Abstract
Aim. To investigate the biomechanical effects of zoledronic acid (ZA) on femurs of female osteoporotic rats after follow-up periods of 9 and 12 months. Methods. Eighty female Wistar rats were prospectively assessed. At 60 days of age, the animals were randomly divided into two groups: bilateral oophorectomy (O) (n = 40) and sham surgery (S) (n = 40). At 90 days of age, groups O and S were randomly subdivided into four groups, according to whether 0.1 mg/kg of ZA or distilled water (DW) was intraperitoneally administered: OZA (n = 20), ODW (n = 20), SZA (n = 20), and SDW (n = 20). The animals were sacrificed at 9 and 12 months after the administration of the substances, and then their right femurs were removed and analyzed biomechanically. Axial compression tests that focused on determining the maximum load (N), yield point (N), and stiffness coefficient (N/mm) of the proximal femur were performed in the biomechanical study. Results. ZA significantly increased the maximum load and yield point, reducing the stiffness coefficient concerning the oophorectomy status and follow-up period. Conclusion. Zoledronic acid, at a dose of 0.1 mg/kg, significantly increased the maximum loads and yield points and reduced the stiffness coefficients in the femurs of female rats with osteoporosis caused by bilateral oophorectomy.
1. Introduction
Osteoporosis can be considered a pandemic. Approximately 1.66 million fractures due to osteoporosis are estimated to occur worldwide every year, and this number is expected to quadruple by the year 2050 [1–3].
Because its insidious and silent evolution, fractures are one of the most frequent symptoms. These fractures result from a loss of bone mass and a fragile microscopic architecture of the bone tissue. Osteoporotic fractures are associated with a direct or indirect force on weakened bone tissue, which induces continuity failures in the trabeculae [4–6]. Some medications, such as bisphosphonates, can stop and even reverse the degenerative disease process in the bone.
Bisphosphonates are derived from pyrophosphate and are used in diseases with high rates of bone remodeling, such as Paget's disease, bone tumors, malignant hypercalcaemia, and osteoporosis [5, 7–9]. Given their high affinity for bone calcium, these substances act specifically on skeletal tissue and reduce its reabsorption by inhibiting osteoclasts. Zoledronic acid (ZA), a new bisphosphonate, has been shown to be the most potent inhibitor of bone reabsorption. Studies have shown that ZA is up to 100 times more effective than alendronate and up to 10,000 times more effective than etidronate, besides its once a year posology [10–13].
Several existing bisphosphonates work on the bone tissue increasing its mass; however, there are not enough studies that explain how those drugs act on bone mechanical performance.
The aim of the present study was to investigate the effects of ZA on the biomechanical properties of femoral bone tissue in osteoporotic female rats.
2. Materials and Methods
Eighty 30-day-old female Wistar rats (Rattus norvergicus albinus) were used. After being clinically evaluated and weighted, they were housed in polypropylene cages with a controlled temperature and light-dark cycle environment, with a rodent diet (Labina, Nestlé Purina PetCare Company) and water ad libitum.
Upon reaching a reproductive age (i.e., at 60 days of age, which was 30 days after their arrival in the laboratory), the rats were equally and randomly assigned in two groups: group O (oophorectomy) (n = 40) and group S (sham surgery) (n = 40). The random assignment was conducted using opaque, sealed envelopes.
An oophorectomy was performed after intraperitoneal anesthesia with 30 mg/kg of 3% sodium pentobarbital and a bilateral dorsolateral trichotomy just below the last rib, under sterile conditions. A longitudinal incision approximately 1.5 cm in length was made on the skin between the last rib and the hip joint. The peritoneal cavity was exposed, and the muscle layer was divulsed for access to the ovary and its surrounding adipose tissue. The ovary was ligated with a 3.0 cotton thread and resected distally to the ligature (Figure 1). The muscle and skin were joined with a 3–0 mono nylon suture, and the same procedures were repeated on the contralateral side to remove the other ovary. The animals in group S were submitted to the same procedure described above, except for the ligation and resection of the ovaries.
Figure 1.
