Abstract
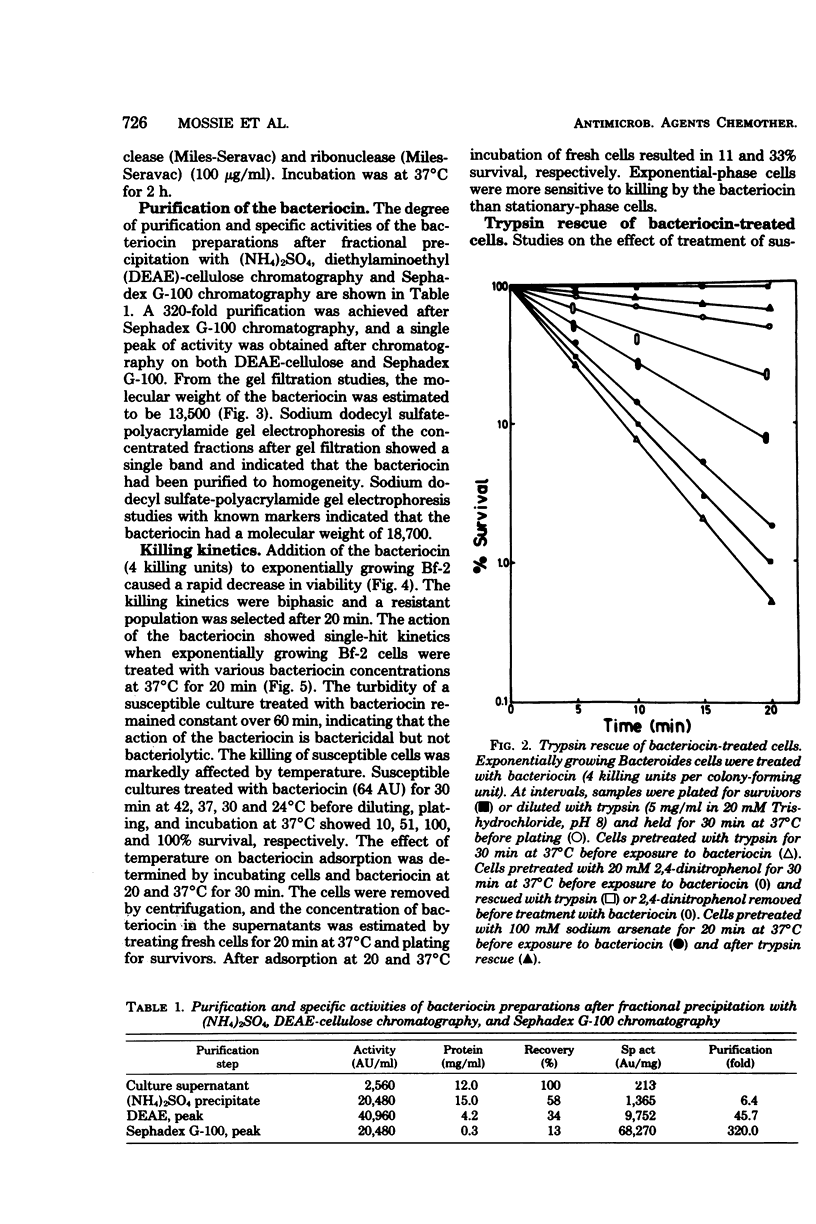
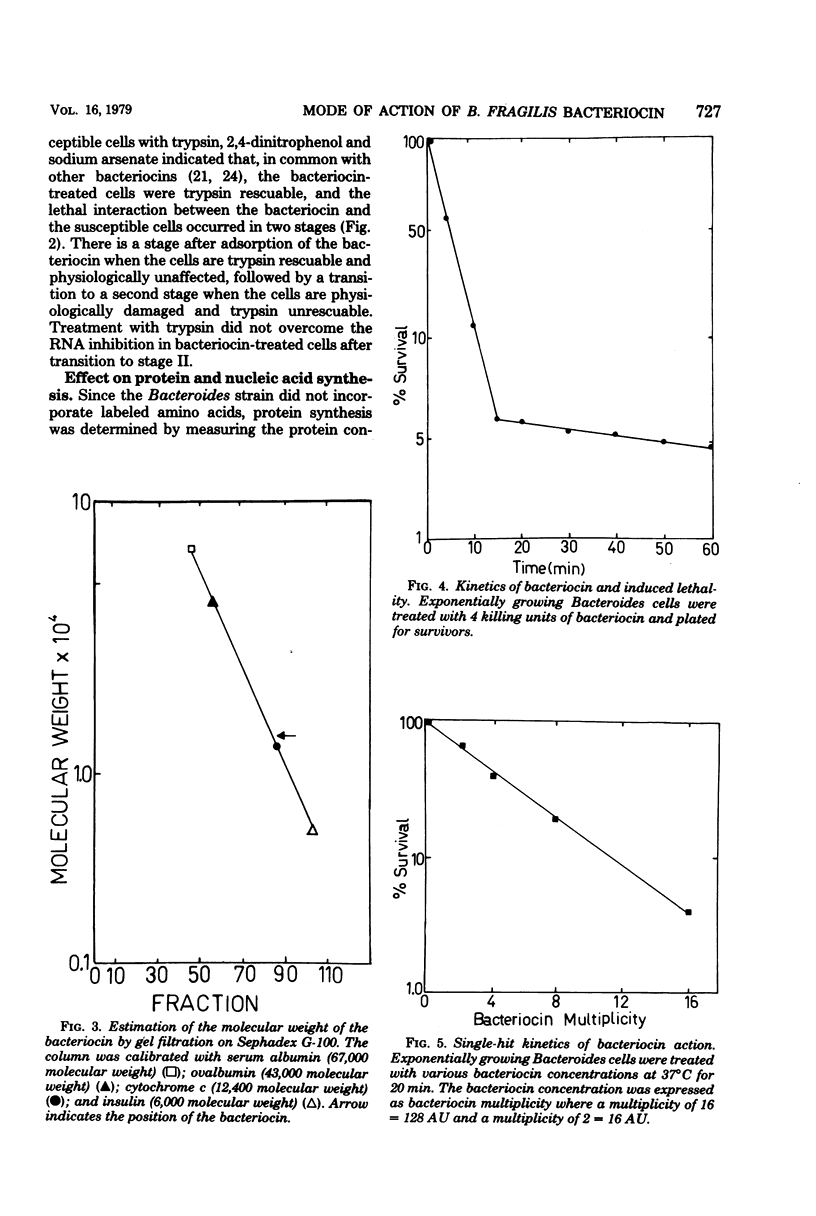
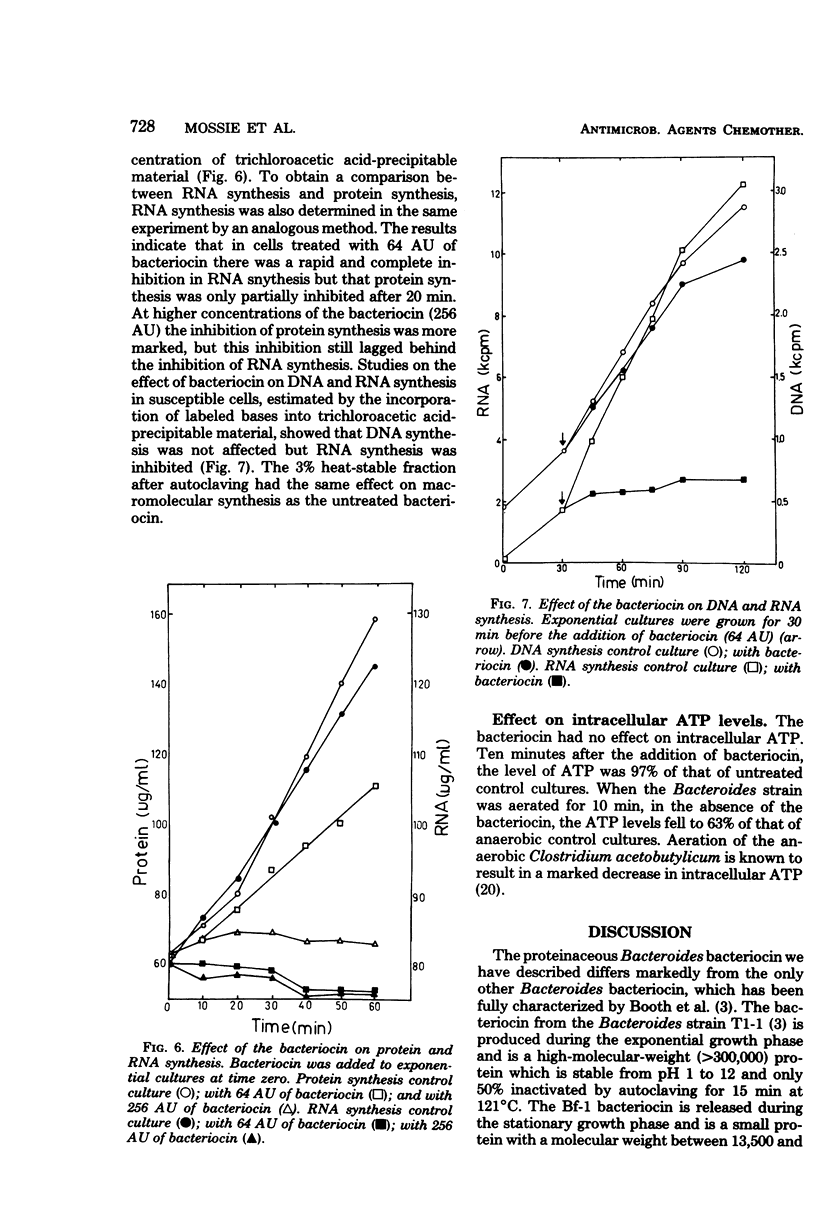
A Bacteroides fragilis strain produces a low-molecular-weight (13,500 to 18,700), proteinaceous bacteriocin during the stationary growth phase. The extracellular bacteriocin is not inducible by ultraviolet light or mitomycin C and is stable between pH 7.5 and 8.2. The majority of the bacteriocin is thermolabile, but a small proportion (3%) of the bacteriocin is stable after autoclaving at 121 degrees C for 15 min. Killing of sensitive bacteroides cells follows single-hit kinetics, and the interaction of a single molecule of bacteriocin with a target cell occurs in two stages. The killing of susceptible cells is affected by temperature and the growth state of the susceptible cells. The bacteriocin is unusual in that the primary event in its mode of action is the inhibition of RNA synthesis. The bacteriocin inhibits RNA synthesis immediately but has no effect on DNA synthesis or intracellular ATP levels. Protein synthesis is inhibited after a delay of 20 min, presumably as a result of the initial inhibition of RNA synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beerens H., Baron G., Tahon Mise en evidence di substances inhibitrices elaborées par les bacteries anaerobies non sporulées. Ann Inst Pasteur Lille. 