Abstract
Inverse association between dietary intake of cruciferous vegetables and cancer risk observed in population-based case-control studies is partly attributable to structurally simple but mechanistically complex phytochemicals with an isothiocyanate (–N=C=S) functional group. Cancer protective role for dietary isothiocyanates (ITCs) is substantiated by preclinical studies in rodent models. A common feature of many naturally occurring ITCs relates to their ability to cause growth arrest and cell death selectively in cancer cells. At the same time, evidence continues to accumulate to suggest that even subtle change in chemical structure of the ITCs can have a profound effect on their activity and mechanism of action. Existing mechanistic paradigm stipulates that ITCs may not only prevent cancer initiation by altering carcinogen metabolism but also inhibit post-initiation cancer development by suppressing many processes relevant to tumor progression, including cellular proliferation, neoangiogenesis, epithelial–mesenchymal transition, and self-renewal of cancer stem cells. Moreover, the ITCs are known to suppress diverse oncogenic signaling pathways often hyperactive in human cancers (e.g. nuclear factor-κB, hormone receptors, signal transducer and activator of transcription 3) to elicit cancer chemopreventive response. However, more recent studies highlight potential adverse effect of Notch activation by ITCs on their ability to inhibit migration of cancer cells. Mechanisms underlying ITC-mediated modulation of carcinogen metabolism, growth arrest, and cell death have been reviewed extensively. This article provides a perspective on bench-cage-bedside evidence supporting cancer chemopreventive role for some of the most promising ITCs. Structure–activity relationship and mechanistic complexity in the context of cancer chemoprevention with ITCs is also highlighted.
Introduction
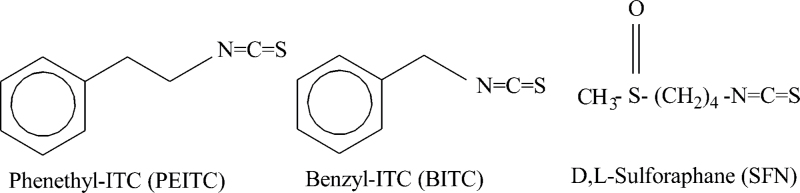
Social and economic burden from cancer is still quite substantial around the world despite increasing awareness of life-style risk factors (e.g. smoking) and screening efforts for early detection of the disease. Novel approaches for prevention of cancer are desirable mainly because many risk factors associated with tumor development are not easily modifiable (e.g. genetic predisposition). Chemoprevention of cancer is feasible as exemplified by selective estrogen receptor modulators and aromatase inhibitors for breast cancer risk reduction (1–3). Data accumulated over the past three decades provides compelling preclinical evidence for cancer protective effect of isothiocyanates (ITCs) derived from edible cruciferous vegetables. Despite convincing preclinical evidence, however, the progress toward clinical translation for ITCs has been rather disappointing probably due to a variety of reasons, including lack of suitably formulated agents for oral administration, regulatory issues requiring investigational new drug application submission and approval from the Federal Drug Administration, and complexities associated with primary prevention clinical trials requiring thousands of subjects and years of follow-up to draw meaningful conclusions. This article summarizes preclinical evidence for cancer preventive role for some of the most promising ITCs, including watercress constituent phenethyl isothiocyanate (PEITC), garden cress constituent benzyl isothiocyanate (BITC) and synthetic racemic analogue of broccoli constituent l-sulforaphane (d,l-sulforaphane; hereafter abbreviated as SFN) (4–6). Chemical structures of BITC, PEITC and SFN are shown in Figure 1.
Fig. 1.
Chemical structure of phenethyl isothiocyanate (PEITC), benzyl isothiocyanate (BITC), and sulforaphane (SFN).
The ITCs are stored as thioglucoside conjugates (commonly known as glucosinolates) in cruciferous vegetables (7,8). For example, the glucosinolate precursor of PEITC is gluconasturtiin, whereas l-sulforaphane is stored as glucoraphanin in cruciferous vegetables (8). Plant tissue damage resulting from cutting or chewing of the cruciferous vegetables releases an enzyme (myrosinase) that is responsible for conversion of the glucosinolates to corresponding ITCs (8). The ITCs can also be generated by intestinal microflora (9). Substantial amounts of glucosinolates are achievable through dietary intake of the cruciferous vegetables. For example, glucosinolate content in edible cruciferous vegetables ranges from 0.5 to 3mg/g and one ounce of watercress is estimated to result in intake of about 37 μmol of PEITC (10).
ITCs are effective inhibitors of chemically induced cancer in experimental rodents
Wattenberg was the first to report inhibition of chemically induced cancer in experimental rodents upon PEITC and BITC administration more than 30 years ago (11). Specifically, the PEITC administration 4 hours before 7,12-dimethylbenz[a]anthracene administration resulted in inhibition of mammary carcinogenesis in rats (11). Since then, a number of studies from different laboratories have documented protective effect of PEITC and BITC against cancer in rodents induced by structurally diverse chemical carcinogens. In this context, contributions of Gary D. Stoner, Late Bandaru S. Reddy, Stephen S. Hecht, Fung-Lung Chung, and Paul Talalay et al. are noteworthy (10,12–15). Some of their seminal studies documenting ITC-mediated prevention of chemically induced cancers are summarized in Table I and exemplified later.
Table I.
Protective effect of isothiocyanates (ITCs) against chemically-induced cancer in rodents
| Compound | Activity | Carcinogen | Rodent species | Reference |
|---|---|---|---|---|
| PEITC | Inhibition of mammary carcinogenesis | DMBA | Female Sprague-Dawley rat | (11) |
| Inhibition of esophageal tumorigenesis | NBMA | Male F344 rat | (12) | |
| Inhibition of lung tumorigenesis | NNK | Male F344 rat | (13) | |
| Inhibition of aberrant crypt foci formation | AOM | Male F344 rat | (28) | |
| Inhibition of colon tumor multiplicity | AOM/DSS | Male C57BL/6 mice | (16) | |
| Inhibition of oral squamous epithelial carcinogenesis | NBMA | Male Syrian golden hamster | (19) | |
| Inhibition of neoplasia of the forestomach | BaP | A/J mouse | (21) | |
| Inhibition of lung tumor multiplicity | BaP+NNK | A/J mouse | (22) | |
| PEITC-NAC | Inhibition of lung tumorigenesis | BaP | A/J mouse | (18) |
| BITC | Inhibition of mammary carcinogenesis | DMBA | Female Sprague-Dawley rat | (11) |
| Inhibition of neoplasia of the breast | DMBA | Female Sprague-Dawley rat | (20) | |
| Inhibition of lung tumorigenesis | BaP | A/J mouse | (21) | |
| Inhibition of lung tumorigenesis | BaP | Female A/J mouse | (23) | |
| Inhibition of neoplasia of the forestomach | DEN | Female A/J mouse | (23) | |
| Inhibition of intestinal carcinogenesis | MAM acetate | Female ACI/N rat | (24) | |
| Inhibition of lung tumorigenesis | 5-MeC+DBahA | A/J mouse | (25) | |
| Inhibition of liver tumorigenesis | DEN | Male ACI/N rat | (26) | |
| Inhibition of pancreatic carcinogenesis | BOP | Male Syrian hamster | (27) | |
| SFN | Inhibition of mammary tumorigenesis | DMBA | Female Sprague-Dawley rat | (14) |
| Inhibition of colonic aberrant crypt foci | AOM | Male F344 rat | (28) | |
| Inhibition of neoplasia of the forestomach | BaP | Female ICR mouse | (29) | |
| Inhibition of skin tumorigenesis | DMBA/TPA | Female CD-1mouse | (31) |
Abbreviations: PEITC, phenethyl isothiocyanate; PEITC-NAC, N-acetylcysteine conjugate of PEITC; BITC, benzyl isothiocyanate; SFN, sulforaphane; DMBA, 7,12-dimethylbenz[a]anthracene; NBMA, N-nitrosobenzylmethylamine; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; AOM, azoxymethane; DSS, dextran sodium sulfate; BaP, benzo[a]pyrene; DEN, diethylnitrosamine; MAM acetate, methylazoxymethanol acetate; 5-MeC, 5-methylchrysene; DBahA, dibenz[a,h]anthracene; BOP, N-nitrosobis(2-oxopropyl)amine; TPA, 12-O-tetradecanoylphorbol 13-acetate
Stoner group showed that the rats fed a diet supplemented with 3 and 6 mmol PEITC/kg diet before (pre-initiation) as well as during treatment with the carcinogen N-nitrosobenzylmethylamine (post-initiation) developed significantly fewer esophageal tumors compared with rats fed a control diet (12). Lung tumorigenesis induced by the tobacco-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in rats was inhibited significantly by dietary administration of 4 and 8 mmol PEITC/kg diet (13). Gavage of PEITC inhibited azoxymethane-induced colonic aberrant crypt foci in rats providing important laboratory evidence for protection against colon cancer (15). Feeding of diet supplemented with 0.05% PEITC before or after azoxymethane initiation resulted in lower tumor incidence, lower colon tumor multiplicities and smaller polyps, as compared with mice fed with the basal diet (16). Notably, Plate and Gallaher (17) failed to observe PEITC-mediated prevention of aberrant crypt foci in rats. Yang et al. (18) showed inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugate of PEITC administered during the post-initiation phase. PEITC administration was also shown to suppress N-nitrosomethylbenzylamine-induced hamster buccal pouch cancer (19). Similar to PEITC, cancer protective role for BITC has been demonstrated in a number of chemically induced rodent cancer models, including 7,12-dimethylbenz[a]anthracene-induced breast cancer and benzo[a]pyrene-induced forestomach cancer and pulmonary adenoma using rats or mice (20–23), diethylnitrosamine-induced forestomach tumor in mice (23), methylazoxymethanol acetate-induced intestinal carcinogenesis in rats (24), 5-methylchrysene and dibenz[a,h]anthracene-induced lung cancer in mice (25), diethylnitrosamine-induced liver cancer in rats (26), and N-nitrosobis(2-oxopropyl)amine-induced pancreatic atypical hyperplasia and adenocarcinoma in hamsters (27).