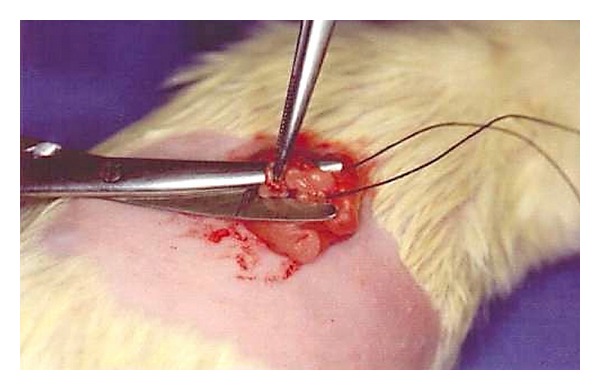
Oophorectomy procedure.
A new sorting was performed at 90 days of age (30 days after the surgical procedures), and groups O and S were further subdivided into four subgroups according to whether they received 0.1 mg/kg of ZA (Aclasta, Novartis Biociências S.A.) or distilled water (DW) intraperitoneally, which yielded the following groups: OZA (n = 20), ODW (n = 20), SZA (n = 20), and SDW (n = 20).
Ten animals were randomly selected from each group and sacrificed after nine or 12 months of receiving the substances (moments 1 and 2) with a lethal intraperitoneal dose of 3% sodium pentobarbital at 80 mg/kg. The animals' right femurs were disarticulated at the proximal (hip) and distal (knee) areas soaked in a 0.9% saline solution, wrapped in aluminum foil, labeled, and stored in a freezer at a controlled temperature of −20°C for 24 hours until the mechanical tests were performed.
Axial compression tests were performed to determine the mechanical properties of the femurs using the Universal Testing Machine EMIC, model DL 10,000, with an accuracy of ±(0.018 + F/3700) kN, calibrated accordingly to ISO 7500-1:2004 and ABNT NBR 6674:1999 standards. The machine interfaced with a computer running Windows 2000 and the Mtest program (DDL, MN, USA), which provided graphical control and was used to record the results.
The femurs were thawed 12 hours before the mechanical tests and kept wrapped in gauze soaked with a 0.9% saline solution. The distal extremities of the specimens were then fixed (in a proportion of 2 : 3) vertically in 35 mL plastic containers containing 30 mL of auto-polymerizing acrylic resin. The vertical fixation of the samples was achieved with the aid of a goniometer positioned in two orthogonal planes with respect to the femurs.
A cleaver with a blunt concave edge (approximately 2 mm diameter), fixed to the assay machine, was manually positioned axially to the femur and aligned with its head (Figure 2), until the computer software indicated an axial preload of 0,1N. Once the test was initiated, the cleaver of the testing machine descended axially on the femoral head at a constant speed of 30 mm/min [14–18] (Figure 3). The M test program interrupted the test at the specimen's breaking point and automatically provided the results.
Figure 2.
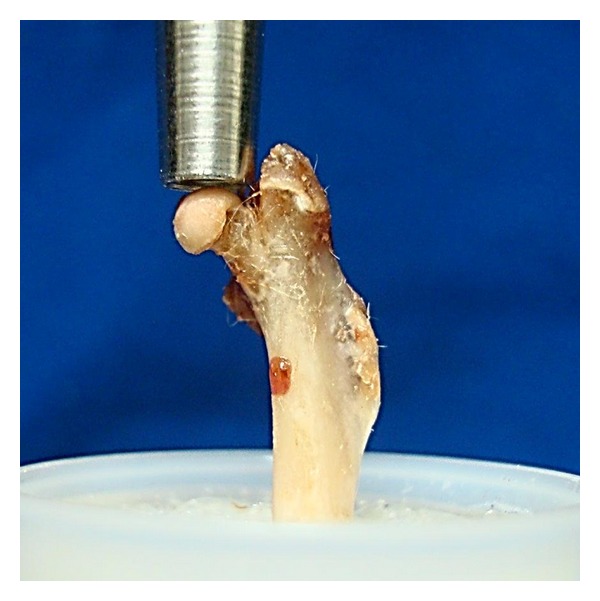
Biomechanical test preparation.
Figure 3.
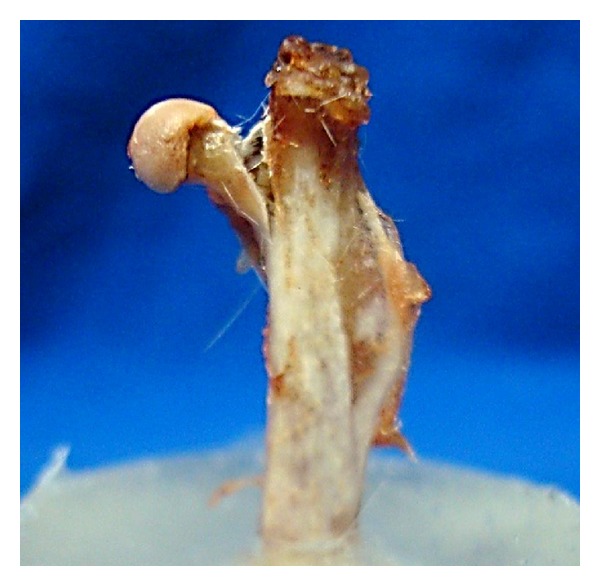
Bone breaking point.
