Abstract
Antiangiogenic treatment using bevacizumab may cause difficulties in distinguishing between antivascular and true antitumor effects when using MRI response criteria based on changes of contrast enhancement (i.e., Macdonald criteria). Furthermore, more precise tumor response assessment criteria (i.e., RANO criteria), which incorporate nonenhancing T2/FLAIR sequences into Macdonald criteria, may be influenced by other causes of T2/FLAIR hyperintensity (e.g., radiation-induced gliosis). The authors present discrepant MR and [18F]fluoroethyl-L-tyrosine PET imaging findings in a patient with bevacizumab treatment failure.
Key Words: Volume-of-interest analysis, Metabolically active tumor volume, Amino acid PET, [18F]Fluoroethyl-L-tyrosine, RANO criteria
Introduction
Bevacizumab is thought to normalize tumor vasculature and restore the blood-brain barrier, decreasing contrast enhancement and peritumoral edema [1]. Conventional measurements of tumor response using MRI rely upon dimensions of enhancing tumor. Antiangiogenic treatment targeting the VEGF pathway using bevacizumab causes a rapid decrease in T1 contrast-enhancing tumor parts, with high radiographic response rates ranging between 30–60% [1]. However, in one third of all patients, glioblastomas are more prone to progress as nonenhancing tumor after bevacizumab treatment [2]. Recently defined RANO (Response Assessment in Neuro-Oncology) criteria for glioma progression recommend fluid-attenuated inversion recovery (FLAIR)/T2 hyperintensity on MRI as a surrogate for nonenhancing tumor; however, nonenhancing tumor can be difficult to differentiate from other causes of FLAIR/T2 hyperintensity (e.g., radiation-induced gliosis, peritumoral edema) [3]. Positron emission tomography using the radiolabeled amino acid O-(2-[18F]fluoroethyl)-L-tyrosine (18F-FET PET) may offer an improvement of the detection of ‘true’, i.e., metabolically active tumor extent of high-grade glioma over and above the information provided by contrast enhancement and FLAIR hyperintensity on MRI alone.
Case Report
A 58-year-old patient suffered from aphasia and agitation 4 weeks prior to admission. Initial MRI revealed a contrast-enhancing, tumor-suspicious lesion in the left temporal lobe, and a biopsy was performed stereotactically. Histopathologic findings confirmed a glioblastoma. Molecular analysis of prognostic factors revealed that the O6-methylguanine methyltransferase promoter (MGMT) was not methylated and isocitrate dehydrogenases 1 and 2 (IDH1/IDH2) were not mutated. In order to achieve a macroscopically complete tumor resection, a fluorescence-guided resection using 5-aminolevulinic acid (5-ALA) was performed. After surgery, fractionated external radiation therapy was initiated. Within 2 weeks after tumor resection, the aphasia improved substantially. After stratification for molecular markers, the patient was treated with concomitant radiotherapy up to a dosage of 60 Gy, and afterwards with adjuvant bevacizumab [4] every 2 weeks over 8 months. Due to a severe allergic reaction, the combination therapy with irinotecan had to be discontinued after the first application. During bevacizumab treatment, clinical controls and follow-up MRIs in 8- to 12-week intervals showed no signs of tumor recurrence.
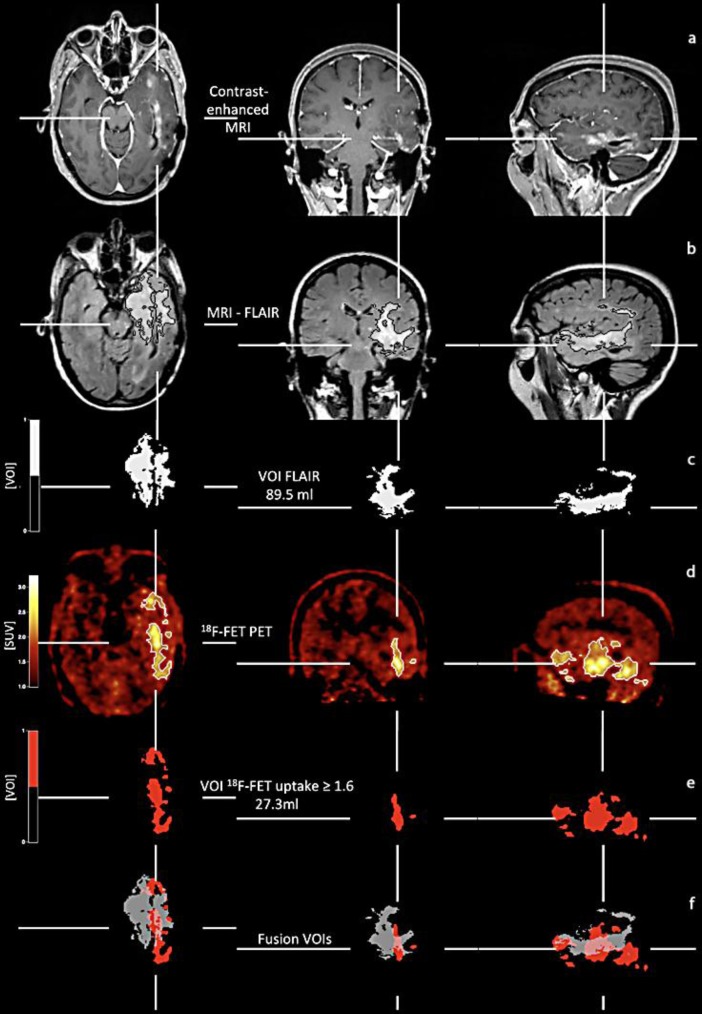
After 8 months of single bevacizumab therapy, hemiparesis of the right side and deterioration of the aphasia occurred. The follow-up T1-weighted MRI (fig. 1a) demonstrates slight contrast enhancement within the left temporal lobe. In contrast, the hyperintensity on the FLAIR-weighted MRI (fig. 1b, encircled within black lines) is significantly larger, suggesting signs of nonenhancing tumor progression during bevacizumab treatment [2, 5]. To further evaluate the MRI results, 18F-FET PET (fig. 1d) was performed. Metabolically active tumor volume of 18F-FET PET uptake at a threshold =1.6 and volume of the hyperintense FLAIR signal were calculated [6, 7] (fig. 1c, e).
