The paper by Tentori at al confirms previous findings about the positive impact of treatment time on outcome […], taking into account that urea kinetics is not very representative for the kinetics of other solutes […]. The main challenge nowadays is to characterize a better marker reflecting dialysis adequacy and/or outcome.
Keywords: DOPPS, hemodialysis, outcomes, survival, treatment length
Abstract
Background
Longer dialysis session length (treatment time, TT) has been associated with better survival among hemodialysis (HD) patients. The impact of TT on clinical markers that may contribute to this survival advantage is not well known.
Methods
Using data from the international Dialysis Outcomes and Practice Patterns Study, we assessed the association of TT with clinical outcomes using both standard regression analyses and instrumental variable approaches. The study included 37 414 patients on in-center HD three times per week with prescribed TT from 120 to 420 min.
Results
Facility mean TT ranged from 214 min in the USA to 256 min in Australia–New Zealand. Accounting for country effects, mortality risk was lower for patients with longer TT {hazard ratio for every 30 min: all-cause mortality: 0.94 [95% confidence interval (CI): 0.92–0.97], cardiovascular mortality: 0.95 (95% CI: 0.91–0.98) and sudden death: 0.93 (95% CI: 0.88–0.98)}. Patients with longer TT had lower pre- and post-dialysis systolic blood pressure, greater intradialytic weight loss, higher hemoglobin (for the same erythropoietin dose), serum albumin and potassium and lower serum phosphorus and white blood cell counts. Similar associations were found using the instrumental variable approach, although the positive associations of TT with weight loss and potassium were lost.
Conclusions
Favorable levels of a variety of clinical markers may contribute to the better survival of patients receiving longer TT. These findings support longer TT prescription in the setting of in-center, three times per week HD.
Introduction
The morbidity and mortality rate of patients receiving three times per week hemodialysis (HD) remain unacceptably high [1]. Compared to ‘standard’ dialysis, daily in-center and long nightly home dialysis have been associated with better outcomes and quality of life in small cohorts of selected patients [2–4]. In the recent Frequent Hemodialysis Network trial, six times per week dialysis was associated with favorable outcomes compared to the standard regimen [5]. While extended dialysis schedules may lead to better clinical outcomes, logistical, financial, and other impediments remain for their use for the majority of HD patients.
Most HD patients worldwide receive conventional three times per week dialysis with a duration of <5 h [1]. Even in this setting, shorter dialysis session length (treatment time, TT) has been associated with worse survival [6–11]. While the association of TT with survival is independent of dialysis dose, most prior studies did not provide a mechanistic insight through assessment of the association of TT with clinical markers (e.g. hemoglobin, serum phosphorus, blood pressure) which may contribute to morbidity and mortality in this population. Furthermore, despite the wide range of adjustments and analytic techniques [11], prior studies failed to completely address differences in the health status of patients receiving longer versus shorter TT. The present study highlights international differences in TT, presents associations of TT with intermediate measures and applies an instrumental variable approach to account, in part, for unmeasured confounders that may bias the associations of TT with clinical outcomes.
Materials and methods
Data sources
The Dialysis Outcomes and Practice Patterns Study (DOPPS) is a prospective cohort study of in-center HD patients. Details on the study design have been described previously [12, 13].
The current study included data from seven countries (France, Germany, Italy, Japan, Spain, the UK and the USA) in DOPPS 1 (1996–2001) and from an additional five countries (Australia, Belgium, Canada, New Zealand and Sweden) in DOPPS 2 (2002–04) and 3 (2005–08). Selected data are presented within the geographic regions: North America (USA + Canada); Eur/ANZ (European countries + Australia and New Zealand) and Japan. Detailed case-mix and comorbid data were collected at study entry. Cause-specific mortality and hospitalization events were collected during the study follow-up. Informed patient consent was obtained as indicated in accordance with local requirements.
TT was defined as the prescribed HD session length and was analyzed as a continuous variable (per 30 min longer TT) as well as a categorical variable ( < 200, 200–225, 226–250 and > 250 min). Because TT for the great majority of patients was at exactly 30 min intervals, we used 180, 210, 240 and 270–300 min as respective surrogate names for the categories. Mortality included all-cause mortality, cardiovascular death and sudden death (mortality due to cardiac arrhythmia, cardiac arrest or hyperkalemia). Hospitalizations included all-cause hospitalization, hospitalizations due to cardiovascular events and congestive heart failure or fluid overload. Intermediate outcomes included intradialytic weight loss, pre- and post-dialysis systolic blood pressure (SBP) and laboratory values [hemoglobin, white blood cell count (WBC), serum phosphorus, potassium, albumin and ferritin] measured at study enrollment. Sensitivity analyses were conducted using the delivered TT (i.e. the HD session length as actually received).
Statistical analysis
Differences in patient characteristics across TT categories were assessed using a test for trends. Linear mixed models for continuous outcomes and the Generalized Estimating Equation method with logit link function for dichotomized outcomes were used to examine the associations between TT and patient intermediate outcomes. Models were adjusted for patient characteristics, DOPPS country and study phase and accounted for facility clustering, assuming a compound symmetry covariance structure. Cox models were used to estimate the associations of TT with mortality/hospitalization risk, were stratified by country and study phase and accounted for facility clustering using robust sandwich covariance estimators. The proportional hazard assumption was tested and satisfied.
In order to partially account for patient-level unmeasured confounders which may impact the relationship between TT and outcomes, we also conducted a separate set of analyses applying an instrumental variable approach that used the dialysis facility as the instrument [14–17]. For patient intermediate outcomes, we conducted the standard two-stage least square instrumental variable method for continuous outcomes and an extended, two-stage instrumental variable method with a linear model as the first stage and logistic regression as the second stage for dichotomized outcomes. For risk of mortality/hospitalization, we used an extended instrumental variable approach that uses a linear model first stage and a Cox model second stage [18]. Since the F-statistic in all the first-stage models was > 25, we rejected the null hypothesis of weak instruments with the interpretation that the instrumental variable estimates are less biased [15, 19, 20].