Talalay et al. are credited with sparking research interest in SFN by demonstrating preventive activity against 9,10-dimethyl-1,2-benzanthracene-induced breast cancer in rats (14). Subsequently, chemopreventive response to SFN was extended to other chemical carcinogens. For example, both pre- and post-initiation administration of SFN resulted in suppression of colonic aberrant crypt foci in rats induced by azoxymethane (28). However, SFN–N-acetylcysteine conjugate exhibited activity only against post-initiation cancer development (28). SFN-mediated inhibition of benzo[a]pyrene-induced forestomach cancer in mice has also been demonstrated (29). SFN and its N-acetylcysteine conjugate inhibited malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice (30). SFN treatment was shown to prevent chemically induced skin cancer during the stage of promotion (31). It is important to mention that some studies have used the naturally occurring l-isomer whereas others have utilized synthetic racemic compound in these studies (14,28–31). Nevertheless, it is now obvious that ITCs can offer protection against cancer in experimental rodents induced by structurally diverse chemical carcinogens.
Bladder carcinogenesis/promotion in rats is concerning for some ITCs
A few studies, albeit inconsistently, have suggested that some ITCs might act as a bladder carcinogen or promote chemically induced bladder tumorigenesis at least in rats. Using a rather complex multiorgan carcinogenesis model in rats involving a single intraperitoneal injection of diethylnitrosamine, 4 intraperitoneal injections of N-methylnitrosourea as well as N-butyl-N-(4-hydroxybutyl)nitrosamine administration in the drinking water during the first 2 weeks followed by 4 subcutaneous injections of dimethylhydrazine as well as 2,2’-dihydroxy-di-n-propylnitrosamine in the drinking water over 2 weeks, PEITC administration lowered the induction of esophageal hyperplasia, kidney atypical tubules, and liver glutathione S-transferase placental form-positive foci when given during the initiation period but enhanced the development of liver glutathione S-transferase placental form-positive foci and urinary bladder tumors if administered in the post-initiation period (32). Additional work from this same group showed that PEITC-induced papillary or nodular hyperplasia, dysplasia, and transitional cell carcinoma in a dose-dependent manner but only after initiation with diethylnitrosamine and N-butyl-N-(4-hydrozybutyl)nitrosamine (33). Whether PEITC alone acts as a carcinogen in the urinary bladder is also controversial. Some studies indicate that PEITC can promote proliferation in normal looking epithelium leading to dysplasia (34). Other studies indicated that PEITC treatment had only limited potential to initiate abnormal growth and did not effectively induce irreversible lesion in urinary bladder (35). Similar inconsistencies are discernible for BITC in the context of bladder cancer in rats. In experiments designed to determine the post-initiation effect of BITC against hepatocarcinogenesis and urinary bladder carcinogenesis, rats were pretreated with diethylnitrosamine and N-butyl-N-(4-hydroxybutyl)-nitrosamine prior to dietary BITC administration (36). Incidence of papillary or nodular hyperplasia and carcinoma were significantly elevated in the BITC treatment group (36). Interestingly, BITC potently inhibited induction of these lesions when given simultaneously with the carcinogen (37). In a follow-up study, simultaneous treatment with BITC and a lower dose of the same carcinogen did not inhibit, but rather enhanced rat urinary bladder carcinogenesis (38).
These observations will undoubtedly pose difficulties in regulatory approval of PEITC or BITC as a potential chemopreventive agent for long-term administration in high-risk but otherwise normal healthy subjects. At the same time, a few arguments lend support for long-term administration of these agents for cancer chemoprevention purpose. First, bladder carcinogenesis or promotion of chemically induced bladder cancer by PEITC or BITC has not been reported in any other rodent species (e.g. mouse). Second, PEITC/BITC-containing cruciferous vegetables are consumed by humans on a daily basis in some cultures, yet epidemiological studies have suggested an inverse association between intake of these vegetables and bladder cancer risk and survival (39–41). It is possible that rats are unusually sensitive to bladder carcinogenesis by PEITC and BITC. Finally, human relevance of the multiorgan rat carcinogenesis models employed to demonstrate bladder tumor promoting effects of PEITC and BITC (32,36) is unclear if not questionable. Notably, at least two studies have documented clastogenic effects of BITC (42,43). Furthermore, BITC treatment exhibited genotoxic effects in HepG2 cells but substantially weaker effects were obtained in vivo (44). Clearly, toxicological evaluations after long-term administration of PEITC and BITC in different species are needed to advance these agents in clinical translational studies.
Bladder carcinogenesis or promotion of chemically induced bladder cancer has not been reported for SFN. In fact, a recent study showed that SFN administration inhibited DNA adduct formation induced by a bladder carcinogen, 4-aminobiphenyl, in a NF-E2 related factor-2-dependent manner in bladder cells and tissues (45). Furthermore, dietary administration of a freeze-dried aqueous extract of broccoli sprouts, which is a rich source of glucoraphanin, conferred significant and dose-dependent protection against bladder cancer development in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine (46). It is important to note that the broccoli sprout extract itself did not cause any histologic changes in the bladder (46).
ITCs prevent oncogene-driven cancer development in transgenic mice
Availability of transgenic mouse models in recent years has enabled determination of chemopreventive efficacy of the ITCs against spontaneous cancer development. Key studies documenting cancer chemoprevention by ITCs in transgenic mouse models are summarized in Table II. For example, the ApcMin/+ mice fed a diet supplemented with 0.05% PEITC for 3 weeks developed significantly less (31.7% reduction) and smaller polyps than those fed basal diet (47). Dietary feeding of 8 mmol PEITC/kg diet to polyoma middle-T antigen transgenic mice resulted in smaller mammary cancer lesions, although there was no effect on lung metastasis or survival (48). Feeding of a diet supplemented with 0.05% PEITC alone or 0.025% PEITC in combination with 1% curcumin, a constituent of turmeric, significantly decreased incidence of prostate tumor in transgenic adenocarcinoma of mouse prostate (TRAMP) mice (49). Recent studies from our laboratory have revealed that administration of 3 mmol PEITC/kg diet decreases incidence as well as burden (affected area) of poorly differentiated tumors in the dorsolateral prostate of TRAMP mice (50). Dietary administration of 3 mmol BITC/kg diet for 25 weeks markedly suppressed the incidence and/or burden of mammary hyperplasia and carcinoma in female MMTV-neu transgenic mice without causing weight loss or affecting neu protein level (51). The BITC-mediated prevention of mammary carcinogenesis in MMTV-neu mice was shown to be associated with T-cell infiltration and induction of E-cadherin (51). Mammary cancer in MMTV-neu mice is also suppressed by dietary administration of 3 mmol PEITC/kg diet (S.V.Singh et al., unpublished results). Long-term administration of SFN in the diet resulted in suppression of tumor development in Apc min mice (52). Dietary administration of SFN (300 and 600 ppm) for 3 weeks to ApcMin/+ mice resulted in suppression of polyps in the small intestine in a dose-dependent manner (53). Oral gavage of 6 μmol SFN thrice per week beginning at 6 weeks of age significantly inhibited prostate intraepithelial neoplasia and pulmonary metastasis in TRAMP mice (54). Consistent with these results, 8-week-old TRAMP mice fed with 240mg of broccoli sprouts/mouse/day exhibited a significant retardation of prostate tumor growth in another study (55).
Table II.
