Abstract
Spinal cord injuries above mid-thoracic levels can lead to a potentially life-threatening hypertensive condition termed autonomic dysreflexia that is often triggered by distension of pelvic viscera (bladder or bowel). This syndrome is characterized by episodic hypertension due to sudden, massive discharge of sympathetic preganglionic neurons in the thoracolumbar spinal cord. This hypertension is usually accompanied by bradycardia, particularly if the injury is caudal to the 2nd to 4th thoracic spinal segments. The development of autonomic dysreflexia is correlated with aberrant sprouting of peptidergic afferent fibers into the spinal cord below the injury. In particular, sprouting of nerve growth factor-responsive afferent fibers has been shown to have a major influence on dysreflexia, perhaps by amplifying the activation of disinhibited sympathetic neurons. Using a model of noxious bowel distension after complete thoracic spinal transection at the 4th thoracic segment in rats, we selectively altered C-fiber sprouting, at specified spinal levels caudal to the injury, with microinjections of adenovirus encoding the growth-promoting nerve growth factor or the growth-inhibitory semaphorin 3A. This was followed by assessment of physiological responses to colorectal distension and subsequent histology. Additionally, anterograde tract tracers were injected into the lumbosacral region to compare the extent of labeled propriospinal rostral projections in uninjured cords to those incords after complete 4th thoracic transection. In summary, over-expression of chemorepulsive semaphorin 3A impeded C-fiber sprouting in lumbosacral segments and mitigated hypertensive autonomic dysreflexia, whereas the opposite results were obtained with nerve growth factor over-expression. Furthermore, compared to naïve rats there were significantly more labeled lumbosacral propriospinal projections rostrally after thoracic injury. Collectively, our findings suggest that distension of pelvic viscera increases the excitation of expanded afferent terminals in the disinhibited lumbosacral spinal cord. This, in turn, triggers excitation and sprouting of local propriospinal neurons to relay visceral sensory stimuli and amplify the activation of sympathetic preganglionic neurons in the thoracolumbar cord, to enhance transmission in the spinal viscero-sympathetic reflex pathway. These responses are manifested as autonomic dysreflexia.
Keywords: nerve growth factor, semaphorin3A, sprouting, sympathetic, neurotrophin, propriospinal, gene therapy
Autonomic dysreflexia is a clinical syndrome that develops following spinal cord injury above the sixth thoracic (T) vertebral level (T6). It is present after complete as well as incomplete injuries (Karlsson, 1999) with an incidence of up to 70% after complete injuries (Snow et al., 1978). The condition is commonly triggered by distension of the pelvic viscera (bowel and bladder) and is manifested by often debilitating hypertension and sweating, dizziness, nausea and often severe headaches (Snow et al., 1978; Lindan et al., 1980; Harati, 1997; Karlsson, 1999). A reflex bradycardia often accompanies these episodes of hypertension, particularly if the injury is caudal to the spinal segments providing sympathetic control of the heart (T1–4). During distension of the bladder and/or bowel the consequent afferent barrage into the dorsal horn of the lumbosacral spinal cord ultimately results in massive sympathetic reflex discharge below the level of the injury. This results in vasoconstriction of the muscular, splanchnic, and cutaneous vascular beds (reviewed in Karlsson, 1999). The resultant paroxysmal hypertension produces a baroreceptor-mediated reflex bradycardia accompanied by withdrawal of sympathetic activity above the lesion level with resultant vasodilatation that produces adverse symptoms such as headaches and skin flushing.
The dysreflexic hypertension stems, in part, from injury-induced loss of tonic control of sympathetic preganglionic neurons in the intermediolateral cell column of the thoracolumbar spinal cord by medullo-spinal neurons in the rostral and caudal ventrolateral medulla (Finestone and Teasell, 1993; Zagon and Smith, 1993). Anatomical and physiological changes that occur in sympathetic preganglionic neurons and sympathetically-related interneurons as descending inputs degenerate following high thoracic spinal cord transection have been the subject of considerable attention (Weaver et al., 1997; Krenz and Weaver, 1998b; Klimaschewski, 2001). Additionally, the influence of injury-induced sprouting of primary afferent fibers into the thoracolumbar spinal cord has been the focus of investigations (Krenz and Weaver, 1998a; Weaver et al., 2001). Although autonomic dyreflexia can be elicited by even non-noxious stimuli below the injury level (Marsh and Weaver, 2004), a model for consistently inducing autonomic dysreflexia employs colorectal distension in spinal injured rats. This model is designed to mimic the common clinical manifestation of noxious fecal impaction (Krassioukov and Weaver, 1995). We have used this preparation to investigate the undefined relationships among visceral sensory afferents, lumbosacral relay neurons, and sympathetic preganglionic neurons that trigger dysreflexic hypertension.
A contributing factor underlying autonomic dysreflexia is the injury-induced elevation in spinal levels of nerve growth factor (Brown et al., 2004) and the subsequent sprouting of calcitonin gene-related peptide (CGRP)+ afferent fibers in the thoracolumbar spinal cord (Krenz and Weaver, 1998a; Krenz et al., 1999; Weaver et al., 2001; Marsh et al., 2002). It is important to note that CGRP immunoreactivity has been reported in Aβ, Aδ and C fiber afferent projections (McCarthy and Lawson, 1990; Lawson et al., 1993; Lawson et al., 1996). Because these CGRP+ fibers also can be labeled with various neurotransmitter markers, the identity of specific afferent fiber populations that sprout after spinal cord injury remains uncertain. For example, it is reported that subpopulations of CGRP+ fibers sprouting distal to spinal cord injury sites are nociceptive primary afferents. This could be the anatomical substrate for the development and maintenance of chronic pain syndromes after spinal cord injury (Ondarza et al., 2003). Conversely, there is evidence for a lack of significant substance P+ fiber sprouting in parallel with increased CGRP+ fiber sprouting in a rat model of autonomic dysreflexia (Marsh and Weaver, 2004). Since substance P content can be used to distinguish subpopulations of unmyelinated C fibers from myelinated Aδ fibers, the authors suggest that sprouting of Aβ and Aδ fibers, and not C fibers, contributed to increased sympathetic outflow after injury. Accordingly, non-noxious cutaneous and colonic stimulation below the injury was also reported to elicit dysreflexic hypertension, albeit to a lesser degree, than with noxious stimulation.