A multiple imputation method was used to correct for potential biases that could be caused by missing values using the standard software IVEware [21]. All statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC). The authors have followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement guidelines for reporting observational studies [22].
Results
Study sample
This study included 37 414 patients receiving three HD treatments per week with prescribed TT from 120 to 420 min at study enrollment; 15 442 patients were from 308 facilities in DOPPS 1, 11 553 patients were from 322 facilities in DOPPS 2 and 10 419 patients were from 300 facilities in DOPPS 3. The mean follow-up was 19 months. During the study period, 8961 patients died (mortality rate: 0.15/year).
Distribution of TT across DOPPS countries and over time
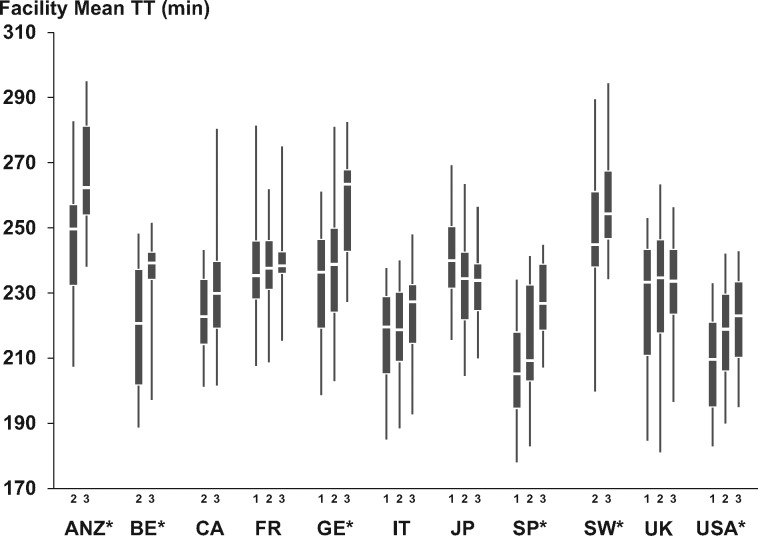
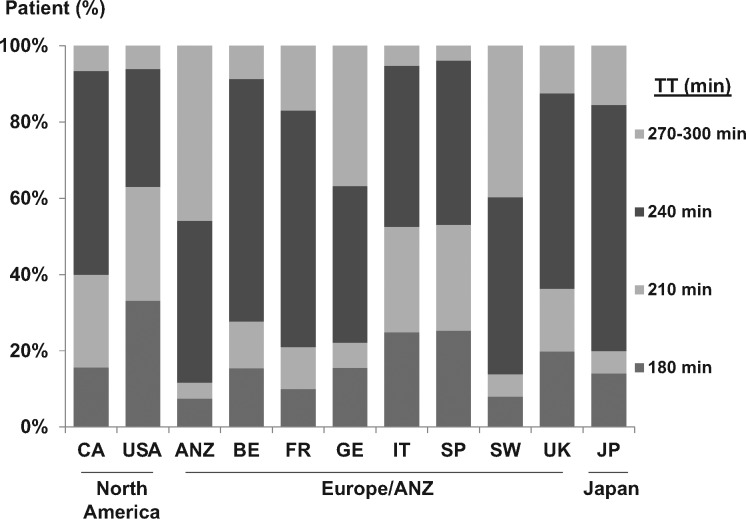
Distributions of facility mean TT (FMTT) by DOPPS country and phase are presented in Figure 1. Large differences in FMTT were observed across countries (P < 0.001), with the longest average FMTT (256 ± 23 min) in ANZ and the shortest in the USA (214 ± 17 min). Overall, FMTT increased over time from DOPPS 1 to DOPPS 3 (P < 0.001). A significant increase in FMTT over time was found in ANZ, Belgium, Germany, Spain, Sweden and the USA (P < 0.05), and a significant decrease was found in Japan (P = 0.01). Distributions of patient prescribed TT in DOPPS 1–3 by country are presented in Figure 2. Significant differences in the distribution of TT were found across countries (P < 0.001). The prevalence of TT < 200 min was highest in the USA (33.1%) and lowest in Australia/New Zealand (7.5%).
Fig. 1.
Distribution of facility mean prescribed TT by DOPPS country and by phase. The box shows the 25th–75th and the whiskers the 5th–95th percentile ranges. *P < 0.05 for increase over time. DOPPS Phase 1 (1996–2001), Phase 2 (2002–04) and Phase 3 (2005–08). ANZ, Australia and New Zealand; BE, Belgium; CA, Canada; FR, France; GE, Germany; IT, Italy; JP, Japan; SP, Spain; SW, Sweden; UK, United Kingdom; USA, United States of America.
Fig. 2.
Distribution of patient-level prescribed TT categories by DOPPS country. DOPPS Phase 1 (1996–2001), Phase 2 (2002–04) and Phase 3 (2005–08). ANZ, Australia and New Zealand; BE, Belgium; CA, Canada; FR, France; GE, Germany; IT, Italy; JP, Japan; SP, Spain; SW, Sweden; UK, United Kingdom; USA, United States of America.
Patient characteristics by TT categories
Patient characteristics by prescribed TT at study enrollment within each DOPPS region are shown in Table 1. Patients with longer TT were younger, more likely male, had longer end-stage renal disease (ESRD) duration and higher body weight (P < 0.001 for all). The prevalence of comorbidities within each TT category varied across the DOPPS regions. In all regions, patients with longer TT had higher hemoglobin and serum albumin levels, were less likely to use a catheter as vascular access, had higher blood flow rates and were more likely to be treated with high-flux dialyzers (P ≤ 0.01 for all).
Table 1.
