Abstract
A major goal of modern MRI development is to image neural circuits in the central nervous system. Critical to this mission is the ability to describe a number of important parameters associated with neural circuits. These include functional neural architecture, functional activation of neural circuits, anatomical and functional connectivity of neural circuits, as well as factors that may alter neural circuits such as trafficking of immune cells and brain precursor cells in the brain. Remarkably, a variety of work in human and animal brain has demonstrated that all of these features of neural circuits can be visualized with MRI. Here a brief summary is given of new directions that should prove useful in analysis of the normal and pathological human brain and for studies of relevant pre-clinical animal models of neurological and psychiatric disorders. At present there is little work imaging neural circuits in the heart with MRI, however, where applicable present developments and prospects for the future are discussed.
Introduction
Over the last two and a half decades, Magnetic Resonance Imaging (MRI) has become a critical tool for the diagnosis and management of human disease, especially of disorders of the central nervous system (CNS). The impact of MRI owes to its ability to make high resolution images that have contrast to pathology. In addition, gadolinium based MRI contrast agents in routine clinical use are relatively safe and sensitive to specific pathology such as disruption of the blood brain barrier caused by brain tumors [1] or multiple sclerosis [2] and delayed accumulation or enhancement caused by cell death in heart [3].
The past ten years has seen an explosion in the range of applications of MRI. This has been due to the development of new “functional” MRI techniques in the late 1980’s and early 1990’s that have enabled contrast to be generated to a wide range of physiological processes. Sensitizing MRI to water diffusion has enabled an early view of tissue damage, providing important information for the diagnosis and treatment of stroke [4, 5]. The anisotropy of water diffusion in white matter had led to rapidly increasing use of MRI to study white matter tracts in normal and diseased brain [4, 6, 7]. In addition, there have been attempts to use diffusion anisotropy to map fiber orientation in the heart [8]. Finally, the sensitivity of MRI to blood volume [9], blood flow [10], and most importantly blood oxygenation [11] has led to detailed mapping of perfusion in the heart and brain activity during a wide range of cognitive tasks [12, 13]. Similarly, in the heart, quantitative perfusion techniques are having a broad impact [14]. Thus, there has been a transformation in MRI from applications aimed primarily at detecting anatomical markers of disease processes, towards approaches that include the acquisition of functional information. Together with continued technological advancement and the development of sophisticated MR contrast agents, this has led to improved understanding of normal and abnormal organ function that has begun to have widespread clinical impact.
MRI is continuing to develop at a fast pace and recent developments in technology are enabling the anatomical and functional basis of neural circuits to be visualized at increased resolution with novel contrast sensitive to specific aspects of neural circuits. The majority of this work has progressed in the CNS, however, it is anticipated that the lessons learned will have growing application in the peripheral nervous system. The goal of this short review is to emphasize those newer developments which are predicted to have increasing impact on studying the normal and pathological brain in the next five to ten years. Throughout the history of MRI of brain, fruitful new directions have arisen from work on animal models as well as the human brain. Current developments that are presently only applicable to animal models, such as development of novel contrast agents, are discussed as well. When applicable, the relevance to imaging the heart is discussed.
MRI of Brain is Beginning to Visualize Functional Architecture Directly
In the CNS, MRI contrast has traditionally been used to distinguish grey matter, white matter and CSF. However, it is well known from cytoarchitectural studies using a wide range of histological stains that there is remarkable degree of heterogeneity in white and grey matter structures. Indeed, this heterogeneity in histological stains has been used for decades to subdivide the brain into functional areas [15-17]. Thus, it would be a great step forward for defining neural circuits in vivo if MRI could directly visualize this functional heterogeneity. The ability to detect cortical myelin based on well established MRI contrast was originally described by Clark and co-workers [18] as an approach for using MRI to accomplish cytoarchitectonics in vivo. This idea has received renewed attention [19-21] but has not yet reached the point of widespread application. In animal brains, reports of being able to detect laminar structures with MRI have been limited to the olfactory bulb [22]. For detecting functional neuroanatomy there has been much interest in using MnCl2, as an MRI contrast agent administered systemically to rodents [23, 24]. About a day after delivering MnCl2 image features that define architectural boundaries are readily apparent. The laminar structure of the hippocampus, olfactory bulb, cerebellum, and cortex have been readily observed (Fig 1). It should be straightforward to use this laminar contrast to subdivide the brain into many functionally specific regions. This near histological contrast is beginning to find application in studying the changes in brain architecture caused by disease in pre-clinical models of a wide range of neurological disorders [25]. MnCl2 has been used to image heart and shown to be able to delineate ischemic regions [26].
Figure 1.
