Abstract
Background
The prevalence of frontotemporal lobar degeneration (FTLD) in Japan is unknown. An epidemiological survey study of FTLD was undertaken in Tottori Prefecture, a district in the western region of Japan.
Methods
Hospitals in Tottori Prefecture were surveyed by a two-step questionnaire in 2010, and the prevalence of FTLD per 100,000 inhabitants was calculated using the actual number of patients and inhabitants in Tottori Prefecture on the prevalence day of October 1, 2010.
Results
In this survey, 66 patients were diagnosed with FTLD. The subtypes of FTLD were as follows: 62 cases of frontotemporal dementia (FTD), 3 cases of progressive nonfluent aphasia, and 1 case of semantic dementia. Among the FTD cases, 5 cases were FTD with motor neuron disease and 1 case was FTD with parkinsonism linked to chromosome 17. The prevalence of FTD in the total population of Tottori Prefecture was 11.2 per 100,000 inhabitants. Based on these results, the prevalence of FTLD in Japan in 2008 was estimated to be 9.5 per 100,000 individuals.
Conclusions
Our epidemiological survey results suggest that there are at least 12,000 FTLD patients in Japan, indicating that FTLD is not a rare disease.
Key Words: Prevalence, Frontotemporal dementia, Progressive nonfluent aphasia, Semantic dementia, Tau gene
Introduction
Frontotemporal lobar degeneration (FTLD) is a neurodegenerative disorder predominantly affecting the frontal and temporal lobes. Two major clinical types are recognized in FTLD: behavioral variant frontotemporal dementia (FTD) and progressive aphasia. The latter is divided into progressive nonfluent aphasia (PNFA) and semantic dementia (SD) [1]. Few epidemiological surveys have been conducted concerning FTLD in Japan, and no epidemiological study focusing particularly on FTLD has been reported. In small community-based studies on the prevalence of dementia in individuals 65 years of age or older, a small percentage of the dementia cases were attributed to FTLD [2,3]. In clinic-based survey studies, FTLD was the most third frequent cause of early-onset dementia in patients less than 65 years of age [4,5]. The prevalence of FTLD in Japan is unknown. Here, we report a survey study of FTLD in Tottori Prefecture, the least populated district in Japan.
Methods
We used a questionnaire to perform a retrospective surveillance study of cases of FTLD in Tottori Prefecture. Tottori Prefecture is located in a rural area of western Japan (fig. 1); it had a population of 587,772 (280,602 males and 307,170 females) on the prevalence day of October 1, 2010.
Fig. 1.
Location of the surveyed hospitals in Tottori Prefecture. Tottori Prefecture is located in western Japan. The marked hospitals and clinics (circles) were included in our survey.
In 2010, we sent inquiries with registration criteria for each category of FTLD to the departments of neurology and psychiatry in the 47 hospitals in Tottori Prefecture where patients with dementia were treated, asking if they had admitted or examined any cases of FTLD during the past year. We then sent a second questionnaire to the departments who responded affirmatively enquiring about the type of FTLD, sex and age of patients, age of onset, symptoms, neuroimaging results, and treatment. If permission was obtained, board-certificated neurologists (K.W.-I., S.I., S.N., and M.Y.) visited the hospitals to examine the patients.
The diagnosis of FTLD was based on the consensus criteria by Neary et al. [1] and the criteria of the International Behavioural Variant FTD Criteria Consortium [6]. Structural neuroimaging [cerebral computed tomography (CT) or magnetic resonance imaging (MRI)] was performed to support the clinical diagnosis. Functional imaging data [cerebral blood flow evaluated by single-photon emission computed tomography (SPECT)] were obtained, if available. Patients were diagnosed as having probable or possible amyotrophic lateral sclerosis using the El Escorial criteria [7,8].
The prevalence of FTLD per 100,000 inhabitants and 95% confidence interval (CI) were calculated using the actual number of patients and inhabitants in Tottori Prefecture on the prevalence day, October 1, 2010.
This study was planned and conducted in accordance with the Declaration of Helsinki. The Ethics Committee of the Tottori University Faculty of Medicine approved the study prior to its implementation.
Results
Survey Results
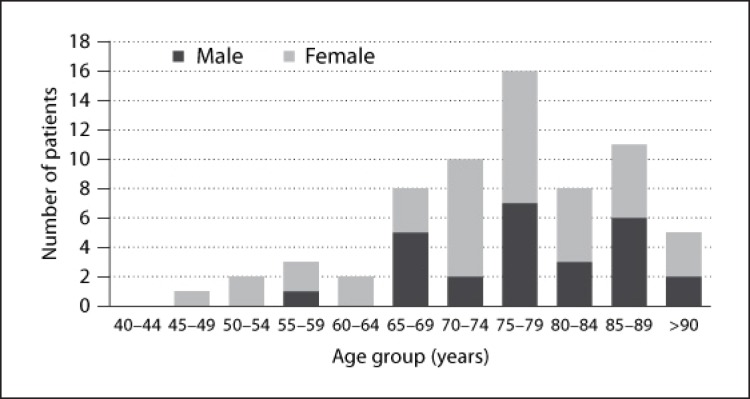
Sixty-six patients were diagnosed with FTLD in Tottori Prefecture on the prevalence day (fig. 2). The subtypes of FTLD were as follows: 62 cases of FTD, 3 cases of PNFA, and 1 case of SD. Among the FTD cases, 5 were FTD with motor neuron disease, and 1 was FTD with parkinsonism linked to chromosome 17 (FTDP-17). Overall, the mean age of patients with FTD was 76.5 ± 11.0 years. The mean age of FTD patients with motor neuron disease was 65.8 ± 14.3 years, less than the mean age of FTD patients without motor neuron disease. The mean age of the 3 patients with PNFA was 71.7 ± 8.1 years, and the age of the patient with SD was 72 years. Three patients with FTLD had a family history of the disease. Genetic analysis revealed that the patient with FTDP-17 had an intronic mutation IVS10 C>T in the microtubule-associated protein tau (MAPT) gene.
