Abstract
The cytokine transforming growth factor-beta (TGF-β) has multiple effects in both physiological and pathological conditions. TGF-β is secreted as part of a tripartite complex from which it must be released in order to bind to its receptor. Sequestration of latent TGF-β in the extracellular matrix (ECM) is crucial for proper mobilization of the latent cytokine and its activation. However, contrary to expectation, loss-of-function mutations in genes encoding certain matrix proteins that bind TGF-β yield elevated, rather than decreased, TGF-β levels, posing a ‘TGF-β paradox.’ In this review, we discuss recent findings concerning the relationship of TGF-β, ECM molecules, and latent TGF-β activation and propose a model to resolve the ‘TGF-β paradox.’
Keywords: elastic microfibrils, extracellular matrix, integrin, TGF-β paradox, transforming growth factor-β
Transforming growth factor-beta (TGF-β) is the prototype of a family of cytokines that includes bone morphogenetic proteins (BMPs), activin and inhibin, nodal and growth differentiation factors. TGF-β plays important roles in embryogenesis, development and normal tissue homeostasis, as it affects cell proliferation, fate determination, differentiation, immune regulation and matrix synthesis by inducing the production of ECM molecules such as fibronectin, collagen and proteoglycan (1, 2). Disruption of TGF-β activity is associated with pathological conditions, such as autoimmune and inflammatory diseases, connective tissue diseases and cancer (3). An example of the role of TGF-β in both normal and pathological processes is epithelial-mesenchymal transition, which is critical for normal development but may be usurped during tumour development (4).
TGF-β’s functional diversity is attributable to several features of its biology: (i) three different isoforms of TGF-β (1, 2 and 3) exist and all are secreted in a latent state either bound or unbound to a second protein, the latent TGF-β binding protein (LTBP); (ii) three different isoforms of LTBP bind to latent TGF-β, thereby facilitating its secretion and directing its ECM localization; (iii) multiple mechanisms are utilized to release TGF-β from the latent complex, in a process called activation; (iv) TGF-β receptor downstream signalling is complex and includes both the canonical Smad pathway and several non-canonical pathways, as summarized in Fig. 1. Thus, the unique temporal and spatial expression patterns of the TGF-β isoforms, combined with the different expression profiles of both LTBPs and activators of the latent cytokine, yield multiple mechanisms controlling TGF-β’s bioavailability and action.
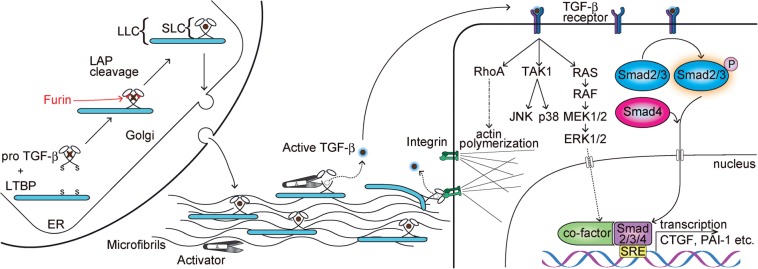
Fig. 1.
TGF-β latency, activation and signal transduction. Pro TGF-β forms a homodimer after synthesis and binds to LTBP via disulfide bonds, followed by a cleavage from LAP by furin proteases. LAP remains associated with mature TGF-β and forms SLC and LLC, conferring latency. Mature TGF-β is shown as a circle after LAP cleavage. After secretion, LLCs are incorporated into ECM, as LTBPs interact with FBNs. Proteolytic degradation of LAP or mechanical stretching by cell-surface integrins releases active TGF-β molecules that bind to their receptors. Active TGF-β is represented as with a blue halo. Cells respond to TGF-β through the canonical Smad-dependent pathway and non-canonical pathways, in which the mitogen-activated protein kinase (MAPK) pathways and the activation of RhoA are involved. Activated phosphoSmads form a ternary complex and, together with co-factors, induce transcription of TGF-β-responsive genes. Non-canonical pathways also influence gene expressions, and the activation of Rho kinases induces the polymerization of actin fibres. ER, endoplasmic reticulum; SRE, Smad response element.
Advances in our understanding of the biochemistry of the ECM have revealed several molecules, including LTBPs, which associate with latent TGF-β and have the potential to modulate its signalling. Since TGF-β stimulates the expression of multiple ECM components, several of which bind latent TGF-β (2), TGF-β directs the synthesis of molecules that govern its own accessibility and signalling. Therefore, the ECM is not simply a storage depot for TGF-β, but a site at which cytokine availability is modulated to ensure proper crosstalk between TGF-β-responsive cells and the products of TGF-β-responsive genes.