To investigate further the role of nerve growth factor and CGRP+ afferent fiber sprouting in the development of autonomic dysreflexia, we precisely manipulated injury-induced CGRP+ C-fiber sprouting in the dorsal horns with bilateral microinjections of a well-characterized replication-defective, temperature-sensitive recombinant adenovirus encoding growth-promoting nerve growth factor (Romero et al., 2001). The principal goal was to target growth factor over-expression to selected regions of the dorsal gray matter of the spinal cord, caudal to a complete T4 transection, to identify sites instrumental to augmenting dysreflexic responses to colorectal distension two weeks after injury (Maiorov et al., 1998). Once the critical spinal levels were identified, with subsequent immunohistochemical analyses for CGRP+ afferent fiber sprouting (Krenz et al., 1999; Weaver et al., 2001), we used recombinant adenovirus to over-express C-fiber growth-inhibitory semaphorin 3A (Tang et al., 2004) after injury in an attempt to prevent sprouting and mitigate dysreflexic hypertension. Regarding the aforementioned variability in phenotypes of post-traumatic sprouting afferent fibers, Tang et al. (2004) showed that over-expression of nerve growth factor within the spinal cord directly increased sprouting of CGRP+ axons without altering neuropeptide expression or sprouting of other sensory axon populations. These populations included myelinated and unmyelinated, glial cell line-derived neurotrophic factor-responsive subpopulations of nociceptive axons. Moreover, over-expression of semaphorin 3A, a repulsive guidance molecule for nerve growth factor-responsive C-fibers, reduced sprouting of CGRP+ and substance P+ axons compared with over-expression of control green fluorescent protein.
The sympathetic component of the intermediolateral cell column extends from T1 to L2, and primary afferent sprouting has been reported both close to and distant from a T4 transection (Krenz et al.1998a, 1999). We first tested whether increasing primary afferent sprouting just caudal to at T4 transection would augment hypertension evoked by colorectal distension (Cameron et al., 2004). Two weeks after we over-expressed nerve growth factor in the T5/6 dorsal horns (n=5), there was copious central sprouting of CGRP+ primary afferent fibers into the mid-thoracic spinal cord dorsal horns compared to control injured rats injected with green fluorescent protein adenovirus at T5/6 (n=3). Notably, we found that sympathetic preganglionic neurons, pre-labeled with FluoroGold (Anderson and Edwards, 1994), were completely surrounded by sprouting CGRP+ fibers (not shown). Physiological recordings of mean arterial blood pressure and heart rate in response to colorectal distension showed that evoked hypertension was no greater compared to injured controls (Figure 2), even though nerve growth factor induced robust afferent fiber sprouting into the intermediolateral cell column. Since this implied that neither the actions of nerve growth factor nor sprouting CGRP+ fibers directly influenced the sympathetic discharge in response to colorectal distension, we chose to similarly manipulate injury-induced sprouting at more caudal levels, based on the evidence that sensory input from the pelvic viscera enter the spinal cord caudal to T5/6.
Figure 2.
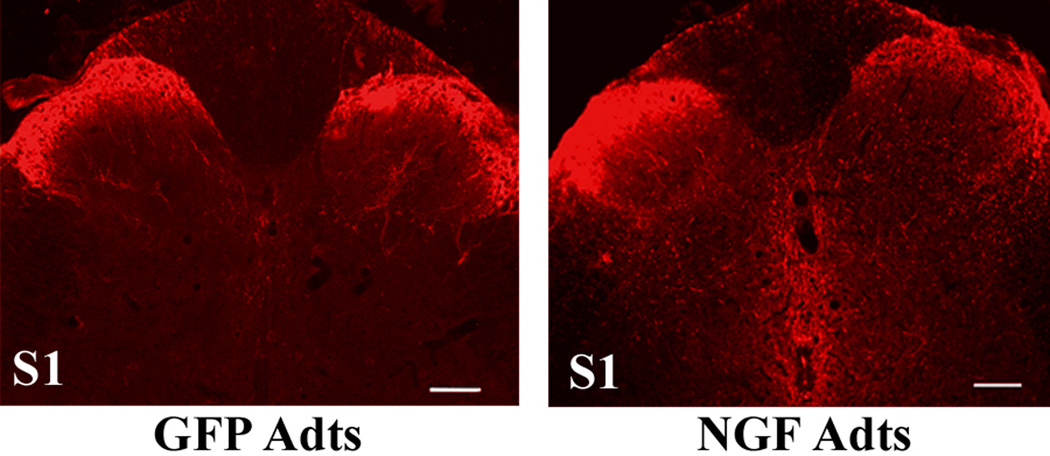
Over-expression of nerve growth factor (NGF) in lumbosacral spinal levels augments calcitonin gene-related peptide (CGRP)+ afferent fiber sprouting 2 weeks post injury. Photomicrographs showing CGRP+ staining in S1 spinal segments injected with adenovirus expressing control green fluorescent protein (GFP Adts - left column) or NGF Adts (right column). Note the robust CGRP+ afferent fiber sprouting throughout the S1 injection site in response to NGF over-expression compared to GFP controls. Scale bars = 100 µm.
