[…] commonly used equations for eGFR should not be applied in AKI patients, neither at the debut of AKI nor during its recovery. The common equations for eGFR will markedly overestimate GFR and may potentially result in overdosing of drugs with renal excretion and risk of side effects. […]
Keywords: acute kidney injury, continuous renal replacement therapy, creatinine generation, critical care nephrology, mortality
Abstract
Background
Existing systems for grading severity of acute kidney injury (AKI) rely on a change of serum creatinine concentration over a defined time interval. The rate of change in serum creatinine increases by degree of reduction in glomerular filtration rate, but is mitigated by low creatinine generation rate (CGR). Failure to appreciate variation in CGR may lead to erroneous conclusions regarding severity of AKI and distorted predictions regarding patient outcomes based on AKI severity.
Methods
Cohort study of 103 patients who received continuous venovenous hemodialysis (CVVHD) over a 2-year period in a tertiary care hospital setting. Study participants entered the cohort when they were anuric, receiving a stable and uninterrupted dose of CVVHD with serum creatinine in steady state. They were followed until hospital discharge. CGR was measured based on dialyzate effluent volume and effluent creatinine concentration (prospective cohort) and via effluent volume and serum creatinine concentration (retrospective cohort).
Results
CGR (mean 10.5, range 1.7–22.4 mg/kg/day) was substantially lower in this patient population than what would be predicted from existing equations. Correlates of CGR in multivariable analysis included the length of hospitalization prior to measurement and presence of an oncologic diagnosis. Lower CGR was independently associated with in-hospital mortality in unadjusted analysis and after multivariable adjustment for measures of severity of illness.
Conclusions
Grading systems for severity of AKI fail to account for variation in CGR, limiting their ability to predict relevant outcomes. Calculation of CGR is superior to other risk metrics in predicting hospital mortality in this population.
Introduction
Acute kidney injury (AKI) affects an estimated 4–5% of hospital inpatients, and is a potentially devastating condition, with mortality rates approaching 20% [1]. Until recently, the study of AKI was hampered by a lack of clear consensus definitions. The Risk, Injury, Failure, Loss, End-stage kidney disease (RIFLE) and subsequent Acute Kidney Injury Network (AKIN) criteria for AKI severity have ushered in a new era of AKI research [2, 3]. These scoring systems define AKI by a change in serum creatinine concentration or reductions in urine output over a defined time period. Urine output may be unreliable due to inaccuracies in measurement and confounded by diuretic usage [4, 5], while increases in serum creatinine concentration depend not only on the degree of reduction of glomerular filtration rate (GFR) but also on the patients' underlying creatinine generation rate (CGR)—with higher CGR leading to more rapid increases in serum creatinine. The CGR is itself dependent on the non-enzymatic hydrolysis of creatine, which is produced primarily in the liver but stored almost entirely in skeletal muscle. Progressive stages of kidney injury in both the RIFLE and AKIN frameworks predict mortality [4–10], but studies in stable outpatients demonstrate that higher CGR independently associates with better survival [11]. Similar findings have been published in chronic hemodialysis patients [12]. Animal studies have demonstrated acute reductions in CGR in the setting of sepsis [13]. As lower CGR would lead to assignment of lower RIFLE or AKIN stages for any given level of GFR decrement, heterogeneity in CGR may limit the ability of the RIFLE and AKIN criteria to capture the true severity of AKI and to accurately predict mortality. Few studies have examined CGR in an acutely ill population, and none have described its association with mortality [14–16].
Measurement of CGR in AKI is difficult for several reasons. Patients are often not in steady state with regard to serum creatinine, making urinary collections for creatinine excretion uninterpretable. In addition, unmeasurable changes in the volume of distribution of creatinine may occur due to fluid administration and accumulation [17]. Finally, the logistical apparatus needed to prospectively identify and enroll a large number of subjects with AKI is costly and cumbersome.
We identified a population of patients undergoing continuous venovenous hemodialysis (CVVHD) in order to overcome the above challenges. The patients studied were all in steady state with regard to serum creatinine, receiving a stable and continuous CVVHD dose and were anuric, allowing an accurate calculation of CGR based on effluent volume and serum creatinine, as first described by Clark et al. [18]. In this study, we sought to describe CGR in a critically ill cohort with AKI and measure its association with inpatient mortality. We hypothesized that CGR would be lower than predicted by existing equations and that lower CGR would be associated with higher inpatient mortality.