The maximum load (ML) (Newtons-N) upon rupture and the load-deformation curve were automatically provided by the program (Figure 4). The other variables, including the yield point (YP) (Newtons-N) and stiffness coefficient (SC) (Newtons per millimeters-N/mm), were calculated by applying Johnson's method to the results provided by the program.
Figure 4.
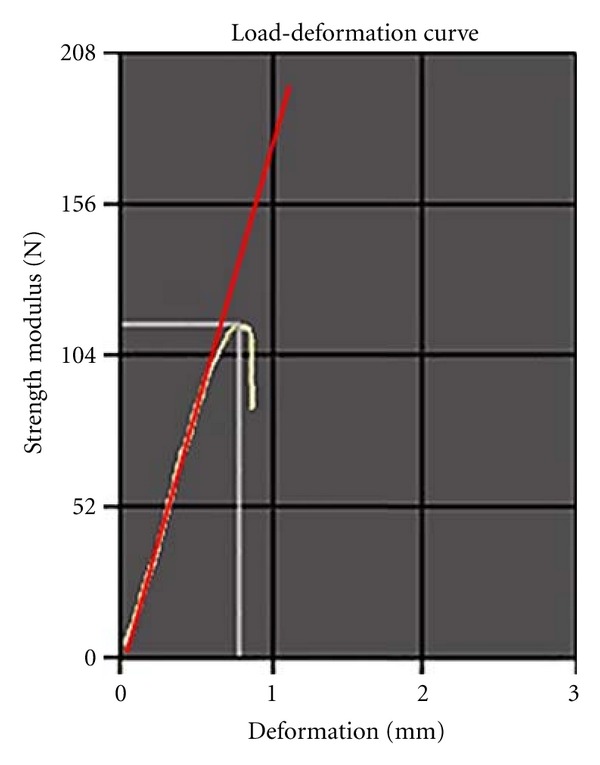
Load-deformation curve.
3. Statistical Analysis
The statistical analysis comprised a completely randomized analysis of variance model and Tukey's multiple comparison tests in the SigmaStat version 3.5.1.2 (Systat Software, Inc., Germany, 2006) and Minitab version 15.1.30.0 (Minitab Inc., 2007) statistical software. The variables were assessed using a 2 × 2 × 2 analysis of variance factorial design with the following three factors (each with two levels):
surgical procedure (oophorectomy versus sham surgery);
substance administered (ZA acid versus DW);
moment of euthanasia (nine versus 12 months).
Both longitudinal and cross-sectional statistical studies were performed, in order to investigate the biomechanical parameters and conclude if (a) there was a “time influence,” (b) if there was a “drug influence” and (c) if there was a “surgery influence.” A repeated measurement two-way analysis of variance (ANOVA) for the longitudinal study design over the biomechanical parameters were used. If F values for a given variable were found to be significant, a paired Student's t-test was employed. Equally, the cross-sectional study design used a two-way ANOVA analysis. When F values were found to be significant, a Holm-Sidak's post hoc test was used.
For all the comparisons, differences were deemed to be statistically significant at P < 0.05.
4. Results
All the biomechanical measurements, averages, and P values are displayed in Tables 1, 2, 3, and 4.
Table 1.
Maximum load (N) at 9 and 12 months.
