Abstract
Introduction
Ewing sarcoma (ES) is the most common chest wall malignancy in adolescents. Current therapy incorporates chemotherapy to treat systemic disease and radiation to assist with local control. We sought to evaluate the timing of surgery and role of adjuvant radiation.
Methods
We reviewed the SJCRH chest wall ES experience from 1979-2009. Patient demographics, tumor characteristics, treatment variables, and outcomes were analyzed with respect to timing of surgery and use of adjuvant radiation.
Results
Our cohort consisted of 36 patients with chest wall ES; median follow-up of 14.2 years and 15-year estimate of overall survival (OS) was 66%. In patients with localized disease, the timing of surgery (up-front versus delayed) did not impact margin negativity or the use of adjuvant radiation, but did decrease the extent of chest wall resection. When considering radiation in patients with localized disease, we found that patients who did not receive radiation had smaller tumor size (median, 6 versus 10cm) (p=0.04), and were more likely to have had negative margins (p=0.01) than patients who received adjuvant radiation. One patient in each group developed a locoregional recurrence. The 15-year estimated of OS for patients who received adjuvant radiation was 80% versus 100% for those who did not.
Conclusion
Delayed surgery decreased the extent of chest wall resection and helped define a patient population with favorable tumor biology. Patients with complete pathologic responses to chemotherapy, and those with tumors < 8cm and negative surgical margins may be spared adjuvant radiation without any decrement in OS.
Introduction
Ewing sarcoma is the most common pediatric malignant chest wall tumor; about 10% of Ewing sarcoma arise from the chest wall.1 Treatment of Ewing sarcoma of the chest wall involves intense local therapy with surgical resection and/or radiation therapy, and chemotherapy to treat systemic disease. Historically, Ewing sarcoma of the chest wall was treated with extensive chest wall resection involving multiple ribs and adjuvant radiation.2 This treatment strategy resulted in a significant numbers of incomplete surgical resections and complications such as scoliosis, chest wall deformities, pulmonary fibrosis, and secondary malignancies; thus, many began to advocate the use of preoperative chemotherapy.2,3,4,5 Complete surgical resection after neoadjuvant chemotherapy yielded excellent outcomes and quickly became the standard of care.6,5
Radiation therapy has long been considered a necessary component of treatment for Ewing sarcoma of the chest wall. As long-term survival from Ewing sarcoma has improved, more attention has been paid to the potential long-term complications of radiation, including pulmonary fibrosis, cardiac toxicity, and secondary malignancies.7,8,9 Radiation therapy is at times used as the sole treatment modality for local control, but is more frequently used in combination with surgical resection. Despite the prevalence of radiation therapy there are not well agreed upon indication for its use in the adjuvant setting. Thus, the decision to administer or not administer adjuvant radiation is usually determined on a case-by-case basis.
We sought to review the SJCRH single-institution experience with Ewing sarcoma of the chest wall to evaluate the timing of surgical resection (up-front versus delayed) and use of adjuvant radiation on local control, event-free survival (EFS) and overall survival.
Methods
A retrospective chart review was approved by the Institutional Review Board for all patients with Ewing sarcoma of the chest wall treated at SJCRH from 1979 to 2009. During that time period there were a total of 45 patients treated. All patients were staged with either CT scan or MRI, with bone scan serving as an adjunct to cross-sectional imaging. Patients were considered to have Ewing sarcoma of the chest wall if they had a chest wall tumor that was pathologically diagnosed as Ewing sarcoma (osseous or extraosseous) or primitive neuroectodermal tumor of bone or soft tissue. All patients received multi-agent chemotherapy, either vincristine, actinomycin D, cyclophosphamide, and doxorubicin (VACA) or VACA with alternating doses of ifosfamide and etoposide. Also, all patients received local therapy consisting of surgical resection, radiation, or both. We excluded 3 patients whose tumors were secondary primaries, 3 patients referred to SJCRH after recurrence/progression for salvage therapy, 2 patients referred for bone marrow transplant late in their disease course, and 1 patient who received chemotherapy only while visiting Memphis,TN. Data regarding demographics (age, sex, and race), presentation, primary tumor characteristics (location and size), staging, chemotherapy (timing, agents, and doses), radiation (timing, type, and doses), surgery (timing, extent, and margin status), recurrence (locoregional and distant), survival, cause of death, and follow-up were recorded.