The general view has been that matrix binding of latent TGF-β complexes is necessary for proper TGF-β function, and impaired binding to the ECM yields a TGF-β-deficient state. This hypothesis is supported by evidence showing that a mutation in the Tgfb1 gene that interferes with latent TGF-β matrix association yields mice with a phenotype associated with decreased TGF-β levels (5). These studies concluded that interaction of latent TGF-β with specific ECM components sequesters and concentrates the cytokine for future use. Additionally, localization may shield the latent complex from some activators but may be required for the action of other activators. Several recent papers, however, have reported that defective ECM results in enhanced TGF-β signalling rather than decreased TGF-β signalling, creating what we call a ‘TGF-β paradox.’
In this review, we discuss the composition of the latent TGF-β complex as well as the effects of normal and defective ECM molecules on the binding and activation of latent TGF-β with a primary focus on those features of ECM-TGF-β interaction revealed by rare genetic disorders of connective tissue. We also discuss the activators of latent TGF-β and how activation may be coupled to matrix interaction. Finally, we propose a model that resolves some of the issues comprising the ‘TGF-β paradox.’
Latent TGF-β complex composition and activation
Mature TGF-β is a covalent 25-kD homodimer produced after intracellular proteolytic cleavage from its propeptide dimer (latency associated peptide (LAP)) (Fig. 1). LAP remains bound non-covalently to the mature TGF-β dimer, and TGF-β and LAP are secreted together forming the small latent complex (SLC). The association of LAP with TGF-β confers latency by preventing the mature cytokine from binding to its receptor. Within the endoplasmic reticulum (ER), most SLCs become disulfide bound to an LTBP through LAP (6), forming the large latent complex (LLC). Because LTBPs are incorporated into the ECM (7, 8), TGF-β, as part of an LLC, is targeted to the matrix, which stores the latent cytokine for future mobilization and activation. Knock-in mice expressing a mutant TGF-β1 (p.C33S) that is unable to bind LTBP exhibit decreased levels of active TGF-β (5), indicating that formation of LLC is critical for normal TGF-β biology and suggesting that soluble SLC is poorly activated.
Of the four LTBPs, LTBP-1 and LTBP-3 bind efficiently to all three TGF-β LAP isoforms, whereas LTBP-4 binds poorly and only to TGF-β1 LAP and LTBP-2 does not bind LAP (9).
Latent TGF-β activators include proteases (8, 10, 11), thrombospondin-1 (12), reactive oxygen species (ROS) (13) and integrins (14, 15). These activators release TGF-β either by degrading LAP and/or LTBPs or by modifying the conformation of the latent complex. The recently elucidated molecular structure of TGF-β1 SLC shows both that protease-sensitive sites are located in the accessible LAP surface sequences and that mechanical pulling of LAP by integrins may stretch the LAP latency lasso and release active TGF-β (16). It is also clear that there is an intimate relationship between TGF-β and inflammation (17). The inflammatory response may alter TGF-β levels directly by specific mediators affecting gene transcription. There are also data indicating that inflammatory mediators perturb the level of specific activators of latent TGF-β (18).
ECM components and regulation of TGF-β action
TGF binding molecules
LTBPs
LTBPs are large ECM molecules structurally related to fibrillins (FBNs). LTBP-1 and LTBP-4 exist in two major isoforms, short (S) and long (L), transcribed from different promoters (19). Since SLCs are not effectively targeted to the ECM and free SLCs are not efficiently activated, LTBP loss should result in decreased TGF-β activity. Indeed, LTBP-1L-deficient mice display phenotypes associated with TGF-β signalling, including impaired development of the cardiac outflow tract and valves (20–23). Consistent with decreased TGF-β signalling, phosphorylation of the intracellular TGF-β signal transmitter Smad2 and the expression of TGF-β-responsive genes, such as connective tissue growth factor (CTGF) and periostin, are decreased in specific cell populations during heart development of Ltbp1L−/− mice. Ltbp3−/− mice show bone abnormalities and defective septation of the terminal pulmonary air sacs (24, 25), phenotypes observed with loss of TGF-β. Nuclear staining of pSmad2 is decreased in lungs of 4-day old mutant mice, indicating decreased TGF-β signalling. Thus, the phenotypes of Ltbp1L and Ltbp3 null mice are consistent with decreased active TGF-β.
Unlike LTBP-1 and -3, LTBP-4 may have a dual role, both as a regulator of TGF-β signalling and as a promoter of elastogenesis. Ltbp4−/− mice display developmental emphysema, cardiac hypertrophy and colorectal cancer with diminished ECM deposition of TGF-β1 and decreased phosphorylation of Smad2 in the lungs and colon (26). In agreement with findings with Ltbp1L and Ltbp3 null mice, TGF-β activation was decreased in fibroblasts isolated from lungs of adult Ltbp4−/− animals, although the secretion of latent TGF-β complexes was increased (27). Interestingly, mutant cells expressed increased levels of certain TGF-β-responsive gene products (i.e. CTGF and plasminogen activator inhibitor-1 (PAI-1)), due to BMP-4 overproduction. In other studies, however, fibroblasts from LTBP4−/− human skin activated more latent TGF-β than controls (28). In support of this observation, Dabovic et al. reported that pSmad2 staining was increased in the lungs of 7-day old Ltbp4−/− mice, and the inhibition of TGF-β signalling by introduction of Tgfb2 null alleles normalized the emphysematous septation phenotype (29). Interestingly, abnormal elastogenesis was not reversed in Ltbp4−/−; Tgfb2−/− lungs suggesting that defective elastogenesis was not TGF-β2-mediated. These discrepancies in TGF-β production observed by different groups with Ltbp4−/− mice are part of the ‘TGF-β paradox.’