| Group | Maximum Load | |||||
|---|---|---|---|---|---|---|
| 9 months | 12 months | |||||
| Animal | Measurement (N) | Mean ± SD | Animal | Measurement (N) | Mean ± SD | |
| ODW | 1 | 134.5 | 124.2 ± 12.1 | 11 | 119.1 | 112.6 ± 8.9 |
| 2 | 111.4 | 12 | 95.4 | |||
| 3 | 105.8 | 13 | 111.5 | |||
| 4 | 110.5 | 14 | 113.1 | |||
| 5 | 133.2 | 15 | 116.8 | |||
| 6 | 137.1 | 16 | 120.5 | |||
| 7 | 135.4 | 17 | 124.2 | |||
| 8 | 125.1 | 18 | 107.5 | |||
| 9 | 115.7 | 19 | 101.7 | |||
| 10 | 133.1 | 20 | 116.3 | |||
|
| ||||||
| OZA | 31 | 128.8 | 141.4 ± 9.8 | 21 | 125.4 | 138.7 ± 9.1 |
| 32 | 133.2 | 22 | 131.7 | |||
| 33 | 152.5 | 23 | 147.2 | |||
| 34 | 151.7 | 24 | 149.3 | |||
| 35 | 137.0 | 25 | 133.1 | |||
| 36 | 137.4 | 26 | 135.8 | |||
| 37 | 158.4 | 27 | 153.0 | |||
| 38 | 139.0 | 28 | 140.7 | |||
| 39 | 132.8 | 29 | 129.4 | |||
| 40 | 143.0 | 30 | 141.2 | |||
|
| ||||||
| SDW | 41 | 126.0 | 140.0 ± 6.6 | 51 | 109.0 | 118.4 ± 10.5 |
| 42 | 138.9 | 52 | 111.0 | |||
| 43 | 149.4 | 53 | 125.5 | |||
| 44 | 135.7 | 54 | 110.3 | |||
| 45 | 141.4 | 55 | 116.8 | |||
| 46 | 136.8 | 56 | 110.6 | |||
| 47 | 147.1 | 57 | 124.1 | |||
| 48 | 144.2 | 58 | 140.9 | |||
| 49 | 138.4 | 59 | 109.7 | |||
| 50 | 142.0 | 60 | 126.3 | |||
|
| ||||||
| SZA | 71 | 142.5 | 149.7 ± 4.3 | 61 | 142.7 | 151.0 ± 9.7 |
| 72 | 152.9 | 62 | 150.4 | |||
| 73 | 151.7 | 63 | 154.1 | |||
| 74 | 147.1 | 64 | 148.9 | |||
| 75 | 152.8 | 65 | 137.6 | |||
| 76 | 154.9 | 66 | 170.7 | |||
| 77 | 149.2 | 67 | 150.2 | |||
| 78 | 154.2 | 68 | 140.4 | |||
| 79 | 143.7 | 69 | 158.8 | |||
| 80 | 148.5 | 70 | 156.7 | |||
Table 2.
Yield point (N) at 9 and 12 months.
| Group | Yield Point | |||||
|---|---|---|---|---|---|---|
| 9 months | 12 months | |||||
| Animal | Measurement (N) | Mean ± SD | Animal | Measurement (N) | Mean ± SD | |
| ODW | 1 | 122.8 | 113.0 ± 11.4 | 11 | 113.3 | 105.7 ± 9.0 |
| 2 | 110.5 | 12 | 90.8 | |||
| 3 | 120.3 | 13 | 102.4 | |||
| 4 | 97.5 | 14 | 106.3 | |||
| 5 | 117.1 | 15 | 107.6 | |||
| 6 | 97.7 | 16 | 118.1 | |||
| 7 | 118.5 | 17 | 115.2 | |||
| 8 | 123.5 | 18 | 98.6 | |||
| 9 | 97.5 | 19 | 95.4 | |||
| 10 | 125.0 | 20 | 110.6 | |||
|
| ||||||
| OZA | 31 | 127.4 | 129.7 ± 6.0 | 21 | 100.5 | 119.8 ± 9.7 |
| 32 | 131.8 | 22 | 111.4 | |||
| 33 | 134.2 | 23 | 124.5 | |||
| 34 | 123.5 | 24 | 130.6 | |||
| 35 | 125.1 | 25 | 119.2 | |||
| 36 | 122.5 | 26 | 119.5 | |||
| 37 | 131.8 | 27 | 134.0 | |||
| 38 | 127.5 | 28 | 122.2 | |||
| 39 | 130.4 | 29 | 112.6 | |||
| 40 | 143.2 | 30 | 123.7 | |||
|
| ||||||
| SDW | 41 | 113.5 | 125.3 ± 9.1 | 51 | 124.0 | 112.6 ± 6.2 |
| 42 | 119.4 | 52 | 103.7 | |||
| 43 | 118.2 | 53 | 114.1 | |||
| 44 | 119.6 | 54 | 107.3 | |||
| 45 | 131.1 | 55 | 106.5 | |||
| 46 | 133.2 | 56 | 111.6 | |||
| 47 | 135.7 | 57 | 116.2 | |||
| 48 | 129.8 | 58 | 115.2 | |||
| 49 | 137.9 | 59 | 108.3 | |||
| 50 | 114.6 | 60 | 118.1 | |||
|
| ||||||
| SZA | 71 | 133.3 | 137.0 ± 10.7 | 61 | 103.9 | 124.6 ± 16.8 |
| 72 | 124.4 | 62 | 128.5 | |||
| 73 | 141.0 | 63 | 111.4 | |||
| 74 | 135.8 | 64 | 126.7 | |||
| 75 | 149.5 | 65 | 120.0 | |||
| 76 | 152.0 | 66 | 152.1 | |||
| 77 | 139.8 | 67 | 110.4 | |||
| 78 | 116.1 | 68 | 128.7 | |||
| 79 | 137.2 | 69 | 112.2 | |||
| 80 | 141.7 | 70 | 152.3 | |||
Table 3.