Materials and methods
Study population
In this cohort study, we identified all patients at our institution (a tertiary care medical center serving an urban and suburban population) who received CVVHD from 1 July 2008 to 30 June 2010 (n = 525) using an electronic medical record database. Patients were eligible for inclusion if they were >17 years of age, were anuric and had achieved a steady state serum creatinine on a stable and uninterrupted dose of CVVHD. To be considered in steady state, subjects were required to have at least three serum creatinine measurements within 10% of each other within a 24-h period. The first and last creatinine measurements were required to be at least 12 h apart. Steady-state creatinine was defined as the arithmetic mean of all creatinine concentrations obtained during the 24-h period. Furthermore, the subsequent serum creatinine concentration after the 24-h window (whenever measured) was required to be within 10% of the steady-state creatinine. Patients with end-stage renal disease were excluded. Among the 107 patients who met eligibility requirements, 92 had complete dialysis flow sheet data and were included in the primary analysis. In addition, 11 patients were prospectively studied (see below) and also included in the primary analysis for a total of 103 studied subjects. In all patients, indications for and administration of CVVHD were determined by the treating nephrologist independent of the study team. It is our institutional practice that all patients are prescribed a blood flow of 300 mL/min, a dialyzate flow of 2–3 L/h and a dialyzate temperature of 36.5°C. CVVHD was performed using a NxStage machine with a high flux polyethersulfone membrane-integrated cartridge system (NxStage Medical, Lawrence, MA) [19].
The 11 prospectively identified patients provided informed consent for measurement of serum and effluent creatinine concentrations. This study was approved by the Institutional Review Board of the University of Pennsylvania.
Data collection
Demographic variables were assessed at hospital admission. Laboratory and clinical variables were assigned on the first day of steady-state analysis. Patient weights, typically from a hospital bed scale, were obtained from CVVHD flow sheets and were carried forward from the last measured weight if missing on the day of steady-state analysis (36/103 patients). Patient height was recorded by the admitting nurse, typically based on self-report or report of family members, and was available on all patients. Ideal body weights (IBW) were calculated to normalize patients' height to a body mass index (BMI) of 22, without adjustment for demographic or anthropometric factors. We generated a modified Sequential Organ Failure Assessment (SOFA) score for each patient based on clinically available data, excluding the nervous system categorizations due to unreliable reporting of Glasgow Coma Scale [20]. Serum and effluent creatinine concentrations were measured in our clinical laboratory using the Jaffe rate method [21].
As patients were anuric and in steady state, CGR was assumed to be equal to the amount of creatinine cleared via CVVHD over a 24-h period. This procedure is exactly analogous to measuring the amount of creatinine in a 24-h urine specimen; a standard test performed in outpatients in order to calculate a measured creatinine clearance. Effluent volume was calculated as the sum of dialyzate volume and ultrafiltrate volume, which is recorded on an hourly basis during treatment. In the prospective cohort, we measured spot serum and effluent creatinine concentrations simultaneously during steady state to validate the assumption that they are equivalent. We timed spot effluent collection to coincide with scheduled phlebotomy, but required that the patient had already met the steady state requirements described above before collection. We planned a priori to exclude patients whose serum creatinine concentration was no longer within 10% of steady state concentration at the time of simultaneous blood/effluent sampling but this scenario did not occur. Based on these results and on a prior study documenting complete equilibration of creatinine across the same dialysis membrane at similar dialyzate flow rates [22], effluent creatinine concentration was assumed to be equal to serum creatinine concentration at steady state in the retrospective cohort. CGR was calculated as the product of serum creatinine and effluent volume in this group. When expressed in terms of milligram per kilogram, IBW was used for all formulas.
Outcome measures
The primary outcome was in-hospital mortality. Patients discharged to hospice care services were categorized as alive. Sensitivity analysis counting those patients as deceased did not significantly alter the results.
Statistical analysis
Patient characteristics are presented as means and SDs for continuous variables that were normally distributed and as medians and interquartile ranges for non-normally distributed variables. Measured CGR was compared to that predicted by existing equations using paired t-tests. Predictors of CGR were assessed using univariable and multivariable linear regression. Correlations were measured with Pearson's or Spearman's correlation coefficient as appropriate. Associations with in-hospital mortality were assessed using multivariable logistic regression. Due to the low number of non-events (survivors), we performed a series of trivariate analyses in addition to the full multivariable regression to account for instability in odds ratios (ORs) that can occur when non-events are rare. This is analogous to situations where events are very rare, limiting the number of covariates that can be fit to a multivariable model. Potential predictors of in-hospital mortality were assessed using receiver operator characteristic (ROC) curves. Count data were analyzed using Fisher's exact or chi-square testing as appropriate. Continuous variables were partitioned in analysis based on clinically relevant values that approximated the SDs or interquartile ranges of the variables. Model goodness-of-fit was assessed with the Akaike information criterion. Analyses were performed using Stata v11.2 (College Station, TX).
Results
Patient characteristics are summarized in Table 1. The mean age was 59 years; 64% were male; 21% were black and 36% had diabetes. All patients were in the intensive care unit (ICU), and the vast majority of patients were mechanically ventilated. There was a similar representation of medical and surgical patients. BMIs reflect an overweight to obese population, which is consistent with the demographics of our catchment area. Dialysate parameters were consistent with our institutional practice of using dialyzate flow rates of 2–3 L/h; all blood flow rates were 300 mL/min during the study period.
Table 1.