Electrophysiological (Al-Chaer et al., 1997) and anatomical studies (Birder et al., 1991; Keast and De Groat, 1992; Vizzard, 2000) report that the primary afferents supplying the pelvic viscera (bladder, distal colon, rectum) in rats run in the pelvic nerve and distribute mainly to the L6 and S1 dorsal root ganglia and spinal cord segments. A smaller percentage of afferents run in the hypogastric nerve to the T13 and L1 dorsal root ganglia and spinal cord segments, and neurons responsive to colorectal distension are located in the superficial dorsal horns of the T13-L2 spinal segments (Ness and Gebhart, 1988; Ness and Gebhart, 1989). Within the L6/S1 spinal regions, however, lamina X and V-VIII contain most of the neurons responding to visceral stimulation (Al-Chaer et al., 1997). These projection neurons respond physiologically to colorectal distension (Martinez et al., 1998; Landrum et al., 2002), as well as to cutaneous stimulation.
Two groups of lumbosacral spinal interneurons relay visceral information rostrally to supraspinal targets, each through a separate pathway. One group of neurons resides primarily in the dorsal commissure of L6/S1 segments (Willis et al., 1999; Vizzard et al., 2000) and projects axons in the medial part of the dorsal columns (Hirshberg et al., 1996; Wang et al., 1999). Most of these neurons respond physiologically to colorectal distension and their axons terminate in the medullary gracile nucleus (Al-Chaer et al., 1996; Al-Chaer et al., 1999). The other group of neurons lies in both the dorsal commissure and lateral parasympathetic nucleus and their axons project through the ventrolateral white matter to the thalamus (Ness and Gebhart, 1987). These neurons are activated by colorectal distension as well as somatic (cutaneous) stimulation.
Propriospinal neurons connect lamina X/dorsal commissure at multiple rostro-caudal levels of the cord (Petkó and Antal, 2000) and injections of anterograde tract tracers into lamina X/dorsal commissure in the lumbosacral segments (L6-S2) labels terminals in lamina X throughout the cord up to cervical levels (Matsushita, 1998). These studies show that long-ranging propriospinal neurons exist in the dorsal commissure/deep dorsal horn of the lumbosacral spinal cord. Such relays extending the entire length of the cord could potentially send off collaterals after spinal cord injury and influence the activity of sympathetic preganglionic neurons in the intermediolateral cell column. Since the number of activated neurons expressing c-Fos in response to colorectal distension is much greater after a chronic spinal cord transection than after an acute injury (Landrum et al., 2002), it suggests that larger numbers of lumbosacral propriospinal neurons can be activated by visceral stimuli following injury. In view of these findings and our preliminary results, we reasoned that autonomic dysreflexia may be mediated primarily by visceral afferent activation of propriospinal interneurons that project from the lumbosacral to the thoracic spinal cord.
Therefore, we injected T4 spinal-transected rats with adenovirus encoding nerve growth factor into the T13/L1 (n=8) or L6/S1 (n=9) segments versus green fluorescent protein controls (n=7 per level) to augment sprouting specifically and, potentially, to augment dysreflexic hypertension (Cameron et al., 2004). After two weeks of nerve growth factor over-expression within either caudal spinal level, hypertension induced by colorectal distension was greater in magnitude (~35 mm Hg) than green fluorescent protein-injected injured controls (~20 mm Hg) (Figure 1). Conversely, L6/S1 over-expression of semaphorin 3A, a chemorepulsive factor for nerve growth factor-responsive primary afferent fibers, led to a marked reduction in evoked hypertension compared to cord-injured controls (~10 mm Hg) (not shown).
Figure 1.

Physiological responses to colorectal distension (CRD) two weeks after T4 spinal transection. Illustrative traces of pulsatile arterial pressure (PAP), mean arterial pressure (MAP) and heart rate (HR) before, during and after one minute CRD with rectal balloon catheter inflation (indicated by arrows) from injured rats with bilateral L6/S1 injections of (A) adenovirus expressing control green fluorescent protein (GFP Adts) or (B) nerve growth factor (NGF Adts). Note that both injured groups show autonomic dysreflexia with hypertension accompanied by bradycardia, but the severity of hypertension is almost two-fold greater and more prolonged with nerve growth factor over-expression.
Subsequent histological processing of the lumbosacral spinal cord confirmed that over-expression of nerve growth factor in the S1 segment elicited profuse sprouting of CGRP+ fibers two weeks after T4 cord transection compared to modest sprouting in response to control green fluorescent protein over-expression (Figure 2). In contrast, over-expression of semaphorin 3A in the S1 segment caused diminished sprouting compared to controls (not shown). Linear regression analysis of percent CGRP+ fiber area covered in the dorsal horns plotted against reflex-induced hypertensive changes revealed a positive correlation between the extent of C-fiber sprouting into the lumbosacral spinal level and the severity of autonomic dysreflexia among the three treatment groups (Cameron et al., 2004). This suggests that, in this model of autonomic dysreflexia induced by noxious colorectal distension, nerve growth-factor induced sprouting of primary afferents in the region where colonic afferents enter the spinal cord causes abnormal activation of sympathetic preganglionic neurons in the thoracolumbar spinal cord via putative propriospinal pathways (Matsushita, 1998; Petkó and Antal, 2000).