Stiffness coefficient (N/mm) at 9 and 12 months.
| Group | Stiffness Coefficient | |||||
|---|---|---|---|---|---|---|
| 9 months | 12 months | |||||
| Animal | Measurement (N/mm) | Mean ± SD | Animal | Measurement (N/mm) | Mean ± SD | |
| ODW | 1 | 207.4 | 214.2 ± 19.0 | 11 | 218.4 | 236.2 ± 24.4 |
| 2 | 201.0 | 12 | 272.4 | |||
| 3 | 198.8 | 13 | 209.8 | |||
| 4 | 219.2 | 14 | 235.6 | |||
| 5 | 257.1 | 15 | 273.0 | |||
| 6 | 211.6 | 16 | 222.5 | |||
| 7 | 190.7 | 17 | 201.7 | |||
| 8 | 225.9 | 18 | 236.9 | |||
| 9 | 203.8 | 19 | 254.2 | |||
| 10 | 226.5 | 20 | 237.5 | |||
|
| ||||||
| OZA | 31 | 160.2 | 158.2 ± 9.1 | 21 | 175.1 | 176.4 ± 6.7 |
| 32 | 150.4 | 22 | 178.0 | |||
| 33 | 157.5 | 23 | 179.8 | |||
| 34 | 142.8 | 24 | 163.3 | |||
| 35 | 175.0 | 25 | 182.4 | |||
| 36 | 162.4 | 26 | 169.8 | |||
| 37 | 166.0 | 27 | 187.9 | |||
| 38 | 149.3 | 28 | 176.4 | |||
| 39 | 161.1 | 29 | 177.3 | |||
| 40 | 157.7 | 30 | 174.3 | |||
|
| ||||||
| SDW | 41 | 180.6 | 171.7 ± 15.3 | 51 | 212.1 | 219.1 ± 7.0 |
| 42 | 171.7 | 52 | 231.7 | |||
| 43 | 141.9 | 53 | 225.8 | |||
| 44 | 176.1 | 54 | 217.6 | |||
| 45 | 174.4 | 55 | 215.9 | |||
| 46 | 165.0 | 56 | 213.3 | |||
| 47 | 180.1 | 57 | 221.6 | |||
| 48 | 154.3 | 58 | 221.3 | |||
| 49 | 176.4 | 59 | 215.7 | |||
| 50 | 196.5 | 60 | 216.4 | |||
|
| ||||||
| SZA | 71 | 134.3 | 130.1 ± 29.0 | 61 | 144.6 | 153.4 ± 12.3 |
| 72 | 109.6 | 62 | 155.4 | |||
| 73 | 130.9 | 63 | 148.5 | |||
| 74 | 118.2 | 64 | 136.5 | |||
| 75 | 109.1 | 65 | 147.2 | |||
| 76 | 133.8 | 66 | 156.4 | |||
| 77 | 164.7 | 67 | 170.4 | |||
| 78 | 112.7 | 68 | 141.2 | |||
| 79 | 95.9 | 69 | 158.8 | |||
| 80 | 191.9 | 70 | 174.9 | |||
Table 4.