Chemopreventive efficacy of isothiocyanates (ITCs) in transgenic mouse models
| Agent | Transgenic mouse model | Experimental protocol | Effect | Reference |
|---|---|---|---|---|
| PEITC | ApcMin/+ mouse model of gastrointestinal cancer | Dietary administration of 0.05% PEITC | Inhibition of intestinal polyp development and reduced intestinal tumor size | (47) |
| Polyoma middle-T antigen (PyMT) transgenic mouse model of breast cancer | Dietary feeding of 8 mmol PEITC/kg | Reduced size of mammary cancer lesions | (48) | |
| Transgenic adenocarcinoma of mouse prostate (TRAMP) model of prostate cancer | Dietary feeding of 0.05% PEITC alone or 0.025% PEITC + 1% curcumin | Inhibition of prostate tumor incidence | (49) | |
| Transgenic adenocarcinoma of mouse prostate (TRAMP) model of prostate cancer | Dietary administration of 3 mmol PEITC/kg | Inhibition of incidence and burden of poorly-differentiated prostate tumor | (50) | |
| BITC | MMTV-neu transgenic mouse model of breast cancer | Dietary administration of 3 mmol BITC/kg | Suppression of incidence and/or burden of hyperplasia and carcinoma | (51) |
| SFN | ApcMin mouse model of gastrointestinal cancer | Dietary feeding of ~6 μmol SFN/day | Suppression of polyp formation | (52) |
| ApcMin/+ mouse model of gastrointestinal cancer | Dietary feeding of 300 or 600 ppm of SFN | Suppression of polyps in the small intestine | (53) | |
| Transgenic adenocarcinoma of mouse prostate (TRAMP) model of prostate cancer | Oral gavage of 6 μmol SFN thrice a week | Inhibition of prostate intraepithelial neoplasia and pulmonary metastasis | (54) | |
| Transgenic adenocarcinoma of mouse prostate (TRAMP) model of prostate cancer | Feeding with 240mg broccoli sprouts/mouse/day | Inhibition of prostate tumor growth | (55) |
Abbreviations: PEITC, Phenethyl isothiocyanate; BITC, Benzyl isothiocyanate; SFN, Sulforaphane
ITCs inhibit processes relevant to cancer progression
Unregulated cellular proliferation, evasion of apoptosis, and neo-angiogenesis (formation of new blood vessels) are hallmarks of cancer progression. Interestingly, all these processes are sensitive to inhibition by ITCs. For example, PEITC, BITC and SFN inhibit cellular proliferation in association with G2/M phase cell-cycle arrest and apoptosis induction. Mechanistic complexity underlying growth arrest and cell death by PEITC is exemplified in Figure 2. Hasegawa et al. (56) were the first to report G2/M phase cell-cycle arrest upon treatment with PEITC and BITC in HeLa cells. Except for a few reports, most studies have shown G2/M phase cell-cycle arrest in cancer cells upon treatment with PEITC and BITC (57–59). For example, G2/M phase cell-cycle arrest after treatment with BITC was shown in leukemia (60), pancreatic (61) and breast cancer cells (62). However, a few studies have shown arrest in other phases of the cell cycle upon treatment with BITC (63,64). Similar discrepancies are also found for SFN (65–68). For example, SFN treatment was shown to cause G2/M phase cell-cycle arrest in HT29 colon cancer cells (65) and androgen- independent PC-3 prostate cancer cells (67), whereas androgen-responsive LNCaP prostate cancer cells exposed to SFN were arrested in both S and G2/M phases of the cell cycle (68). Mechanisms by which ITCs cause cell-cycle arrest have been reviewed elsewhere (4–6). As an example, using PC-3 cells as a model we demonstrated important contribution of checkpoint kinase-2 activation leading to phosphorylation and cytoplasmic sequestration of cell division cycle 25C in SFN-mediated G2/M phase cell-cycle arrest (67).
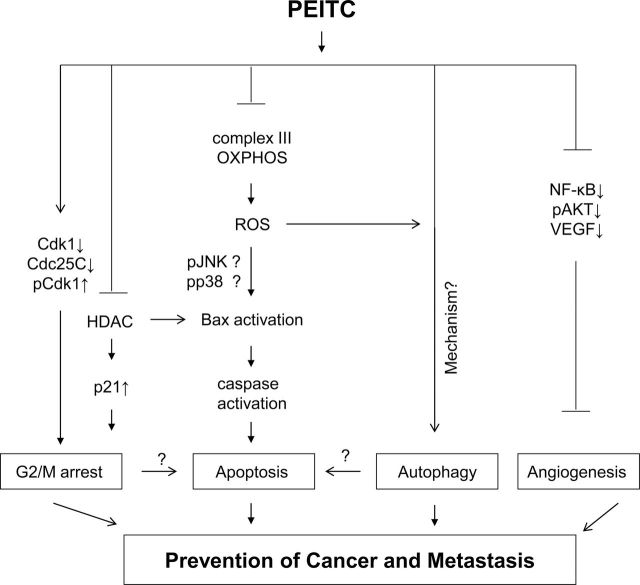
Fig. 2.
Mechanisms underlying inhibition of post-initiation cancer development by PEITC. Post-initiation cancer chemoprevention by PEITC probably involves (i) growth arrest at G2/M phase of the cell cycle due to downregulation of cyclin-dependent kinase 1 (Cdk1) and cell division cycle 25C (Cdc25C) leading to accumulation of tyrosine-15 phosphorylated (inactive) Cdk1 (57); inhibition of histone deacetylase (HDAC) leading to induction of p21 may also contribute to cell cycle arrest by PEITC and SFN (6), (ii) apoptosis mediated by reactive oxygen species (ROS) resulting from inhibition of complex III of the mitochondrial respiratory chain (81); the PEITC-induced ROS results in Bax activation leading to caspase activation and ultimately cell death (81); the ROS-mediated Bax activation may be mediated by activation of c-Jun-N-terminal kinases (JNK) and/or p38 mitogen activated protein kinase similar to that reported for benzyl isothiocyanate (80), (iii) induction of autophagic death that is partly dependent on ROS (100); mechanism underlying PEITC-induced autophagy is still unclear, and (iv) inhibition of angiogenesis due to suppression of nuclear factor-κB, phosphorylated (active) AKT, and vascular endothelial growth factor (VEGF) signaling (87,112). This illustration does not fully capture every mechanistic alteration resulting from PEITC exposure but signifies mechanistic complexity by which PEITC may prevent post-initiation cancer development.
One of the unique properties of ITCs is their ability to selectively cause apoptosis in cancer cells. Differential sensitivity of cancer cells versus normal epithelial cells to apoptosis induction has been noted for PEITC (4,69), BITC (5,62) and SFN (70). Research over the past decade reveals that the molecular circuitry of apoptosis induction by ITCs is complex and utilizes a wide range of molecular mechanisms, including alterations in Bcl-2 family protein expression, activation of mitogen-activated protein kinases, suppression of oncogenic signaling pathways and activation of caspases (reviewed in refs. 4–6,71). Yu et al. (72) were the first to show apoptosis induction by PEITC in HeLa cells. During the same time period, Huang et al. (73) used mouse embryonic fibroblasts to demonstrate a critical role for p53 in regulation of PEITC-induced apoptosis. This association was found to be unique to the mouse embryonic fibroblasts because PEITC was later shown to cause apoptosis in p53-deficient cancer cells (74). Lack of p53 involvement in apoptosis induction by BITC has also been demonstrated (75). Interestingly some ITCs selectively deplete mutant p53, but not the wild-type p53, via a transcription-independent mechanism (76). Direct p53 binding followed by conformational change is implicated in depletion of mutant p53 by some ITCs (76). Mechanistic complexity of apoptosis induction by PEITC, BITC and SFN has been reviewed previously (4–6,71) but production of reactive oxygen species appears to be a common link in apoptosis induction by PEITC, BITC and SFN (77–82). Interestingly, normal cells are resistant to ROS production by ITCs (78,81). The mechanism of ROS production and signal transduction downstream of ROS production in execution of apoptosis by PEITC, BITC and SFN involves inhibition of mitochondrial respiratory chain and activation of multidomain proapoptotic protein Bax, respectively (80–82). The mechanism underlying differential sensitivity of cancer cells and normal cells to apoptosis induction by ITCs is still unclear but PEITC treatment has been shown to differentially alter expression of oxidative stress and antioxidant defense genes in PC-3 (a prostate cancer cell line) versus PrEC cells (a normal prostate epithelial cell line) (83). A role for the adapter protein p66Shc has also been established in ROS production and apoptosis induction by PEITC as well as SFN (84,85).