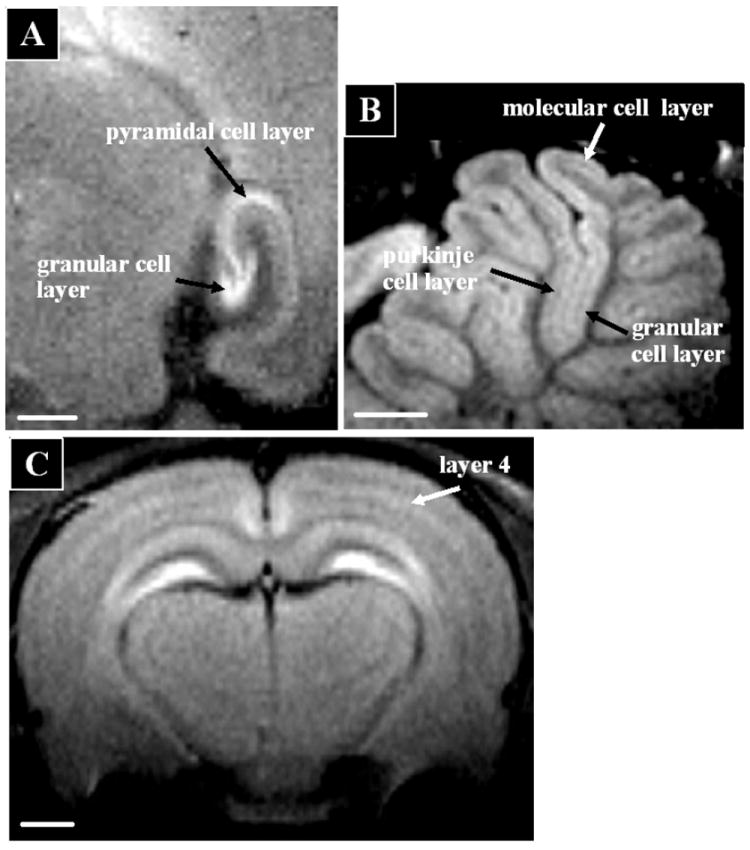
Manganese enhanced MRI enables functional neuroarchitecture to be detected in the in vivo rat brain. Images were taken 24-48 hours after intravenous infusion of MnCl2 at 100 micron resolution using and 11.7 T animal MRI system. Scalebar = 1mm. A) Cell layers of the hippocampus; B) Layers in the cerebellum, and C) layers in cerebral cortex. Images adapted from [24].
A shortcoming of using MnCl2 is that it is a know neurotoxin. Mn2+ is also an essential heavy metal, therefore, results from the animal can be translated to human only after careful consideration of safety. However, very recent work using high field MRI indicates that there exists endogenous contrast that will be sensitive to details of functional neuroarchitecture [27]. The ability of MRI to study brain anatomy is primarily determined by resolution and contrast, both of which have seen dramatic improvements in recent years. A large part of this improvement relates to the advent of high field MRI, and the improvements in detector technology in recent years [28, 29]. At the NIH, sensitivity gains due to increases in magnetic field strength to 7.0 T and parallel detectors with up to 32 elements are leading to increases in sensitivity ranging from 10-25 fold (depending on brain area) over data obtained using single detector receivers at 1.5 T. This order of magnitude improvement in sensitivity can be used to increase spatial resolution and in recent MRI of human brain, pixel volumes of around 50 nl (equivalent to 350 micron isotropic voxels) have been acquired [27, 30]. At these high magnetic fields, substantial contrast improvement can be achieved with so called T2*-weighted or magnetic susceptibility contrast. This type of MRI contrast is generated primarily by local variations in myelin, iron, and deoxy-hemoglobin concentration and forms the basis for BOLD based fMRI and has been used previously to map venous architecture in the brain [31]. Interestingly and surprisingly, at the high sensitivity and resolution afforded at 7 T, susceptibility contrast allows direct visualization of brain architecture. Figure 2a shows a slice from a normal volunteer taken with susceptibility weighted contrast. A large degree of contrast variation is observed throughout the slice. Specific major fiber bundles are readily detected in white matter (Figure 2b). This high resolution identification of fiber bundles nicely complements the lower resolution information currently available with diffusion tensor MRI [6]. It will be interesting in future work to see if individual fiber bundles can be followed to enable tractography between functional areas of the brain Susceptibility contrast has also recently been shown to vary across cortical layers [27], opening up the possibility to robustly detect individual layers in grey matter (Fig. 2c). This contrast was an order of magnitude higher than that described previously using T1 weighted MRI [19]. The robust detection of cortical heterogeneity should enable classification of functional brain regions in individuals. Furthermore, the high contrast and resolution available with susceptibility contrast at high field make this technique promising for the study of diseases in which myelin, iron or deoxyhemoglobin is locally altered, such as Multiple Sclerosis (MS) [32] (Fig 2d), Alzheimer’s Disease (AD) [33-35], brain tumors [36], and stroke [37].
Figure 2.