Patient characteristics by patient TT categories within each DOPPS region
| Patient TT |
P-valuea | |||||
|---|---|---|---|---|---|---|
| 180 min | 210 min | 240 min | 270–300 min | |||
| Number of patients | All regions | 8411 (22%) | 7282 (19%) | 16 795 (45%) | 4926 (13%) | |
| North America (%) | 4924 (32%) | 4587 (29%) | 5124 (33%) | 948 (6%) | ||
| Eur/ANZ (%) | 2556 (17%) | 2306 (15%) | 7389 (49%) | 2950 (19%) | ||
| Japan (%) | 931 (14%) | 389 (6%) | 4282 (65%) | 1028 (16%) | ||
| Demographics | ||||||
| Age (years) | North America | 64.5 | 62.7 | 58.7 | 54.2 | <0.0001 |
| Eur/ANZ | 64.3 | 65.3 | 62.7 | 59.1 | <0.0001 | |
| Japan | 66.3 | 64.2 | 60.5 | 56.5 | <0.0001 | |
| Sex (male, %) | North America | 45 | 52 | 63 | 80 | <0.0001 |
| Eur/ANZ | 53 | 52 | 59 | 70 | <0.0001 | |
| Japan | 58 | 56 | 61 | 69 | <0.0001 | |
| Vintage (years) | North America | 1.9 | 2.3 | 2.4 | 3.7 | <0.0001 |
| Eur/ANZ | 2.2 | 3.8 | 3.7 | 5.1 | <0.0001 | |
| Japan | 1.8 | 4.1 | 7.0 | 10.7 | <0.0001 | |
| Target weight (kg) | North America | 67.6 | 72.1 | 79.8 | 97.3 | <0.0001 |
| Eur/ANZ | 65.4 | 64.6 | 68.7 | 77.4 | <0.0001 | |
| Japan | 50.7 | 51.3 | 52.8 | 55.6 | <0.0001 | |
| Comorbidities | ||||||
| Diabetes (%) | North America | 47 | 52 | 53 | 58 | <0.0001 |
| Eur/ANZ | 28 | 26 | 29 | 34 | <0.0001 | |
| Japan | 41 | 43 | 31 | 21 | 0.13 | |
| Hypertension (%) | North America | 85 | 87 | 86 | 89 | 0.08 |
| Eur/ANZ | 79 | 78 | 78 | 82 | <0.0001 | |
| Japan | 75 | 71 | 67 | 56 | 0.04 | |
| Coronary artery disease (%) | North America | 54 | 57 | 54 | 58 | 0.79 |
| Eur/ANZ | 39 | 38 | 42 | 51 | 0.92 | |
| Japan | 27 | 31 | 29 | 25 | 0.83 | |
| Congestive heart failure (%) | North America | 45 | 48 | 46 | 47 | 0.31 |
| Eur/ANZ | 27 | 27 | 33 | 36 | <0.0001 | |
| Japan | 26 | 23 | 17 | 13 | 0.36 | |
| Labs | ||||||
| Hemoglobin (g/dL) | North America | 10.7 | 10.9 | 11.0 | 11.6 | <0.0001 |
| Eur/ANZ | 10.5 | 11.2 | 11.2 | 11.5 | <0.0001 | |
| Japan | 9.4 | 10.0 | 9.9 | 10.3 | <0.0001 | |
| Albumin (g/dL) | North America | 3.6 | 3.6 | 3.6 | 3.8 | <0.0001 |
| Eur/ANZ | 3.6 | 3.7 | 3.7 | 3.8 | <0.0001 | |
| Japan | 3.6 | 3.7 | 3.8 | 3.9 | <0.0001 | |
| Dialysis treatment | ||||||
| Catheter use (%) | North America | 42 | 39 | 42 | 29 | <0.0001 |
| Eur/ANZ | 31 | 20 | 23 | 14 | <0.0001 | |
| Japan | 9 | 2 | 1 | 0 | <0.0001 | |
| Blood flow (mL/min) | North America | 363.4 | 383.9 | 384.1 | 414.0 | <0.0001 |
| Eur/ANZ | 273.1 | 307.4 | 301.9 | 308.6 | <0.0001 | |
| Japan | 173.2 | 192.3 | 193.9 | 203.5 | <0.0001 | |
| High-flux dialyzer useb (%) | North America | 55 | 55 | 50 | 66 | 0.01 |
| Eur/ANZ | 33 | 39 | 39 | 51 | <0.0001 | |
| Japan | 62 | 74 | 73 | 76 | <0.0001 | |
aTest of trend adjusted for country and phase and accounted for facility clustering.
bHigh flux percent calculated after excluding those with missing flux information (22%).
TT and mortality and hospitalization risk
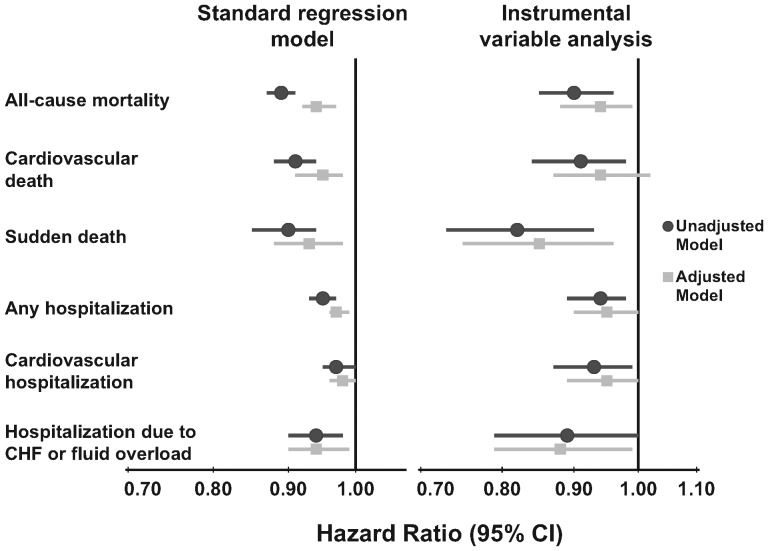
Table 2 shows that longer TT was associated with lower mortality and hospitalization risk, both in unadjusted and adjusted standard regression models. Because most trends across categories were approximately linear, we also examined TT as a continuous variable. In Figure 3, the adjusted standard regression models show a significantly decreased risk of both mortality [hazard ratio (HR) = 0.94, 95% confidence interval (CI): 0.92–0.97] and hospitalization (HR = 0.97, 95% CI: 0.96–0.99) per 30 min longer TT. Results of the instrumental variable analyses yielded qualitatively similar estimates with (as expected) less precision.