1966;17:1–20. [PubMed] [Google Scholar]
- Booth S. J., Johnson J. L., Wilkins T. D. Bacteriocin production by strains of Bacteroides isolated from human feces and the role of these strains in the bacterial ecology of the colon. Antimicrob Agents Chemother. 1977 Apr;11(4):718–724. doi: 10.1128/aac.11.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Elgat M., Ben-Gurion R. Mode of action of pesticin. J Bacteriol. 1969 May;98(2):359–367. doi: 10.1128/jb.98.2.359-367.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. G. Colicinogeny and related phenomena. Bacteriol Rev. 1975 Dec;39(4):464–515. doi: 10.1128/br.39.4.464-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Fukui S., Ishikawa S. Initial events caused by colicin K infection--cation movement and depletion of ATP pool. J Biochem. 1969 May;65(5):843–847. doi: 10.1093/oxfordjournals.jbchem.a129088. [DOI] [PubMed] [Google Scholar]
- Holdeman L. V., Good I. J., Moore W. E. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Energy requirement for the initiation of colicin action in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):12–22. doi: 10.1016/0005-2728(75)90048-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moodie H. L., Woods D. R. Anaerobic R factor transfer in Escherichia coli. J Gen Microbiol. 1973 Jun;76(2):437–440. doi: 10.1099/00221287-76-2-437. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Plate C. A., Luria S. E. Stages in colicin K action, as revealed by the action of trypsin. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2030–2034. doi: 10.1073/pnas.69.8.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Reeves P. R. Kinetics of adsorption of colicin CA42-E2 and reversal of its bactericidal activity. J Bacteriol. 1969 Oct;100(1):301–309. doi: 10.1128/jb.100.1.301-309.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallehn G., Krämer J. Studies on mode of action of a bacteriocin from Clostridium septicum. Can J Microbiol. 1976 Mar;22(3):435–437. doi: 10.1139/m76-066. [DOI] [PubMed] [Google Scholar]
- Schaller K., Nomura M. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Stouthamer A. H. Mode of action of a bacteriocin produced by Enterobacter cloacae. J Gen Microbiol. 1969 Mar;55(3):13–14. [PubMed] [Google Scholar]