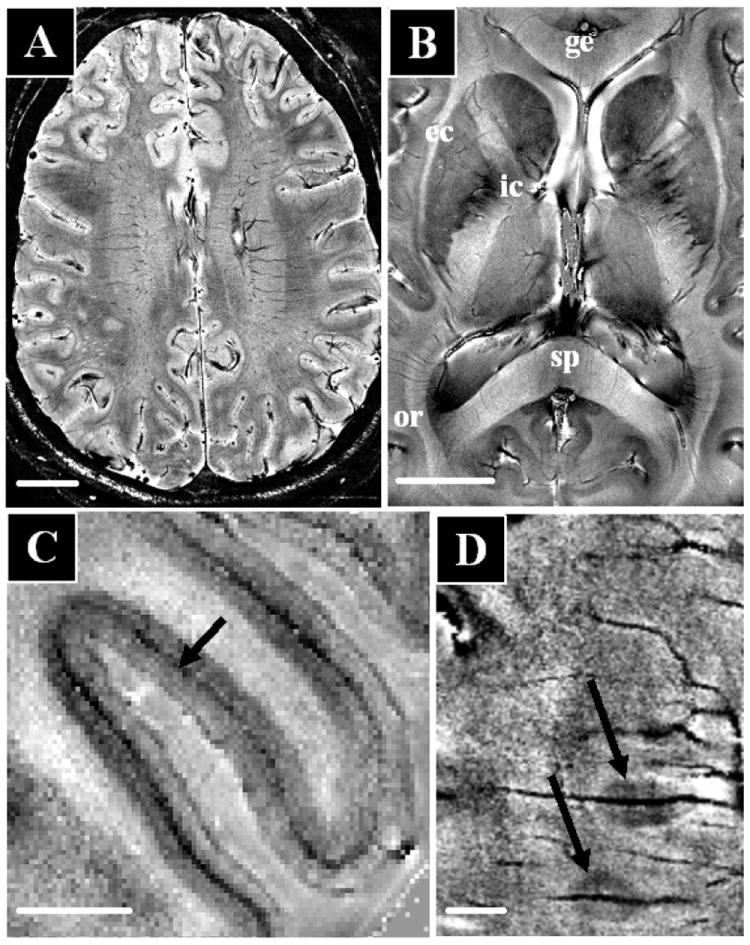
High resolution in-vivo human brain anatomy using multi-channel detectors at 7.0 T and susceptibility contrast using signal magnitude (A) and phase (C-D) at 25-50 nl resolution An axial brain slice (A, scale bar 20 mm) shows strong contrast variation throughout the image. Substantial contrast variations are also seen in the major fiber bundles (B, scale bar 20 mm) including the internal and external capsule (ic, ec), the genu and splenium of the corpus callosum (ge, sp), and the optic radiation (or). Within the visual cortex (C, scalebar 5 mm) intracortical detail allows identification of the line of Gennari (darkening in central layer). In MS (D, scalebar 5 mm), signal phase allow high resolution imaging of small peri-vascular lesions (image courtesy of Francesca Bagnato and Henry McFarland, NIH).
T2* contrast has been applied to the heart as well as to the brain. By adding exogenous T2* contrast agents, Vignaud et al. [38] have been able to map capillary orientation by looking at signal intensity as a function of orientation with respect to the magnetic field. T2* images of the rodent heart have shown exquisite anatomical features such as fiber orientation and the microvasculature [39]. While not finding widespread application to the heart as yet, the wide availability of high field MRI should increase the general usefulness of these approaches.
Functional MRI Can Detect Correlated Network Activity
Increasingly, MRI studies of the brain are aimed at the collection of functional information. The purpose of these studies is to develop a general understanding of normal brain function, and to detect functional abnormalities in disease. These methods rely on contrast intrinsic to the physiologic processes that underlie brain function. An important aspect of this is that there are local increases in blood flow to regions of increased neuronal activity. This forms the basis of functional MRI (fMRI), which either directly, through perfusion fMRI [10], or indirectly, through blood oxygen level dependent (BOLD) fMRI [11], allows detection of activity dependent blood flow changes. This ability to non-invasively map brain activity has had a tremendous impact on the field of cognitive neuroscience. Localization of areas of the brain involved in complex cognitive tasks is now routine and has enabled definition of the neuronal networks involved in a wide range of normal and abnormal behavior. The temporal resolution of fMRI is presently too slow to map the flow of information through neural circuits, however, modeling and work that combines EEG with fMRI show great promise [40, 41]. While most fMRI work relies on analysis of group data, it has had large implications for understanding the cause and pathophysiology of diseases of the brain. Exciting new results providing an understanding of brain plasticity after injury and using fMRI for biofeedback make it likely that clinical applications will grow. Recently the FDA has approved use of fMRI for pre-surgical planning on individuals.
There have been some fMRI studies investigating input from the CNS into autonomic control of cardiovascular function. These studies have helped to define the role of the anterior cingulate cortex, the medial prefrontal cortex, and the insular cortex in regulating sympathetic outflow to the heart [42]. There is growing evidence that there are connections between cortical dysfunction and dysfunction of autonomic control of the heart [43]. Future studies correlating fMRI with vagal activity should improve the understanding the role of the CNS in cardiovascular disease.