Patient characteristics measured at steady statea
| Total (N = 103) | |
|---|---|
| Age (SD) | 59 (14.3) |
| Male (%) | 68 (64) |
| Black (%) | 23 (21) |
| Diabetes mellitus (%) | 39 (36) |
| Medical ICU (%) | 52 (51) |
| Surgical ICU (%) | 51 (50) |
| Oncologic diagnosis (%) | 14 (14) |
| ABW, kg | 88.6 (74.7–106.2) |
| BMI, kg/m2 | 30.1 (25.8–37.1) |
| IBW, kg | 63.7 (56.3–69.5) |
| Obese (%) | 57 (57) |
| Pressor support (%) | 75 (73) |
| Mechanical ventilation (%) | 90 (84) |
| Hospital stay pre-CRRT, days | 5 (2–13) |
| Time from AKI onset to CRRT initiation, days | 2 (0–4) |
| Serum creatinine at CRRT initiation, mg/dL | 3.6 (2.7–4.8) |
| BUN at CRRT Initiation, mg/dL | 70 (47–99) |
| Time from CRRT initiation to steady state, hours | 60 (43–107) |
| Serum albumin, g/dL | 1.9 (1.7–2.6) |
| Serum total bilirubin, mg/dL | 2.0 (1.1–5.5) |
| Weight change from admission to CRRT initiation, kg | 4.9 (0–10.9) |
| Dialysate flow rate, L/h | 2.0 (2.0–2.5) |
aAll data median (interquartile range) unless otherwise specified. AKI onset defined as first day when creatinine was >50% of baseline value. Albumin available in 63/103 patients. Total bilirubin available on 96/103 patients. BUN, blood urea nitrogen.
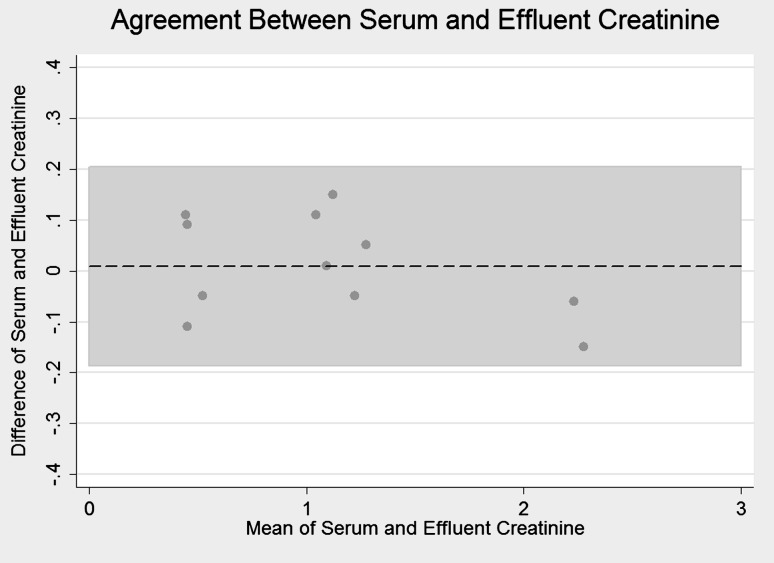
Serum and effluent creatinine were measured simultaneously in the prospective cohort (Figure 1). The correlation between the two measures was very strong (r = 0.99), and the signed-rank test did not demonstrate a significant difference between paired measures (P = 0.69), confirming near complete equilibration of creatinine across the dialysis membrane.
Fig. 1.
Bland–Altman plot showing agreement of serum and effluent creatinine concentrations. A positive direction on the Y-axis indicates a higher serum than effluent value. X-axis is the average of the two measurements. Dotted line represents mean difference (0.009 mg/dL). Shaded area represents 95% CIs of agreement.
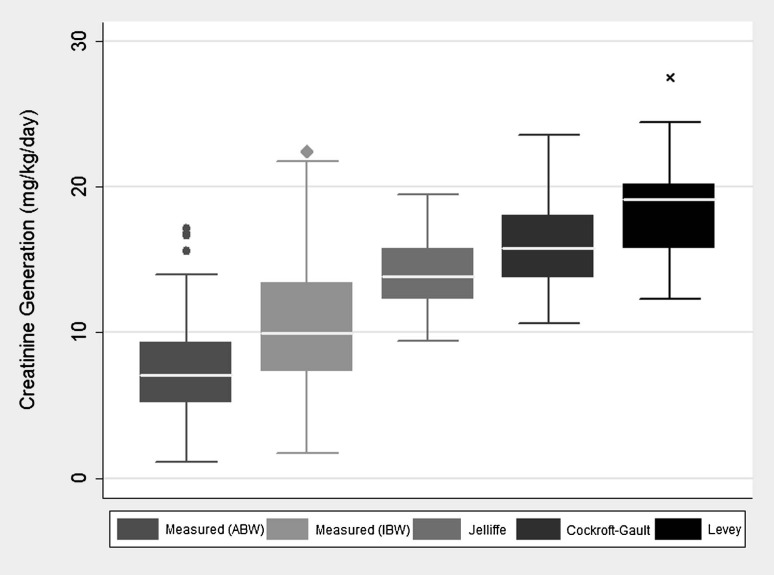
The mean (SD) steady state serum creatinine concentration in our cohort was 1.2 (0.5) mg/dL. This was achieved after a median of 60 h on CVVHD. The mean CGR was 10.5 (4.2) mg/kg/day using IBW. We compared this rate to the rate that would be predicted by actual body weight (ABW), the Cockroft-Gault formula, a modification of the Jelliffe formula and a recently published formula by Levey et al. [23–25]. The Cockroft-Gault formula was derived in 249 male Caucasian outpatients with and without chronic kidney disease (CKD), while the Levey equation was developed in 2466 outpatients with CKD. The Jelliffe equation was developed mathematically to estimate GFR in patients with changing serum creatinine concentration due to AKI. CGR was significantly less than predicted by each of these equations (P < 0.001 for all comparisons) (Figure 2).