Overview of the biomechanical parameters over time.
| Group | Biomechanical parameter | 9 months (mean ± SD) | P | 12 months (mean ± SD) |
|---|---|---|---|---|
| ODW | ML (N) | 124.2 ± 12.1 | † P = 0.002 | 112.6 ± 8.9 |
| YP (N) | 113.0 ± 11.4 | NS | 105.7 ± 9.0 | |
| SC (N/mm) | 214.2 ± 19.0 | † P = 0.002 | 236.2 ± 24.4 | |
| § P < 0.001 | ||||
|
| ||||
| OZA | ML (N) | 141.4 ± 9.8 | † P = 0.003 | 138.7 ± 9.1 |
| YP (N) | 129.7 ± 6.0 | † P = 0.02 | 119.8 ± 9.7 | |
| SC (N/mm) | 158.2 ± 9.1 | † P < 0.001 | 176.4 ± 6.7 | |
|
| ||||
| SDW | ML (N) | 140.0 ± 6.6 | †§ P < 0.001 | 118.4 ± 10.5 |
| YP (N) | 125.3 ± 9.1 | † P = 0.01 | 112.6 ± 6.2 | |
| SC (N/mm) | 171.7 ± 15.3 | † P < 0.001 | 219.1 ± 7.0 | |
|
| ||||
| SZA | ML (N) | 149.7 ± 4.3 | NS | 151.0 ± 9.7 |
| YP (N) | 137.0 ± 10.7 | NS | 124.6 ± 16.8 | |
| SC (N/mm) | 130.1 ± 29.0 | § P < 0.001 | 153.4 ± 12.3 | |
| † P = 0.01 | ||||
†Longitudinal study design (paired t-test).
§Cross-sectional study design (ANOVA; Holm-Sidak's post hoc).
NS: no significance.
The analysis of variance revealed a significant increase in the maximum load and yield point and a significant decrease in the stiffness coefficient of the femurs of those animals receiving ZA (the OZA and SZA groups), as compared to those that received DW (the ODW and SDW groups).
A significant decrease in the maximum load and yield point and a significant increase in the stiffness coefficient were observed after nine months (moment 1) in the animals that were submitted to the oophorectomy procedure and received DW (group ODW) as compared to the nonoophorectomized group (group SDW). However, this difference was no longer present at 12 months (moment 2).
The analysis of variance showed that the nonoophorectomized animals that received DW (group SDW) had significantly higher maximum load and lower stiffness coefficients at nine months (moment 1) than at twelve months (moment 2). The yield point was not affected by the moment of euthanasia.
5. Discussion
Zoledronic acid was selected in this study due to its high rates of positive bone balance with only one annual dose, decreasing patients' noncompliance with the treatment [19, 20]. The trabecular bone is the main site of action of these drugs, preserving the thickness and density of the bone connections and increasing the resistance of the bone to fractures [21–23]. Patients with severe postmenopausal osteoporosis, that have a high osteoclastic activity, may greatly benefit from this third generation bisphosphonate, simply by keeping their bone mass [22].
Bone tissue has the following three important mechanical properties: bone resistance, which roughly translates to the amount of calcium in the tissue and is defined by the maximum load; stiffness, which represents the fragility of the material and is defined by the stiffness coefficient; and yield point, which is the limit at which irreversible structural damage occurs without the possibility of restoring the original shape. These properties can only be studied experimentally by observing bone behavior when subjected to loading forces. The equipment used in this study, which provided highly accurate results, was developed for testing isotropic materials (e.g., metals); however, biological materials are viscoelastic in nature. The results obtained were not absolute, given the limitations of the measurement techniques.
For determination of biomechanical properties, the assay machine applied to the femurs an axial load under the speed of 30 mm/min, considered by other authors [14–18] as the ideal average speed for viscoelastic materials (bone). It was also taken into consideration the capacity and type of the used assay machine, which does not allow high speed or impact testing. Since the scope of this study was to evaluate only fractures around the hip, the femurs were distally fixated in the acrylic resin in a proportion of 2 : 3; in others studies that considered fractures of the femurs at lower sites (diaphysis and lower diaphysis fractures), femurs were fixated in a proportion of 1 : 3 [14, 15].
The maximum load, defined as the maximum load supportable before the breaking point, was automatically determined by the software; it represents the highest point in the load-deformation curve. Analyzing this variable is essential for studying the biomechanics of any material. The region of interest (ROI) chosen in the present study was the proximal femur, which is a region that is greatly affected by osteoporosis due to the high concentration of trabecular bone. The significantly increased maximum load in the ZA groups (OZA and SZA) can probably be explained by inhibition of osteoclast activity, which diminished the osteoporotic process even in the oophorectomized group (OZA). In similar biomechanical investigations [14, 15], compressive tests conducted on bone regions with lower concentrations of trabecular bone, such as the tibial diaphysis, produced nonsignificant results. The hormonal deficit caused by a bilateral oophorectomy results in a loss of bone mass and consequent decrease in the maximum load. The animals in the present study that received ZA (the OZA group), even those subjected to oophorectomy, had maximum loads equivalent to those of the nonoophorectomized group (the SZA group).