An interesting new application of fMRI is the study of functional brain connectivity which shows much promise for determining the network connectivity of activated brain regions. Recent studies have shown that spontaneous blood flow variations in the brain occur in distinct spatial patterns, in which functionally related regions vary in a temporally correlated fashion. These “resting state fluctuations” in absence of external stimuli were first described in motor cortex and showed that motor areas in different hemispheres co-varied in synchrony [44]. There has been much work trying to determine if these associated regions arise due to artifacts associated with MRI or are of vascular origin. Many groups have now developed robust ways to remove potential MRI artifacts that can arise due to breathing or cardiac pulsations [45]. Importantly, it has been shown that the metabolic costs of these resting state fluctuations are the same as that found for task induced fMRI changes, strongly arguing for a neuronal contribution [46]. Whole-brain analysis of these resting state correlations have shown that multiple independent spatial patterns exist that potentially provide information about the brain’s intrinsic communication and connectivity (Figure 3, [47]). Preliminary clinical studies have shown that these patterns are altered in various diseases including AD [48] and MS [49], suggesting a possible diagnostic role for fMRI resting state connectivity studies.
Figure 3.
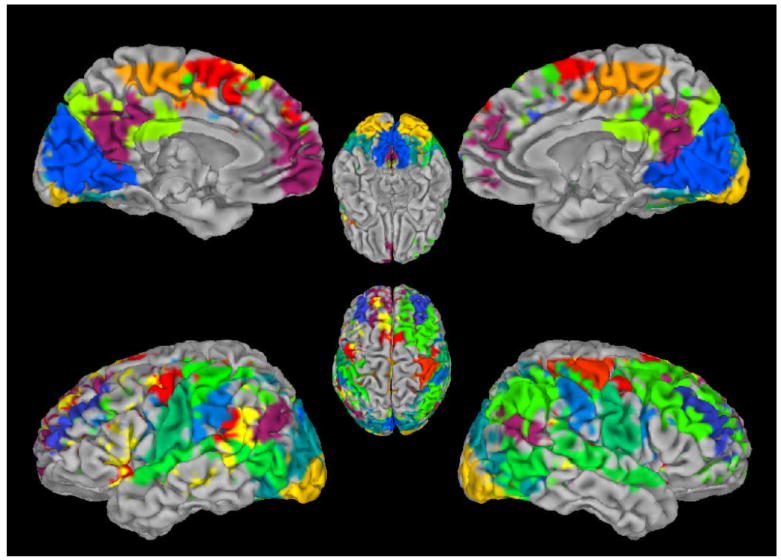
Spatial patterns of correlated BOLD fMRI activity in humans during rest. Spontaneous activity occurs in distinct spatial patterns, many of which show hemispheric symmetry. Each color represents a single activity pattern that occurred consistently over 11 subjects (for methodological details see [71]. Image courtesy of Masaki Fukunaga, LFMI, NINDS, NIH.
A drawback of fMRI studies is that they rely on vascular changes for contrast and so are dependent on proper neurovascular control. It has become clear that the biological basis of neuro-vascular control is quite complex [50] and therefore MRI techniques that can more directly measure neuronal activity would be important for imaging neural circuits. There are two areas where there have been attempts to make a more direct measure of neuronal activity. One is based on the use of Mn2+ ion as a surrogate of calcium influx. It has been appreciated for many years that Mn2+ can enter excitable cells via voltage gated calcium channels, thus influx and accumulation of Mn2+ in an area of the brain has been shown to allow mapping active regions of the rat cortex [51]. This early work required breaking the blood brain barrier to enable rapid access of Mn2+ to the brain. Many groups working in a large range of animal models have used the activity dependence of manganese enhanced MRI to map brain regions involved in a wide range of tasks. Recent work in the mouse auditory system [52] and rat hypothalmus [53] indicates that it may be possible to make maps of activity without breaking the blood brain barrier. Furthermore, it has been shown that Mn2+ uptake into the heart is affected by cardiac workload, enabling MRI to estimate calcium influx into the heart [54].
First attempts have been described to make MRI contrast agents that can detect the rise in calcium that is known to occur with activity in analogy to fluorescence indicators of calcium. Agents that respond to changes in calcium based on controlling the relaxivity of gadolinium chelates [55] or controlling the aggregation of iron oxide [56] have been proposed and are promising tools for measuring brain activity. Finally, there has been an interest in using MRI to directly detect the magnetic field produced by neuronal currents [57]. While there have been preliminary demonstrations of using MRI to directly measure neuronal currents, these results remain controversial. Models that can predict the signal changes that may arise from neuronal currents will be critical [58]. While still far from being well established, these innovative approaches to measuring neuronal activity offer promise for increasing the specificity of MRI to measure brain function.