Table 2.
Associations between prescribed TT categories and risk of mortality/hospitalizationa
| Categorical TT (standard regression model) |
||||
|---|---|---|---|---|
| 180 min | 210 min | 240 min | 270–300 min | |
| All-cause mortality | ||||
| Unadjustedb | 1.30 (1.22–1.40) | 1.18 (1.11–1.26) | 1.00 (reference) | 0.78 (0.71–0.85) |
| Adjustedc | 1.16 (1.07–1.24) | 1.06 (0.99–1.13) | 1.00 (reference) | 0.90 (0.83–0.98) |
| Cardiovascular death | ||||
| Unadjustedb | 1.29 (1.17–1.42) | 1.18 (1.07–1.29) | 1.00 (reference) | 0.87 (0.77–0.98) |
| Adjustedc | 1.18 (1.06–1.31) | 1.06 (0.96–1.17) | 1.00 (reference) | 0.97 (0.86–1.10) |
| Sudden death | ||||
| Unadjustedb | 1.30 (1.13–1.49) | 1.10 (0.96–1.26) | 1.00 (reference) | 0.76 (0.63–0.91) |
| Adjustedc | 1.19 (1.02–1.38) | 0.99 (0.86–1.14) | 1.00 (reference) | 0.84 (0.70–1.01) |
| Any hospitalization | ||||
| Unadjustedb | 1.14 (1.08–1.20) | 1.01 (0.96–1.06) | 1.00 (reference) | 0.93 (0.89–0.98) |
| Adjustedc | 1.10 (1.04–1.16) | 1.00 (0.94–1.05) | 1.00 (reference) | 0.99 (0.94–1.05) |
| Cardiovascular hospitalization | ||||
| Unadjustedb | 1.08 (1.01–1.16) | 1.05 (0.98–1.13) | 1.00 (reference) | 0.99 (0.92–1.07) |
| Adjustedc | 1.07 (1.00–1.15) | 1.04 (0.97–1.11) | 1.00 (reference) | 1.01 (0.93–1.10) |
| Hospitalization due to CHF or fluid overload | ||||
| Unadjustedb | 1.26 (1.11–1.43) | 1.12 (0.99–1.27) | 1.00 (reference) | 0.92 (0.79–1.08) |
| Adjustedc | 1.24 (1.09–1.42) | 1.10 (0.97–1.24) | 1.00 (reference) | 0.94 (0.80–1.11) |
aHRs (95% CI) shown for each outcome. CHF, congestive heart failure.
bModel stratified by country and study phase and accounted for facility clustering.
cModel stratified by country and study phase, adjusted for age, sex, race, time on dialysis, BMI, 13 summary comorbid conditions, residual kidney function, prescribed blood flow rate and catheter use and accounted for facility clustering.
Fig. 3.
Association between prescribed TT (per 30 min longer) and risks of mortality and hospitalization. Adjusted model: adjusted for age, sex, race, time on dialysis, BMI, 13 summary comorbid conditions, residual kidney function, prescribed blood flow rate and catheter use, stratified by country and phase of study and accounted for facility clustering. CHF, congestive heart failure.
A sensitivity analysis adjusting for patient height and target weight rather than body mass index was consistent (all-cause mortality HR = 0.94, 95% CI: 0.91–0.96). Stratifying by the median target weight (66 kg) provided similar results below (HR = 0.92, 95% CI: 0.89–0.95) and above (HR = 0.95, 95% CI: 0.92–0.98) the median (P for interaction = 0.42). Additionally, all-cause mortality results were consistent in models that excluded patients (i) using a catheter as vascular access [HR = 0.93 (95% CI: 0.90–0.95) per 30 min longer TT], (ii) with TT >240 min [HR = 0.95 (95% CI: 0.92–0.98) per 30 min longer TT] or (iii) who had been on dialysis for <12 months [HR = 0.94 (95% CI: 0.91–0.97) per 30 min longer TT]. In addition to prescribed TT, we evaluated the association of delivered (versus prescribed) TT with outcomes and found consistent results [all-cause mortality HR = 0.92 (95% CI: 0.89–0.95) per 30 min TT]. A sensitivity analysis adjusting for single pool Kt/V rather than blood flow rate attenuated the effect slightly, as expected [all-cause mortality HR = 0.96 (95% CI: 0.93–0.99) per 30 min longer TT], due to collinearity with TT.