Neoangiogenesis is critical not only for tumor growth but also for metastatic spread (86). Several groups have thoroughly investigated anti-angiogenic effect of ITCs. For example, PEITC treatment decreased expression of vascular endothelial growth factor and inhibited capillary-like tube structure formation (a measure of neoangiogenesis) and migration in human umbilical vain endothelial cells (87). Furthermore, the PEITC treatment inhibited angiogenesis ex vivo as revealed by chicken egg chorioallantoic membrane assay (87). PEITC is an effective inhibitor of hypoxia inducible factor (88), a proangiogenic transcription factor. Because angiogenesis plays an important role in metastasis, a previous study from our group determined the effect of PEITC administration on incidence and multiplicity of pulmonary metastasis in TRAMP model (50). Overall incidence of pulmonary metastasis did not differ between the control and the PEITC-treated mice but the number of lung metastasis per mouse in the mice fed PEITC-supplemented diet was about 38% lower than that in the mice fed control diet (50). In MDA-MB-231 xenografts, analysis of the vasculature in the tumors from BITC-treated mice indicated smaller vessel area compared with control tumors based on immunohistochemistry for angiogenesis marker CD31 (89). The BITC-mediated inhibition of angiogenesis in vivo correlated with downregulation of vascular endothelial growth factor receptor 2 protein levels in the tumor (89). Oral BITC treatment reduced hemoglobin content, CD31 and vascular endothelial growth factor expression in vivo (90). The BITC-mediated inhibition of neoangiogenesis in rat aorta and chicken-chorioallantoic membrane models was also shown (91). The inhibitory effect of SFN on endothelial cell function essential for angiogenesis were shown using HMEC-1, an immortalized human microvascular endothelial cell line (92). The SFN treatment suppressed angiogenesis and disrupted endothelial mitotic progression and microtubule polymerization (93). Anti-angiogenic effect for ITCs has been reviewed by Cavell et al. (94). In summary, it is reasonable to conclude that inhibition of post-initiation cancer development by ITCs is probably achieved by suppression of multiple processes relevant to cancer progression.
Functional significance of autophagy induction by PEITC/BITC versus SFN
Autophagy is an evolutionarily conserved process for bulk degradation of macromolecules including organelles (e.g. mitochondria) (95). Autophagy is considered a valid cancer chemotherapeutic target (95,96). Our laboratory was the first to document autophagy induction by SFN in prostate cancer cells (97). Autophagy induction by SFN was since then confirmed by other investigators in different cellular systems (98,99). Even though autophagy induction is not unique to SFN as this process is activated upon treatment of cancer cells with PEITC (100) and BITC (101) and the autophagic response to all three agents is partially linked to ROS production (81,82,101), outcome of this cellular response is strikingly different for PEITC and BITC versus SFN (97,100,101). Autophagy serves to inhibit apoptosis induction by SFN by preventing release of cytochrome c from mitochondria to the cytosol (97). To the contrary, autophagy induction contributes to cell death by PEITC and BITC (100,101). Previous studies from our laboratory have also provided in vivo correlative evidence for autophagy induction by PEITC and BITC (50,100,101). Similar to apoptosis, normal epithelial cells are significantly more resistant to induction of autophagy by PEITC and BITC compared with cancer cells (100,101). Mechanism underlying autophagy induction by PEITC or SFN is still unresolved, but BITC-mediated autophagy is associated with increased acetylation of FoxO1 (101).
ITCs inhibit self-renewal of cancer stem cells
Evidence continues to accumulate to suggest that resistance of cancer stem cells to conventional therapy (e.g. chemotherapy, hormonal therapy, radiotherapy) is a major cause of disease recurrence (102,103). It was shown recently that SFN can suppress self-renewal of breast cancer stem cells characterized by a significant decrease in aldehyde dehydrogenase 1-positive cell population and reduction in the size and number of primary mammospheres (104). The SFN-mediated inhibition of self-renewal of breast cancer stem cells correlated with suppression of Wnt/β-catenin pathway (104). Furthermore, daily injection with 50mg/kg SFN for 2 weeks reduced aldehyde dehydrogenase 1-positive cells by >50% in a breast cancer xenograft model (104). Unpublished studies from our laboratory also indicate inhibitory effect of BITC on self-renewal of breast cancer stem cells (S.V.Singh, unpublished results). Efficacy of PEITC against cancer stem cells and that of BITC or SFN against cancer stem cells of other organs is yet to be determined.
BITC inhibits epithelial to mesenchymal transition in breast cancer cells
Using human breast cancer cells (MDA-MB-231 and MCF-7) as a model, we have demonstrated previously that BITC inhibits epithelial to mesenchymal transition (EMT) (105). The EMT is essential for normal physiological processes such as embryonic development, tissue remodeling and wound healing (106). During EMT, epithelial phenotype characterized by tight cell–cell junctions and polarity changes to a mesenchymal phenotype typified by disruption of the cell–cell contact with conversion to fibroblastic morphology and increased motility (106). The EMT is implicated in progression of cancers to invasive state (106). The BITC-mediated inhibition of EMT in breast cancer cells was characterized by upregulation of E-cadherin and downregulation of mesenchymal markers, including vimentin and fibronectin (105). Our observations of EMT inhibition by BITC have since been confirmed by other investigators (107). The mechanism by which BITC inhibits EMT is still unresolved, but the BITC-mediated growth retardation of MDA-MB-231 xenograft in vivo is associated with induction of E-cadherin and suppression of vimentin and fibronectin protein levels in the tumor (105). Studies are needed to determine if anti-EMT effect is unique to BITC.
Lack of target-specificity is probably beneficial for cancer chemoprevention by ITCs
Lack of target-specificity is a frequently voiced sentiment for naturally occurring chemopreventive agents, and ITCs are no exception to this potential criticism. In our view, ability to target multiple pathways is a desirable attribute for chemopreventive agents because pathogenesis of cancer is complex often characterized by deregulation of multiple checkpoints and activation of several oncogenic pathways. Agents selective against a single pathway/molecule may have limited clinical utility as exemplified by the estrogen receptor antagonists (1,2). An overall mechanistic model emerging from research over the past two decades stipulates that ITCs, including PEITC, BITC and SFN, have the ability to not only inhibit cancer initiation by decreasing carcinogen activation and/or increasing detoxification of the activated carcinogenic intermediates but also prevent post-initiation cancer development by affecting a variety of processes relevant to cancer progression. Alteration of carcinogen metabolism as a likely mechanism for the ITC-mediated inhibition of cancer initiation has been reviewed extensively elsewhere (71,108–110), but some of the notable mechanisms potentially contributing to post-initiation cancer chemoprevention by ITCs include: (i) inhibition of histone deacetylase (111); (ii) inhibition of oncogenic transcription factors (e.g. nuclear factor-κB, signal transducer and activator of transcription 3, androgen receptor and estrogen receptor-α) (61,112–116); (iii) protein binding (117) and (iv) inhibition of cap-dependent translation (118). However, relative contribution of these mechanisms to cancer chemoprevention by ITCs is hard to predict.
Even a subtle change in the ITC structure can have a profound impact on its activity
A potential misperception about ITCs is that they share common mechanism of action. On one hand, this argument has some validity considering most ITCs are inducers of phase 2 enzymes contributing to their pre-initiation chemopreventive effects (4-6,10,108). At the same time, examples exist to illustrate that even subtle difference in the ITC structure can translate into striking mechanistic divergence. For example, we have shown recently that apoptosis induction by PEITC is mediated by Bim in MCF-7 and MDA-MB-231 human breast cancer cells (119). Surprisingly, Bim is dispensable for proapoptotic response to BITC in the same cell lines (120). Autophagy is another example to highlight mechanistic differences between ITCs (97,100,101). Noticeable differences in efficacy of ITCs for prevention of chemically induced as well as spontaneous cancers in rodents have also been reported. For instance, SFN and PEITC seem to inhibit different stages of prostate cancer development in the TRAMP model (50,54). Although SFN treatment inhibits incidence of prostatic intraepithelial neoplasia and well-differentiated cancer (54), PEITC is an effective suppressor of poorly differentiated prostate cancer in TRAMP model (50). Interestingly, prostate cancer prevention by SFN in TRAMP model is associated with increased lytic activity of natural killer cells (54). On the other hand, PEITC administration has no effect on activity of natural killer cells (S.V.Singh, unpublished results).