Emerging MRI Approaches Relevant to Imaging Neural Circuits
In vivo neuronal tract tracing
While diffusion tensor based MRI tools are enabling detailed studies of white matter connectivity [4], they do not as yet enable acquiring the kind of detailed anatomical connectivity that classical neuronal tract tracers yield. A major drawback of these classical tracers is that they all require histological procedures or optical imaging which is restricted to surface regions. The Mn2+ ion has been discussed above because it leads to anatomical MRI contrast and because it can accumulate in active regions. A third property of Mn2+ is that it is transported in an anterograde direction in neurons and can cross synapses making it possible to use manganese enhanced MRI to trace connections in the brain in a manner analogous to classical tract tracers. The first demonstration of the use of manganese enhanced MRI for neuronal tract tracing was in the rodent olfactory and visual pathway [59]. It has now been used in many species such as non-human primates and song birds [60]. Manganese enhanced MRI has been used to visualize regional connectivity from a wide variety of brain regions, attesting to the general applicability of this approach. Recently, it has been shown that the tracing properties of Mn2+ will enable functional brain structures to be resolved. Connections from the thalamus to the cortex imaged with Mn2+ allow visualization of the known preference for input into specific cortical layers [61] and individual glomeruli have been resolved as Mn2+ tracks through the olfactory nerves into the glomerular layer of the olfactory bulb (Figure 4). The tract tracing properties of Mn2+ have enabled manganese enhanced MRI to be applied to a number of pre-clinical animal models of a range of diseases such as AD [62], stroke [63], and spinal chord injury [64]. There have been no reported attempts to use Mn2+ to trace neural circuits in the heart, however, such studies should be possible.
Figure 4.
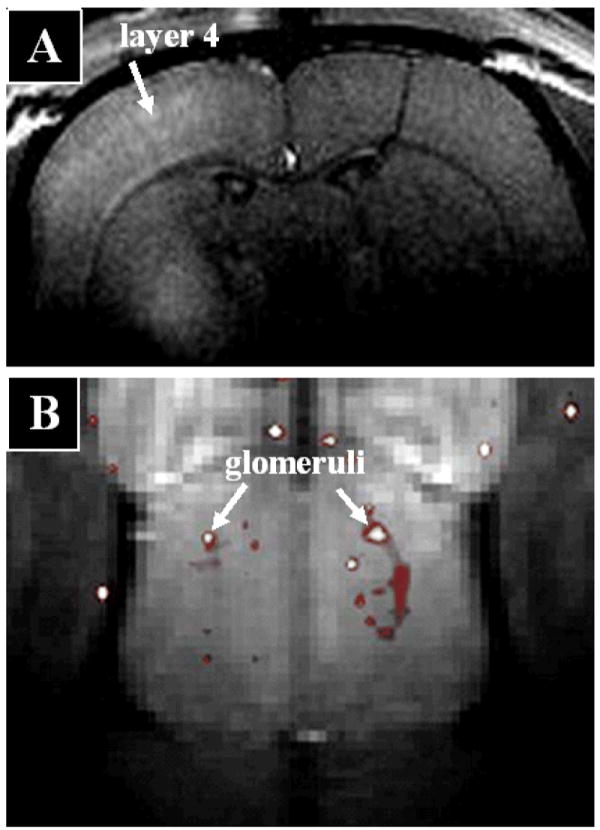
Injection of MnCl2 into specific brain regions enables MRI of neuronal tracts at the level of functional units of the brain. A) Layer specific tracing from rat thalamus to cortex showing enhancement primarily in layer 4 and with less enhancement in deep layer 5 consistent with the known input layers [61] (Image courtesy of Jason Tucciarone, LFMI, NINDS, NIH). B) Individual glomeruli from the surface of the bulb imaged using manganese enhanced MRI after administration of MnCl2 to the nose and presentation of a specific odor (Image courtesy of Kai-Chuang Hsiang, LFMI, NINDS, NIH). Both images were acquired from an 11.7 animal MRI system using resolutions of 100 μm (A) and 75 μm (B).
Tracking Cells Important to Brain Function by MRI
Another area that is having a large impact in pre-clinical MRI studies of animals is cell tracking. It has been shown by a number of groups that specific cells can be robustly labeled with a variety of MRI contrast agents and that trafficking of these cells can be followed [65]. Studies have shown that immune cell infiltration into the brain can be detected [66] and that stem cells injected into the brain can be followed [67]. The first human applications of this exciting new frontier are beginning to appear [67, 68]. Using iron oxide contrast, it has been shown that it is possible to detect single cells with MRI both in vitro and in vivo [69]. Furthermore, judicious injection of MRI contrast near the site of production of neural progenitors in the subventricular zone of the rodent enables imaging the migration of these cells to the olfactory bulb [70]. There results taken together indicate that it will be possible to use MRI to understand the important emerging role of trafficking of cells in remodeling neural circuits in the normal and diseased brain. The early results in humans using approved iron oxide contrast agents indicate that this approach will have a large impact on the clinical uses of MRI.