Fig. 2.
Box plot demonstrating measured versus predicted CGR in the cohort. Prediction equations appear in appendix [23–25].
Table 2 demonstrates the unadjusted and adjusted associations of clinical parameters with CGR. The classic predictors of CGR, i.e. gender, race and age, were not associated with CGR in this cohort. CGR was lower by a small but statistically significant amount for each additional hospital day spent prior to the measurement. To assess for delay in initiation of CVVHD due to lower CGR, we analyzed the association between serum creatinine at the start of CVVHD and the time to CVVHD initiation, and found no significant relationship (P = 0.15). Patients with CKD Stage ≥3 (n = 55) had significantly higher CGR than their non-CKD counterparts, although CKD status could not be determined in 16 patients. Patients who carried an oncologic diagnosis had a significantly lower CGR than non-oncologic patients. There was no difference in CGR between medical and surgical patients. There was no association between CGR and serum total bilirubin concentration (r = 0.04, P = 0.67) or between steady state creatinine concentration and serum total bilirubin (r = 0.11, P = 0.28).
Table 2.
Patient variables and associations with CGRa
| Variable | Difference in creatinine generation (mg/kg/day) | 95% CI | P |
|---|---|---|---|
| Univariable associations with CGR | |||
| Male sex | 1.4 | − 0.29 to 3.10 | 0.10 |
| Hospital days prior to measurement (per day) | − 0.08 | − 0.12 to − 0.04 | 0.01 |
| CKD ≥ Stage 3 | 2.6 | 0.74 to 4.43 | 0.01 |
| Oncologic diagnosis | − 2.7 | − 5.04 to − 0.29 | 0.03 |
| Black (versus other races) | 2.02 | − 0.01 to 4.06 | 0.05 |
| Age (per 10 years greater) | − 0.25 | − 0.85 to 0.35 | 0.41 |
| Log albumin (per 1 log unit) | 3.99 | 0.87 to 7.11 | 0.01 |
| Obese | 0.90 | − 0.76 to 2.56 | 0.28 |
| Diabetes | 1.18 | − 0.53 to 2.90 | 0.17 |
| Ventilated | − 1.30 | − 3.53 to 0.94 | 0.25 |
| Transplanted organ | − 0.39 | − 2.30 to 1.53 | 0.69 |
| pH (per 0.1 increase) | − 0.56 | − 1.55 to 0.42 | 0.26 |
| Pressor medications (per 1 additional) | 0.001 | -0.77 to 0.77 | 0.99 |
| Modified SOFA score (per 1 additional point) | − 0.08 | − 0.43 to 0.26 | 0.63 |
| Medical patient (versus surgical patient) | 0.81 | − 0.84 to 2.47 | 0.33 |
| Multivariable associations with CGR | |||
| Male sex | 1.08 | − 0.48 to 2.65 | 0.17 |
| Hospital days prior to measurement (per day) | − 0.08 | − 0.12 to − 0.04 | < 0.001 |
| Oncologic diagnosis | − 2.44 | − 4.62 to − 0.27 | 0.03 |
| Black (versus other races) | 1.61 | − 0.27 to 3.49 | 0.09 |
| Age (per 10 years greater) | − 0.40 | − 0.95 to 0.15 | 0.16 |
aUnivariable and Multivariable associations with CGR. CKD status and albumin not included in multivariable analysis due to missing data. Subgroup analysis on patients with available CKD status data did not alter results.
Multivariable linear regression models were fit with CGR as the outcome and variables associated with CGR in unadjusted analyses and those that have been previously described as associated with CGR. Sensitivity analyses (assuming patients with missing data were entirely without CKD, entirely with CKD or with the population mean) did not substantially alter the univariate or multivariable relationship between CKD and CGR. Shorter hospital stay prior to our calculation of CGR and lack of oncologic diagnosis were significantly associated with higher CGR in the multivariable model.