These results are consistent with a nerve growth factor-dependent increase in the number of primary afferent terminals. This may amplify the synaptic action of colonic afferents, during colorectal distension, on lumbosacral dorsal horn neurons that project axons rostrally in the gray matter and dorsal columns (Wang et al., 1999; Willis et al., 1999) and may connect with, and influence the activity of preganglionic sympathetic neurons in the intermediolateral cell column (Llewellyn-Smith and Weaver, 2001). Intrathecal delivery of nerve growth factor antibody following spinal cord injury reduces the elevated nerve growth factor protein levels in L6 and S1 dorsal root ganglia and spinal cord of cord-injured rats (Seki et al., 2002). It seems likely that nerve growth factor-mediated plasticity of CGRP+ afferents causes hyperactivity of lumbosacral propriospinal neurons and, as a result, excitation of sympathetically-correlated interneurons in the intermediolateral cell column. In support of this, colorectal distension in acute and chronic spinal transected rats activates sympathetically-correlated neurons in the T10 segment, most likely via sacral-thoracic interneurons (Chau et al., 2000; Krassioukov et al., 2002).
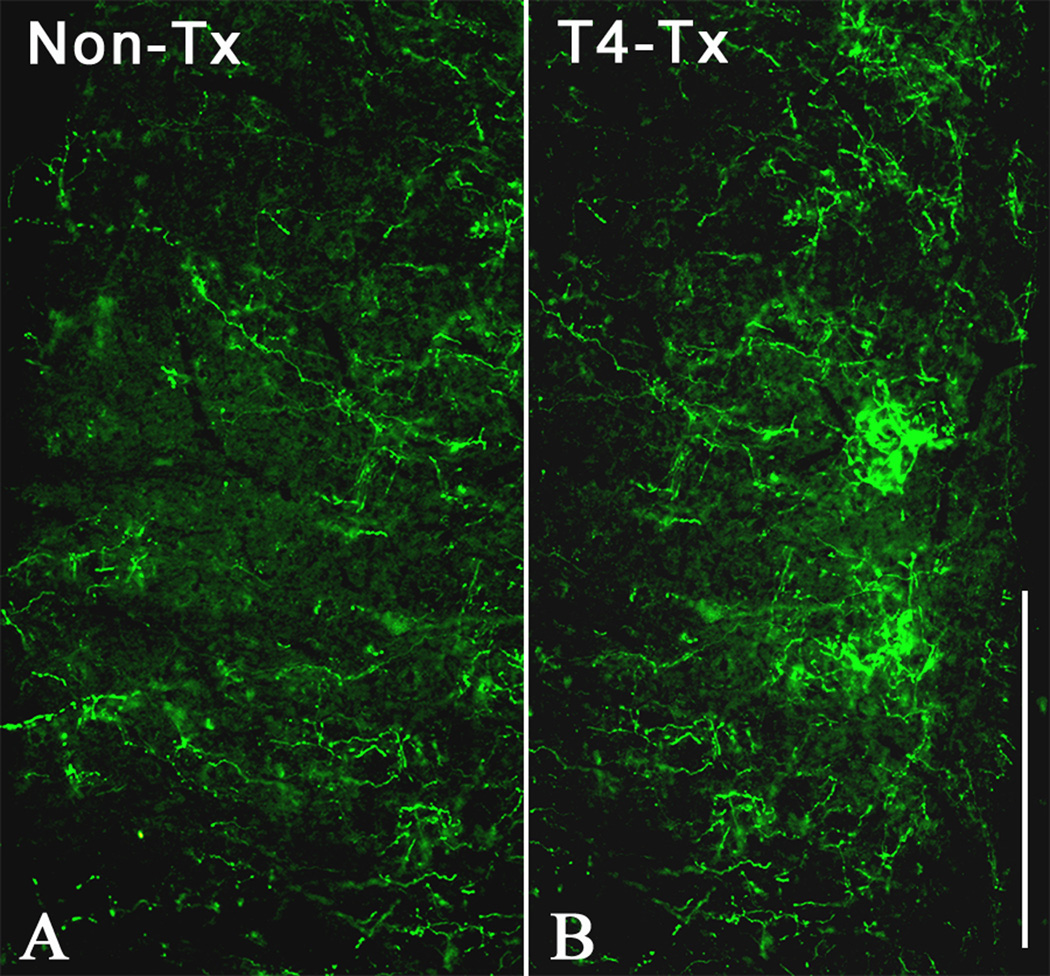
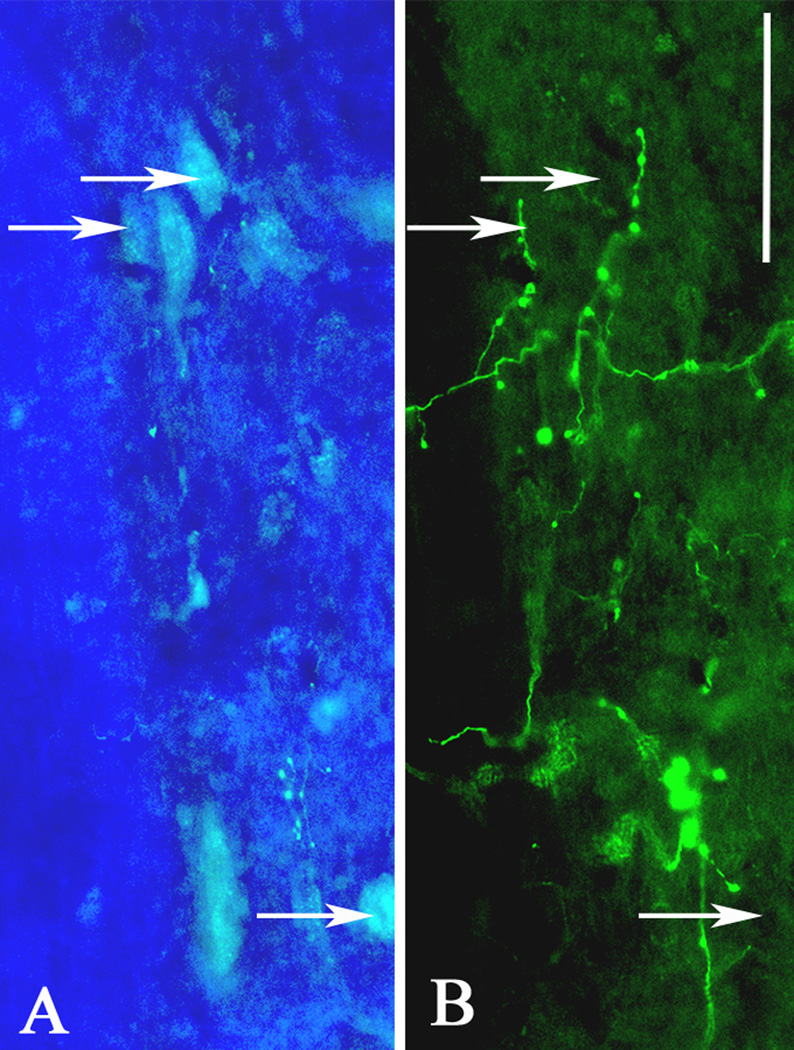
We investigated the potential influence of intraspinal sprouting of the axons of lumbosacral projection interneurons for activating sympathetically-correlated interneurons after complete high thoracic spinal cord injury. To do this we injected the anterograde tracer biotinylated dextran amine (BDA) into the S1 dorsal horn of acute T4 spinal-transected animals and to uninjured controls (Cameron et al., 2004). Two weeks later, there were conspicuously more labeled ipsilateral (and contralateral) propriospinal projections in the rostral thoracic gray matter in the injured spinal cords (Figure 3). In fact, many of these projections were found in proximity to FluoroGold-labeled sympathetic preganglionic neurons (Figure 4). Additionally, we found that transection of the L5 spinal cord segment alone, or in addition to T4, markedly reduced colorectal distension-induced hypertension. This suggests that relay neurons arising in the L6/S1 segments are necessary for increased hypertension elicited by pelvic visceral distension. Moreover, transection of the T11 cord segment above the entry of the visceral afferents in the hypogastric nerve, alone or in addition to T4 transection, produced a colorectal