Fibrillins
FBNs are the major constituents of microfibrils and bind LTBP (7, 8). Therefore, impairment of microfibril organization due to mutations in fibrillin-1 should influence the incorporation of LLC into the ECM and alter TGF-β signalling. Disruption of microfibril assembly does lead to decreased matrix incorporation of LTBP (30, 31), as proposed for patients with Marfan syndrome (MFS), a condition caused by mutations in the fibrillin-1 gene (FBN1) (32). Several features of MFS, including aortic dilation and dissection, hypertrophic mitral valves and developmental emphysema, are reversed in Fbn1 mutant mice by administration of TGF-β neutralizing antibody (33, 34). Losartan, an angiotensin receptor 1 antagonist that suppresses TGF-β levels, also reverses the pulmonary and cardiovascular abnormalities in MFS mice (35). Consistent with these findings, significant upregulation of TGF-β signalling was revealed by a green fluorescent protein reporter construct introduced into these MFS mice (33). Similar findings were observed with osteoblasts from Fbn1 or Fbn2 null mice, which showed increased TGF-β signalling and impaired maturation reversed by a TGF-β type receptor (activin receptor-like kinase 5 (ALK5)) inhibitor, a TGF-β neutralizing antibody or knockdown of ALK5 (36). Osteoclasts from Fbn2−/− mice also displayed increased osteolytic activity (37). These observations indicate negative regulation of TGF-β action by FBN-1 and FBN-2 during bone formation. Thus, defective incorporation of latent TGF-β into microfibrils yields elevated rather than decreased levels of active TGF-β. The identity and localization of the LLC activator is unclear, but a recent report indicates that activation of latent complexes in MFS is mediated by matrix metalloproteinase (MMP)-2 (38). An important question is whether activator expression is constitutive or enhanced because of the compromised ECM.
Fibulins
Fibulins (FBLNs) comprise a group of seven family members, among which FBLN-3, -4 and -5 play important roles in ECM formation and maintenance; deficiency in these proteins yields impaired elastogenesis (39–43). Smooth muscle-specific conditional knockouts of Fbln4 show enhanced TGF-β signalling through a non-canonical pathway, as inferred from an increase of Erk1/2 phosphorylation in the aortic wall (44). On the other hand, FBLN-2-deficient mice display decreased TGF-β signalling in the peri-ischemic area after myocardial infarction, which correlates with a better survival rate compared with wild type littermates (45). Alterations in TGF-β signalling have not been reported for FBLN-3 or FBLN-5-deficient mice. Studies with Fbln2 and Fbln4 mutants demonstrate that loss of individual FBLNs may yield either increased or decreased TGF-β signalling; a situation reminiscent of the discrepant results with Ltbp mutations.
Fibronectin
Fibronectin is a fibrillar protein that interacts with many ECM components. In cultures of smooth muscle cells and fibroblasts, incorporation of LTBP-1 into matrices is dependent on fibronectin and independent of FBN microfibrils, whereas incorporation of LTBP-3 and -4 is dependent on the presence of FBNs (30). During ECM maturation, LTBP-1 shifts its association from fibronectin to FBN (46). Fibronectin binds to LTBP-1 through the LTBP-1 hinge region (47), and this interaction is indispensable for the integrin αvβ6-dependent activation of TGF-β (47, 48). Failure of LTBP-1 to bind to fibronectin yields decreased levels of active TGF-β1, a result congruous with the Tgfb1−/−like phenotype of Tgfb1C33S/C33S mice (5).
Proteoglycans
The small leucine-rich proteoglycans (SLRPs) are extracellular glycoproteins characterized by a protein core with leucine-rich repeats and various types of glycosaminoglycan side chains (49). The SLRP family members decorin, biglycan, fibromodulin, and asporin bind to and sequester free TGF-β (50). Decorin influences the response of myoblasts to TGF-β by interacting with lipoprotein-receptor-related protein-1 (51). The interaction between decorin and TGF-β also determines the mechanical characteristics of cell-seeded collagen gels (52), a property that may affect TGF-β activation (see below). Biglycan-deficient mice develop spontaneous aortic dissection (53), a phenotype associated with TGF-β dysregulation. Bone marrow derived stromal cells from these mutant mice show reduced response to exogenous TGF-β and produce less collagen, leading to decreased bone mineral density (54). Asporin binds to TGF-β and thereby inhibits TGF-β-induced expression of cartilage marker genes (55). Thus, SLRP proteoglycans may be important modulators of active TGF-β and may maintain appropriate active TGF-β levels.