Impact of CGR on in-hospital mortality
The overall in-hospital mortality rate was 73%. We divided the patients into tertiles of CGR: highest (>12.2 mg/kg/day), middle (8.7–12.2 mg/kg/day) and lowest (<8.7 mg/kg/day). Corresponding death rates were 57, 76 and 85%, respectively (P = 0.01). Adjusted ORs for in-hospital mortality appear in Table 3. Adjustment for additional patient factors including SOFA score, medical versus surgical status, age and race strengthened the association between CGR and in-hospital mortality. Areas under the ROC curve were created for CGR and other potential predictors of mortality. CGR yielded a C-statistic of 0.64 [95% confidence interval (CI) 0.52–0.76]. Other predictors including SOFA score, lactic acid, actual weight, IBW, baseline creatinine, admission creatinine and age performed no better than chance in terms of identifying patients who would meet the primary endpoint.
Table 3.
CGR tertile and odds of inpatient mortalitya
| Model | Highest (n = 35) | Middle (n = 34) | Lowest (n = 34) | P |
|---|---|---|---|---|
| Events/no. at risk % | 20/35 (57) | 26/34 (76) | 29/34 (85) | |
| Unadjusted | 1.00 | 2.12 (1.19–3.79) | 4.52 (1.42–14.4) | 0.01 |
| Age and sex adjusted | 1.00 | 2.34 (1.27–4.34) | 5.50 (1.60–18.8) | 0.01 |
| Age, sex, race adjusted | 1.00 | 2.39 (1.24–4.59) | 5.70 (1.54–21.0) | 0.01 |
| Medical status, SOFA adjusted | 1.00 | 2.23 (1.22–4.10) | 4.99 (1.49–16.74) | 0.01 |
| Medical status, SOFA, LOS adjusted | 1.00 | 3.60 (1.72–7.56) | 12.97 (2.94–57.18) | 0.001 |
| Fully adjustedb | 1.00 | 3.47 (1.57–7.67) | 12.0 (2.47–58.7) | 0.002 |
aValues are OR (95% CI), Highest = 15 ± 2 mg/kg/day, Middle = 10 ± 1 mg/kg/day and Lowest = 6 ± 2 mg/kg/day.
bAdjusted for age, sex, race, oncologic diagnosis, modified SOFA score, medical ICU status and hospital stay prior to measurement.
We explored other independent associations with death in this population. To assure stability of the demonstrated relationships, a series of trivariate analyses were performed in addition to the full multivariable regression (Table 4). In every case, CGR (expressed as a continuous variable) was strongly and independently associated with in-hospital mortality. Other associations with death in multivariable analysis included medical status (versus surgical status) and SOFA score.
Table 4.
Mutually adjusted associations of risk factors for in-hospital mortality
| Risk factor | OR | 95% CI | P |
|---|---|---|---|
| Full multivariable model | |||
| Creatinine generation rate (per 5 mg/kg/day less) | 2.61 | 1.26–5.39 | 0.01 |
| Medical patient | 3.78 | 1.08–13.1 | 0.04 |
| Modified SOFA score (per each additional pt) | 1.37 | 1.04–1.80 | 0.03 |
| Diabetes | 2.65 | 0.81–8.73 | 0.11 |
| Black | 1.23 | 0.32–4.72 | 0.76 |
| Male sex | 0.99 | 0.34–2.90 | 0.98 |
| BMI (per 5 kg/m2 greater) | 0.89 | 0.65–1.22 | 0.48 |
| Age (per 10 years greater) | 0.83 | 0.56–1.24 | 0.37 |
| Oncologic diagnosis | 0.57 | 0.12–2.74 | 0.49 |
| Ventilated | 0.14 | 0.02–1.05 | 0.06 |
| Trivariate models | |||
| Medical patient | 2.96 | 1.02–8.59 | 0.05 |
| Modified SOFA score (per each additional pt) | 1.18 | 0.96–1.45 | 0.13 |
| CGR (per 5 mg/kg/day less) | 1.87 | 1.06–3.30 | 0.03 |
| Medical patient | 1.83 | 0.70–4.75 | 0.22 |
| Vented | 0.57 | 0.14–2.33 | 0.44 |
| CGR (per 5 mg/kg/day less) | 1.82 | 1.06–3.12 | 0.03 |
| Medical patient | 2.05 | 0.81–5.18 | 0.13 |
| Oncologic diagnosis | 0.95 | 0.22–3.99 | 0.95 |
| CGR (per 5 mg/kg/day less) | 1.81 | 1.04–3.18 | 0.04 |
In addition to being a reflection of overall muscle mass, CGR may also be related to humorally-mediated decrements in muscle production of creatine, which is subsequently metabolized to creatinine. While we did not have measurements of inflammatory markers in the cohort, 61% of patients had measurements of serum albumin, a marker of nutrition as well as a negative acute phase reactant, available. Serum albumin was correlated with CGR (Rho = 0.39, P = 0.002), but it was not associated with death (P = 0.63).