Statistical Methods
Survival was defined as the time interval from date of diagnosis to date of death from any cause or to date of last contact. EFS was defined as the time interval from date of diagnosis to date of first event (relapsed or progressive disease or death from any cause) or to date of last contact for patients without events. Survival and EFS were estimated using the method of Kaplan and Meier. Standard errors were calculated using the method of Peto and Pike.10 Outcome estimates are reported ± standard error. Differences in survival and EFS distributions by demographic and treatment factors were examined using the exact log rank test.
Local failure was defined as the time interval from date of diagnosis to date of local or regional disease recurrence or progression. Distant failure was defined as the time interval from date of diagnosis to date of distant disease recurrence or progression. The cumulative incidence of local failure (and of distant failure) was estimated.11 Treatment failure was defined as the time interval from date of diagnosis to date of treatment failure (relapsed or progressive disease or death related to disease). Disease-specific survival was defined as the time interval from date of diagnosis to date of death from disease. The cumulative incidences of treatment failure and disease-specific survival were estimated. Differences in cumulative incidence curves were examined using Gray's test.12
Results
Thirty-six patients with chest wall Ewing sarcoma diagnosed between February 1979 and August 2009 were included in our study. Patient and tumor characteristics are shown in Table 1. The majority of patients were male (n=27, 75%), Caucasian (n=30, 83%), and the median age at diagnosis was 13.1 years (range, 1.1 – 18.4 years). The most common presentations were pain (n=29, 81%) and a palpable mass (n=11, 31%). All patients had tumors arising from a rib or the soft tissue of the chest wall; there were no patients with sternal, vertebral, scapular, or clavicular sites of origin. At diagnosis, the median tumor size was 10 cm (range, 4 – 19 cm). Eleven patients (31%) had tumors less than 8 cm in diameter and 25 patients (69%) had tumors ≥ 8 cm in diameter. At diagnosis, 22 (61%) patients had localized disease and 14 (39%) had metastatic disease. Thirteen of 14 patients with metastatic disease at diagnosis had pulmonary (n=10) or pleural (n=3) metastases. All 36 patients received chemotherapy; 22 patients (61%) received chemotherapy prior to definitive local therapy (7 had a complete pathologic response) and the other 14 patients (39%) underwent up-front surgery and received chemotherapy postoperatively. Thirty-three of 36 patients (92%) underwent surgical resection of their chest wall tumor a median of 2.7 months from diagnosis; the other 3 patients (8%) received XRT for local therapy instead. Negative surgical margins were achieved in 24 of 33 patients (73%). XRT was administered in either a neoadjuvant or adjuvant setting to 25 patients (69%).
Table 1.
Patient and tumor characteristics for the entire cohort of 36 patients with chest wall Ewing sarcoma.
| N (%) | |
|---|---|
| Gender | |
| Male | 27 (75) |
| Female | 9 (25) |
| Race | |
| Caucasian | 30 (83) |
| African American | 4 (11) |
| Other | 2 (6) |
| Age at Diagnosis (years) | |
| Median | 13.1 |
| Range | 1.1–184 |
| Tumnr Size (cm) | |
| Median | 10 |
| Range | 4–19 |
| < 8 | 11 (31) |
| ≥ 8 | 25 (69) |
| Stage | |
| Localized | 22 (61) |
| Metastatic | 14 (39) |
Twenty-two of 36 patients (61%) were alive at the time of analysis with a median follow-up from diagnosis of 14.2 years (range, 1.0 – 30.9 years). The cumulative incidence of local failure and distant failure were estimated to be 21.9% ± 7.7% and 26.1% ± 8.4% at 15 years, respectively. The 15-year estimates of EFS and survival were 56.3% ± 11.2% and 65.6% ± 10.7%, respectively (Figure 1). First events in the cohort included relapsed or progressive disease in 15 patients (6 local-regional, 8 distant, 1 simultaneous local-regional and distant) and death from other causes in 2 patients. Localized disease was marginally associated with improved EFS (p=0.06) (Table 2). Tumor size < 8 cm and localized disease were marginally associated with improved overall survival (p=0.10 and p=0.06, respectively) (Table 2).
