Abstract
We describe a 49-year-old Japanese woman with cutaneous squamous cell carcinoma (SCC) developing from recessive dystrophic epidermolysis bullosa (RDEB). Interestingly, immunohistochemical staining revealed dense infiltration of CD163+ M2 macrophages and numerous Foxp3+ regulatory T cells (Tregs) around the tumor. Since the contribution of immunosuppressive factors (e.g. TGFβ) to the carcinogenesis of SCC from RDEB was recently reported, our present findings suggest one of the possible contributions of immunosuppressive cells, such as CD163+ M2 macrophages and Tregs, to the carcinogenesis of SCC from RDEB.
Key Words: Recessive dystrophic epidermolysis bullosa, Squamous cell carcinoma, Immunosuppressive cells, Carcinogenesis
Introduction
Recessive dystrophic epidermolysis bullosa (RDEB) is a hereditary skin disorder with a high risk of developing aggressive squamous cell carcinoma (SCC) [1, 2]. Indeed, over two-thirds of RDEB patients eventually die from SCC [1]. However, the precise mechanism by which SCC develops from RDEB is still unclear. Recently, Knaup et al. [3] reported the contribution of immunosuppressive factors (e.g. TGFβ) to the carcinogenesis of SCC from RDEB. In this report, we describe a patient with SCC developing from RDEB and investigate the profiles of tumor-infiltrating cells, especially focusing on immunosuppressive cells.
Case Presentation
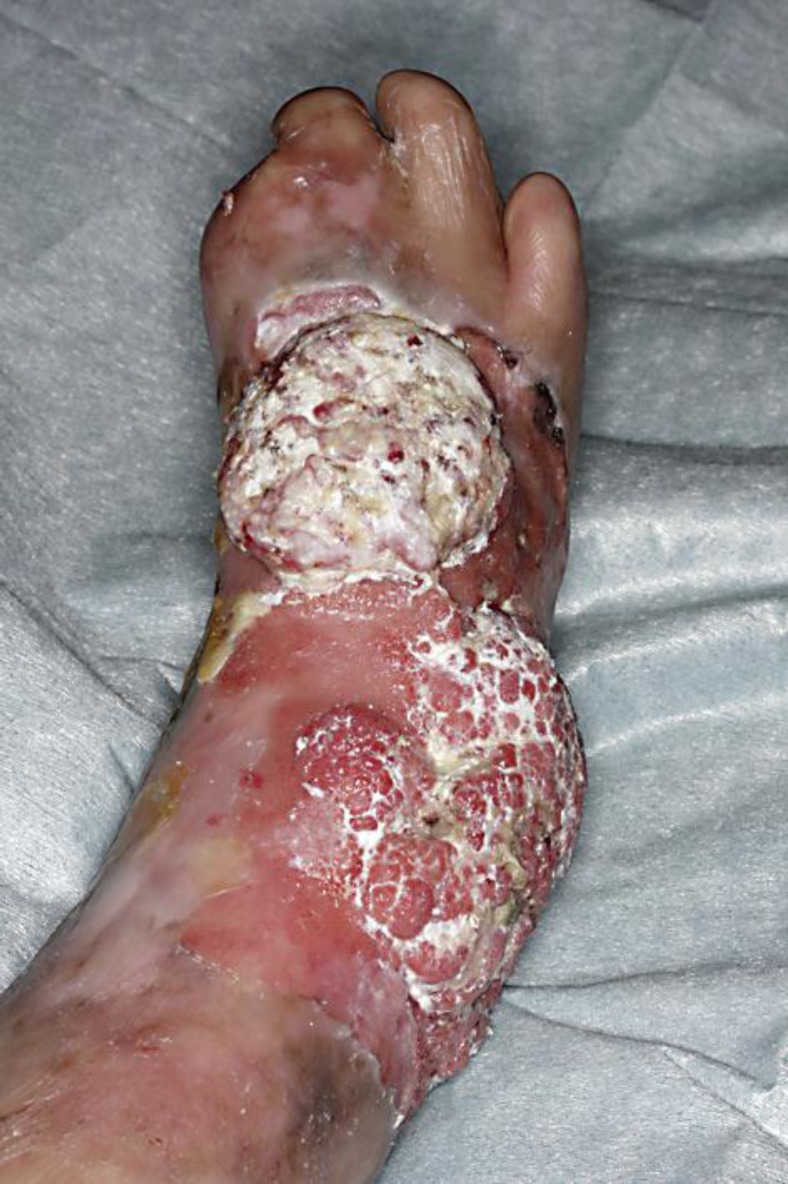
A 49-year-old woman consulted us for a 2-year history of a bleeding nodule on her leg. She had been treated for RDEB for 30 years. On her initial visit, physical examination revealed dome-shaped, 20 × 10 mm and 20 × 20 mm, easy-to-bleed nodules on her right leg (fig. 1). The biopsy specimen revealed that the invasive cell mass extended into the reticular dermis (fig. 2a). The tumor-composing cells were sheets of small cells with hyperchromatic nuclei and individual keratinization (fig. 2b). The SCC antigen was increased (14.5 ng/dl). From the above findings, we diagnosed this patient as developing SCC from RDEB. We excised the tumor with a 2-cm margin. We screened for possible metastatic lesions with positron emission tomography and found no evidence of metastasis.