Fig. 2.
Age-specific number of patients with FTLD.
Prevalence
Table 1 shows the age-specific prevalence of FTLD per 100,000 inhabitants of Tottori Prefecture in 2010. The survey results indicate that the overall prevalence of FTLD in Tottori Prefecture was at least 11.2 per 100,000 inhabitants. Based on the demographics of Japan in 2008, the data suggest an estimated prevalence of FTLD in Japan of 9.5 per 100,000 inhabitants, or an overall prevalence of at least 12,000 individuals.
Table 1.
Age-specific prevalence estimates of FTLD in Tottori Prefecture
| Patients, n | Prevalence (95% CI)1 | |
|---|---|---|
| Age group | ||
| 45-54 years | 3 | 4.0 (-5.3 to 8.6) |
| 55-64 years | 5 | 5.6 (0.7-10.5) |
| 65-74 years | 18 | 25.8 (13.9-37.7) |
| 75-84 years | 24 | 40.5 (24.3-56.7) |
| >85 years | 16 | 64.0 (32.6-95.0) |
| Population ≥45 years | 66 | 20.7 (15.7-26.0) |
| Total Population | 66 | 11.2 (8.5-14.0) |
Per 100,000 inhabitants.
Discussion
We conducted an epidemiological survey focusing on the prevalence of FTLD in Tottori Prefecture of Japan. Our previous epidemiological study in Ama-cho [3], a small island town, revealed that only 1 patient with FTLD was diagnosed among 943 subjects with dementia aged 65 years or older, suggesting that more subjects would be needed to examine the true prevalence of FTLD. Therefore, we decided to carry out an epidemiological survey in Tottori Prefecture, which has a total population of 587,772.
The prevalence of FTLD has also been reported in areas outside of Japan. In population-based studies, the prevalence of FTLD between 45 and 64 years of age has varied from 4.0 per 100,000 individuals in the Zuid-Holland district in the Netherlands to 22 per 100,000 individuals in Brescia, Italy [9,10,11,12]. A nationwide hospital-based clinicoepidemiological study in Germany showed a high estimated prevalence of FTLD of 43.1 per 100,000 individuals between 45 and 64 years of age [13]. In contrast, Ikejima et al. [14] reported the prevalence of restricted FTD patient in Ibaraki Prefecture, Japan, to be 2.0 per 100,000 individuals between 45 and 64 years of age. Taken together with our results, the prevalence of FTLD in individuals under 65 years of age in Japan might be less than that in Europe.
Although FTLD is generally considered to be a presenile dementia, a review of demographic characteristics of 353 FTLD patients by Johnson et al. [15] indicated that approximately one quarter of the patients diagnosed as having FTD and semantic dementia and half of PNFA patients had a disease onset after age 65 years. The prevalence of FTLD was 3.8 per 100,000 individuals between 70 and 79 years of age in the Zuid-Holland district in the Netherlands [9]. A nationwide study in Germany estimated the prevalence of FTLD to be 49.3 per 100,000 individuals between 70 and 79 years of age [11]. Gislason et al. [16] reported a much higher prevalence of FTD of 3% in a cohort of 85-year-old individuals in Gothenburg, Sweden. In the current study, the prevalence of FTLD was 37.6 per 100,000 inhabitants 65 years of age or older. These epidemiological data indicate that the prevalence of FTLD among elderly subjects might be higher than previously described. One challenging aspect of FTLD is that the clinicopathological features of FTLD in elderly patients may differ from those in patients with presenile-onset FTLD. Baborie et al. [17] proposed that FTLD in elderly patients might exist as a separate entity from presenile-onset FTLD in that the characteristics of clinically frequent memory loss and behavioral changes predominate over language and semantic dysfunction. It was suggested that FTLD in elderly patients is under-recognized, and FTLD should be considered in elderly subjects presenting with an ‘atypical Alzheimer's disease’ phenotype.
Although epidemiological studies in Europe have reported that a large percentage of FTLD patients have a family history (29% in the UK, 43% in the Netherlands), only 4.5% of FTLD patients in our current study had a family history. Only 1 patient (1.5%) of 66 FTLD patients in Tottori Prefecture had a mutation of MAPT, compared with 32 (13.8%) of 245 FTD patients in the study in the Zuid-Holland district in the Netherlands. These results suggest that genetic factors for the development of FTLD may have a less important role in the Japanese population.