Biomarkers of ITC exposure/response
Successful clinical realization of a chemopreventive strategy depends on systematic investigations beginning with identification of promising agents and characterization of their mechanisms of action to animal studies focusing not only on bioavailability, safety and efficacy assessments but also on discovery of biomarker(s) associated with exposure and response prior to translation in humans with a pilot biomarker modulation trial in a smaller cohort followed by larger trials with cancer incidence as the primary end point. Biomarker(s) of tissue exposure and/or response are critical for cancer chemopreventive agents because clinical trials with cancer incidence as the primary end point are expensive and laborious requiring years of follow-up and thousands of high-risk subjects. Recent studies have identified some biomarkers potentially useful in future clinical investigations of ITCs. Our own studies have utilized two-dimensional gel electrophoresis followed by mass spectrometry to identify plasma clusterin as a potential biomarker of PEITC exposure and possibly response in TRAMP model (50). Clusterin (also known as apolipoprotein J and testosterone-repressed prostate message-2) is a highly conserved protein expressed in a variety of tissues, secreted in blood, and involved in regulation of apoptosis, cell adhesion, cell-cycle, and DNA repair (121,122). Increased levels of clusterin have been reported in several malignancies including breast, colon, lung, and prostate cancer (121). Moreover, expression of clusterin correlated with Gleason score in prostate cancer patients (122). Future clinical trials will test whether clusterin is a viable biomarker to assess tissue exposure and/or response to PEITC. Stable reaction products with albumin and hemoglobin as biomarkers to monitor ITC exposure in humans have also been identified (123,124). For instance, blood samples collected from a normal healthy volunteer 1 day after ingestion of garden cress (60g), watercress (100g) and broccoli (300g) revealed presence of PEITC-lysine adducts in both albumin and hemoglobin (123).
ITCs are chemotherapy sensitizers
Evidence exists to suggest that ITCs may act as sensitizers of chemotherapeutic agents. Sublethal doses of PEITC sensitized Fas-resistant T24 bladder carcinoma cell line and Bcl-2 overexpressing Jurkat T cells to Fas-mediated apoptosis (125). Inhibition of P-glycoprotein and multidrug-resistance protein 1-mediated efflux of daunomycin, which is a major mechanism of resistance for some anticancer agents, by PEITC has been described (126,127). The PEITC caused sensitization of PC-3 and HeLa cells to adriamycin and etoposide-induced apoptosis by downregulation of protein kinase C and inhibition of telomerase activity (128,129). The PEITC and BITC treatments resulted in sensitization of non-small cell lung cancer cell line NCI-H596 to cisplatin independent of cellular platinum accumulation or DNA platination (130). Studies from our laboratory have revealed that pharmacologic concentrations of PEITC augment Docetaxel-induced apoptosis in PC-3 and DU145 human prostate cancer cells in association with suppression of Bcl-2 and XIAP protein levels and induction of Bax and Bak (131). Pretreatment with BITC resulted in sensitization of BxPC-3 pancreatic cancer cells to gamma-radiation induced cell-cycle arrest and apoptosis due to inhibition of nuclear factor-κB and activation of p38 mitogen-activated protein kinase (132). The BITC treatment increased sensitivity of MIAPaCa-2 and PANC-1 pancreatic cancer cells to X-ray in association with increased apoptosis, suppression of X-linked inhibitor of apoptosis protein and increase in apoptosis protease activating factor-1 (133). The BITC treatment resulted in sensitization of a panel of pancreatic cancer cells to TRAIL-induced apoptosis due to dual activation of extrinsic and intrinsic pathways (134). The SFN potentiated effects of chemotherapy drugs (e.g. cisplatin) on inhibition of clonogenicity and spheroid formation and aldehyde dehydrogenase 1 activity along with Notch-1 and c-Rel expression in pancreatic and prostate cancer cells (135). The SFN and doxorubicin combination reversed resistance in mouse fibroblasts with p53Ser220 mutation (136).
The in vivo relevance of most of these predominantly cellular findings is still unclear, but we have shown previously that PEITC–Docetaxel combination is markedly more efficacious against PC-3 xenograft in vivo compared with PEITC or Docetaxel alone (131). The SFN and chemotherapy drug combination was most effective and totally abolished growth of cancer stem cell xenografts and tumor-initiating potential (135). Similar in vivo studies with other ITC-chemotherapy drugs are necessary to spark interest among clinicians to test such combinations in cancer patients.
Some ITCs activate Notch signaling in cancer cells and normal epithelial cells
Recent studies from our laboratory have demonstrated that PEITC treatment increases cleavage (activation) of Notch1 and Notch2 in prostate cancer cells leading to transcriptional activation of Notch (137). Notch pathway is implicated in tumorigenesis, maintenance of mesenchymal phenotype and self-renewal of cancer stem cells (138–140). The PEITC-mediated activation of Notch is not selective for cancer cells as normal epithelial cells (PrEC) are also sensitive to Notch1 and Notch2 activation by PEITC (137). However, Notch activation may be a double-edge sword in the context of cancer chemoprevention with these ITC compounds. On one hand, PEITC-induced apoptosis in LNCaP and PC-3 cells was attenuated by RNA interference of Notch2, but not Notch1 (137). On the other hand, inhibition of PC-3 and LNCaP cell migration resulting from PEITC exposure was significantly augmented by knockdown of Notch2 as well as pharmacological inhibition of Notch1 activation (137). Further studies are needed to determine whether Notch1 and Notch2 activation by PEITC is unique to prostate cancer cells, and if Notch activation affects cancer chemopreventive response to PEITC in vivo. Activation of Notch1, Notch2, and Notch4 upon treatment with BITC has been observed in human breast cancer cells (141). The BITC-mediated inhibition of breast cancer cell migration was significantly augmented by RNA interference of Notch2, but not Notch1 or Notch4 (141). Once again these observations underscore mechanistic differences between structurally related ITC compounds.
miRNAs targeted by BITC
The miRNA function as either oncogenes or tumor suppressor and can target multiple genes (142). Recent studies have shown that BITC treatment alters the expression of miRNA-221 and miRNA-375 to switch hyperproliferative cancer cells to a hypoproliferative state in pancreatic adenocarcinoma cells (143). The miRNA-221 and miRNA-375 are abnormally expressed in pancreatic cancer patients (144). Ectopic expression of miRNA-375 and silencing of miRNA-221 sensitized cells to antiproliferative effect of BITC (143). Additional work is needed to determine if other ITCs target miRNA as well as to identify other potential miRNA targeted by ITCs.
Human studies are limited to raw cruciferous vegetables or their extracts
Human studies on biological effects of pure ITC compounds are still lacking, but a few studies have attempted to determine the effects of raw cruciferous vegetables or their extracts on certain biological parameters (145–149). For example, consumption of 2 ounce (56.8g) of watercress at each meal for 3 days was shown to inhibit oxidative metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers (145). Consistent with cellular observations (118), phosphorylation of 4E-BP1 was significantly reduced 6 and 8 hours after ingestion of 80g watercress in peripheral blood cells from four participants (146). Another randomized and placebo-controlled trial performed in Qidong, China, utilizing a beverage infused with 3-day-old broccoli sprouts showed an inverse association between excretion of dithiocarbamates and urinary aflatoxin-DNA adducts (148). Ingestion of 68g of broccoli sprouts by humans resulted in a significant decrease in histone deacetylase activity in peripheral blood mononuclear cells (149).
Concluding remarks and future directions
Discovery of cancer chemopreventive potential of ITCs more than 30 years ago sparked tremendous research interest focusing on pharmacokinetic, efficacy, and mechanistic characterization of these compounds. Notably, emerging technologies and research tools (e.g. RNA interference, microarray, proteomics, etc.) have been appropriately utilized to study the mechanism by which ITCs may prevent cancer. Clinical investigation of ITCs for cancer chemoprevention seems more plausible today mainly because of knowledge acquired in the past few decades. However, a few lingering hurdles in clinical development of ITCs are noteworthy. First, formulations of pure ITCs suitable for clinical investigations are not yet available. Second, clinical trial designs must consider rapid clearance of ITCs as corresponding mercapturic acids; a single daily administration schedule may not be effective. Finally, the question of whether PEITC and BITC are promoters of bladder cancer requires further investigation because this may turn out to be a major obstacle in long-term usage of ITCs for cancer prevention in high-risk subjects.
Funding
The work cited from the Singh laboratory was supported by the US PHS grant RO1 CA101753, RO1 CA129347 and RO1 CA115498 awarded by the National Cancer Institute. Acknowledgments Contributions of the present (Eun-Ryeong Hahm, Su-Hyeong Kim, Anuradha Sehrawat, Marie L. Antony, Kozue Sakao, Julie A Arlotti) and past members (Sanjay K. Srivastava, Karen L. Lew, Dong Xiao, Yan Zeng, Sunga Choi, Anna Herman-Antosiewicz, Ajay Bommareddy) of the Singh laboratory to the preclinical studies cited in this article are greatly appreciated.
Acknowledgments
Conflict of Interest: The authors have no conflict of interest.
Glossary
Abbreviations
- BITC
benzyl isothiocyanate
- EMT
epithelial to mesenchymal transition
- ITCs
isothiocyanates
- PEITC
phenethyl isothiocyanate
- ROS
reactive oxygen species
- SFN
d,l-sulforaphane
- TRAMP
transgenic adenocarcinoma of mouse prostate.