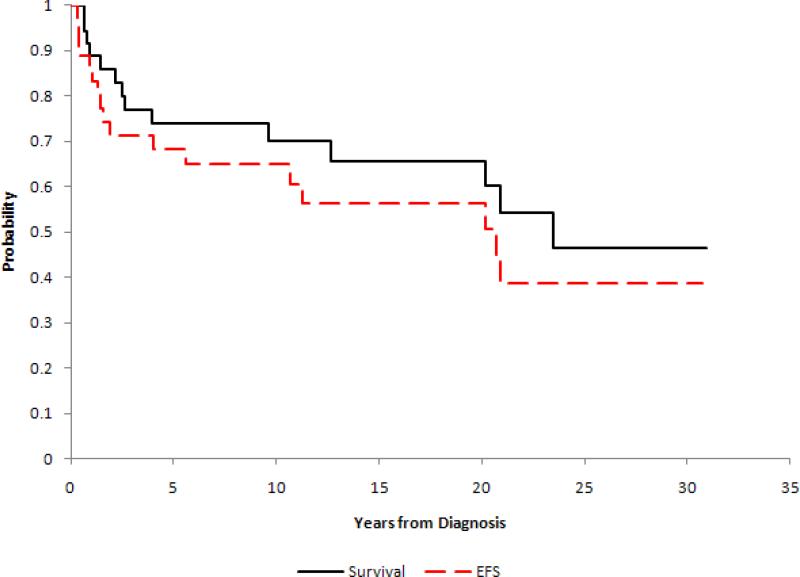
Figure 1.
Kaplan-Meier event-free survival and survival curves for the entire cohort of 36 patients with chest wall Ewing sarcoma. 15-year estimate of EFS 56.3% +/- 11.2% and 65.6% +/- 10.7%.
Table 2.
Potential factors predictive of EFS and survival for the entire cohort of 36 patients with chest wall Ewing sarcoma.
| EFS±SE (%) | Survival±SE (%) | ||||
|---|---|---|---|---|---|
| Factors | n | Year15 | p-value | Year15 | p-value |
| Age at Diagnosis | |||||
| <=13 years | 18 | 49.9±15.8 | 0.47 | 55.9±15.2 | 0.43 |
| >13 years | 18 | 62.8±14.5 | 75.4±13.2 | ||
| Gender | |||||
| Male | 27 | 57.5±13.3 | 0.97 | 69.9±12.1 | 0.62 |
| Female | 9 | 51.9±18.0 | 50.8±17.8 | ||
| Tumor Size | |||||
| <8 cm | 11 | 68.2±15.7 | 0.11 | 79.5±13.6 | 0.10 |
| ≥8 cm | 25 | 52.7±14.8 | 59.7±14.3 | ||
| Stage | |||||
| Localized | 22 | 67.8±11.6 | 0.06 | 79.5±10.0 | 0.06 |
| Metastatic | 14 | 37.7±29.8 | 43.6±32.8 | ||
| Timing of Surgery | |||||
| Delayed surgery | 19 | 73.0±15.5 | 0.66 | 78.3±14.9 | 0.76 |
| Upfront surgery | 14 | 51.6±14.7 | 68.2±13.6 | ||
| Surgical Margin | |||||
| Positive | 8 | 37.5±21.0 | 0.08 | 50.0±25.0 | 0.13 |
| Negative (including CPR) | 25 | 72.2±12.0 | 81.8±10.1 | ||
Timing of Surgical Resection
Twenty-two patients had localized disease at diagnosis and underwent either up-front or delayed therapy for local control. Nine patients underwent surgery prior to receiving any chemotherapy or radiation. The other 13 patients received chemotherapy alone or chemotherapy and radiation prior to definitive local therapy, surgery (n=11) or radiation (n=2). The two patients who received radiation for local therapy instead of surgery had tumors < 8cm in diameter, had complete radiographic responses to neoadjuvant chemotherapy and radiation, and received additional radiation to the tumor bed for local therapy. For both patients, there was a question whether the ribs adjacent to the tumor were actually involved. One of these patients had a distant chest wall recurrence after 1.6 years, disease progression, and died one year later. The other patient suffered a local recurrence after 10 years and died almost 2 years later from disease progression.