distension-induced rise in mean arterial pressure with the same magnitude as T4- and L5-transected animals (~10 mmHg above baseline) (Cameron et al., 2004). This finding indicates that the upper lumbar sympathetic neurons, that are known to constrict hindlimb and visceral blood vessels (Baron et al., 1985; Bahr et al., 1986), are not sufficient to induce significant dysreflexic hypertension. However, activation of the entire sympathetic column after injury does not appear necessary for eliciting modest hypertension during distension of the pelvic viscera.
Figure 3.
Injections of biotinylated dextran amine (BDA) tracer into L6/S1 spinal levels label more ascending projections after T4 transection. Longitudinal, horizontal sections at thoracolumbar spinal levels of (A) non-transected or (B) T4-transected rats approximately 5 mm rostral to 200 nl injections of BDA into the left L6/S1 dorsal commissure. After 2 weeks to allow tracer transport and verify physiologically that the injured rats had developed autonomic dysreflexia, histological processing revealed significantly more BDA-labeled projections (data not shown) within ipsilateral gray matter extending to rostral levels of the thoracic spinal cord. Scale bar = 0.5 mm
Figure 4.
High magnification, dual immunofluorescent images at the mid-thoracic spinal leel two weeks after T4 transection demonstrate close proximity of (A) FluoroGold-labeled sympathetic preganglionic neurons (arrows) in the intermediolateral cell column and (B) biotinylated dextran amine (BDA)-labeled fibers originating from lumbosacral projection interneurons. Scale bar = 50 µm.
Notably, experimental autonomic dysreflexia was not completely eliminated by impeding CGRP+ fiber sprouting with semaphorin3A over-expression, similar to previous findings in which endogenous nerve growth factor was immunologically neutralized after complete T4 spinal transection (Krenz et al., 1999; Marsh et al., 2002). Since the high affinity nerve growth factor trkA receptor is located on cholinergic propriospinal neurons in the deep dorsal horn of the rat spinal cord (Michael et al., 1997), it is possible that, in addition to altering primary afferent plasticity, spinal cord injury provokes both nerve growth factor-dependent and -independent reorganization of propriospinal pathways as well. Such changes may provide a neural substrate for the amplification of minor sensory signals entering the spinal cord, resulting in the synchronous discharge of the preganglionic sympathetic column.