TGF-β activators
Integrins
Integrins are cell-surface heterodimeric proteins that interact with proteins both inside and outside of cells and play multiple roles in cell–ECM interactions and transmembrane signalling. Integrins consist of an α- and a β-subunit and exhibit various distributions and different ligand affinities depending on α-and β-chain composition. TGF-β1 and TGF-β3 LAPs contain the integrin recognition motif RGD and bind to several different integrins (56). The mutation of the RGD to RGE in TGF-β1 LAP yields mice that recapitulate the phenotype of Tgfb1−/− mice indicating the importance of LAP binding to integrins in latent TGF-β1 activation (57).
The integrin αvβ6 binds and activates latent TGF-β1 and 3 (14, 58). Latent TGF-β1 activation by αvβ6 requires LTBP interaction with fibronectin, as soluble latent complexes are not activated (47). The αvβ6-mediated activation process also requires both the cytoplasmic domain of integrin β6 and an organized cytoskeleton, suggesting that mechanical pulling of LLC releases active TGF-β. This process depends upon activation of RhoA, which has a pivotal role in actin fibre assembly (59). A quantitative analysis of the interaction dynamics between LAP and integrins revealed that a single molecule of integrin can produce sufficient force to release TGF from LLC (60). Integrin β6 null mice are resistant to bleomycin-induced pulmonary fibrosis, underscoring the crucial nature of integrin-mediated activation of latent TGF-β1 in inflammation and tissue fibrosis (14). The importance of αvβ6 in fibrosis is underscored by the interest in the development of therapeutic antibodies that block the activity of the integrin (61).
TGF-β is also a ligand for integrin αvβ8 and both the LLC and SLC are activated by this integrin. β8-mediated activation does not require the integrin cytoplasmic domain or cytoskeletal assembly, but does require participation of membrane type 1 matrix metalloprotease (62).
Dermal fibroblasts obtained from the affected area in systemic sclerosis patients show upregulation of integrin αvβ3 and αvβ5 (63, 64). These cells induce the expression of pSmad-responsive genes when co-cultured with reporter cells. This induction is partially blocked by either anti-αvβ3 or anti-αvβ5 antibodies indicating the involvement of these integrins in the activation of latent TGF-β and the potential importance of this mechanism in systemic sclerosis pathogenesis.
β1 integrins also play a role in TGF-β signalling. The incorporation of LTBP-1 into the ECM by cultured dermal fibroblasts requires α5β1, and the inhibition of α5β1 integrin leads to increased phosphorylation of Smad2, suggesting enhanced TGF-β signalling (31). Integrins αvβ1 and α8β1 bind to LAP, but the interactions do not activate latent TGF-β (65, 66). These associations might repress activation by sequestering latent TGF-β complexes.
Integrins may indirectly influence TGF-β signalling via interaction with other activators of latent TGF-β. Cultured mouse embryonic fibroblasts show an integrin-dependent increase of ROS production in the absence of FBLN-5 (67). This was reversed by the addition of FBLN-5 but not by mutant FBLN-5 with an RGD-deletion, indicating that integrin-mediated ROS production is suppressed by the binding of FBLN-5 to integrin. Mice expressing this mutant FBLN-5 display increased ROS production and MMP-9 activity that lead to subclinical pelvic organ prolapse (68). These results suggest that the binding of integrin to FBLN-5 prevents the production of ROS and the subsequent activation of MMP-9, both of which are potential TGF-β activators.
Matrix elasticity
Several reports have described the influence of matrix elasticity on TGF-β activation and signalling (69, 70). Wipff et al. reported that mechanical stress activated latent TGF-β produced by cultured myofibroblasts (69). Activation required a stretch-resistant elastic substrate, an integrin (αvβ5) and an organized cytoskeleton. The rigidity of the ECM also alters the response of cells to TGF-β stimulation via the phosphatidylinositol 3-phosphate/Akt signalling pathway, as well as regulating a switch between apoptosis and epithelial mesenchymal transition (71).
Additional lines of evidence have highlighted the effect of microenvironment stiffness on the behaviour of cells, namely that ECM elasticity may modulate latent TGF-β activation. For example, mesenchymal stem cells differentiate into various cell lineages, such as myogenic, neurogenic and osteogenic, according to the stiffness of the culture substrate (72). Similarly, hematopoietic cells cultured on more elastic substrates proliferate more and repopulate better in irradiated recipient mice (73).
Latent TGF-β activating proteases
Several proteases, including plasmin, MMP-2, -9, BMP-1 and plasma kallikrein are latent TGF-β activators (10, 11, 74, 75). These enzymes activate latent TGF-β directly by digesting LAP. Alternatively, proteolytic cleavage can activate latent TGF-β indirectly by digesting ECM molecules and releasing a truncated LLC (8, 76) that is further processed releasing TGF-β.