For the 20 patients with localized disease who underwent surgery the median tumor size at diagnosis was 9 cm (range, 4-11 cm) for the up-front group and 9 cm (range, 4-17 cm) for the delayed group (p=0.64) (Table 3). Patients with up-front surgery had a median of 3 ribs (range, 0-4) resected compared to a median of 2 ribs (range, 0-3) for the delayed group (Table 3). Synthetic material was used to reconstruct/close the chest wall in 5 of 9 (56%) patients and 5 of 11 (45%) in the delayed group (Table 3). Negative surgical margins were achieved in 6 of 9 (67%) patients in the up-front group and 8 of 11 (73%) patients in the delayed group, 4 of whom demonstrated a complete pathological response. Adjuvant radiation was administered to 3 of 9 (33%) patients who underwent up-front surgery; these 3 patients all had positive margins. Seven of 11 (64%) patients in the delayed surgery group received adjuvant radiation; 3 patients had positive margins, 1 patient had spinal cord compression, 1 patient had less than 20% necrosis seen on the pathologic specimen, 1 patient had “close” margins, and 1 patient underwent pulmonary lobectomy at the initial operation with negative margins. Locoregional failure occurred in 1 of 9 (11%) patients in the up-front group with a pleural-based recurrence, and 1 of 11 (9%) in the delayed group with a local recurrence in the chest wall. Of note, both of these patients had received adjuvant radiation therapy for positive surgical margins. There was no evidence of a significant difference in EFS, (p=0.64). However, there was evidence of a marginally significant difference in survival distributions among localized patients in the up-front and the delayed therapy groups (p=0.058); the 15-year estimate for patients who underwent up-front surgery was 100%, compared to 81.8% ± 14.2% for patients with delayed surgery. However, one patient in the delayed group died from an unrelated nonmalignant cause, and an additional patient died from complications after developing ALL. Thus, when considering only deaths related to Ewing sarcoma, there was no evidence of a significant difference in disease-specific survival among the delayed vs. up-front surgery groups (p=0.42).
Table 3.
Characteristics among Localized Patients^ by Timing of Surgical Resection Characteristics of patients with localized chest wall Ewing sarcoma according to timing of surgical resection (up-front versus delayed).
| Up-front Surgical Resection (n=9) | Delayed Surgical Resection (n=ll) | P-value | |
|---|---|---|---|
| Tumor size (cm) | |||
| Median | 9 | 9 | 0.64 |
| Range | 4–11 | 4–17 | |
| Tumor size | |||
| <8 cm | 4 (44%) | 4 (36%) | 1 |
| =8 cm | 5 (56%) | 7 (64%) | |
| Mumber of ribs resected | |||
| Median | 3 | 2 | 0.36 |
| Range | 0–4 | 0–3 | |
| Use of mesh | |||
| Yes | 5 (56%) | 5 (45%) | 1 |
| No | 4 (44%) | 5 (45%) | |
| Data not available | 0 | 1 (9%) | |
| Margin status | |||
| Positive | 2 (22%) | 3 (27%) | 1 |
| Negative | 7 (78%) | 8 (73%) | |
| Use of adjuvant radiation | |||
| Yes | 3 (33%) | 7 (64%) | 0.37 |
| No | 6 (67%) | 4 (36%) | |
Adjuvant Radiation Therapy
Of the twenty-two patients with localized disease, 9 patients did not receive radiation and 13 patients were treated with radiation. Of the 13 patients receiving radiation, 10 received adjuvant radiation and 3 received neoadjuvant radiation, 2 of whom received additional radiation as definitive local therapy instead of surgery. We excluded the latter 3 patients from the analysis, so we could compare patients who did not receive radiation to those who received adjuvant XRT. There was a significant difference in median tumor size observed between patients who did not receive, 6 cm (range, 4-10 cm), and those who received adjuvant radiation, 10 cm (range, 5-14 cm) (p=0.04) (Table 4). All 9 patients who did not receive radiation had negative surgical margins, with 3 having a complete pathologic response. Four of 10 patients treated with adjuvant radiation had negative margins, and 1 patient had a complete pathologic response. In the no radiation group, one patient with a tumor > 8 cm and negative surgical margins developed a locoregional recurrence. One patient in the adjuvant radiation group developed a local recurrence, he had a tumor > 8 cm and a positive surgical margin. Also, two patients in each group developed distant recurrence, yielding a 15-year estimated EFS of 74.1% ± 15.4% for the no radiation group and 80.0% ± 14.6% for the adjuvant radiation group (p=0.69). The 15-year estimates of survival were 100% for the no radiation group and 80.0% ± 14.6% for the adjuvant radiation group (p=0.35).