Discussion
Prior studies examining creatinine generation in critical illness have been limited by small sample size [18] or have been performed in animal models [13]. One large study found a correlation between creatinine generation and mortality in an outpatient cohort with cardiovascular disease [11]. This study is the first of its kind examining a large population of critically ill adults with AKI.
CGR was markedly reduced in this cohort compared to rates predicted by existing equations that, with the exception of the modified Jelliffe formula, were developed in the outpatient setting. It should be noted that the best performing formula (Jelliffe) is the only formula that includes serum creatinine as a variable (in addition to demographic factors). The Jelliffe equation also accounts for decreased creatinine generation in the setting of uremia. In our cohort, however, serum creatinine levels were quite low, making this adjustment less useful. These observations should reinforce the fact that equations designed to predict GFR or creatinine clearance should not be used in patients with AKI or critical illness as they will in most cases dramatically overestimate true GFR, which can lead to inappropriate drug dosing and delays in appropriate treatment of AKI. Though all patients in the cohort were receiving CVVHD, these findings may extend into other critically ill populations. The mean CGR in this cohort was 10.5 mg/kg/day, but with a wide range of 1.7–22.4 mg/kg/day. This is similar to the mean of 9.0 mg/kg/day seen in the Clark et al. [18] study of 11 critically ill patients with AKI, but less than the 30% of patients with CGR < 10 mg/kg/day in a study of 209 non-AKI ICU patients performed by Pesola et al. [15]. In contrast, the study by Levey et al. [26] examining 2466 stable outpatients found a mean CGR of 17.8 mg/kg/day.
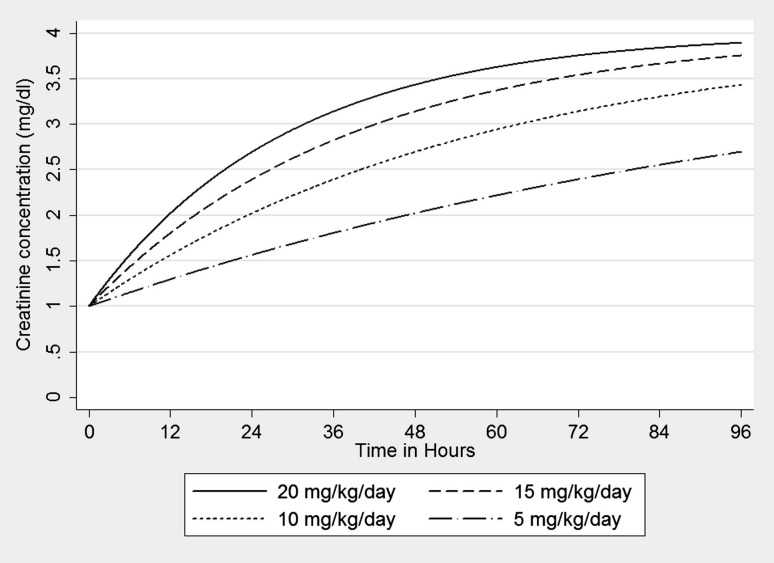
The wide range of CGRs in this cohort implies that the RIFLE and AKIN systems may differentially classify patients with the same reduction in GFR. Figure 3 graphically displays the impact of CGR on the rate of increase in serum creatinine concentration after an acute AKI event (reduction of GFR by 75% at time 0). The calculations are based on mass transfer principles with a single-compartment model of creatinine in a hypothetical 75 kg male with a baseline creatinine of 1. Baseline GFR would vary from 104 mL/min in the 20 mg/kg/day patient to 26 mL/min in the 5 mg/kg/day patient. Note that the time to a doubling of creatinine (AKIN Stage 2) ranges from 12 h to 48 h as CGR decreases. Clinicians may thus misinterpret acute changes in serum creatinine concentration if they fail to account for the CGR on the individual patient level. The creatinine-based definitions of AKI may require incorporation of factors correlated with CGR in order to accurately assess loss of kidney function. The methods described in our study rely on routinely collected clinical information (serum creatinine concentration and effluent volume) and as such can be easily scaled to existing large data sets to craft new prediction equations for CGR in the critically ill. With reliable prediction of CGR and estimates of volume of distribution, it would be possible to derive a non-steady state equation for instantaneous GFR based on a change in serum creatinine over time.
Fig. 3.
Creatinine concentration over time curves for a hypothetical 75 kg male suffering from AKI with a 75% reduction in GFR at time 0. Legend describes varying CGRs.