Peptide–receptor interactions
An interesting role for matrix signalling through the TGF-β type I receptor has been suggested by Garamszegi et al. (77), who described complex formation of integrin β1 and TGF-β type 1 receptor stimulated by collagen, and other ECM peptides and subsequent intracellular signalling. The significance of these findings remains to be understood.
Conclusions and Perspectives
The interplay between multiple ECM components, latent TGF-β complexes and TGF-β activators determines the levels of active TGF-β. Many of these interactions were originally revealed through studies of rare genetic disorders affecting connective tissue, and defects in several of these protein-TGF-β associations may be the basis of the disease pathologies, such as in MFS.
As the number of genetic disorders reported with altered TGF-β levels related to matrix proteins increased, the original model for ECM control of TGF-β activity has become unsatisfactory. In the original scheme latent TGF-β was sequestered in the matrix until needed, at which time matrix perturbations allowed activators to access the latent cytokine and generate mature TGF-β (Fig. 2A). In this vision, impaired matrix sequestration led to failure of latent cytokine activation. Consistent with the hypothesis, loss of SLC binding to LTBP or loss of LTBP-1 or LTBP-3 results in decreased TGF-β levels. However, a number of additional pathological conditions characterized by matrix protein defects result in higher, rather than lower, levels of active TGF-β. This phenomenon was explained by assuming that unanchored latent complexes of TGF-β are inappropriately activated by molecules unavailable to properly sequestered growth factors. Two recent observations indicate that this scheme too is unsatisfactory. The first observation is that a mutant form of FBN-1 (H1Δ), in which the LTBP binding region is deleted and which should, therefore, yield excessive latent TGF-β activation, shows none of the clinical features of MFS (78). The second observation is that LTBP-4 deficiency, in which only the elastin component of the microfibril is perturbed and LLC binding to FBN should be normal, causes an increase in active TGF-β levels (29). We have called these apparently conflicting results a ‘TGF-β paradox.’
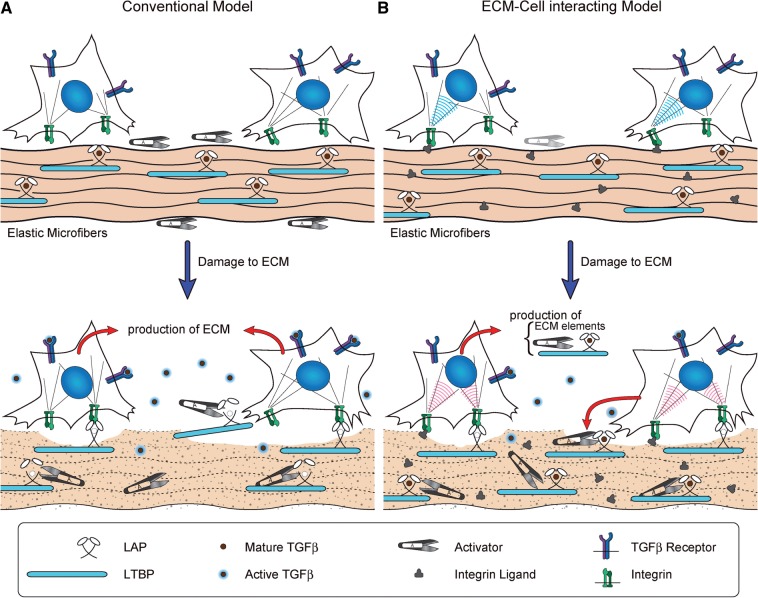
Fig. 2.
Models of the interaction between cells and defective ECM. (A) The conventional model, in which LLCs are sequestered into matrices and protected from activators. Once damage occurs to the ECM, LLCs are exposed and active TGF-β molecules are released. (B) An alternative model, which does not require ready-to-use latent TGF-β activators in the normal state. When the ECM is damaged, surrounding cells detect the alteration through cell-surface integrins, and initiate repair responses producing ECM constituents, SLCs, LLCs, together with their activators.
To resolve this paradox, we propose the model shown in Fig. 2B. Under physiological conditions, latent TGF-β complexes are sequestered, as in the conventional model. However, when ECM integrity is lost by aging, degeneration, inflammation or mutation, mesenchymal cells, such as fibroblasts or smooth muscle cells, detect the defective matrix. The response of the mesenchymal cell is to repair the failed matrix by generating active TGF-β and to produce the required activators of latent TGF-β as part of the repair process (Fig. 2B).
