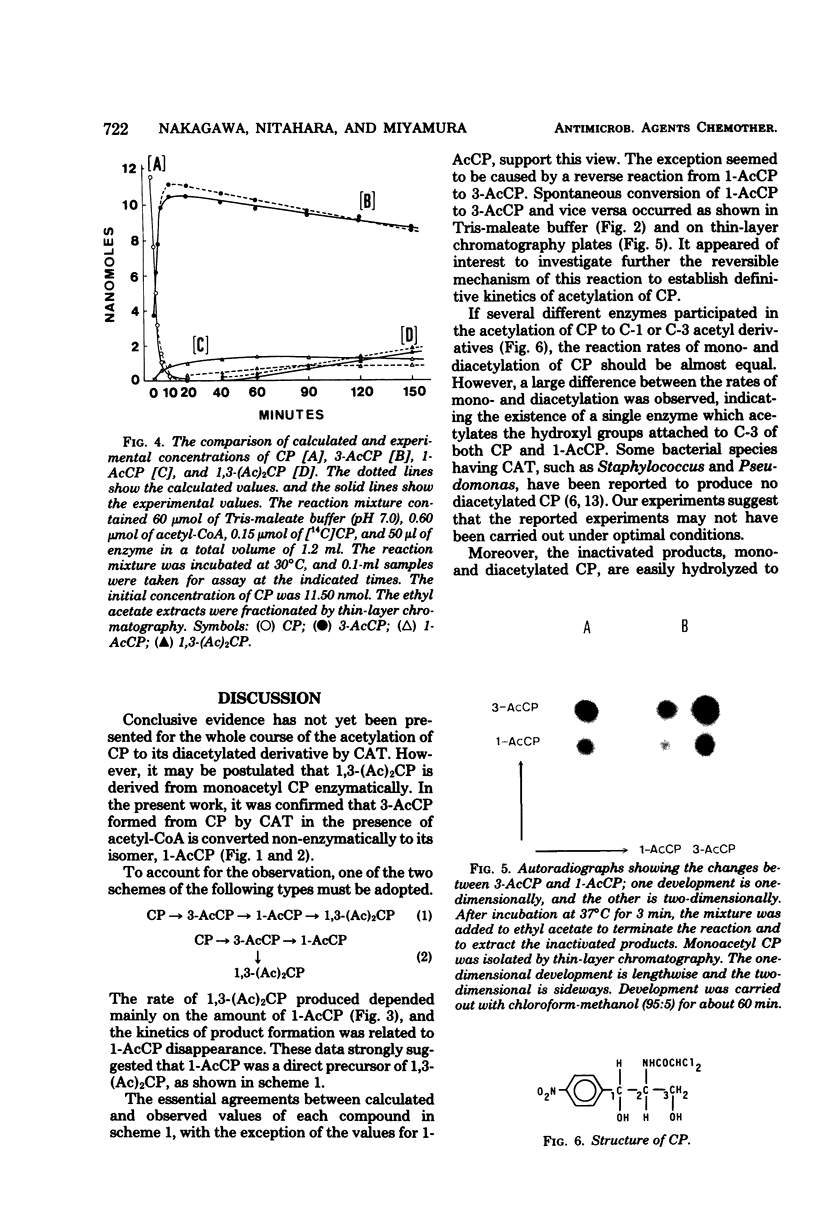
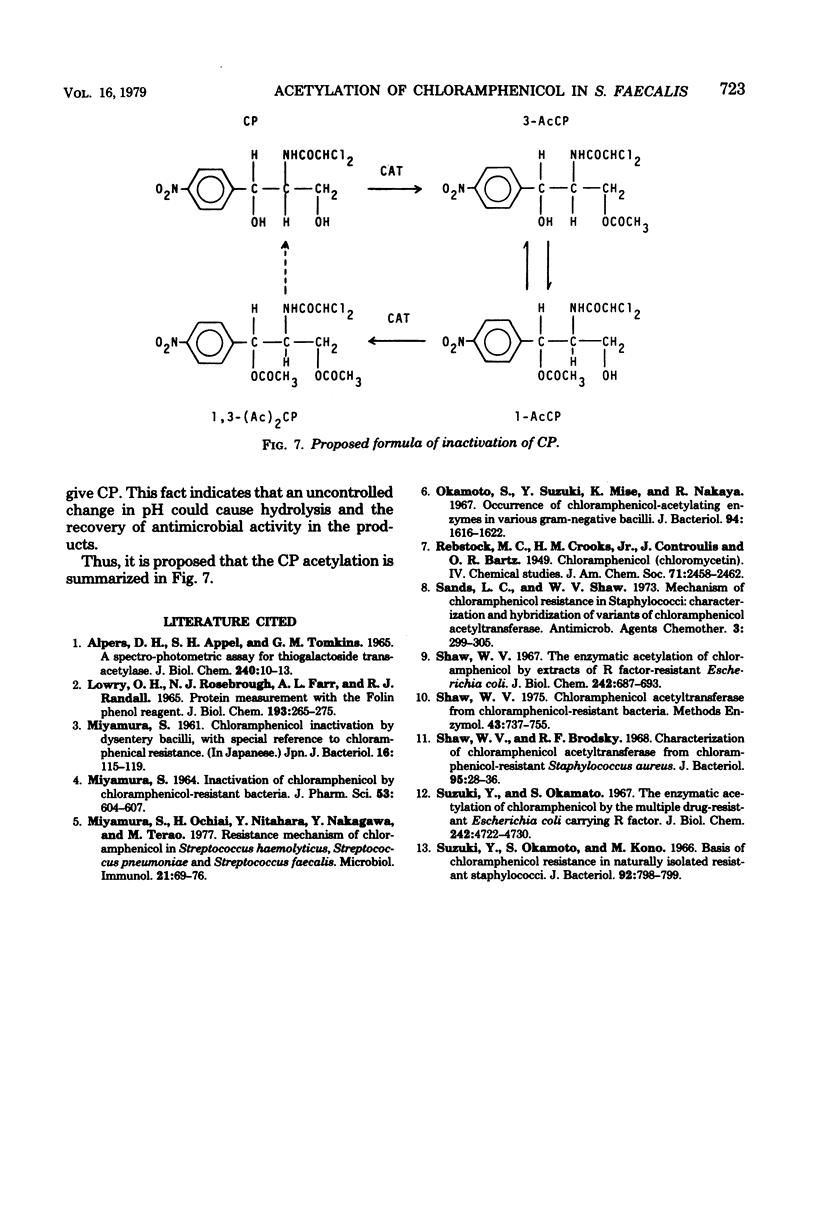
The kinetics of chloramphenicol (CP) acetylation by CP acetyltransferase from Streptococcus faecalis was studied. CP was shown to be acetylated enzymatically to its 3-O-acetyl derivative (3-AcCP) in the presence of acetyl coenzyme A, after which 3-AcCP was converted nonenzymatically to its 1-O-acetyl isomer, 1-O-acetyl CP (1-AcCP). At equilibrium, the 1-AcCP and 3-AcCP were present in a 1:4 ratio. Subsequently the diacetylated product, 1,3-O-O-diacetyl CP [1,3-(Ac)2CP], was enzymatically produced from 1-AcCP by the same enzyme. Theoretical calculation of rate constants (k1, k2, k3) for each successive reaction is as follows: (Formula: see text). This calculation gave k1 = 0.4 min-1, k2 = 0.002 min-1, and k3 = 0.016 min-1. Experimental results agreed closely with these calculated values.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERS D. H., APPEL S. H., TOMKINS G. M. A SPECTROPHOTOMETRIC ASSAY FOR THIOGALACTOSIDE TRANSACETYLASE. J Biol Chem. 1965 Jan;240:10–13. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MIYAMURA S. INACTIVATION OF CHLORAMPHENICOL BY CHLORAMPHENICOL-RESISTANT BACTERIA. J Pharm Sci. 1964 Jun;53:604–607. doi: 10.1002/jps.2600530606. [DOI] [PubMed] [Google Scholar]