There are several limitations in our estimates of the prevalence of FTLD in Tottori Prefecture of Japan. First, the diagnosis of FTLD was dependent on clinical symptoms only, due to the absence of a definitive biomarker. We could not confirm the diagnosis neuropathologically in any case in this survey. Careful clinical examinations are needed because of the similarities in symptoms between syndromes such as corticobasal degeneration, progressive supranuclear palsy, Alzheimer's disease, vascular dementia, and FTLD. In this study, board-certificated neurologists and psychiatrists reported the diagnosis of FTLD patients based on their clinical assessment and results of neuroimaging such as cerebral CT, MRI, or cerebral blood flow by SPECT. Further, board-certificated neurologists who have scientific interest in dementia or neurodegenerative disorders visited the clinic or hospital for assessment of the patients when required. For the diagnosis of FTD, we applied the criteria by Neary et al. [5] as well as the criteria of the International Behavioural Variant FTD Criteria Consortium [6]. The former criteria are thought to be relatively insensitive and difficult to apply in the early stage of FTD, whereas the sensitivity of the latter criteria is reported to be better [6].
Further, only patients diagnosed with FTLD who had a medical consultation with the department of neurology or psychiatry were included in the survey. A clinical survey in an academic hospital indicated that the prevalence of PNFA or SD was similar to that of FTD [4]. In the current survey, the proportion of patients with PNFA or SD was much less than that reported in the previous survey, suggesting that the prevalence of PNFA and SD may have been underestimated.
In conclusion, the results of this study suggest that there are as many as 12,000 patients with FTLD in Japan, indicating that FTLD is not a rare disease at all.
Disclosure Statement
We certify that there is no conflict of interest.
Acknowledgment
We thank the staff at the Department of Neurology at Tottori University for their help in recruiting the patients.
This study was supported in part by the Research Committee of CNS Degenerative Diseases, Ministry of Health, Labor, and Welfare, Japan.
References
- 1.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda M, Hokoishi K, Maki N, et al. Increased prevalence of vascular dementia in Japan: a community-based epidemiological study. Neurology. 2001;57:839–844. doi: 10.1212/wnl.57.5.839. [DOI] [PubMed] [Google Scholar]
- 3.Wada-Isoe K, Uemura Y, Suto Y, et al. Prevalence of dementia in the rural island town of Ama-cho, Japan. Neuroepidemiology. 2009;32:101–106. doi: 10.1159/000177035. [DOI] [PubMed] [Google Scholar]
- 4.Yokota O, Sasaki K, Fujisawa Y, et al. Frequency of early and late-onset dementias in a Japanese memory disorders clinic. Eur J Neurol. 2005;12:782–790. doi: 10.1111/j.1468-1331.2005.01072.x. [DOI] [PubMed] [Google Scholar]
- 5.Shinagawa S, Ikeda M, Toyota Y, et al. Frequency and clinical characteristics of early-onset dementia in consecutive patients in a memory clinic. Dement Geriatr Cogn Disord. 2007;24:42–47. doi: 10.1159/000102596. [DOI] [PubMed] [Google Scholar]
- 6.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial ‘Clinical limits of amyotrophic lateral sclerosis’ workshop contributors. J Neurol Sci. 1994;124(suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 8.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 9.Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126:2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- 10.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 11.Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry. 2003;74:1206–1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borroni B, Alberici A, Grassi M, et al. Is frontotemporal lobar degeneration a rare disorder? Evidence from a preliminary study in Brescia county, Italy. J Alzheimers Dis. 2010;19:111–116. doi: 10.3233/JAD-2010-1208. [DOI] [PubMed] [Google Scholar]
- 13.Ibach B, Koch H, Koller M, Wolfersdorf M, Workgroup for Geriatric Psychiatry of the Psychiatric State Hospitals of Germany. Workgroup for Clinical Research of the Psychiatric State Hospitals of Germany Hospital admission circumstances and prevalence of frontotemporal lobar degeneration: a multicenter psychiatric state hospital study in Germany. Dement Geriatr Cogn Disord. 2003;16:253–264. doi: 10.1159/000072810. [DOI] [PubMed] [Google Scholar]
- 14.Ikejima C, Yasuno F, Mizukami K, Sasaki M, Tanimukai S, Asada T. Prevalence and causes of early-onset dementia in Japan: a population-based study. Stroke. 2009;40:2709–2714. doi: 10.1161/STROKEAHA.108.542308. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 16.Gislason TB, Sjögren M, Larsson L, Skoog I. The prevalence of frontal variant frontotemporal dementia and the frontal lobe syndrome in a population based sample of 85 year olds. J Neurol Neurosurg Psychiatry. 2003;74:867–871. doi: 10.1136/jnnp.74.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baborie A, Griffiths TD, Jaros E, et al. : Frontotemporal dementia in elderly individuals. Arch Neurol 2012, E-pub ahead of print. [DOI] [PubMed]