Table 4. Characteristics among Localized Patients^ by Use of Adjuvant Radiation.
Characteristics of patients with localized chest wall Ewing sarcoma according to use of adjuvant radiation therapy.
| Adjuvant Radiation (n=10) | No Radiation (n=9) | P-value | |
|---|---|---|---|
| Tumor size (cm) | |||
| Median | 10 | 6 | 0.038 |
| Range | 5–14 | 4–10 | |
| Tumor size | |||
| <8 cm | 2 (20%) | 6 (67%) | 0.070 |
| =8 cm | 8 (80%) | 3 (33%) | |
| Margin status | |||
| Positive | 5 (50%) | 0 (0%) | 0.033 |
| Negative | 5 (50%) | 9 (100%) | |
| 15-year Survival Estimate±1 standard error | 80.0±14.6% | 100±0% | 0.35 |
| 15-year EFS Estimate±1 standard error | 80.0±14.6% | 74.1±15.4% | 0.69 |
Discussion
In 1979, a treatment protocol was instituted at SJCRH for patients with Ewing sarcoma which required that multi-agent chemotherapy (vincristine, actinomycin D, cyclophosphamide, and doxorubicin) be given to all patients. Here we present the ensuing 30-years of experience with Ewing sarcoma of the chest wall. Fourteen (39%) patients had metastatic disease upon diagnosis. For the entire cohort, the median follow-up was 14.2 years. The 15-year estimates of EFS and survival were 56% and 66%, respectively. We identified localized disease and tumor size to be factors marginally associated with EFS and survival. Previously, patient age at diagnosis, site of disease, initial tumor size, chemotherapy administered, and tissue of origin have been shown to be associated with outcomes in patients with Ewing sarcoma and/or those with chest wall primaries.13,4,1,14,15,16 Also, Lin et al found that response to chemotherapy was an important predictor of local recurrence after surgical resection of Ewing sarcoma.17
We focused our attention on the 22 patients with localized disease. Two these patients with tumors < 8 cm received radiation therapy as the sole modality for local control. They both demonstrated complete radiographic responses, but ultimately developed disease progression and died, overall survival of 2.5 and 11.5 years. For the remaining 20 patients who all underwent surgical resection the estimated 15-year survival was 90%. Shamberger et al reported an estimated 5-year survival of 61% for patients with nonmetastatic chest wall ES.4,17 We further analyzed the patients with localized disease, specifically addressing the timing of surgical resection and the role of adjuvant radiation.