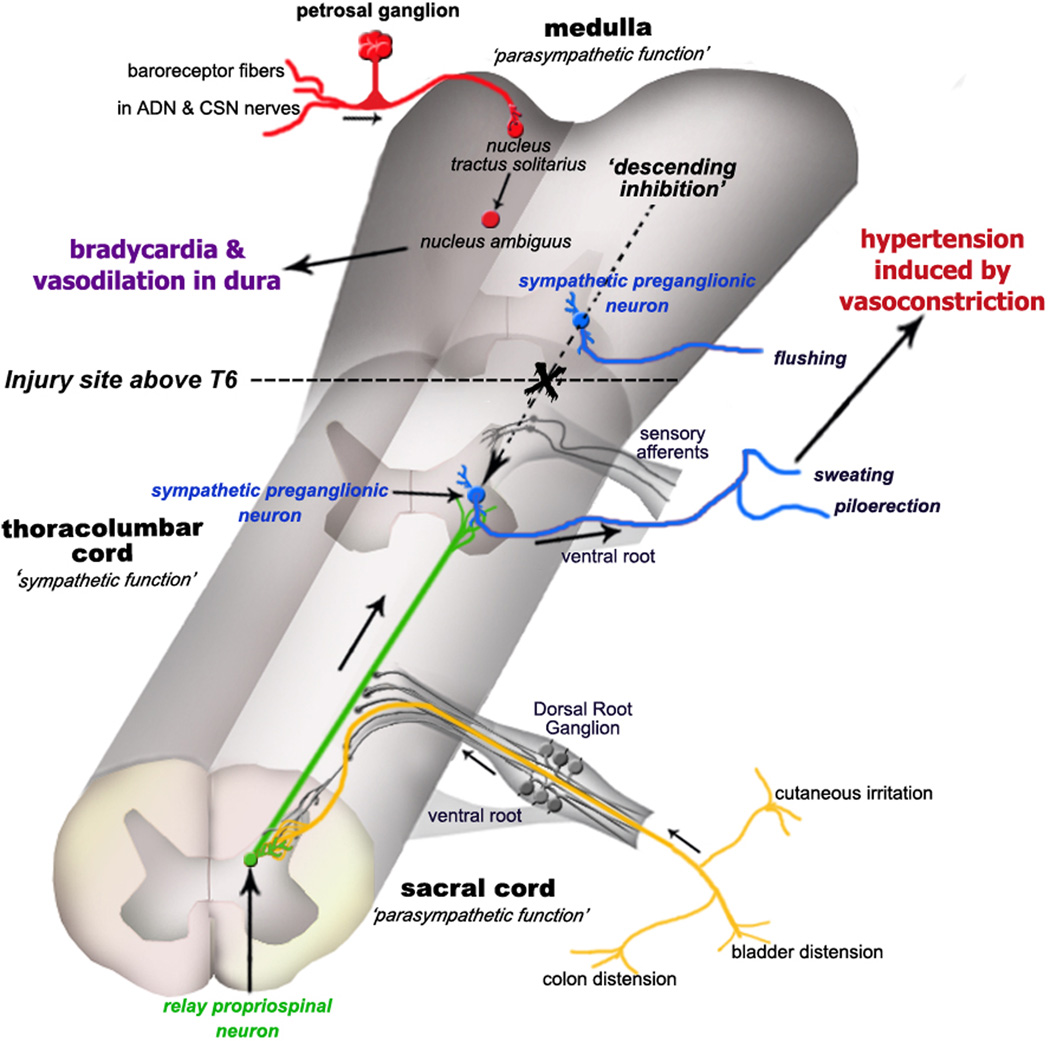
In our attempts to identify neuronal substrates for the autonomic hyperactivity after complete spinal cord injury, we have proposed a model (Cameron et al., 2004) which involves the sprouting of CGRP+ visceral primary afferent C-fibers and of the axons of lumbosacral projection interneurons (Figure 5). We hypothesize that increased nerve growth factor-mediated sprouting of sacral nociceptive C-fibers triggered by complete thoracic spinal cord transection (de Groat et al., 1990) drives larger numbers of propriospinal projection neurons located in the dorsal intercommissural nucleus and amplifies the information relayed from colonic afferents during colorectal distension to lumbosacral dorsal horn neurons. These, in turn, convey the signal to rostral sympathetically-correlated neurons (Chau et al., 2000; Tang et al., 2003) that activate the sympathetic preganglionic neurons to elicit hypertension (Figure 5). Since larger numbers of lumbosacral propriospinal neurons are activated by visceral stimuli in more chronic stages of spinal cord injury (Landrum et al., 2002), this implies that acute functional reorganization occurs which results in signal amplification by spinal circuitry that has presumably been modified as a result of the injury. As a result, following acute thoracic spinal cord transaction, multiple neuronal networks in the thoracolumbar cord generate sympathetic activity upon activation from as yet unidentified neurons in the L6/S1 segments of the cord. The ascending terminals of propriospinal neurons may also undergo morphologic changes including the aberrant convergence and formation of synapses with sympathetically-correlated neurons or sympathetic preganglionic neurons in the intermediolateral cell column (Weaver et al., 1997; Llewellyn-Smith and Weaver, 2001). In various ways the pelvic visceral signal may be conveyed to the ultimate vasoconstrictor postganglionic sympathetic neurons to induce dysreflexia (Figure 5).
Figure 5.
Schematic illustration depicting the etiology of autonomic dysreflexia evoked by pelvic visceral distension and other stimuli. Following complete spinal cord injury above the T6 level, sympathetic preganglionic neurons (blue) are released from descending medullo-spinal control (dashed arrow) and autonomic spinal reflexes are rendered hyperactive. Consequently, pelvic visceral sensory input (yellow) is relayed by propriospinal neurons (green) projecting from the dorsal gray commissure at the lumbosacral level to sympathetic preganglionic neurons and/or sympathetically-correlated interneurons located in the thoracolumbar cord (blue). Post-traumatic C-fiber sprouting into the lumbosacral cord (yellow) further amplifies the central signals (green) to elicit hypertension, ultimately causing profound peripheral vasoconstriction of splanchnic, muscle and cutaneous vascular beds. Subsequent stimulation of aortic depressor nerve (ADN) and carotid sinus nerve (CSN) baroreceptor afferents of the petrosal ganglion (red) is conveyed to the nucleus tractus solitarius that elicits bradycardia via activation of the nucleus ambiguus. Note that flushing is likely the result of inhibition of skin vasoconstrictor preganglionic neurons above the lesion by the baroreceptor reflex, and not due to the activation of sympathetic preganglionic neurons below the lesion by the dysreflexia-inducing stimulus.