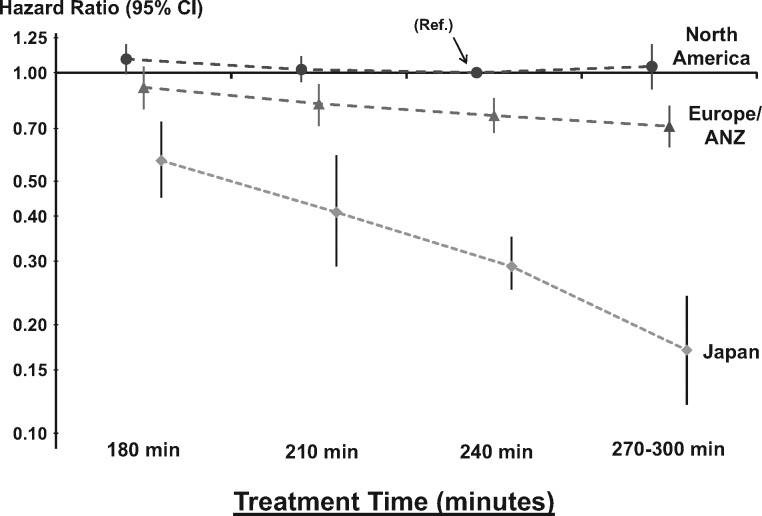
A significant interaction effect (P < 0.001) was found between TT and DOPPS regions (Figure 4). Longer TT was strongly associated with lower mortality in Japan [HR = 0.75 (95% CI: 0.69–0.81) per 30 min longer TT], still significantly associated with lower mortality in Eur/ANZ [HR = 0.94 (95% CI: 0.91–0.97) per 30 min longer TT], but not associated with mortality in North America [HR = 0.98 (95% CI: 0.95–1.02) per 30 min longer TT]. Similar TT effect on mortality was found in each region after adjusting for non-adherence with dialysis prescription (defined as any skipped dialysis session in the 30 days prior to DOPPS enrollment) [23]. Because Japanese patients receiving < 4 h of TT may be a subset of the sickest patients, an analysis restricted to patients with TT ≥240 min was conducted and found similar results in Japan [all-cause mortality HR = 0.75 (95% CI: 0.64–0.88) per 30 min longer TT].
Fig. 4.
Association between prescribed TT and mortality by region. Interaction between TT and region (P < 0.0001). Longer TT was associated with lower mortality in Eur/ANZ [HR = 0.94 (95% CI: 0.91–0.97) per 30 min TT, P = 0.0002] and Japan [HR = 0.75 (95% CI: 0.69–0.81), P < 0.0001] but not in North America [HR = 0.98 (95% CI: 0.95–1.02), P = 0.28]. Model was adjusted for age, sex, race, time on dialysis, BMI, 13 summary comorbid conditions, residual kidney function, prescribed blood flow rate and catheter use, stratified by study phase and accounted for facility clustering. The chosen reference category was for North American patients with prescribed TT at 240 min.
Overall, the protective effect of TT on mortality appeared to be most pronounced among patients with low blood flow rate (P = 0.02 for interaction between TT and blood flow rate). However, this finding was likely confounded by region, as Japan had the lowest blood flow rates and the strongest association between TT and mortality. Within each of the three geographic regions, no significant interaction between TT and blood flow rate was found (all P > 0.1).
TT and intermediate outcomes
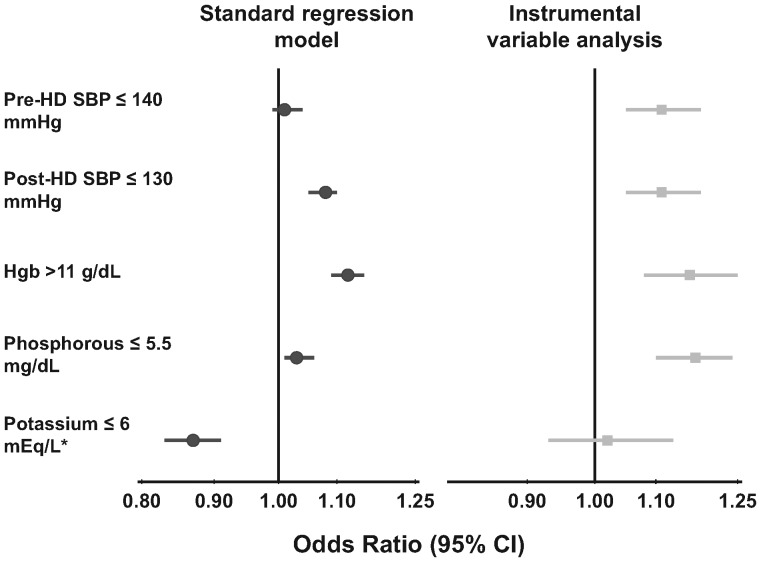
Associations between prescribed TT (both categorically and continuously) and intermediate outcomes are shown in Table 3. Longer TT was associated with levels of intermediate outcomes which are generally considered favorable, including higher hemoglobin [for a given erythropoietin (EPO) dose] and serum albumin, lower WBC and phosphorus. Longer TT was associated with greater weight loss and higher potassium levels in the standard regression models (perhaps due to unmeasured confounding by indication), but these associations were lost in the instrumental variable analysis (intended to lessen the biases resulting from patient-level unmeasured confounders). The associations between TT and achievement of clinical practice targets (most recent at time of manuscript submission) are shown in Figure 5. These associations are in keeping with the findings in Table 3.
Table 3.