Surgical resection of Ewing sarcoma tumors of the chest wall can be performed as the initial therapy (up-front) or after the patient has received neoadjuvant chemotherapy (delayed). Roughly half of our patients with localized disease underwent up-front surgical resection. Patients who underwent up-front surgery had larger chest wall resections with a median of 3 ribs resected compared to 2 ribs in the delayed group (p=0.36). In 1988, Rao et al reported the early SJCRH experience and noted that patients with good responses to neoadjuvant chemotherapy often only required resection of the involved rib and were spared a larger chest wall resection.2 The additional 20+ years of experience here in reported support this initial observation. Comparable margin negativity rates were found between the up-front and delayed groups (67% and 73%, respectively). This 67% margin negativity rate for patients who underwent up-front surgery is higher than the 50% reported by Shamberger et al.5 Larger chest wall resections, including multiple ribs, could explain the high margin negativity rate in our cohort. Consequently, only 3 (33%) patients with up-front surgery received adjuvant radiation. Curiously, 7 (64%) patients with delayed surgical resection received adjuvant radiation. One possible explanation for the fact that fewer patients in the up-front group received adjuvant radiation is that many of these patients underwent their operation early on in our experience when the use of adjuvant radiation was less prevalent. Importantly, 4 of 11 (36%) of the patients who underwent delayed surgical resection were found to have a complete pathologic response. Locoregional failure was infrequent with only one patient in each group developing a local recurrence. We found no significant difference in EFS between the groups. Shamberger et al also found that local recurrence rate and EFS did not significantly differ between patients who underwent up-front and delayed surgical resection.4 As mentioned earlier, patients with localized disease treated with surgical resection (up-front or delayed) and chemotherapy with or without radiation had excellent long-term survival, estimated 15-year survival of 90%.
Next, we wanted to elucidate the impact and role of adjuvant radiation therapy. Among patients with localized disease who underwent surgery, 9 patients did not receive radiation, while 10 received adjuvant radiation. In our cohort, patients who did not receive radiation had significantly smaller tumors and all had negative surgical margins with 3 patients demonstrating a complete pathologic response. One patient in the no adjuvant radiation group developed a pleural based locoregional recurrence; this patient had a 10 cm tumor at diagnosis. EFS did not differ between the no radiation and the adjuvant radiation group (15-year estimates 74% ± 15% and 80% ± 15%, respectively, p=0.69); most of the events were distant recurrences. Schuck et al examined a cohort of patients with localized disease and compared those who received adjuvant radiation to those who did not.14 The radiation group had a higher percentage of patients with tumor pleural infiltration, pleural effusion, and intraoperative tumor contamination of the pleural cavity than the no radiation group. Of note, the no radiation group had significantly larger tumors than the radiation group. The 7-year EFS was 63% for the radiation group and 46% for the no radiation group, (p=0.13). Thus, the authors concluded that adjuvant hemithorax radiation should be recommended.15 When interpreting the results of this study we must keep in mind that the group who did not receive radiation had larger tumors and thus is expected to have a worse EFS. Finally, in our cohort the estimated 15-year survival rate for those who did not receive adjuvant radiation was 100%, compared to 80% for those who received adjuvant radiation. Thus, there is a group of patients who likely receive minimal benefit from adjuvant radiation.
Limitations of our study include the retrospective design. Also, SJCRH is a large referral center, so our patient population may have more advanced or complicated disease than other hospitals, which would ultimately effect treatment strategies and outcomes. Fortunately, the chemotherapeutic regimen has been standardized at SJCRH since 1979 with little variation. But, the use of radiation therapy was less standardized and was frequently evaluated on a case-by-case basis. Despite the fact that our review encompasses the last 30-years of experience at SJCRH the sample size is fairly small with only 36 patients.
In conclusion, Ewing sarcoma of the chest wall is a complex problem requiring a multidisciplinary approach with multimodal therapy, but long-term survival is achievable. High rates of margin negativity can be achieved with up-front surgery, but this requires large chest wall resections. Administering chemotherapy first and performing a delayed surgical resection decreases the extent of chest wall resection and helps define a patient population with favorable tumor biology (i.e. complete pathologic responders). Adjuvant radiation therapy plays a vital role in the treatment of patients with Ewing sarcoma of the chest wall, but there are patient populations who have excellent outcomes in the absence of adjuvant radiation therapy. It seems that in addition to patients with complete pathologic responses to induction chemotherapy, patients with tumors <8 cm and negative surgical margins can safely receive surgical resection as the sole mode of local therapy. In our cohort, these two patient populations included 45% of all patients with localized disease. Thus, a significant portion of patients with Ewing sarcoma of the chest wall could be spared adjuvant radiation therapy and its potential long-term complications. Further prospective studies are needed to determine if it is safe to omit adjuvant radiation from the treatment regimen for these patient populations.