We chose to express CGR in terms of IBW as opposed to ABW. The latter is subject to significant inaccuracy of measurement and is confounded by fluid resuscitation, while the former is less physiologic. On the individual patient level though, deviations from IBW are typically due to accumulation of fluid and/or fat, neither of which play a significant role in creatinine production. IBW associated more strongly with CGR than measured body weight, as we expected (r = 0.45 versus 0.29). IBW was lower than ABW in 96% of our patients, meaning that if ABW was used CGR would appear to be even lower than the values we measured. We followed net fluid balance during the steady-state period, but did not identify a strong relationship to CGR. This is likely due to our selection criteria, as patients with significant fluid shifts would, all else being equal, have a changing serum creatinine concentration reflecting the changing volume of distribution of creatinine, and thus not be included for analysis. Sensitivity analyses using CGR calculated using ABW did not significantly affect the performance of CGR as a predictor of mortality.
CGR was lower in patients in whom our calculation of CGR took place later during their hospital stay. This may be due to progressive decrease in CGR during a hospital stay (with its attendant muscle loss). An additional explanation is that patients with a low CGR are dialyzed later in their hospital course, perhaps due to failure to recognize AKI, as the rate of rise in serum creatinine would be slower. This explanation is supported by the observation that patients dialyzed later in the hospital course had similar serum creatinine concentrations to those dialyzed earlier. Longitudinal measures of CGR in hospitalized patients would help to characterize this finding further.
We did not demonstrate an association of CGR with the classic variables of age, race and gender. This may be due to the fact that our population was so critically ill that race-, sex- and age-specific muscle mass correlations were lost or that these effects were overwhelmed by other factors. It is unclear whether our findings result from a reduction in creatine (and hence creatinine) production as a reflection of reduced muscle mass or a decrease in production of creatine or creatinine from muscle due to systemic factors. An interesting study by Bagshaw et al. [27] suggested a lower CGR among patients with septic versus non-septic AKI, though this was based on nadir serum creatinine concentration rather than quantified CGR. The lack of difference in CGR between our medical (primarily septic-ATN) and surgical (primarily ischemic-ATN) patients suggests that sepsis may not differentially account for reduction in CGR.
The correlation between serum albumin concentration and CGR may be related to their mutual dependence on nutritional intake or perhaps a mutual response to inflammatory mediators. Future studies with closer analysis of nutritional intake and inflammatory markers will help to elucidate this relationship. Our study did not demonstrate an association between albumin concentration and in-hospital mortality, contrary to prior studies, perhaps due to the degree of critical illness and overall low albumin concentrations. As albumin concentration was not associated with in-hospital mortality in this cohort, it does not confound the relationship between CGR and mortality.
CGR (as either a continuous variable or expressed in tertiles) was strongly and independently associated with in-hospital mortality in multiple analyses. We chose to examine a binary outcome (in-hospital mortality) as opposed to a time-to-event analysis. The mortality rate in this population is extremely high in the early part of the hospital stay. We felt that survival to hospital discharge was a good proxy of intermediate to long-term survival. Also, due to the pain and suffering associated with a prolonged ICU stay, we did not feel that a time-to-event analysis was clinically meaningful as it would assign a higher ‘value’ to a longer hospital stay even if that stay results in death. CGR was a more discriminant predictor of in-hospital mortality than many classic measures of critical illness including SOFA score and also outperformed other measures of muscle mass including baseline creatinine, admission creatinine and weight.
This study has several limitations. It was performed in a single center in extremely ill patients and thus may not be broadly generalizable to other geographic areas or into mild forms of AKI. As discussed above, it suffers for a lack of non-events (survivors) to allow adequate stability of estimates in full multivariable models. Although it represents the largest study of its kind in intensive care patients, the number of patients studied was not sufficient to generate or validate a prediction equation for CGR in this population. We did not have access to detailed nutritional data and thus can make no assessments as to the value of nutritional supplementation in terms of its effect on CGR and mortality. There was no control group; we compared overall CGR to those predicted from historical cohorts, limiting our ability to determine causative factors. Finally, our method assumes full equilibration of creatinine across the dialysis membrane. Rather than assume this was true based on prior studies, we confirmed this observation in the prospective cohort. Bland–Altman analysis revealed non-creatinine concentration-dependent variation in effluent creatinine with a 95% CI of ±0.2. This variability is likely due to random variability in laboratory measurements, as an effluent creatinine concentration higher than contemporaneous serum creatinine concentration is very unlikely. It should be noted that failure of full equilibration of creatinine across the membrane would artificially increase our calculation of CGR, meaning true CGR may be even lower than reported here. There is a small amount of gastrointestinal creatinine excretion and elimination that was not accounted for in this study, but this amount is proportional to serum concentration of creatinine, which was quite low in this population at steady state [26]. Patients were only included if anuric, minimizing the potential for renal loss of creatinine. Elevated bilirubin concentration may artificially lower serum creatinine concentration as measured via the Jaffe method [28], which may lead to lower CGR estimates, but we saw no association between serum bilirubin level and CGR or steady state serum creatinine concentration in our study. While failing to account for gastrointestinal or renal creatinine elimination would artificially decrease our calculation of CGR, the relationship to mortality would be unaffected.