References
- 1. Fisher B., et al. (1998). Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 90 1371–1388 [DOI] [PubMed] [Google Scholar]
- 2. Cauley J.A., et al. (2001). Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res. Treat. 65 125–134 [DOI] [PubMed] [Google Scholar]
- 3. Goss P.E., et al. (2011). Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 364 2381–2391 [DOI] [PubMed] [Google Scholar]
- 4. Powolny A.A, et al. (2012). Slow but steady progress in cancer chemoprevention with phenethyl isothiocyanate: fulfilled promises and translational challenges. In: Sarkar F.H. (ed.) Neutraceuticals and Cancer Springer Science+Business Media B.V, The Netherlands: pp. 231–258 [Google Scholar]
- 5. Sehrawat A, et al. (2012). Molecular mechanisms of cancer chemoprevention with benzyl isothiocyanate. In: Kong A.N. (ed.) Inflammation and Cancer: Mechanisms and Dietary Approaches for Cancer Prevention Taylor and Francis; New York: in press [Google Scholar]
- 6. Clarke J.D., et al. (2008). Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 269 291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halkier B.A., et al. (2006). Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57 303–333 [DOI] [PubMed] [Google Scholar]
- 8. Fahey J.W., et al. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56 5–51 [DOI] [PubMed] [Google Scholar]
- 9. Fahey J.W., et al. (2012). Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 5 603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hecht S.S. (2000). Inhibition of carcinogenesis by isothiocyanates. Drug Metab. Rev. 32 395–411 [DOI] [PubMed] [Google Scholar]
- 11. Wattenberg L.W. (1977). Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J. Natl. Cancer Inst. 58 395–398 [DOI] [PubMed] [Google Scholar]
- 12. Stoner G.D., et al. (1991). Inhibitory effects of phenethyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 51 2063–2068 [PubMed] [Google Scholar]
- 13. Chung F.L., et al. (1996). Chemopreventive efficacy of arylalkyl isothiocyanates and N-acetylcysteine for lung tumorigenesis in Fischer rats. Cancer Res. 56 772–778 [PubMed] [Google Scholar]
- 14. Zhang Y., et al. (1994). Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. U.S.A. 91 3147–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morse M.A., et al. (1989). Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 49 549–553 [PubMed] [Google Scholar]
- 16. Cheung K.L., et al. (2010). Differential in vivo mechanism of chemoprevention of tumor formation in azoxymethane/dextran sodium sulfate mice by PEITC and DBM. Carcinogenesis 31 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plate A.Y., et al. (2006). Effects of indole-3-carbinol and phenethyl isothiocyanate on colon carcinogenesis induced by azoxymethane in rats. Carcinogenesis 27 287–292 [DOI] [PubMed] [Google Scholar]
- 18. Yang Y.M., et al. (2002). Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 62 2–7 [PubMed] [Google Scholar]
- 19. Solt D.B., et al. (2003). Phenethyl isothiocyanate inhibits nitrosamine carcinogenesis in a model for study of oral cancer chemoprevention. Cancer Lett. 202 147–152 [DOI] [PubMed] [Google Scholar]
- 20. Wattenberg L.W. (1981). Inhibition of carcinogen-induced neoplasia by sodium cyanate, tert-butyl isocyanate, and benzyl isothiocyanate administered subsequent to carcinogen exposure. Cancer Res. 41 2991–2994 [PubMed] [Google Scholar]
- 21. Lin J.M., et al. (1993). Effects of isothiocyanates on tumorigenesis by benzo[a]pyrene in murine tumor models. Cancer Lett. 74 151–159 [DOI] [PubMed] [Google Scholar]
- 22. Hecht S.S., et al. (2000). Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 150 49–56 [DOI] [PubMed] [Google Scholar]
- 23. Wattenberg L.W. (1987). Inhibitory effects of benzyl isothiocyanate administered shortly before diethylnitrosamine or benzo[a]pyrene on pulmonary and forestomach neoplasia in A/J mice. Carcinogenesis 8 1971–1973 [DOI] [PubMed] [Google Scholar]
- 24. Sugie S., et al. (1994). Inhibitory effects of benzyl thiocyanate and benzyl isothiocyanate on methylazoxymethanol acetate-induced intestinal carcinogenesis in rats. Carcinogenesis 15 1555–1560 [DOI] [PubMed] [Google Scholar]
- 25. Hecht S.S., et al. (2002). Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett. 187 87–94 [DOI] [PubMed] [Google Scholar]
- 26. Sugie S., et al. (1993). Inhibitory effects of benzyl isothiocyanate and benzyl thiocyanate on diethylnitrosamine-induced hepatocarcinogenesis in rats. Jpn. J. Cancer Res. 84 865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuroiwa Y., et al. (2006). Protective effects of benzyl isothiocyanate and sulforaphane but not resveratrol against initiation of pancreatic carcinogenesis in hamsters. Cancer Lett. 241 275–280 [DOI] [PubMed] [Google Scholar]
- 28. Chung F.L, et al. (2000). Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate Carcinogenesis 21 2287–2291 [DOI] [PubMed] [Google Scholar]
- 29. Fahey J.W., et al. (2002). Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. U.S.A. 99 7610–7615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Conaway C.C., et al. (2005). Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 65 8548–8557 [DOI] [PubMed] [Google Scholar]
- 31. Gills J.J., et al. (2006). Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Lett. 236 72–79 [DOI] [PubMed] [Google Scholar]
- 32. Ogawa K., et al. (1998). Stage and organ dependent effects of 1-O-hexyl-2,3,5-trimethylhydroquinone, ascorbic acid derivatives, n-heptadecane-8-10-dione and phenylethyl isothiocyanate in a rat multiorgan carcinogenesis model. Int. J. Cancer 76 851–856 [DOI] [PubMed] [Google Scholar]
- 33. Ogawa K., et al. (2001). Dose-dependent promotion by phenylethyl isothiocyanate, a known chemopreventer, of two-stage rat urinary bladder and liver carcinogenesis. Nutr. Cancer 40 134–139 [DOI] [PubMed] [Google Scholar]
- 34. Sugiura S., et al. (2003). Reversibility of proliferative lesions and induction of non-papillary tumors in rat urinary bladder treated with phenylethyl isothiocyanate. Carcinogenesis 24 547–553 [DOI] [PubMed] [Google Scholar]
- 35. Takagi H., et al. (2005). Limited tumor-initiating activity of phenylethyl isothiocyanate by promotion with sodium L-ascorbate in a rat two-stage urinary bladder carcinogenesis model. Cancer Lett. 219 147–153 [DOI] [PubMed] [Google Scholar]
- 36. Hirose M., et al. (1998). Strong promoting activity of phenylethyl isothiocyanate and benzyl isothiocyanate on urinary bladder carcinogenesis in F344 male rats. Int. J. Cancer 77 773–777 [DOI] [PubMed] [Google Scholar]
- 37. Okazaki K., et al. (2002). Simultaneous treatment with benzyl isothiocyanate, a strong bladder promoter, inhibits rat urinary bladder carcinogenesis by N-butyl-N-(4-hydroxybutyl)nitrosamine. Nutr. Cancer 42 211–216 [DOI] [PubMed] [Google Scholar]
- 38. Okazaki K., et al. (2003). Enhancement of urinary bladder carcinogenesis by combined treatment with benzyl isothiocyanate and N-butyl-N-(4-hydroxybutyl)nitrosamine in rats after initiation. Cancer Sci. 94 948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Michaud D.S., et al. (1999). Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J. Natl. Cancer Inst. 91 605–613 [DOI] [PubMed] [Google Scholar]
- 40. Tang L., et al. (2008). Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol. Biomarkers Prev. 17 938–944 [DOI] [PubMed] [Google Scholar]
- 41. Tang L., et al. (2010). Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol. Biomarkers Prev. 19 1806–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Musk S.R., et al. (1993). The clastogenic effects of isothiocyanates. Mutat. Res. 300 111–117 [DOI] [PubMed] [Google Scholar]
- 43. Musk S.R., et al. (1995). Cytotoxic and clastogenic effects of benzyl isothiocyanate towards cultured mammalian cells. Food Chem. Toxicol. 33 31–37 [DOI] [PubMed] [Google Scholar]
- 44. Kassie F., et al. (1999). Genotoxic effects of benzyl isothiocyanate, a natural chemopreventive agent. Mutagenesis 14 595–604 [DOI] [PubMed] [Google Scholar]
- 45. Ding Y., et al. (2010). Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissues. Carcinogenesis 31 1999–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Munday R., et al. (2008). Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 68 1593–1600 [DOI] [PubMed] [Google Scholar]
- 47. Khor T.O., et al. (2008). Chemoprevention of familial adenomatous polyposis in Apc Min/+ mice by phenethyl isothiocyanate (PEITC). Mol. Carcinog. 47 321–325 [DOI] [PubMed] [Google Scholar]