The yield point is the weight limit that the body safely supports before plastic deformation occurs, (i.e., an irreversible deformation or microstructure rearrangement that does not allow the bone to return to its initial shape and dimension). This variable is defined as the last point in the linear phase (elastic phase) of the load-deformation curve. As the yield point is a rarely studied variable, few studies are available for comparison. ZA increased the maximum yield point values in this study, which increased the safety zone over which the bone microstructure remained intact and within the limits of elastic deformation. Choosing an ROI with a high concentration of trabecular bone may have positively influenced these results [13–15].
The stiffness coefficient is one of the major biomechanical evaluation parameters; it indicates the stability or rigidity of the material under the maximum load. The stiffness coefficient is obtained from the relationship between the applied load and body deformation. A higher stiffness should be interpreted as a greater propensity to break under a fixed deformation, which is typical of brittle materials, such as glass. The stiffness coefficient is calculated from the linear portion of the elastic phase of the load-deformation curve (Figure 4). Zoledronic acid reduced the stiffness coefficient in all groups. In other words, ZA reduced femoral stiffness and rendered the bone to be more ductile and less likely to fracture. In a similar study of human tibiae at different patients ages, Burstein et al. [24] concluded that the resistance (maximum load) was similar in young and elderly individuals; however, the “old” bone was more “brittle” and characteristic of “fragile” materials; thus, it had a greater stiffness. Hormonal deficiency tends to increase the stiffness coefficient, making the bone more fragile or brittle. Similar to previous results [8, 25, 26], ZA could maintain and even reduce the bone stiffness coefficients of the oophorectomized animals in the present study (group OZA) to levels similar to those of the non-oophorectomized animals (group SZA). These results suggest that the drug maintained the absolute (i.e., organic and inorganic) bone integrity, which would explain the decrease in the femoral stiffness coefficient.
6. Conclusions
Zoledronic acid administered intraperitoneally at a single dose of 0.1 mg/kg significantly increased the maximum load and yield point and reduced the stiffness coefficient in the proximal region of the femur of oophorectomized rats after nine and 12 months of observation.
Conflict of Interests
The authors have no conflict of interests to declare.
References
- 1.Meirik O, Rowe PJ. WHO—World Health Organization. Annual Technical Report 1998. Geneva, Switzerland: WHO Press; 1999. Research on fertility regulation. Safety and efficacy of existing methods of fertility regulation; pp. 151–169. [Google Scholar]
- 2.Borah B, Gross GJ, Dufresne TE, Smith TS, Cockman MD, et al. Three-dimensional microimaging (MRmI and mCT), finite element modeling, and rapid prototyping provide unique insights into bone architecture in osteoporosis. Anatomical Record. 2001;265:101–110. doi: 10.1002/ar.1060. [DOI] [PubMed] [Google Scholar]
- 3.OMS/WHO—World Health Organization. Report of a Joint WHO/FAO Expert consultation. [WHO technical Report Series] 916. Geneva, Switzerland: WHO Press; 2003. Diet, nutrition and the prevention of chronic diseases. [PubMed] [Google Scholar]
- 4.Pinto Neto AM, Soares A, Urbanetz AA, Souza ACA, Ferrari AEM, Amaral B, et al. Consenso brasileiro de osteoporose 2002. Revista Brasileira de Reumatologia. 2002;42(6):343–354. [Google Scholar]
- 5.Nguyen TV, Blangero J, Eisman JA. Genetic epidemiological approaches to the search for osteoporosis genes. Journal of Bone and Mineral Research. 2000;15(3):392–401. doi: 10.1359/jbmr.2000.15.3.392. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. Journal of the American Medical Association. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Mori S, Kaji Y, Mashiba T, Kawanishi J, Norimatsu H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. Journal of Bone and Mineral Research. 1999;14(6):969–979. doi: 10.1359/jbmr.1999.14.6.969. [DOI] [PubMed] [Google Scholar]