Conclusion
Further studies with gene delivery, and perhaps pharmacological agents that mimic plasticity-altering agents, are needed to clarify the relative contribution of changes in primary afferent versus interneuronal/propriospinal systems to autonomic dysreflexia. Such approaches may also address intraspinal plasticity in other forms of dysfunction and/or recovery after spinal cord injury, such as chronic pain and locomotion. These results are the first demonstration that the remodeling of endogenous circuitry, which may underlie the development of autonomic dysreflexia, can be altered by genetic manipulation of axon guidance and inhibitory molecules to modulate plasticity-induced autonomic pathophysiology after complete spinal cord injury.
Acknowledgements
The author thanks Dr. Adrian A. Cameron for contributions to the text and Drs. David C. Randall and George M. Smith for critical review. Additionally, Kainath Durre, Johnna Shipp, Igor Voskresensky and Leslie Schwindel are appreciated for expert technical assistance. AGR is supported by grants from the International Spinal Research Trust, the Kentucky Spinal Cord and Head Injury Research Trust, and the NIH/NINDS (R01 NS-049901-01).
References
- Al-Chaer ED, Westlund KN, Willis WD. Sensitization of postsynaptic dorsal column neuronal responses by colon inflammation. Neuroreport. 1997;8:3267–3273. doi: 10.1097/00001756-199710200-00016. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Feng Y, Willis WD. Comparative study of viscerosomatic input onto postsynaptic dorsal column and spinothalamic tract neurons in the primate. J. Neurophysiol. 1999;82:1876–1882. doi: 10.1152/jn.1999.82.4.1876. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J. Neurophysiol. 1996;76:2675–2690. doi: 10.1152/jn.1996.76.4.2675. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Edwards SL. Intraperitoneal injections of Fluorogold reliably labels all sympathetic preganglionic neurons in the rat. J. Neurosci. Methods. 1994;53:137–141. doi: 10.1016/0165-0270(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Bahr R, Bartel B, Blumberg H, Janig W. Functional characterization of preganglionic neurons projecting in the lumbar splanchnic nerves: vasoconstrictor neurons. J. Auton. Nerv. Syst. 1986;15:131–140. doi: 10.1016/0165-1838(86)90009-3. [DOI] [PubMed] [Google Scholar]
- Baron R, Janig W, McLachlan EM. The afferent and sympathetic components of the lumbar spinal outflow to the colon and pelvic organs in the cat. III. The colonic nerves, incorporating an analysis of all components of the lumbar prevertebral outflow. J. Comp. Neurol. 1985;238:158–168. doi: 10.1002/cne.902380204. [DOI] [PubMed] [Google Scholar]
- Birder LA, Roppolo JR, Iadarola MJ, de Groat WC. Electrical stimulation of visceral afferent pathways in the pelvic nerve increases c-fos in the rat lumbosacral spinal cord. Neurosci. Lett. 1991;129:193–196. doi: 10.1016/0304-3940(91)90459-7. [DOI] [PubMed] [Google Scholar]
- Brown A, Ricci MJ, Weaver LC. NGF message and protein distribution in the injured rat spinal cord. Exp. Neurol. 2004;188:115–127. doi: 10.1016/j.expneurol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of afferent fiber sprouting following spinal cord injury modulates the severity of autonomic dysreflexia. J. Neurotrauma. 2004;21:1271. [Google Scholar]
- Chau D, Johns DG, Schramm LP. Ongoing and stimulus-evoked activity of sympathetically correlated neurons in the intermediate zone and dorsal horn of acutely spinalized rats. J. Neurophysiol. 2000;83:2699–2707. doi: 10.1152/jn.2000.83.5.2699. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst. 1990;30(Suppl):S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- Finestone HM, Teasell RW. Autonomic dysreflexia after brainstem tumor resection. A case report. Am. J. Phys. Med. Rehabil. 1993;72:395–397. [PubMed] [Google Scholar]
- Harati Y. Autonomic disorders associated with spinal cord injury. In: Low PA, editor. Clinical autonomic disorders. 2nd Edition. Philadelphia: Lippincott-Raven; 1997. pp. 455–461. [Google Scholar]
- Hirshberg RM, Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Is there a pathway in the posterior funiculus that signals visceral pain? Pain. 1996;67:291–305. doi: 10.1016/0304-3959(96)03127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37:383–391. doi: 10.1038/sj.sc.3100867. [DOI] [PubMed] [Google Scholar]
- Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J. Comp. Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L. Increased innervation of rat preganglionic sympathetic neurons by substance P containing nerve fibers in response to spinal cord injury. Neurosci. Lett. 2001;307:73–76. doi: 10.1016/s0304-3940(01)01922-x. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Weaver LC. Episodic hypertension due to autonomic dysreflexia in acute and chronic spinal cord-injured rats. Am. J. Physiol. 1995;268:H2077–H2083. doi: 10.1152/ajpheart.1995.268.5.H2077. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Johns DG, Schramm LP. Sensitivity of sympathetically correlated spinal interneurons, renal sympathetic nerve activity, and arterial pressure to somatic and visceral stimuli after chronic spinal injury. J. Neurotrauma. 2002;19:1521–1529. doi: 10.1089/089771502762300193. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998a;85:443–458. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Changes in the morphology of sympathetic preganglionic neurons parallel the development of autonomic dysreflexia after spinal cord injury in rats. Neurosci. Lett. 1998b;243:61–64. doi: 10.1016/s0304-3940(98)00101-3. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J. Neurosci. 1999;19:7405–7414. doi: 10.1523/JNEUROSCI.19-17-07405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrum LM, Jones SL, Blair RW. The expression of Fos-labeled spinal neurons in response to colorectal distension is enhanced after chronic spinal cord transection in the rat. Neuroscience. 2002;110:569–578. doi: 10.1016/s0306-4522(01)00548-6. [DOI] [PubMed] [Google Scholar]