- 48. McCune K., et al. (2010). Loss of ERa and FOXA1 expression in a progression model of luminal type breast cancer: insights from PyMT transgenic mouse model. Oncol. Rep. 24 1233–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barve A., et al. (2008). Murine prostate cancer inhibition by dietary phytochemicals–curcumin and phenyethylisothiocyanate. Pharm. Res. 25 2181–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Powolny A.A., et al. (2011). Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J. Natl. Cancer Inst. 103 571–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Warin R., et al. (2009). Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res. 69 9473–9480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Myzak M.C., et al. (2006). Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc min mice. FASEB J. 20 506–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu R., et al. (2006). Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis 27 2038–2046 [DOI] [PubMed] [Google Scholar]
- 54. Singh S.V., et al. (2009). Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 69 2117–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keum Y.S., et al. (2009). Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm. Res. 26 2324–2331 [DOI] [PubMed] [Google Scholar]
- 56. Hasegawa T., et al. (1993). Isothiocyanates inhibit cell cycle progression of HeLa cells at G2/M phase. Anticancer. Drugs 4 273–279 [DOI] [PubMed] [Google Scholar]
- 57. Xiao D., et al. (2004). Proteasome-mediated degradation of cell division cycle 25C and cyclin-dependent kinase 1 in phenethyl isothiocyanate-induced G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells. Mol. Cancer Ther. 3 567–575 [PubMed] [Google Scholar]
- 58. Visanji J.M., et al. (2004). Dietary isothiocyanates inhibit Caco-2 cell proliferation and induce G2/M phase cell cycle arrest, DNA damage, and G2/M checkpoint activation. J. Nutr. 134 3121–3126 [DOI] [PubMed] [Google Scholar]
- 59. Jakubíková J., et al. (2005). Effects of MEK1 and PI3K inhibitors on allyl-, benzyl- and phenylethyl-isothiocyanate-induced G2/M arrest and cell death in Caco-2 cells. Int. J. Oncol. 27 1449–1458 [PubMed] [Google Scholar]
- 60. Miyoshi N., et al. (2004). Benzyl isothiocyanate modifies expression of the G2/M arrest-related genes. Biofactors 21 23–26 [DOI] [PubMed] [Google Scholar]
- 61. Srivastava S.K., et al. (2004). Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis 25 1701–1709 [DOI] [PubMed] [Google Scholar]
- 62. Xiao D., et al. (2006). Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol. Cancer Ther. 5 2931–2945 [DOI] [PubMed] [Google Scholar]
- 63. Zhang Y., et al. (2003). Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol. Cancer Ther. 2 1045–1052 [PubMed] [Google Scholar]
- 64. Wu C.L., et al. (2011). Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J. Orthop. Res. 29 1199–1209 [DOI] [PubMed] [Google Scholar]
- 65. Gamet-Payrastre L., et al. (2000). Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 60 1426–1433 [PubMed] [Google Scholar]
- 66. Jackson S.J., et al. (2004). Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis 25 219–227 [DOI] [PubMed] [Google Scholar]
- 67. Singh S.V., et al. (2004). Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J. Biol. Chem. 279 25813–25822 [DOI] [PubMed] [Google Scholar]
- 68. Herman-Antosiewicz A., et al. (2007). Induction of p21 protein protects against sulforaphane-induced mitotic arrest in LNCaP human prostate cancer cell line. Mol. Cancer Ther. 6 1673–1681 [DOI] [PubMed] [Google Scholar]
- 69. Sakao K., et al. (2012). Phenethyl isothiocyanate suppresses inhibitor of apoptosis family protein expression in prostate cancer cells in culture and in vivo. Prostate 72 1104–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Choi S., et al. (2005). Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res. 65 2035–2043 [DOI] [PubMed] [Google Scholar]
- 71. Cheung K.L., et al. (2010). Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 12 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yu R., et al. (1998). Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 58 402–408 [PubMed] [Google Scholar]
- 73. Huang C., et al. (1998). Essential role of p53 in phenethyl isothiocyanate-induced apoptosis. Cancer Res. 58 4102–4106 [PubMed] [Google Scholar]
- 74. Xiao D., et al. (2002). Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 62 3615–3619 [PubMed] [Google Scholar]
- 75. Kim S.H., et al. (2010). p53-Independent apoptosis by benzyl isothiocyanate in human breast cancer cells is mediated by suppression of XIAP expression. Cancer Prev. Res. 3 718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang X, et al. (2011). Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships J. Med. Chem., 54 809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rose P., et al. (2005). β-phenylethyl isothiocyanate mediated apoptosis; contribution of Bax and the mitochondrial death pathway. Int. J. Biochem. Cell Biol. 37 100–119 [DOI] [PubMed] [Google Scholar]
- 78. Trachootham D., et al. (2006). Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 10 241–252 [DOI] [PubMed] [Google Scholar]
- 79. Singh S.V., et al. (2005). Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J. Biol. Chem. 280 19911–19924 [DOI] [PubMed] [Google Scholar]
- 80. Xiao D., et al. (2008). Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J. Biol. Chem. 283 30151–30163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xiao D., et al. (2010). Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J. Biol. Chem. 285 26558–26569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xiao D., et al. (2009). Cellular responses to cancer chemopreventive agent D,L-sulforaphane in human prostate cancer cells are initiated by mitochondrial reactive oxygen species. Pharm. Res. 26 1729–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Powolny A.A., et al. (2010). Differential response of normal (PrEC) and cancerous human prostate cells (PC-3) to phenethyl isothiocyanate-mediated changes in expression of antioxidant defense genes. Pharm. Res. 27 2766–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xiao D., et al. (2010). p66Shc is indispensable for phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Cancer Res. 70 3150–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sakao K., et al. (2012). D,L-sulforaphane-induced apoptosis in human breast cancer cells is regulated by the adapter protein p66Shc. J. Cell. Biochem. 113 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Folkman J. (1971). Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285 1182–1186 [DOI] [PubMed] [Google Scholar]
- 87. Xiao D., et al. (2007). Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 67 2239–2246 [DOI] [PubMed] [Google Scholar]
- 88. Wang X.H., et al. (2009). Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochem. Pharmacol. 78 261–272 [DOI] [PubMed] [Google Scholar]
- 89. Warin R., et al. (2010). Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Mol. Carcinog. 49 500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kim E.J., et al. (2011). Oral administration of benzyl-isothiocyanate inhibits solid tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. Breast Cancer Res. Treat. 130 61–71 [DOI] [PubMed] [Google Scholar]
- 91.Boreddy S.R., et al. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-a/VEGF/Rho-GTPases: pivotal role of STAT-3. PLoS ONE. (2011);6:e25799. doi: 10.1371/journal.pone.0025799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bertl E., et al. (2006). Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol. Cancer Ther. 5 575–585 [DOI] [PubMed] [Google Scholar]
- 93. Jackson S.J., et al. (2007). Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vascul. Pharmacol. 46 77–84 [DOI] [PubMed] [Google Scholar]
- 94. Cavell B.E., et al. (2011). Anti-angiogenic effects of dietary isothiocyanates: mechanisms of action and implications for human health. Biochem. Pharmacol. 81 327–336 [DOI] [PubMed] [Google Scholar]
- 95. Dikic I., et al. (2010). Selective autophagy in cancer development and therapy. Cancer Res. 70 3431–3434 [DOI] [PubMed] [Google Scholar]
- 96. Gibson S.B. (2010). A matter of balance between life and death: targeting reactive oxygen species (ROS)-induced autophagy for cancer therapy. Autophagy 6 835–837 [DOI] [PubMed] [Google Scholar]
- 97. Herman-Antosiewicz A., et al. (2006). Sulforaphane causes autophagy to inhibit release of cytochrome c and apoptosis in human prostate cancer cells. Cancer Res. 66 5828–5835 [DOI] [PubMed] [Google Scholar]
- 98. Nishikawa T., et al. (2010). Inhibition of autophagy potentiates sulforaphane-induced apoptosis in human colon cancer cells. Ann. Surg. Oncol. 17 592–602 [DOI] [PubMed] [Google Scholar]
- 99. Naumann P., et al. (2011). Autophagy and cell death signaling following dietary sulforaphane act independently of each other and require oxidative stress in pancreatic cancer. Int. J. Oncol. 39 101–109 [DOI] [PubMed] [Google Scholar]
- 100. Bommareddy A., et al. (2009). Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 69 3704–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao D., et al. Benzyl isothiocyanate causes FoxO1-mediated autophagic death in human breast cancer cells. PLoS ONE. (2012);7:e32597. doi: 10.1371/journal.pone.0032597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vinogradov S., et al. (2012). Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine 7 597–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Alison M.R., et al. (2012). Cancer stem cells: In the line of fire. Cancer Treat. Rev. 38 589–598 [DOI] [PubMed] [Google Scholar]