In conclusion, this study demonstrates a marked reduction in CGR among a cohort of critically ill patients. It uses a novel and easily scalable methodology to assess CGR in similar populations. CGR performed better as a predictor of in-hospital mortality than a variety of other factors. Clinicians should be aware that in the critically ill, small changes in serum creatinine concentration may reflect large decrements in GFR due to low CGR.
Funding
This study had no external funding. Conflict of interest statement. None declared.
(See related article by Heimburger et al. The enigma of decreased creatinine generation in acute kidney injury. Nephrol Dial Transplant 2012; 27: 3973–3974.)
Acknowledgments
The authors wish to thank Dr Harold Feldman for his insight regarding this manuscript.
We have had no involvements that might raise the question of bias in the work reported or in the conclusions, implications or opinions stated.
All authors maintain responsibility for the integrity of the manuscript and publication.
Appendix
Prediction Equations (solved for CGR):
Cockroft–Gault [23]:
CGR = [28 − (0.2×ages)] × weight × 0.85 (if female).
Levey [25]:
CGR = 879:89 + 12.51 × weight × age + (34.51if black) −; (379.42 if female).
CGR = [29.305 − 0.203 × age)] × weight × [1.037
if mean or 7.765 if female)].
References
- 1.Himmelfarb J, Ikizler TA. Acute kidney injury: changing lexicography, definitions, and epidemiology. Kidney Int. 2007;71:971–976. doi: 10.1038/sj.ki.5002224. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 5.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 7.Arnaoutakis GJ, George TJ, Robinson CW, et al. Severe acute kidney injury according to the RIFLE (risk, injury, failure, loss, end stage) criteria affects mortality in lung transplantation. J Heart Lung Transplant. 2011;10:1161–1168. doi: 10.1016/j.healun.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuhaci B. More data on epidemiology and outcome of acute kidney injury with AKIN criteria: benefits of standardized definitions, AKIN and RIFLE classifications. Crit Care Med. 2009;37:2659–2661. doi: 10.1097/CCM.0b013e3181ad76c2. [DOI] [PubMed] [Google Scholar]
- 10.Kohli HS, Bhat A, Jairam A, et al. Predictors of mortality in acute renal failure in a developing country: a prospective study. Ren Fail. 2007;29:463–469. doi: 10.1080/08860220701260651. [DOI] [PubMed] [Google Scholar]
- 11.Ix JH, de Boer IH, Wassel CL, et al. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation. 2010;121:1295–1303. doi: 10.1161/CIRCULATIONAHA.109.924266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beddhu S, Pappas LM, Ramkumar N, et al. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 13.Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells M, Lipman J. Measurements of glomerular filtration in the intensive care unit are only a rough guide to renal function. S Afr J Surg. 1997;35:20–23. [PubMed] [Google Scholar]
- 15.Pesola GR, Akhavan I, Carlon GC. Urinary creatinine excretion in the ICU: low excretion does not mean inadequate collection. Am J Crit Care. 1993;2:462–466. [PubMed] [Google Scholar]
- 16.Hoste EA, Damen J, Vanholder RC, et al. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Trasnplant. 2005;20:747–753. doi: 10.1093/ndt/gfh707. [DOI] [PubMed] [Google Scholar]
- 17.Ikizler TA, Sezer MT, Flakoll PJ, et al. Urea space and total body water measurements by stable isotopes in patients with acute renal failure. Kidney Int. 2004;65:725–732. doi: 10.1111/j.1523-1755.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 18.Clark WR, Mueller BA, Kraus MA, et al. Quantification of creatinine kinetic parameters in patients with acute renal failure. Kidney Int. 1998;54:554–560. doi: 10.1046/j.1523-1755.1998.00016.x. [DOI] [PubMed] [Google Scholar]
- 19.Clark WR, Turk JE., Jr The NxStage System One. Semin Dial. 2004;17:167–170. doi: 10.1111/j.0894-0959.2004.17220.x. [DOI] [PubMed] [Google Scholar]
- 20.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Fabiny DL, Ertinghausen G. Automated reaction-rate method for determination of serum creatinine with CentrifiChem. Clin chem. 1971;17:696–700. [PubMed] [Google Scholar]
- 22.Leypoldt JK, Kamerath CD, Gilson JF, et al. Dialyzer clearances and mass transfer-area coefficients for small solutes at low dialysate flow rates. ASAIO J. 2006;52:404–409. doi: 10.1097/01.mat.0000227687.88929.08. [DOI] [PubMed] [Google Scholar]
- 23.Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Bouchard J, Macedo E, Soroko S, et al. Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2010;25:102–107. doi: 10.1093/ndt/gfp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ix JH, Wassel CL, Stevens LA, et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6:184–191. doi: 10.2215/CJN.05030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–490. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 27.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 28.Daugherty NA, Hammond KB, Osberg IM. Bilirubin interference with the kinetic Jaffe method for serum creatinine. Clin Chem. 1978;24:392–393. [PubMed] [Google Scholar]