- 8.Chen T, Berenson J, Vescio R, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. Journal of Clinical Pharmacology. 2002;42(11):1228–1236. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel C, Bartsch R, Hussian D, et al. Zoledronate in a patient with pamidronate refractory hypercalcemia syndrome. Supportive Care in Cancer. 2004;12(9):678–681. doi: 10.1007/s00520-004-0645-y. [DOI] [PubMed] [Google Scholar]
- 10.Pataki A, Müller K, Green JR, Ma YF, Li QN, Jee WSS. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anatomical Record. 1997;249:458–468. doi: 10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Cheer SM, Noble S. Zoledronic acid. Drugs. 2001;61(6):799–805. doi: 10.2165/00003495-200161060-00010. [DOI] [PubMed] [Google Scholar]
- 12.Glatt M, Pataki A, Evans GP, Hornby SB, Green JR. Loss of vertebral bone and mechanical strength in estrogen-deficient rats is prevented by long-term administration of zoledronic acid. Osteoporosis International. 2004;15(9):707–715. doi: 10.1007/s00198-004-1588-3. [DOI] [PubMed] [Google Scholar]
- 13.Wise LM, Waldman SD, Kasra M, et al. Effect of zoledronate on bone quality in the treatment of aseptic loosening of hip arthroplasty in the dog. Calcified Tissue International. 2005;77(6):367–375. doi: 10.1007/s00223-005-0062-3. [DOI] [PubMed] [Google Scholar]
- 14.Palacio EP, Jabob EM, Campi TB, Müller SS. O zoledronato no tratamento da osteoporose umeral em ratas. Estudo prospectivo e randomizado. Acta Ortopédica Brasileira. 2010;18:90–95. [Google Scholar]
- 15.Pereira FRA, Dutra RC, Olímpio TCR, Müller SS, Palacio EP. Efeito do ácido zoledrônico em tíbias de ratas ooforectomizadas. Estudo prospectivo e randomizado. Revista Brasileira de Ortopedia. 2009;44:61–68. doi: 10.1016/S2255-4971(15)30051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller SS, Curcelli EC, Sardenberg T, Zuccon A, Crudis JL, Jr., Padovani CR. Ánalise clínica e biomecânica do efeito do diclofenaco sódico na consolidação da fratura da tíbia no rato. Acta Ortopédica Brasileira. 2004;4:197–204. [Google Scholar]
- 17.Smith BA, Livesay GA, Woo SLY. Biology and biomechanics of the anterior cruciate ligament. Clinics in Sports Medicine. 1993;12(4):637–670. [PubMed] [Google Scholar]
- 18.Smith EL, Raab DM. Osteoporosis and physical activity. Acta Medica Scandinavica. 1986;220(711):149–156. doi: 10.1111/j.0954-6820.1986.tb08944.x. [DOI] [PubMed] [Google Scholar]
- 19.Amanat N, Brown R, Bilston LE, Little DG. A single systemic dose of pamidronate improves bone mineral content and accelerates restoration of strength in a rat model of fracture repair. Journal of Orthopaedic Research. 2005;23(5):1029–1034. doi: 10.1016/j.orthres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. Journal of Pharmacology and Experimental Therapeutics. 2001;296(2):235–242. [PubMed] [Google Scholar]
- 21.Wise LM, Waldman SD, Kasra M, et al. Effect of zoledronate on bone quality in the treatment of aseptic loosening of hip arthroplasty in the dog. Calcified Tissue International. 2005;77(6):367–375. doi: 10.1007/s00223-005-0062-3. [DOI] [PubMed] [Google Scholar]
- 22.Amanat N, Brown R, Bilston LE, Little DG. A single systemic dose of pamidronate improves bone mineral content and accelerates restoration of strength in a rat model of fracture repair. Journal of Orthopaedic Research. 2005;23(5):1029–1034. doi: 10.1016/j.orthres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. Journal of Pharmacology and Experimental Therapeutics. 2001;296(2):235–242. [PubMed] [Google Scholar]
- 24.Burstein AH, Reilly DT, Martens M. Aging of bone tissue: mechanical properties. Journal of Bone and Joint Surgery A. 1976;58(1):82–86. [PubMed] [Google Scholar]
- 25.Guo XE, Goldstein SA. Vertebral trabecular bone microscopic tissue elastic modulus and hardness do not change in ovariectomized rats. Journal of Orthopaedic Research. 2000;18(2):333–336. doi: 10.1002/jor.1100180224. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho DCL, Cliquet A. Ação do ultra-som de baixa intensidade sobre ossos de ratas osteopênicas. Acta Ortopédica Brasileira. 2003;11:17–24. [Google Scholar]