Conclusions
The development of fMRI and diffusion tensor based MRI tractography has led to an immense activity in imaging neural circuits in the human brain. There have been a large number of applications of these tools to study the normal brain and a wide range of diseases of the brain. MRI applied to visualization of the anatomy and function of neural circuits in the heart is much more challenging. However, it is clear that a number of interesting avenues are being explored that have the potential to greatly expand MRI of neural circuits. Emerging high resolution, susceptibility weighted MRI of the human brain has demonstrated the potential to routinely measure the wide range of functional anatomy that exists in the brain. Similar results have been obtained from animal brain using contrast agents and, in particular, manganese enhanced MRI. While much less work has been done in the heart, it is clear that these novel MRI approaches will enable better delineation of cardiac anatomy, especially fiber orientation and remodeling due to ischemia.
fMRI is progressing from analyzing activation to specific tasks to looking at intrinsically correlated activity throughout the brain. All of this work is limited by its dependence on the complexities of using vascular responses to infer neural activity. It is expected that there will be a growing amount of work using fMRI techniques to study the role of the CNS in autonomic control. Attempts to find MRI contrast more directly related to neuronal activity is receiving increasing attention. Finally, there has been a large increase in developing functional contrast or molecular contrast agents for imaging neural circuits. Manganese enhanced MRI to perform neural tract tracing and the rapidly growing application of MRI to visualize cell migration and are two promising areas that have already impacted pre-clinical animal studies and are ripe for widespread application to the human brain. These new molecular imaging approaches are most actively being developed for studying cancer and brain, however extension to the heart should be possible. Taken together we would argue that MRI is poised to continue develop rapidly to enable more and more sophisticated analysis of neural circuits in health and disease.
References
- 1.Essig M, Weber MA, von Tengg-Kobligk H, Knopp MV, Yuh WT, Giesel FL. Contrast-enhanced magnetic resonance imaging of central nervous system tumors: agents, mechanisms, and applications. Top Magn Reson Imaging. 2006;17:89–106. doi: 10.1097/01.rmr.0000245464.36148.dc. [DOI] [PubMed] [Google Scholar]
- 2.Rovaris M, Filippi M. Contrast enhancement and the acute lesion in multiple sclerosis. Neuroimaging Clin N Am. 2000;10:705–16. viii–ix. [PubMed] [Google Scholar]
- 3.Marcu CB, Nijveldt R, Beek AM, Van Rossum AC. Delayed contrast enhancement magnetic resonance imaging for the assessment of cardiac disease. Heart Lung Circ. 2007;16:70–8. doi: 10.1016/j.hlc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 5.Heiss WD, Sorensen AG. Advances in imaging, 2006. Stroke. 2007;38:238–40. doi: 10.1161/01.STR.0000254934.95188.a7. [DOI] [PubMed] [Google Scholar]
- 6.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–39. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Sundgren PC, Dong Q, Gomez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46:339–50. doi: 10.1007/s00234-003-1114-x. [DOI] [PubMed] [Google Scholar]
- 8.Tseng WY, Wedeen VJ, Reese TG, Smith RN, Halpern EF. Diffusion tensor MRI of myocardial fibers and sheets: correspondence with visible cut-face texture. J Magn Reson Imaging. 2003;17:31–42. doi: 10.1002/jmri.10223. [DOI] [PubMed] [Google Scholar]
- 9.Belliveau JW, Cohen MS, Weisskoff RM, Buchbinder BR, Rosen BR. Functional studies of the human brain using high-speed magnetic resonance imaging. J Neuroimaging. 1991;1:36–41. doi: 10.1111/jon19911136. [DOI] [PubMed] [Google Scholar]
- 10.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Esposito M. Functional neuroimaging of cognition. Semin Neurol. 2000;20:487–98. doi: 10.1055/s-2000-13182. [DOI] [PubMed] [Google Scholar]
- 13.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 14.Hsu LY, Rhoads KL, Holly JE, Kellman P, Aletras AH, Arai AE. Quantitative myocardial perfusion analysis with a dual-bolus contrast-enhanced first-pass MRI technique in humans. J Magn Reson Imaging. 2006;23:315–22. doi: 10.1002/jmri.20502. [DOI] [PubMed] [Google Scholar]
- 15.Brodmann K. Vergleichende Lokalisationlehre der Grosshirnrinde. Leipzig: Barth-Verlag; 1909. [Google Scholar]
- 16.Vogt O. Die Myeloarchitektonik des Isocortex parietalis. J Psychol Neurol. 1911;18:107–18. [Google Scholar]
- 17.Annese J, Pitiot A, Dinov ID, Toga AW. A myelo-architectonic method for the structural classification of cortical areas. Neuroimage. 2004;21:15–26. doi: 10.1016/j.neuroimage.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Clark VP, Courchesne E, Grafe M. In vivo myeloarchitectonic analysis of human striate and extrastriate cortex using magnetic resonance imaging. Cereb Cortex. 1992;2:417–24. doi: 10.1093/cercor/2.5.417. [DOI] [PubMed] [Google Scholar]