Fig. 1.
Dome-shaped, 20 × 10 mm and 20 × 20 mm, easy-to-bleed nodules on the right leg.
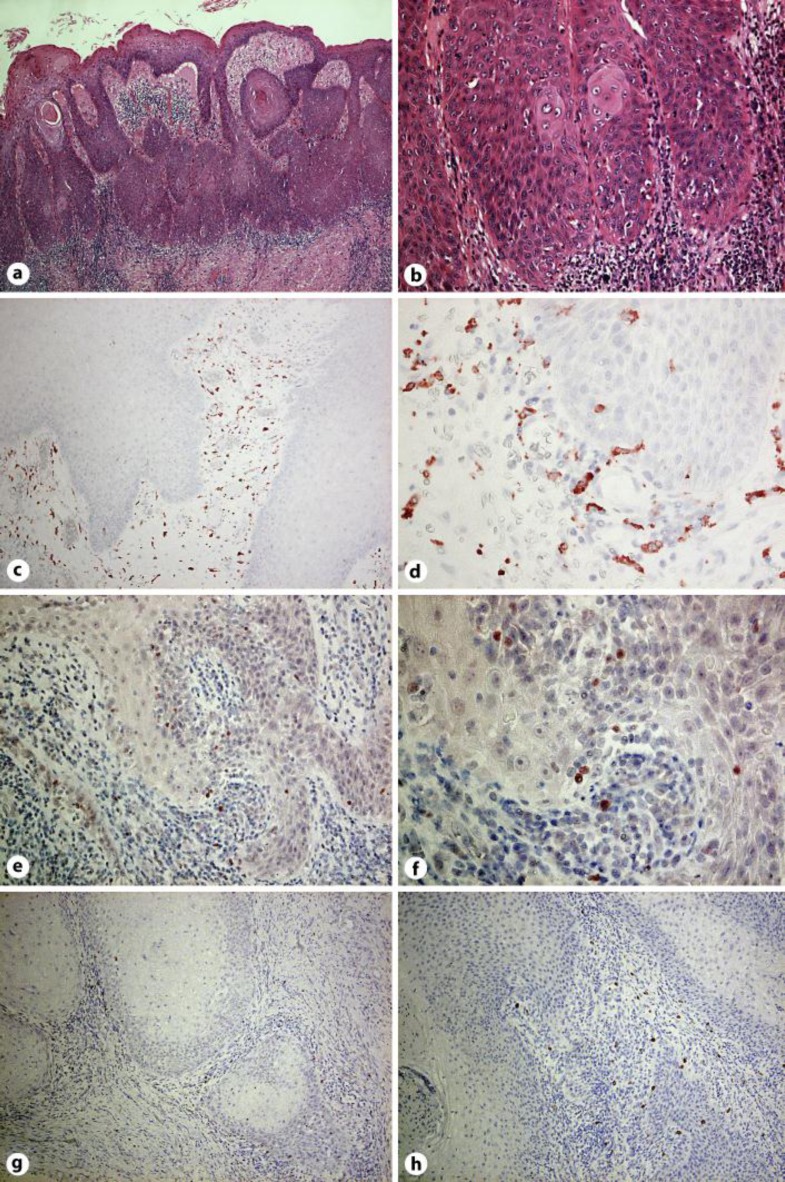
Fig. 2.
Invasive cell mass extends into the reticular dermis (a). Tumor-composing cells were sheets of small cells with hyperchromatic nuclei and individual keratinization (b). Paraffin-embedded tissue samples from the patient were stained as follows: the sections were developed with new fuchsin for CD163 (c, d), Foxp3 (e, f), granulysin (g) and IL-17 (h). Original magnification ×100 (a, c, g), ×200 (e, h) and ×400 (b, d, f).