In this model, defects in elastic fibre assembly, such as those observed with LTBP-4 and FBLN-4 deficiencies, have the same effect as defects in microfibres in MFS. We suggest that the abnormalities caused by LTBP-1 and LTBP-3 null mutations reflect either decreased extracellular levels of LLC due to impaired secretion or failure of SLC to be activated. It is unclear why FBLN-5 mutations do not result in heightened levels of TGF-β, as these mutations cause major abnormalities in elastogenesis. We also suggest that the lack of an MFS-like phenotype in H1Δ mice indicates that the mutant FBN-1 is not perceived by the sensing system of the cell as being defective.
Integrins play key roles in cell responses to their environment and many ECM components, such as FBN-1, -2, LTBP-1, -2 and FBLN-5 bear RGD motifs. Therefore, integrins are likely to be the molecules through which cells sense ECM integrity and transmit an intracellular signal. The observation that the FBLN-5 RGE mutation influences the integrin-dependent increase of ROS production supports this notion (67). We propose that differences in integrin activation and/or signalling should be apparent if cells on normal and mutant ECM are compared.
This sensing system allows cells to transmit signals to the cytosol via transmembrane receptors (integrins) in response to alterations in ECM. These signals may be relayed to the nucleus via cytoplasmic structures such as actin–myosin assemblies (79). This explains why mutations in myosin heavy chain and α-smooth muscle actin gene (MYH11 and ACTA2) yield increased levels of active TGF-β (80). Therefore, elastic microfibrils, integrins and the actomyosin cytoskeleton function as an integrated signalling pathway from the ECM to the nucleus.
Over 1000 distinct mutations scattered throughout the FBN1 gene cause MFS. As most of these mutations result in similar phenotypes with enhanced TGF-β signalling, integrin sensing of the altered ECM provides a common mechanism by which defective elastic microfibres promote latent TGF-β activation and/or stimulate expression of TGF-β. In contrast to MFS, mutations of FBN1 that cause stiff skin syndrome, a congenital scleroderma-like condition localized to the dermis and associated with increased TGF-β signalling, are found only in the fourth TGF-β-binding protein-like domain (81); the domain containing the single RGD motif in FBN-1. The difference in stiff skin syndrome and MFS implies that cell-specific differences in interactions with abnormal microfibrils lead to different cellular responses with distinct clinical manifestations. Alternatively, the phenotypes associated with certain other mutations, such as FBN1 mutations that yield Weill–Marchesani syndrome or mutations in FBN1 or ADAMTSL2 (gene of a disintegrin and metalloproteinase with thrombospondin motifs-like 2) that yield Geleophysic Dysplasia may reflect the interactions of additional modifying proteins or signalling molecules.
This model has several advantages over the current model. First, the diversity of the TGF-β response to mutations of ECM molecules can be attributed to the diversity of the responses of mesenchymal cells and other modifying factors. Second, the presence of TGF-β activators in extracellular space does not have to be constitutive. Rather latent TGF-β activators are produced as part of the matrix repair response. Third, this model implies that it will be possible to target the pathways upstream of the activation of latent TGF-β resulting from enhanced matrix repair, thereby providing novel therapeutic strategies for treatments of ECM-related disorders, including not only genetic diseases but also acquired conditions such as osteoarthritis. Further investigations into the crosstalk between matrix and cells from the viewpoint of defective ECM sensing are warranted.
Funding
This work was supported by the National Cancer Institute [CA034282]; National Institute of Arthritis and Musculoskeletal and Skin Diseases [AR049698] and a grant from the National Marfan Foundation to D.B.R.
Conflict of interest
None declared.
Acknowledgements
We apologize for not citing all of relevant references due to space limitations. M.H. was supported by Banyu Life Science Foundation International and The Uehara Memorial Foundation.