- 19.Barbier EL, Marrett S, Danek A, Vortmeyer A, van Gelderen P, Duyn J, Bandettini P, Grafman J, Koretsky AP. Imaging cortical anatomy by high-resolution MR at 3.0T: detection of the stripe of Gennari in visual area 17. Magn Reson Med. 2002;48:735–8. doi: 10.1002/mrm.10255. [DOI] [PubMed] [Google Scholar]
- 20.Bridge H, Clare S. High-resolution MRI: in vivo histology? Philos Trans R Soc Lond B Biol Sci. 2006;361:137–46. doi: 10.1098/rstb.2005.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters NB, Egan GF, Kril JJ, Kean M, Waley P, Jenkinson M, Watson JD. In vivo identification of human cortical areas using high-resolution MRI: an approach to cerebral structure-function correlation. Proc Natl Acad Sci U S A. 2003;100:2981–6. doi: 10.1073/pnas.0437896100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci U S A. 2003;100:11029–34. doi: 10.1073/pnas.1832864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe T, Natt O, Boretius S, Frahm J, Michaelis T. In vivo 3D MRI staining of mouse brain after subcutaneous application of MnCl2. Magn Reson Med. 2002;48:852–9. doi: 10.1002/mrm.10276. [DOI] [PubMed] [Google Scholar]
- 24.Aoki I, Wu YJ, Silva AC, Lynch RM, Koretsky AP. In vivo detection of neuroarchitecture in the rodent brain using manganese-enhanced MRI. Neuroimage. 2004;22:1046–59. doi: 10.1016/j.neuroimage.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 25.Alvestad S, Goa PE, Qu H, Risa O, Brekken C, Sonnewald U, Haraldseth O, Hammer J, Ottersen OP, Haberg A. In vivo mapping of temporospatial changes in manganese enhancement in rat brain during epileptogenesis. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Wendland MF. Applications of manganese-enhanced magnetic resonance imaging (MEMRI) to imaging of the heart. NMR Biomed. 2004;17:581–94. doi: 10.1002/nbm.943. [DOI] [PubMed] [Google Scholar]
- 27.Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. From the Cover: High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007;104:11796–801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Zwart JA, Ledden PJ, van Gelderen P, Bodurka J, Chu R, Duyn JH. Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn Reson Med. 2004;51:22–6. doi: 10.1002/mrm.10678. [DOI] [PubMed] [Google Scholar]
- 29.Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216–23. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- 30.Li TQ, van Gelderen P, Merkle H, Talagala L, Koretsky AP, Duyn J. Extensive heterogeneity in white matter intensity in high-resolution T2*-weighted MRI of the human brain at 7.0 T. Neuroimage. 2006;32:1032–40. doi: 10.1016/j.neuroimage.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 31.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–7. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 32.Craelius W, Migdal MW, Luessenhop CP, Sugar A, Mihalakis I. Iron deposits surrounding multiple sclerosis plaques. Arch Pathol Lab Med. 1982;106:397–9. [PubMed] [Google Scholar]
- 33.Benveniste H, Einstein G, Kim KR, Hulette C, Johnson GA. Detection of neuritic plaques in Alzheimer’s disease by magnetic resonance microscopy. Proc Natl Acad Sci U S A. 1999;96:14079–84. doi: 10.1073/pnas.96.24.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Wengenack TM, Reyes DA, Garwood M, Curran GL, Borowski BJ, Lin J, Preboske GM, Holasek SS, Adriany G, Poduslo JF. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer’s transgenic mice. J Neurosci. 2005;25:10041–8. doi: 10.1523/JNEUROSCI.2588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Yarowsky P, Gordon MN, Di Carlo G, Munireddy S, van Zijl PC, Mori S. Detection of amyloid plaques in mouse models of Alzheimer’s disease by magnetic resonance imaging. Magn Reson Med. 2004;51:452–7. doi: 10.1002/mrm.10730. [DOI] [PubMed] [Google Scholar]
- 36.Sehgal V, Delproposto Z, Haacke EM, Tong KA, Wycliffe N, Kido DK, Xu Y, Neelavalli J, Haddar D, Reichenbach JR. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22:439–50. doi: 10.1002/jmri.20404. [DOI] [PubMed] [Google Scholar]
- 37.Wycliffe ND, Choe J, Holshouser B, Oyoyo UE, Haacke EM, Kido DK. Reliability in detection of hemorrhage in acute stroke by a new three-dimensional gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography: a retrospective study. J Magn Reson Imaging. 2004;20:372–7. doi: 10.1002/jmri.20130. [DOI] [PubMed] [Google Scholar]
- 38.Vignaud A, Rodriguez I, Ennis DB, DeSilva R, Kellman P, Taylor J, Bennett E, Wen H. Detection of myocardial capillary orientation with intravascular iron-oxide nanoparticles in spin-echo MRI. Magn Reson Med. 2006;55:725–30. doi: 10.1002/mrm.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohler S, Hiller KH, Waller C, Bauer WR, Haase A, Jakob PM. Investigation of the microstructure of the isolated rat heart: a comparison between T*2- and diffusion-weighted MRI. Magn Reson Med. 2003;50:1144–50. doi: 10.1002/mrm.10636. [DOI] [PubMed] [Google Scholar]