To evaluate the profiles of infiltrating cells in the tumor area, we employed immunohistochemical staining for Foxp3 and CD163, as well as CD8, granulysin and IL-17. CD163+ macrophages (fig. 3c, d) and Foxp3high+ regulatory cells (fig. 3e, f) were densely infiltrated around the tumor. Dense infiltration of CD8+ cells was observed (data not shown), but few granulysin+ cells were detected in the tumor area (fig. 3g). In contrast to immunosuppressive cells, proinflammatory cells, including few IL-17-producing cells, were scattered around the tumor area (fig. 2h).
Discussion
RDEB is a hereditary skin disorder characterized by mechanical fragility of the skin, resulting in blistering and chronic wounds. A previous report suggested that patients with RDEB had a high risk of developing aggressive SCC [1, 2]. Indeed, over two-thirds of RDEB patients eventually die from SCC [1]. However, reports of the possible mechanisms for carcinogenesis of SCC from RDEB are limited. Among them, Knaup et al. [3] reported that RDEB keratinocytes showed elevated levels of TGFβ1, which suggested the contribution of immunosuppressive factors to the carcinogenesis of SCC from RDEB.
Evaluating the immunological environment is indispensable for the assessment of oncogenesis [4]. Indeed, strong evidence for an association between bacterial infectious disease, such as chronic osteomyelitis and hidradenitis suppurativa, with oncogenesis has been reported [5]. Chronic irritation of the skin and secondary bacterial infection lead to proliferative epidermal changes. From the immunological point of view, bacterial infection (i.g. Staphylococcus aureus, Streptococcus pyogenes) strongly induces proinflammatory cytokines, including IL-1β, IL-6, IL-17 and PGE2 [6, 7]. These proinflammatory cytokines induce myeloid-derived suppressor cells, which, together with regulatory T cells (Tregs), are known to play a central role in the induction of peripheral tolerance [4]. In aggregate, in our present case, the long-term skin wound or cutaneous scar caused by RDEB might be one of the possible triggers in the development of SCC.
To test this hypothesis, we performed immunohistochemical staining for SCC from RDEB, especially focusing on immunosuppressive cells as well as proinflammatory IL-17-producing cells. As we expected, in our case, CD163+ M2 macrophages and Foxp3high+ Tregs were prominent around the tumor. Together with immunosuppressive macrophages, Tregs promote an immunosuppressive environment in the tumor-bearing host [4, 8, 9]. IL-17-producing cells did not aggregate, but were scattered in the superficial dermis surrounded by CD163+ M2 macrophages. In addition, in contrast to immunosuppressive cells, few granulysin-bearing cells, which have been reported to correlate with the prognosis of cancer patients [10], were detected. Though we did not further investigate for these phenotypically immunosuppressive cells, our present findings suggest the contribution of CD163+ M2 macrophages and Tregs to the carcinogenesis of SCC arising from RDEB.
References
- 1.Reed WB, College J, Jr, Francis MJ, Zachariae H, Mohs F, Sher MA, Sneddon IB. Epidermolysis bullosa dystrophica with epidermal neoplasms. Arch Dermatol. 1974;110:894–902. [PubMed] [Google Scholar]
- 2.South AP, O'Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171–178. doi: 10.1016/j.det.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Knaup J, Gruber C, Krammer B, Ziegler V, Bauer J, Verwanger T. TGFβ-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst) 2011;34:339–353. doi: 10.3233/ACP-2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimura T, Mahnke K, Enk AH. Myeloid derived suppressor cells and their role in tolerance induction. J Dermatol Sci. 2010;59:1–6. doi: 10.1016/j.jdermsci.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Samaras V, Rafailidis PI, Mourtzoukou EG, Peppas G, Falagas ME. Chronic bacterial and parasitic infections and cancer: a review. J Infect Dev Ctries. 2010;4:267–281. doi: 10.3855/jidc.819. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed EB, Wang T, Daniels M, Alegre ML, Chong AS. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant. 2011;11:936–946. doi: 10.1111/j.1600-6143.2011.03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimura T, Yamasaki K, Hidaka T, Ito Y, Aiba S. A synthetic NOD2 agonist, muramyl dipeptide (MDP)-Lys (L18) and IFN-β synergistically induce dendritic cell maturation with augmented IL-12 production and suppress melanoma growth. J Dermatol Sci. 2011;62:107–115. doi: 10.1016/j.jdermsci.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, Yang PC, Kuo ML, Jee SH. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T cells (Treg) stimulate B7-H1 expression in myeloid derived suppressor cells (MDSC) in ret melanomas. J Invest Dermatol. 2012;132:1239–1246. doi: 10.1038/jid.2011.416. [DOI] [PubMed] [Google Scholar]
- 10.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]