Glossary
Abbreviations
- ADAMTSL
ADAMTS (A disintegrin and metalloproteinase with thrombospondin motifs)-like
- ALK5
activin receptor-like kinase 5
- BMP
bone morphogenetic protein
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- FBLN
fibulin
- FBN
fibrillin
- LAP
latency associated peptide
- LLC
large latent complex
- LTBP
latent TGF-β binding protein
- MAPK
mitogen-activated protein kinase
- MFS
Marfan syndrome
- MMP
matrix metalloproteinase
- PAI-1
plasminogen activator inhibitor-1
- ROS
reactive oxygen species
- SLC
small latent complex
- SLRP
small leucine-rich proteoglycan
- SRE
Smad response element
- TGF-β
transforming growth factor-β
References
- 1.Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 2.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J. Invest. Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 3.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 2010;15:169–190. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshinaga K, Obata H, Jurukovski V, Mazzieri R, Chen Y, Zilberberg L, Huso D, Melamed J, Prijatelj P, Todorovic V, Dabovic B, Rifkin DB. Perturbation of transforming growth factor (TGF)-beta1 association with latent TGF-beta binding protein yields inflammation and tumors. Proc. Natl Acad. Sci. USA. 2008;105:18758–18763. doi: 10.1073/pnas.0805411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J. Biol. Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 7.Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- 8.Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J. Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol. Biol. Cell. 2000;11:2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 11.Ge G, Greenspan DS. BMP1 controls TGF beta1 activation via cleavage of latent TGF beta-binding protein. J. Cell Biol. 2006;175:111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J. Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol. Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 14.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 15.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur. J. Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markovics JA, Araya J, Cambier S, Somanath S, Gline S, Jablons D, Hill A, Wolters PJ, Nishimura SL. Interleukin-1beta induces increased transcriptional activation of the transforming growth factor-beta-activating integrin subunit beta8 through altering chromatin architecture. J. Biol. Chem. 2011;286:36864–36874. doi: 10.1074/jbc.M111.276790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todorovic V, Rifkin DB. LTBPs, more than just an escort service. J. Cell. Biochem. 2012;113:410–418. doi: 10.1002/jcb.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todorovic V, Frendewey D, Gutstein DE, Chen Y, Freyer L, Finnegan E, Liu F, Murphy A, Valenzuela D, Yancopoulos G, Rifkin DB. Long form of latent TGF-beta binding protein 1 (Ltbp1L) is essential for cardiac outflow tract septation and remodeling. Development. 2007;134:3723–3732. doi: 10.1242/dev.008599. [DOI] [PubMed] [Google Scholar]
- 21.Todorovic V, Finnegan E, Freyer L, Zilberberg L, Ota M, Rifkin DB. Long form of latent TGF-beta binding protein 1 (Ltbp1L) regulates cardiac valve development. Dev. Dyn. 2011;240:176–187. doi: 10.1002/dvdy.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhary B, Ito Y, Makita T, Sasaki T, Chai Y, Sucov HM. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev. Biol. 2006;289:420–429. doi: 10.1016/j.ydbio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development. 2006;133:4585–4593. doi: 10.1242/dev.02597. [DOI] [PubMed] [Google Scholar]
- 24.Dabovic B, Chen Y, Colarossi C, Obata H, Zambuto L, Perle MA, Rifkin DB. Bone abnormalities in latent TGF-[beta] binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-[beta] bioavailability. J. Cell Biol. 2002;156:227–232. doi: 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colarossi C, Chen Y, Obata H, Jurukovski V, Fontana L, Dabovic B, Rifkin DB. Lung alveolar septation defects in Ltbp-3-null mice. Am. J. Pathol. 2005;167:419–428. doi: 10.1016/S0002-9440(10)62986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, von Melchner H. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koli K, Wempe F, Sterner-Kock A, Kantola A, Komor M, Hofmann WK, von Melchner H, Keski-Oja J. Disruption of LTBP-4 function reduces TGF-beta activation and enhances BMP-4 signaling in the lung. J. Cell Biol. 2004;167:123–133. doi: 10.1083/jcb.200403067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urban Z, Hucthagowder V, Schürmann N, Todorovic V, Zilberberg L, Choi J, Sens C, Brown CW, Clark RD, Holland KE, Marble M, Sakai LY, Dabovic B, Rifkin DB, Davis EC. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am. J. Hum. Genet. 2009;85:593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis EC, Rifkin DB. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. J. Cell. Physiol. 2009;219:14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Courousse T, Sakai LY, Rifkin DB. Specificity of latent TGF-beta binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J. Cell Physiol. 2012;227:3828–3836. doi: 10.1002/jcp.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, McGovern A, Baldock C, Shuttleworth CA, Kielty CM. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta. J. Cell Sci. 2010;123:3006–3018. doi: 10.1242/jcs.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 33.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 34.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Carta L, Ono RN, Sengle G, Arteaga-Solis E, Levasseur R, Ducy P, Sakai LY, Karsenty G, Ramirez F. Fibrillin-1 and -2 differentially modulate endogenous TGF-{beta} and BMP bioavailability during bone formation. J. Cell Biol. 2010;190:1107–1121. doi: 10.1083/jcb.201003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Ramirez F. Extracellular microfibrils control osteoblast-supported osteoclastogenesis by restricting TGF{beta} stimulation of RANKL production. J. Biol. Chem. 2010;285:34126–34133. doi: 10.1074/jbc.M110.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong W, Meisinger T, Knispel R, Worth JM, Baxter BT. MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ. Res. 2012;110:e92–e101. doi: 10.1161/CIRCRESAHA.112.268268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 40.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol. Cell Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLaughlin PJ, Bakall B, Choi J, Liu Z, Sasaki T, Davis EC, Marmorstein AD, Marmorstein LY. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum. Mol. Genet. 2007;16:3059–3070. doi: 10.1093/hmg/ddm264. [DOI] [PubMed] [Google Scholar]