Associations between prescribed TT and intermediate outcomesa
| Categorical TT (standard regression model) |
Continuous TT |
|||||
|---|---|---|---|---|---|---|
| 180 min | 210 min | 240 min | 270–300 min | Standard regression model (per 30 min) | Instrumental variable approach (per 30 min) | |
| Weight loss (kg) | − 0.61 (− 0.70 to− 0.52) | − 0.21 (− 0.29 to− 0.12) | 0.00 (reference) | 0.38 (0.28 to 0.47) | 0.26 (0.23 to 0.29) | − 0.01 (− 0.08 to 0.06) |
| Pre-HD SBP (mmHg) | 0.50 (− 0.29 to 1.29) | 0.45 (− 0.31 to 1.22) | 0.00 (reference) | 0.21 (− 0.67 to 1.09) | − 0.29 (− 0.56 to− 0.01) | − 1.35 (− 2.09 to− 0.61) |
| Post-HD SBP (mmHg) | 1.32 (0.52 to 2.12) | 0.38 (− 0.40 to 1.15) | 0.00 (reference) | − 2.18 (− 3.07 to− 1.28) | − 1.01 (− 1.28 to− 0.73) | − 1.49 (− 2.27 to− 0.70) |
| Hemoglobin (g/dL) | − 0.24 (− 0.29 to− 0.19) | − 0.07 (− 0.12 to− 0.02) | 0.00 (reference) | 0.19 (0.13 to 0.24) | 0.11 (0.09 to 0.13) | 0.13 (0.08 to 0.19) |
| Albumin (g/dL) | − 0.02 (− 0.04 to− 0.01) | − 0.01 (− 0.03 to 0.01) | 0.00 (reference) | 0.05 (0.03 to 0.07) | 0.02 (0.01 to 0.02) | 0.05 (0.03 to 0.08) |
| WBC (1000/mL) | 0.10 (0.02 to 0.19) | 0.05 (− 0.03 to 0.13) | 0.00 (reference) | − 0.06 (− 0.15 to 0.03) | − 0.04 (− 0.07 to− 0.01) | − 0.09 (− 0.15 to− 0.03) |
| Ferritin (ng/mL) | − 18.8 (− 33.3 to− 4.3) | − 15.6 (− 29.0 to− 2.3) | 0.00 (reference) | 1.7 (− 13.6 to 17.0) | 6.7 (1.7 to 11.7) | 17.7 (− 2.4 to 37.8) |
| Phosphorous (mg/dL) | 0.05 (− 0.01 to 0.11) | 0.00 (− 0.06 to 0.06) | 0.00 (reference) | − 0.03 (− 0.09 to 0.04) | − 0.04 (− 0.06 to− 0.02) | − 0.16 (− 0.22 to− 0.11) |
| Potassium (mEq/L)b | − 0.11 (− 0.13 to− 0.08) | − 0.03 (− 0.06 to− 0.01) | 0.00 (reference) | 0.10 (0.07 to 0.13) | 0.05 (0.04 to 0.06) | 0.00 (− 0.03 to 0.02) |
aEstimate (95% CI) shown is the difference in each outcome associated with prescribed TT categories, compared to the reference category. Models adjusted for country and study phase, age, sex, race, time on dialysis, BMI, 13 summary comorbid conditions, residual kidney function, prescribed blood flow rate and catheter use and accounted for facility clustering.
bModel also adjusted for dialyzate K.
Fig. 5.
Association between 30 min longer prescribed TT and achievement of clinical targets. Clinical targets are based on the Kidney Disease Outcomes Quality Initiative clinical practice guidelines for cardiovascular disease in dialysis patients [24], bone metabolism and disease in chronic kidney disease [25] and anemia [26]. Model adjusted for age, sex, race, time on dialysis, BMI, 13 summary comorbid conditions, residual kidney function, prescribed blood flow rate, catheter use, country and study phase and accounted for facility clustering; *model also adjusted for dialyzate K.
Discussion
The present study examined a large cohort of patients receiving in-center, three times per week maintenance HD at 930 facilities in 12 countries participating in the DOPPS (1996–2008). TT prescription varied across countries, with the longest average TT in ANZ (255 ± 41 min) and the shortest in the USA (212 ± 32 min). These large differences must be interpreted along with consideration of other clinical practices, such as the use of high-flux dialyzers and delivered dialysis dose (which were both higher in North America). Overall, prescribed TT increased over the study period. The mean TT reported for US DOPPS participants is consistent with those recently reported by two large US dialysis organizations [10, 11]. The trend toward longer TT we observed in the USA was also reported by a US dialysis organization between 1996 and 2008 [10]. However, the increase in TT over time in both our study (from 208 ± 32 to 221 ± 31 min) and that publication (from 201 ± 61 to 213 ± 59 min) was relatively small and may not have had an impact on clinical outcomes. In fact, among US DOPPS participants in 2005–08, only 12% had a TT >250 min, while 23% were dialyzed for <200 min.
In the present study, patients with longer TT had lower risk of all-cause and cardiovascular mortality. A new interesting finding is the strong association between longer TT and lower risk of sudden death, which remained after adjusting for patient comorbidities (like diabetes and atrial fibrillation) that are risk factors for sudden death [27]. It is likely that the smaller plasma dialyzate electrolyte gradients, less dramatic volume shifts and less sympathetic hyperactivity during longer dialysis sessions may contribute to the lower risk of sudden death.
Patients treated with frequent HD may experience lower mortality [5]. The National Cooperative Dialysis Study is the only randomized controlled trial conducted among patients on three times per week HD that assessed the impact of TT on outcomes. Despite a trend toward higher hospitalization risk observed in the short TT arm, no effect of TT on mortality was found [28]. However, the trial was terminated early and thus did not test effect of TT on mortality. Two cohort studies from the early 1990s also failed to find any association between TT and mortality [29, 30]. It is likely that other clinical practices (e.g. dialyzer type) in use at the time these studies were conducted were different than current practices; their findings may not be applicable to the current HD population. Several observational studies have reported higher mortality risk for patients receiving shorter TT [6–11]. Our findings also indicate a higher mortality risk, especially sudden death, among DOPPS participants receiving shorter TT; these findings were confirmed in instrumental variable analyses based on the premise that patients are ‘assigned’ to dialysis facilities that prescribe different TTs on average. While Table 1 shows that patients prescribed a longer TT are generally healthier overall, the instrumental variable analysis results reduce the biases resulting from unmeasured patient-level confounding and still show a significant survival benefit of longer TT.
As reported in a prior DOPPS analysis [8], the association of TT with mortality differed across geographic region, being the strongest in Japan, intermediate in Eur/ANZ and no longer significant in North America. This finding is consistent with a recent analysis of US HD patients that reported no improved survival for patients with TT >4 h [10]. Since TT >4 h is relatively uncommon in North America, we conducted a sensitivity analysis among patients with TT ≤4 h and found very consistent results (overall findings and regional differences). Our sensitivity analyses indicate that the variability in the association of TT with mortality across regions is not explained by differences in blood flow or vascular access and suggest that other factors may play a role. Other differences that may vary across regions and impact the association of TT with mortality potentially include both patient characteristics and dialysis practices, and additional study is warranted.