- Lawson SN, McCarthy PW, Prabhakar E. Electrophysiological properties of neurones with CGRP-like immunoreactivity in rat dorsal root ganglia. J. Comp. Neurol. 1996;365:355–366. doi: 10.1002/(SICI)1096-9861(19960212)365:3<355::AID-CNE2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res. Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- Lindan R, Joiner E, Freehafer AA, Hazel C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia. 1980;18:285–292. doi: 10.1038/sc.1980.51. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Weaver LC. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J. Comp. Neurol. 2001;435:226–240. doi: 10.1002/cne.1204. [DOI] [PubMed] [Google Scholar]
- Maiorov DN, Fehlings MG, Krassioukov AV. Relationship between severity of spinal cord injury and abnormalities in neurogenic cardiovascular control in conscious rats. J. Neurotrauma. 1998;15:365–374. doi: 10.1089/neu.1998.15.365. [DOI] [PubMed] [Google Scholar]
- Marsh DR, Weaver LC. Autonomic dysreflexia, induced by noxious or innocuous stimulation, does not depend on changes in dorsal horn substance p. J. Neurotrauma. 2004;21:817–828. doi: 10.1089/0897715041269605. [DOI] [PubMed] [Google Scholar]
- Marsh DR, Wong ST, Meakin SO, MacDonald JI, Hamilton EF, Weaver LC. Neutralizing intraspinal nerve growth factor with a trkA-IgG fusion protein blocks the development of autonomic dysreflexia in a clip-compression model of spinal cord injury. J. Neurotrauma. 2002;19:1531–1541. doi: 10.1089/089771502762300201. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Mayer E, Tache Y. Proximal colon distention increases Fos expression in the lumbosacral spinal cord and activates sacral parasympathetic NADPHd-positive neurons in rats. J. Comp. Neurol. 1998;390:311–321. [PubMed] [Google Scholar]
- Matsushita M. Ascending propriospinal afferents to area X (substantia grisea centralis) of the spinal cord in the rat. Exp. Brain Res. 1998;119:356–366. doi: 10.1007/s002210050351. [DOI] [PubMed] [Google Scholar]
- McCarthy PW, Lawson SN. Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience. 1990;34:623–632. doi: 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Kaya E, Averill S, Rattray M, Clary DO, Priestley JV. TrkA immunoreactive neurones in the rat spinal cord. J. Comp. Neurol. 1997;385:441–455. [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J. Neurophysiol. 1987;57:1867–1892. doi: 10.1152/jn.1987.57.6.1867. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13-L2 spinal cord of the rat. J. Neurophysiol. 1988;60:1419–1438. doi: 10.1152/jn.1988.60.4.1419. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of superficial T13-L2 dorsal horn neurons encoding for colorectal distension in the rat: comparison with neurons in deep laminae. Brain Res. 1989;46:301–309. doi: 10.1016/0006-8993(89)90516-7. [DOI] [PubMed] [Google Scholar]
- Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp. Neurol. 2003;184:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Petkó M, Antal M. Propriospinal afferent and efferent connections of the lateral and medial areas of the dorsal horn (laminae I–IV) in the rat lumbar spinal cord. J. Comp. Neurol. 2000;422:312–325. doi: 10.1002/(sici)1096-9861(20000626)422:2<312::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J. Neurosci. 2001;21:8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J. Urol. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- Snow JC, Sideropoulos HP, Kripke BJ, Freed MM, Shah NK, Schlesinger RM. Autonomic hyperreflexia during cystoscopy in patients with high spinal cord injuries. Paraplegia. 1978;15:327–332. doi: 10.1038/sc.1977.49. [DOI] [PubMed] [Google Scholar]
- Tang X, Neckel ND, Schramm LP. Locations and morphologies of sympathetically correlated neurons in the T(10) spinal segment of the rat. Brain Res. 2003;976:185–193. doi: 10.1016/s0006-8993(03)02601-5. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J. Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Brisson M, de Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res. 2000;299:9–26. doi: 10.1007/s004419900128. [DOI] [PubMed] [Google Scholar]
- Wang CC, Willis WD, Westlund KN. Ascending projections from the area around the spinal cord central canal: A Phaseolus vulgaris leucoagglutinin study in rats. J. Comp. Neurol. 1999;415:341–367. doi: 10.1002/(sici)1096-9861(19991220)415:3<341::aid-cne3>3.0.co;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LC, Cassam AK, Krassioukov AV, Llewellyn-Smith IJ. Changes in immunoreactivity for growth associated protein-43 suggest reorganization of synapses on spinal sympathetic neurons after cord transection. Neuroscience. 1997;81:535–551. doi: 10.1016/s0306-4522(97)00151-6. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J. Neurotrauma. 2001;18:1107–1119. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proc. Natl. Acad. Sci. U S A. 1999;96:7675–7679. doi: 10.1073/pnas.96.14.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon A, Smith AD. Monosynaptic projections from the rostral ventrolateral medulla oblongata to identified sympathetic preganglionic neurons. Neuroscience. 1993;54:729–743. doi: 10.1016/0306-4522(93)90243-9. [DOI] [PubMed] [Google Scholar]