- MIYAMURA S. [Chloramphenicolase in dysentery bacilli, with special reference to chloramphenicol resistance]. Nihon Saikingaku Zasshi. 1961 Feb;16:115–119. doi: 10.3412/jsb.16.115. [DOI] [PubMed] [Google Scholar]
- Miyamura S., Ochiai H., Nitahara Y., Nakagawa Y., Terao M. Resistance mechanism of chloramphenicol in Streptococcus haemolyticus, Streptococcus pneumoniae and Streptococcus faecalis. Microbiol Immunol. 1977;21(2):69–76. doi: 10.1111/j.1348-0421.1977.tb02809.x. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y., Mise K., Nakaya R. Occurrence of chloramphenicol-acetylating enzymes in various gram-negative bacilli. J Bacteriol. 1967 Nov;94(5):1616–1622. doi: 10.1128/jb.94.5.1616-1622.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands L. C., Shaw W. V. Mechanism of chloramphenicol resistance in staphylococci: characterization and hybridization of variants of chloramphenicol acetyltransferase. Antimicrob Agents Chemother. 1973 Feb;3(2):299–305. doi: 10.1128/aac.3.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S., Kono M. Basis of chloramphenicol resistance in naturally isolated resistant staphylococci. J Bacteriol. 1966 Sep;92(3):798–799. doi: 10.1128/jb.92.3.798-799.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S. The enzymatic acetylation of chloramphenicol by the multiple drug-resistant Escherichia coli carrying R factor. J Biol Chem. 1967 Oct 25;242(20):4722–4730. [PubMed] [Google Scholar]