- 104. Li Y., et al. (2010). Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 16 2580–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sehrawat A., et al. (2011). Benzyl isothiocyanate inhibits epithelial-mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev. Res. 4 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thiery J.P. (2002). Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2 442–454 [DOI] [PubMed] [Google Scholar]
- 107. Chen S.C, et al. (2012). Hepatoma-derived growth factor regulates breast cancer cell invasion by modulating epithelial mesenchymal transition J. Pathol. Doi: 10.1002/path.3988 [DOI] [PubMed] [Google Scholar]
- 108. Conaway C.C., et al. (2002). Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr. Drug Metab. 3 233–255 [DOI] [PubMed] [Google Scholar]
- 109. Fimognari C., et al. (2008). Chemoprevention of cancer by isothiocyanates and anthocyanins: mechanisms of action and structure-activity relationship. Curr. Med. Chem. 15 440–447 [DOI] [PubMed] [Google Scholar]
- 110. Juge N., et al. (2007). Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell. Mol. Life Sci. 64 1105–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dashwood R.H., et al. (2008). Dietary agents as histone deacetylase inhibitors: sulforaphane and structurally related isothiocyanates. Nutr. Rev. 66 (Suppl. 1) S36–S38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xu C, et al. (2005). Suppression of NF-κB and NF-κB-regulated gene expression by sulforaphane and PEITC through IκBα, IKK pathway in human prostate cancer PC-3 cells Oncogene 24 4486–4495 [DOI] [PubMed] [Google Scholar]
- 113. Sahu R.P., et al. (2009). The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J. Natl. Cancer Inst. 101 176–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hahm E.R., et al. (2010). Sulforaphane inhibits constitutive and interleukin-6-induced activation of signal transducer and activator of transcription 3 in prostate cancer cells. Cancer Prev. Res. 3 484–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim S.H., et al. (2009). D,L-Sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol. Cancer Ther. 8 1946–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kang L., et al. (2009). Isothiocyanates repress estrogen receptor alpha expression in breast cancer cells. Oncol. Rep. 21 185–192 [PMC free article] [PubMed] [Google Scholar]
- 117. Mi L., et al. (2011). Proteins as binding targets of isothiocyanates in cancer prevention. Carcinogenesis 32 1405–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hu J., et al. (2007). Phenethyl isothiocyanate, a cancer chemopreventive constituent of cruciferous vegetables, inhibits cap-dependent translation by regulating the level and phosphorylation of 4E-BP1. Cancer Res. 67 3569–3573 [DOI] [PubMed] [Google Scholar]
- 119. Hahm E.R., et al. (2012). Bim contributes to phenethyl isothiocyanate-induced apoptosis in breast cancer cells. Mol. Carcinog. 51 465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Antony M.L., et al. Critical role of p53 upregulated modulator of apoptosis in benzyl isothiocyanate-induced apoptotic cell death. PLoS ONE. (2012);7:e32267. doi: 10.1371/journal.pone.0032267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shannan B., et al. (2006). Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 13 12–19 [DOI] [PubMed] [Google Scholar]
- 122. Miyake H., et al. (2006). Expression of clusterin in prostate cancer correlates with Gleason score but not with prognosis in patients undergoing radical prostatectomy without neoadjuvant hormonal therapy. Urology 68 609–614 [DOI] [PubMed] [Google Scholar]
- 123. Kumar A., et al. (2010). New biomarkers for monitoring the levels of isothiocyanates in humans. Chem. Res. Toxicol. 23 756–765 [DOI] [PubMed] [Google Scholar]
- 124. Kumar A., et al. (2010). Determination of new biomarkers to monitor the dietary consumption of isothiocyanates. Biomarkers 15 739–745 [DOI] [PubMed] [Google Scholar]
- 125. Pullar J.M., et al. (2004). The chemopreventive agent phenethyl isothiocyanate sensitizes cells to Fas-mediated apoptosis. Carcinogenesis 25 765–772 [DOI] [PubMed] [Google Scholar]
- 126. Tseng E., et al. (2002). Effect of organic isothiocyanates on the P-glycoprotein- and MRP1-mediated transport of daunomycin and vinblastine. Pham. Res. 19 1509–1515 [DOI] [PubMed] [Google Scholar]
- 127. Hu K., et al. (2004). Effects of benzyl-, phenethyl-, and alpha-naphthyl isothiocyanates on P-glycoprotein- and MRP1-mediated transport. J. Pharm. Sci. 93 1901–1911 [DOI] [PubMed] [Google Scholar]
- 128. Mukherjee S., et al. (2009). Targeting protein kinase C (PKC) and telomerase by phenethyl isothiocyanate (PEITC) sensitizes PC-3 cells towards chemotherapeutic drug-induced apoptosis. J. Environ. Pathol. Toxicol. Oncol. 28 269–282 [DOI] [PubMed] [Google Scholar]
- 129. Mukherjee S., et al. (2009). Isothiocyanates sensitize the effect of chemotherapeutic drugs via modulation of protein kinase C and telomerase in cervical cancer cells. Mol. Cell. Biochem. 330 9–22 [DOI] [PubMed] [Google Scholar]
- 130. Di Pasqua A.J., et al. (2010). Sensitization of non-small cell lung cancer cells to cisplatin by naturally occurring isothiocyanates. Chem. Res. Toxicol. 23 1307–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xiao D., et al. (2010). Phenethyl isothiocyanate sensitizes androgen-independent human prostate cancer cells to docetaxel-induced apoptosis in vitro and in vivo. Pharm. Res. 27 722–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sahu R.P., et al. (2009). Benzyl isothiocyanate sensitizes human pancreatic cancer cells to radiation therapy. Front. Biosci. 1 568–576 [DOI] [PubMed] [Google Scholar]
- 133. Ohara M., et al. (2011). Benzyl isothiocyanate sensitizes human pancreatic cancer cells to radiation by inducing apoptosis. Int. J. Mol. Med. 28 1043–1047 [DOI] [PubMed] [Google Scholar]
- 134. Wicker C.A, et al. (2010). BITC sensitizes pancreatic adenocarcinomas to TRAIL-induced apoptosis Cancer Growth Metastasis 2 45–55 [PMC free article] [PubMed] [Google Scholar]
- 135. Kallifatidis G., et al. (2011). Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol. Ther. 19 188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Fimognari C., et al. (2007). Combination of doxorubicin and sulforaphane for reversing doxorubicin-resistant phenotype in mouse fibroblasts with p53Ser220 mutation. Ann. N. Y. Acad. Sci. 1095 62–69 [DOI] [PubMed] [Google Scholar]
- 137.Kim S.H., et al. Notch activation by phenethyl isothiocyanate attenuates its inhibitory effect on prostate cancer cell migration. PLoS ONE. (2011);6:e26615. doi: 10.1371/journal.pone.0026615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dang T.P. (2012). Notch, apoptosis and cancer. Adv. Exp. Med. Biol. 727 199–209 [DOI] [PubMed] [Google Scholar]
- 139. Hu Y.Y., et al. (2012). Notch signaling pathway and cancer metastasis. Adv. Exp. Med. Biol. 727 186–198 [DOI] [PubMed] [Google Scholar]
- 140. Wang J., et al. (2012). Notch signaling in cancer stem cells. Adv. Exp. Med. Biol. 727 174–185 [DOI] [PubMed] [Google Scholar]
- 141. Kim S.H, et al. (2012). Notch2 activation by benzyl isothiocyanate impedes its inhibitory effect on breast cancer cell migration Breast Cancer Res. Treat. doi: 10.1007/S10549-012–2043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Croce C.M. (2009). Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 10 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Basu A., et al. (2011). MicroRNA-375 and MicroRNA-221: Potential Noncoding RNAs Associated with Antiproliferative Activity of Benzyl Isothiocyanate in pancreatic cancer. Genes Cancer 2 108–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Szafranska A.E., et al. (2007). MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene 26 4442–4452 [DOI] [PubMed] [Google Scholar]
- 145. Hecht S.S., et al. (1995). Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiol. Biomarkers Prev. 4 877–884 [PubMed] [Google Scholar]
- 146. Syed Alwi S.S., et al. (2010). In vivo modulation of 4E binding protein 1 (4E-BP1) phosphorylation by watercress: a pilot study. Br. J. Nutr. 104 1288–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Shapiro T.A, et al. (2006). Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase 1 study Nutr. Cancer 55 53–62 [DOI] [PubMed] [Google Scholar]
- 148. Kensler T.W., et al. (2005). Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 14 2605–2613 [DOI] [PubMed] [Google Scholar]
- 149. Myzak M.C., et al. (2007). Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp. Biol. Med. 232 227–234 [PMC free article] [PubMed] [Google Scholar]