Better control of anemia, blood pressure, fluid overload and phosphorus levels as well as improved nutrition, left ventricular function and quality of life have been reported in small cohorts of patients receiving daily in-center and long nightly dialysis [2–4]. Improved blood pressure and phosphorus control decreases in left ventricular mass and improvement in physical health were recently reported among Frequent Hemodialysis Network participants randomized to frequent dialysis [5].
Our study demonstrates an association between longer TT and better intermediate outcomes (with ‘better’ referring to generally accepted clinical targets). Longer dialysis sessions provide greater clearance of both small and larger molecules [31]. Greater clearance may, for example, improve inflammatory status as indicated by the lower WBC count. This may, in turn, improve anemia control and lower EPO requirements. Finally, the longer dialysis sessions allow for slower ultrafiltration rates and tolerance of greater fluid removal, leading to improved control of hypertension [32, 33]. This is indicated in instrumental variable analyses where longer TT was associated with lower SBP levels both before and after dialysis as well as with better achievement of current clinical guidelines for BP control [34]. In support of this finding, longer TT was also associated with lower risk of hospital admission for fluid overload or congestive heart failure, presumably due to volume overload. These data confirm the findings of improved volume control with the change to longer TT in a study of 17 patients published in the 1980s [35].
Overall, it is reasonable to postulate that the improvement of one or more of these clinical markers may contribute to better survival for patients receiving longer dialysis sessions. To our knowledge, our results provide support indicating that several pathophysiological mechanisms may link longer TT with longer survival and fewer hospitalizations.
A strength of the current study is that it applies both standard regression and instrumental variable approaches. The latter uses the dialysis facility as an instrument to lessen confounding by indication caused by unmeasured patient-level confounders [14–17]. Both types of analyses yielded generally corroborative associations between TT and patient outcomes. Results of prior patient-based studies may have been biased by differences between patients receiving long versus short TT that may not have been completely taken into account, despite extensive model adjustments. For example, only more adherent patients may be willing to undergo the longer sessions; these patients are likely more adherent with medication prescription and dietary restrictions and may survive longer. On the other hand, patients who are sicker may be prescribed longer dialysis sessions. The instrumental variable methodology partially addresses this issue and is being applied to several fields of medical research [18, 36–40].
The established DOPPS infrastructure and representative sampling approach across 12 countries represents another strength, while raising regional differences in the association of TT with survival as a topic for further study. The extensive DOPPS data set allowed us to describe the association between delivered TT with outcomes, yielding very similar results as the prescribed TT analyses.
The limitations of the study are related to its observational design. Despite the extensive adjustments and the use of an instrumental variable approach, the potential for residual confounders remain and our results do not prove a causal effect between longer TT and better clinical outcomes.
Facilities delivering longer dialysis sessions may face higher costs [41], and shortening dialysis treatments may be associated with cost savings in certain payment environments [42]. The current US Centers for Medicare & Medicaid Services clinical performance measures and the planned Quality Incentive Program are based on delivered dialysis dose rather than TT [43]. Therefore, the pressures that dialysis providers in the USA will be facing with the implementation of the bundled ESRD prospective payment system [44] may incentivize shorter dialysis sessions as long as adequate urea clearance is provided. These incentives contrast with other countries, such as the Japanese reimbursement structure that favors at least 4 h of TT in all but the sickest patients and the German Qualitaetssicherungs-Richtlinie Dialyse that bases reimbursement, in part, on achieved TT of at least 4 h [45]. Of note, TT in Germany has risen dramatically as this financial incentive has been implemented (W. Kleophas, personal communication).
In the absence of randomized controlled clinical trials, we encourage health care providers to take into account findings from observational studies as well as supportive principles of dialysis, when making decisions regarding the duration of dialysis sessions. Similarly, policy makers and developers of quality measures worldwide may consider the current evidence about the duration of dialysis session when creating policies or guidelines that may affect TT.
In summary, our study confirms generally favorable clinical outcomes with longer TT and demonstrates associations of longer TT with better anemia, phosphorus and blood pressure control indicating possible mechanisms for improved clinical outcomes. These findings support longer TT prescription in the setting of three times per week HD.
Conflict of interest statement
F.T., J.Z., A.K., F.P., R.P. and B.R. are employees of Arbor Research Collaborative for Health which is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi/Genzyme (since 2009), Abbott (since 2009) and Baxter (since 2011), without restrictions on publications. F.T. was supported by Award Number K01DK087762-01A1 from the National Institute Of Diabetes And Digestive And Kidney Diseases. Y.L, R.S., J.B. and T.A. have no disclosures to make. P.K. is on advisory boards for Fresenius and Baxter as well as Amgen and Genzyme.
(See related article by Eloot et al. Less water for haemodialysis: is multiple pass the future pace to go? Nephrol Dial Transplant 2012; 27: 3975–3978.)
Acknowledgments
This paper received editorial support from Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health. The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi/Genzyme (since 2009), Abbott (since 2009), Vifor Fresenius Renal Pharma (since 2011) and Baxter (since 2011), without restrictions on publications.