- 40.Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ. Dynamic causal models of neural system dynamics:current state and future extensions. J Biosci. 2007;32:129–44. doi: 10.1007/s12038-007-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritter P, Villringer A. Simultaneous EEG-fMRI. Neurosci Biobehav Rev. 2006;30:823–38. doi: 10.1016/j.neubiorev.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 2007;35:698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Colivicchi F, Bassi A, Santini M, Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004;35:2094–8. doi: 10.1161/01.STR.0000138452.81003.4c. [DOI] [PubMed] [Google Scholar]
- 44.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 45.Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–64. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 46.Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Duyn JH. Brain metabolic activity in absence of stimuli. Proceedings of VIIth International Conference on Quantification of Brain Function with PET; June 7-11, 2005; 2005. p. S63. [Google Scholar]
- 47.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–92. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- 50.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–9. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- 51.Lin YJ, Koretsky AP. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magn Reson Med. 1997;38:378–88. doi: 10.1002/mrm.1910380305. [DOI] [PubMed] [Google Scholar]
- 52.Yu X, Wadghiri YZ, Sanes DH, Turnbull DH. In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat Neurosci. 2005;8:961–8. doi: 10.1038/nn1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo YT, Herlihy AH, So PW, Bell JD. Manganese-enhanced magnetic resonance imaging (MEMRI) without compromise of the blood-brain barrier detects hypothalamic neuronal activity in vivo. NMR Biomed. 2006;19:1028–34. doi: 10.1002/nbm.1070. [DOI] [PubMed] [Google Scholar]
- 54.Hu TC, Pautler RG, MacGowan GA, Koretsky AP. Manganese-enhanced MRI of mouse heart during changes in inotropy. Magn Reson Med. 2001;46:884–90. doi: 10.1002/mrm.1273. [DOI] [PubMed] [Google Scholar]
- 55.Li WH, Parigi G, Fragai M, Luchinat C, Meade TJ. Mechanistic studies of a calcium-dependent MRI contrast agent. Inorg Chem. 2002;41:4018–24. doi: 10.1021/ic0200390. [DOI] [PubMed] [Google Scholar]
- 56.Atanasijevic T, Shusteff M, Fam P, Jasanoff A. Calcium-sensitive MRI contrast agents based on superparamagnetic iron oxide nanoparticles and calmodulin. Proc Natl Acad Sci U S A. 2006;103:14707–12. doi: 10.1073/pnas.0606749103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Applied Magn Reson. 2005;28:1–30. [Google Scholar]
- 58.Blagoev KB, Mihaila B, Travis BJ, Alexandrov LB, Bishop AR, Ranken D, Posse S, Gasparovic C, Mayer A, Aine CJ, Ulbert I, Morita M, Muller W, Connor J, Halgren E. Modelling the magnetic signature of neuronal tissue. Neuroimage. 2007;37:137–48. doi: 10.1016/j.neuroimage.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 59.Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn Reson Med. 1998;40:740–8. doi: 10.1002/mrm.1910400515. [DOI] [PubMed] [Google Scholar]
- 60.Pautler RG. Biological applications of manganese-enhanced magnetic resonance imaging. Methods Mol Med. 2006;124:365–86. doi: 10.1385/1-59745-010-3:365. [DOI] [PubMed] [Google Scholar]
- 61.Silva AC, Lee J, Wu CH, Tucciarone J, Pelled G, Aoki I, Koretsky AP. Detection of cortical laminar architecture using manganese enhanced MRI. J Neurosc Meth. 2007 doi: 10.1016/j.jneumeth.2007.08.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith KD, Kallhoff V, Zheng H, Pautler RG. In vivo axonal transport rates decrease in a mouse model of Alzheimer’s disease. Neuroimage. 2007;35:1401–8. doi: 10.1016/j.neuroimage.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Zijden JP, Wu O, van der Toorn A, Roeling TP, Bleys RL, Dijkhuizen RM. Changes in neuronal connectivity after stroke in rats as studied by serial manganese-enhanced MRI. Neuroimage. 2007;34:1650–7. doi: 10.1016/j.neuroimage.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Bilgen M, Dancause N, Al-Hafez B, He YY, Malone TM. Manganese-enhanced MRI of rat spinal cord injury. Magn Reson Imaging. 2005;23:829–32. doi: 10.1016/j.mri.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002;22:899–907. doi: 10.1097/00004647-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Petry KG, Boiziau C, Dousset V, Brochet B. Magnetic resonance imaging of human brain macrophage infiltration. Neurotherapeutics. 2007;4:434–42. doi: 10.1016/j.nurt.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Politi LS. MR-based imaging of neural stem cells. Neuroradiology. 2007;49:523–34. doi: 10.1007/s00234-007-0219-z. [DOI] [PubMed] [Google Scholar]
- 68.Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007;16:461–6. doi: 10.1089/scd.2007.0083. [DOI] [PubMed] [Google Scholar]
- 69.Shapiro EM, Gonzalez-Perez O, Manuel Garcia-Verdugo J, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage. 2006;32:1150–7. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shapiro EM, Medford-Davis LN, Fahmy TM, Dunbar CE, Koretsky AP. Antibody-mediated cell labeling of peripheral T cells with micron-sized iron oxide particles (MPIOs) allows single cell detection by MRI. Contrast Media Mol Imaging. 2007;2:147–53. doi: 10.1002/cmmi.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]