Reference List
- 1.Cotterill SJ, Ahrens S, Paulussen M, Jurgens HF, Voute PA, Gadner H, et al. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 2.Rao BN, Hayes FA, Thompson EI, Kumar AP, Fleming ID, Green AA, et al. Chest wall resection for Ewing's sarcoma of the rib: an unnecessary procedure. Ann Thorac Surg. 1988;46:40–44. doi: 10.1016/s0003-4975(10)65849-3. [DOI] [PubMed] [Google Scholar]
- 3.Rao BN, Hayes FA, Thompson EI, Kumar AP, Fleming ID, Green AA, et al. Chest wall resection for Ewing's sarcoma of the rib: an unnecessary procedure. 1988. Updated in 1995. Ann Thorac Surg. 1995;60:1454–1455. doi: 10.1016/0003-4975(95)00474-y. [DOI] [PubMed] [Google Scholar]
- 4.Shamberger RC, LaQuaglia MP, Krailo MD, Miser JS, Pritchard DJ, Gebhardt MC, et al. Ewing sarcoma of the rib: results of an intergroup study with analysis of outcome by timing of resection. J Thorac Cardiovasc Surg. 2000;119:1154–1161. doi: 10.1067/mtc.2000.106330. [DOI] [PubMed] [Google Scholar]
- 5.Shamberger RC, LaQuaglia MP, Gebhardt MC, Neff JR, Tarbell NJ, Marcus KC, et al. Ewing sarcoma/primitive neuroectodermal tumor of the chest wall: impact of initial versus delayed resection on tumor margins, survival, and use of radiation therapy. Ann Surg. 2003;238:563–567. doi: 10.1097/01.sla.0000089857.45191.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krasin MJ, Davidoff AM, Rodriguez-Galindo C, Billups CA, Fuller CE, Neel MD, et al. Definitive surgery and multiagent systemic therapy for patients with localized Ewing sarcoma family of tumors: local outcome and prognostic factors. Cancer. 2005;104:367–373. doi: 10.1002/cncr.21160. [DOI] [PubMed] [Google Scholar]
- 7.Corn BW, Trock BJ, Goodman RL. Irradiation-related ischemic heart disease. J Clin Oncol. 1990;8:741–750. doi: 10.1200/JCO.1990.8.4.741. [DOI] [PubMed] [Google Scholar]
- 8.Horning SJ, Adhikari A, Rizk N, Hoppe RT, Olshen RA. Effect of treatment for Hodgkin's disease on pulmonary function: results of a prospective study. J Clin Oncol. 1994;12:297–305. doi: 10.1200/JCO.1994.12.2.297. [DOI] [PubMed] [Google Scholar]
- 9.Strong LC, Herson J, Osborne BM, Sutow WW. Risk of radiation-related subsequent malignant tumors in survivors of Ewing's sarcoma. J Natl Cancer Inst. 1979;62:1401–1406. [PubMed] [Google Scholar]
- 10.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalbfleisch JD, PR The statistical analysis of failure time data. 1980. p. 169.
- 12.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 13.Krasin MJ, Rodriguez-Galindo C, Billups CA, Davidoff AM, Neel MD, Merchant TE, et al. Definitive irradiation in multidisciplinary management of localized Ewing sarcoma family of tumors in pediatric patients: outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 2004;60:830–838. doi: 10.1016/j.ijrobp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Schuck A, Ahrens S, Paulussen M, Kuhlen M, Konemann S, Rube C, et al. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys. 2003;55:168–177. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 15.Schuck A, Ahrens S, Konarzewska A, Paulussen M, Frohlich B, Konemann S, et al. Hemithorax irradiation for Ewing tumors of the chest wall. Int J Radiat Oncol Biol Phys. 2002;54:830–838. doi: 10.1016/s0360-3016(02)02993-0. [DOI] [PubMed] [Google Scholar]
- 16.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 17.Lin PP, Jaffe N, Herzog CE, Costelloe CM, Deavers MT, Kelly JS, et al. Chemotherapy response is an important predictor of local recurrence in Ewing sarcoma. Cancer. 2007;109:603–611. doi: 10.1002/cncr.22412. [DOI] [PubMed] [Google Scholar]