References
- 1.US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. [Google Scholar]
- 2.Suri RS, Nesrallah GE, Mainra R, et al. Daily hemodialysis: a systematic review. Clin J Am Soc Nephrol. 2006;1:33–42. doi: 10.2215/CJN.00340705. [DOI] [PubMed] [Google Scholar]
- 3.Walsh M, Culleton B, Tonelli M, et al. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int. 2005;67:1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 4.Charra B. Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int. 2007;11:21–31. doi: 10.1111/j.1542-4758.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Held PJ, Levin NW, Bovbjerg RR, et al. Mortality and duration of hemodialysis treatment. JAMA. 1991;265:871–875. [PubMed] [Google Scholar]
- 7.Lowrie EG, Li Z, Ofsthun N, et al. Measurement of dialyzer clearance, dialysis time, and body size: death risk relationships among patients. Kidney Int. 2004;66:2077–2084. doi: 10.1111/j.1523-1755.2004.00987.x. [DOI] [PubMed] [Google Scholar]
- 8.Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69:1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 9.Marshall MR, Byrne BG, Kerr PG, et al. Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int. 2006;69:1229–1236. doi: 10.1038/sj.ki.5000188. [DOI] [PubMed] [Google Scholar]
- 10.Miller JE, Kovesdy CP, Nissenson AR, et al. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55:100–112. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunelli SM, Chertow GM, Ankers ED, et al. Shorter dialysis times are associated with higher mortality among incident hemodialysis patients. Kidney Int. 2010;77:630–636. doi: 10.1038/ki.2009.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57(Suppl 74):S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 13.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study: design, data elements, and methodology. Am J Kidney Dis. 2004;44(Suppl 2):S7–S15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91:444–455. [Google Scholar]
- 15.Wooldridge JM. Introductory Econometrics. 4th edn. Mason, OH: South-Western College Publishing; 2002. chapter 15. [Google Scholar]
- 16.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29:722–729. doi: 10.1093/ije/29.4.722. [DOI] [PubMed] [Google Scholar]
- 18.Pisoni RL, Arrington CJ, Albert JM, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis. 2009;53:475–491. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Stock JH, Wright JH, Yogo M. A survey of weak instruments and weak identification in generalized method of moments. J Bus Econ Stat. 2002;20:518–529. [Google Scholar]
- 20.Burgess S, Thompson SG CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–764. doi: 10.1093/ije/dyr036. doi:10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 21.Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation and Variance Estimation Software. 2002 Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan. [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 23.Saran R, Bragg-Gresham JL, Rayner HC, et al. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64:254–262. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 24.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 26.KDOQI Workgroup. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(5 Suppl 3):S11–145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Genovesi S, Valsecchi MG, Rossi E, et al. Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24:2529–2536. doi: 10.1093/ndt/gfp104. [DOI] [PubMed] [Google Scholar]
- 28.Lowrie EG, Laird NM, Parker TF, et al. Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med. 1981;305:1176–1181. doi: 10.1056/NEJM198111123052003. [DOI] [PubMed] [Google Scholar]
- 29.Owen WF, Jr, Lew NL, Liu Y, et al. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329:1001–1006. doi: 10.1056/NEJM199309303291404. [DOI] [PubMed] [Google Scholar]
- 30.Held PJ, Port FK, Wolfe RA, et al. The dose of hemodialysis and patient mortality. Kidney Int. 1996;50:550–556. doi: 10.1038/ki.1996.348. [DOI] [PubMed] [Google Scholar]
- 31.Eloot S, Van Biesen W, Dhondt A, et al. Impact of hemodialysis duration on the removal of uremic retention solutes. Kidney Int. 2008;73:765–770. doi: 10.1038/sj.ki.5002750. [DOI] [PubMed] [Google Scholar]
- 32.Charra B, Calemard M, Laurent G. Importance of treatment time and blood pressure control in achieving long-term survival on dialysis. Am J Nephrol. 1996;16:35–44. doi: 10.1159/000168968. [DOI] [PubMed] [Google Scholar]
- 33.McGregor DO, Buttimore AL, Nicholls MG, et al. Ambulatory blood pressure monitoring in patients receiving long, slow home haemodialysis. Nephrol Dial Transplant. 1999;14:2676–2679. doi: 10.1093/ndt/14.11.2676. [DOI] [PubMed] [Google Scholar]
- 34.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 35.Wizemann V, Kramer W. Short-term dialysis—long-term complications. Ten years experience with short-duration renal replacement therapy. Blood Purif. 1987;5:193–201. doi: 10.1159/000169471. [DOI] [PubMed] [Google Scholar]
- 36.Stukel TA, Fisher ES, Wennberg DE, et al. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneeweiss S, Seeger JD, Landon J, et al. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358:771–783. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 38.Brookhart MA, Rassen JA, Wang PS, et al. Evaluating the validity of an instrumental variable study of neuroleptics: can between-physician differences in prescribing patterns be used to estimate treatment effects? Med Care. 2007;45(10 Suppl 2):S116–S122. doi: 10.1097/MLR.0b013e318070c057. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez SP, Albert JM, Blayney MJ, et al. Rosiglitazone is associated with mortality in chronic hemodialysis patients. J Am Soc Nephrol. 2009;20:1094–1101. doi: 10.1681/ASN.2008060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tentori F, Albert JM, Young EW, et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2009;24:963–972. doi: 10.1093/ndt/gfn592. [DOI] [PubMed] [Google Scholar]
- 41.Hirth RA, Held PJ, Orzol SM, et al. Practice patterns, case mix, Medicare payment policy, and dialysis facility costs. Health Serv Res. 1999;33:1567–1592. [PMC free article] [PubMed] [Google Scholar]
- 42.Held PJ, García JR, Pauly MV, et al. Price of dialysis, unit staffing, and length of dialysis treatments. Am J Kidney Dis. 1990;15:441–450. doi: 10.1016/s0272-6386(12)70362-1. [DOI] [PubMed] [Google Scholar]
- 43. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. United States, 2008, pp. 60–67.
- 44.Medicare Coverage for End-Stage Renal Disease Patients. Compilation of the Social Security Laws Including the Social Security Act, as Amended, and Related Enactments Through January 1, 2009. http://www.ssa.gov/OP_Home/ssact/title18/1881.htm#ft576. (1st December 2011, date last accessed) [Google Scholar]
- 45.NN: Qualitaetssicherungs-Richtlinie Dialyse (German) Bundesanzeiger. 2006;58:115a. [Google Scholar]