- 43.Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc. Natl Acad. Sci. USA. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, Yanagisawa H. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ. Res. 2010;106:583–592. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuda T, Wu J, Gao E, Joyce J, Markova D, Dong H, Liu Y, Zhang H, Zou Y, Gao F, Miller T, Koch W, Ma X, Chu ML. Loss of fibulin-2 protects against progressive ventricular dysfunction after myocardial infarction. J. Mol. Cell. Cardiol. 2012;52:273–282. doi: 10.1016/j.yjmcc.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- 47.Fontana L, Chen Y, Prijatelj P, Sakai T, Fässler R, Sakai LY, Rifkin DB. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- 48.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J. Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J. Biol. Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hildebrand A, Romarís M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem. J. 1994;302(Pt. 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J. Biol. Chem. 2007;282:18842–18850. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- 52.Ferdous Z, Wei VM, Iozzo R, Höök M, Grande-Allen KJ. Decorin-transforming growth factor-interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J. Biol. Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 53.Heegaard A-M, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115:2731–2738. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 54.Chen XD, Shi S, Xu T, Robey PG, Young MF. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J. Bone Miner. Res. 2002;17:331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- 55.Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, Mabuchi A, Kotani A, Kawakami A, Yamamoto S, Uchida A, Nakamura K, Notoya K, Nakamura Y, Ikegawa S. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat. Genet. 2005;37:138–144. doi: 10.1038/ng1496. [DOI] [PubMed] [Google Scholar]
- 56.Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb. Perspect. Biol. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J. Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 2002;511:65–68. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 59.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J. Clin. Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. The single-molecule mechanics of the latent TGF-beta1 complex. Curr. Biol. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 61.Allison M. Stromedix acquisition signals growing interest in fibrosis. Nat. Biotechnol. 2012;30:375–376. doi: 10.1038/nbt0512-375. [DOI] [PubMed] [Google Scholar]
- 62.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J. Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 64.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 2005;52:2897–2905. doi: 10.1002/art.21246. [DOI] [PubMed] [Google Scholar]
- 65.Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol. Biol. Cell. 1998;9:2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM. Integrin alpha8beta1 mediates adhesion to LAP-TGFbeta1. J. Cell Sci. 2002;115:4641–4648. doi: 10.1242/jcs.00145. [DOI] [PubMed] [Google Scholar]
- 67.Schluterman MK, Chapman SL, Korpanty G, Ozumi K, Fukai T, Yanagisawa H, Brekken RA. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Dis. Model. Mech. 2010;3:333–342. doi: 10.1242/dmm.003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Budatha M, Roshanravan S, Zheng Q, Weislander C, Chapman SL, Davis EC, Starcher B, Word RA, Yanagisawa H. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. J. Clin. Invest. 2011;121:2048–2059. doi: 10.1172/JCI45636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32:3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-beta1-induced apoptosis and epithelial-mesenchymal transition. Mol. Biol. Cell. 2012;23:781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 73.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, Rasko JEJ. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 2010;28:1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 74.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J. Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akita K, Okuno M, Enya M, Imai S, Moriwaki H, Kawada N, Suzuki Y, Kojima S. Impaired liver regeneration in mice by lipopolysaccharide via TNF-alpha/kallikrein-mediated activation of latent TGF-beta. Gastroenterology. 2002;123:352–364. doi: 10.1053/gast.2002.34234. [DOI] [PubMed] [Google Scholar]
- 76.Unsöld C, Hyytiäinen M, Bruckner-Tuderman L, Keski-Oja J. Latent TGF-beta binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J. Cell Sci. 2001;114:187–197. doi: 10.1242/jcs.114.1.187. [DOI] [PubMed] [Google Scholar]
- 77.Garamszegi N, Garamszegi SP, Samavarchi-Tehrani P, Walford E, Schneiderbauer MM, Wrana JL, Scully SP. Extracellular matrix-induced transforming growth factor-beta receptor signaling dynamics. Oncogene. 2010;29:2368–2380. doi: 10.1038/onc.2009.514. [DOI] [PubMed] [Google Scholar]
- 78.Charbonneau NL, Carlson EJ, Tufa S, Sengle G, Manalo EC, Carlberg VM, Ramirez F, Keene DR, Sakai LY. In vivo studies of mutant fibrillin-1 microfibrils. J. Biol. Chem. 2010;285:24943–24955. doi: 10.1074/jbc.M110.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 80.Renard M, Callewaert B, Baetens M, Campens L, Macdermot K, Fryns JP, Bonduelle M, Dietz HC, Gaspar IM, Cavaco D, Stattin EL, Schrander-Stumpel C, Coucke P, Loeys B, De Paepe A, De Backer J. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFbeta signaling in FTAAD. Int. J. Cardiol. 2012 doi: 10.1016/j.ijcard.2011.08.079. doi: 10.1016/j.ijcard.2011.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loeys BL, Gerber EE, Riegert-Johnson D, Iqbal S, Whiteman P, McConnell V, Chillakuri CR, Macaya D, Coucke PJ, De Paepe A, Judge DP, Wigley F, Davis EC, Mardon HJ, Handford P, Keene DR, Sakai LY, Dietz HC. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci. Transl Med. 2010;2:23ra20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]