Fig. 1.
Coregistered MR, 18F-FET PET, and superimposed images of the metabolically active tumor volume (red) at an 18F-FET uptake index threshold of =1.6 (red) and volume of FLAIR hyperintensity (white). FLAIR hyperintensity is not always located exclusively within the subareas of the tumor positive on 18F-FET PET. On superimposed pictures (bottom), FLAIR hyperintensity (grey) is eccentric and located partially outside the metabolically active tumor volume (red). VOI = Volume of interest; SUV = standardized uptake value.
Coregistrations of 18F-FET PET scan (fig. 1d), contrast-enhanced and FLAIR-weighted MRI (fig. 1a, b), and volume of the FLAIR lesion (fig. 1c) and the metabolically active tumor (fig. 1d, encircled within white lines; e) show that the volume of the FLAIR hyperintensity is considerably larger than the metabolically active tumor volume. However, on superimposed images (fig. 1f), the volume of the FLAIR hyperintensity (grey) shows no correspondence in size and spatial configuration to the 18F-FET PET metabolically active tumor volume. Moreover, in comparison to the FLAIR hyperintensity, 18F-FET uptake at a threshold =1.6 (red) reveals additional subareas of metabolically active tumor (fig. 1f) in transaxial, coronal, and sagittal orientation.
For treatment of tumor recurrence, chemotherapy with dose-intensified temozolomide was initiated according to a 1 week on/1 week off regimen [8]. Clinically, chemotherapy response could not be observed; during follow-up an exacerbation of the aphasia and hemiparesis occurred. Due to a severe impairment of the patient's clinical condition, palliative care was initiated 3 months after tumor recurrence.
Discussion
The differences in MRI and 18F-FET PET might be explained by a complex morphologic heterogeneity of glioblastoma, leading to difficult interpretation of standard MRI sequences. There are highly malignant tumor parts with and without contrast enhancement, and, furthermore, T2-based signal hyperintensity is a combination of infiltrating tumor cells, necrotic areas, tumor edema, and treatment-related leukoencephalopathy, e.g., due to radiotherapy [3]. In contrast, 18F-FET PET shows metabolically active tumor independent of any anatomic or pathophysiologic changes and may therefore reflect tumor extension more accurately than MRI [6]. In this context, localization of the 18F-FET PET metabolically active tumor does not always match the pathological findings seen on MRIs. For example, in a small group of patients with recurrent high-grade glioma treated with bevacizumab and irinotecan, which partially responded according to RANO criteria without response based on 18F-FET PET imaging findings, tumor progression could be detected earlier by 18F-FET PET [9].
In summary, during bevacizumab treatment, 18F-FET PET in addition to contrast-enhanced and FLAIR-/T2-weighted MRI might add important information for assessment of tumor recurrence, as compared with standard MRI alone.
Disclosure Statement
None of the authors has any financial disclosures regarding the present article.
References
- 1.Chamberlain MC. Emerging clinical principles on the use of bevacizumab for the treatment of malignant gliomas. Cancer. 2010;116:3988–3999. doi: 10.1002/cncr.25256. [DOI] [PubMed] [Google Scholar]
- 2.Wick W, Wick A, Weiler M, Weller M. Patterns of progression in malignant glioma following anti-VEGF therapy: perceptions and evidence. Curr Neurol Neurosci Rep. 2011;11:305–312. doi: 10.1007/s11910-011-0184-0. [DOI] [PubMed] [Google Scholar]
- 3.Ahluwalia MS, Wen PY. Antiangiogenic therapy for patients with glioblastoma: current challenges in imaging and future directions. Expert Rev Anticancer Ther. 2011;11:653–656. doi: 10.1586/era.11.35. [DOI] [PubMed] [Google Scholar]
- 4.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 5.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 6.Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, Zilles K, Coenen HH, Langen KJ. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–687. doi: 10.1093/brain/awh399. [DOI] [PubMed] [Google Scholar]
- 7.Galldiks N, Kracht LW, Dunkl V, Ullrich RT, Vollmar S, Jacobs AH, Fink GR, Schroeter M. Imaging of non- or very subtle contrast-enhancing malignant gliomas with [(11)C]-methionine positron emission tomography. Mol Imaging. 2011;10:453–459. [PubMed] [Google Scholar]
- 8.Wick W, Steinbach JP, Kuker WM, Dichgans J, Bamberg M, Weller M. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62:2113–2115. doi: 10.1212/01.wnl.0000127617.89363.84. [DOI] [PubMed] [Google Scholar]
- 9.Hutterer M, Nowosielski M, Putzer D, Waitz D, Tinkhauser G, Kostron H, Muigg A, Virgolini IJ, Staffen W, Trinka E, Gotwald T, Jacobs AH, Stockhammer G. O-(2–18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52:856–864. doi: 10.2967/jnumed.110.086645. [DOI] [PubMed] [Google Scholar]