Abstract
Background
A growing proportion of men diagnosed with localized prostate cancer detected through prostate-specific antigen testing are dying from causes other than prostate cancer. Temporal trends in specific causes of death among prostate cancer patients have not been well described.
Methods
We analyzed causes of death among all incident prostate cancer cases recorded in the nationwide Swedish Cancer Registry (1961–2008; n = 210 112) and in the US Surveillance, Epidemiology, and End Results Program (1973–2008; n = 490 341). We calculated the cumulative incidence of death due to seven selected causes that accounted for more than 80% of the reported deaths (including ischemic heart disease and non–prostate cancer) and analyzed mortality trends by calendar year and age at diagnosis and length of follow-up.
Results
During follow-up through 2008, prostate cancer accounted for 52% of all reported deaths in Sweden and 30% of reported deaths in the United States among men with prostate cancer; however, only 35% of Swedish men and 16% of US men diagnosed with prostate cancer died from this disease. In both populations, the cumulative incidence of prostate cancer–specific death declined during follow-up, while the cumulative incidences of death from ischemic heart disease and non–prostate cancer remained constant. The 5-year cumulative incidence of death from prostate cancer among all men was 29% in Sweden and 11% in the United States.
Conclusions
In Sweden and the United States, men diagnosed with prostate cancer are less likely to die from prostate cancer than from another cause. Because many of these other causes of death are preventable through changes in lifestyle, interventions that target lifestyle factors should be integrated into prostate cancer management.
Over the past few decades in most Western countries, the probability that a man who was recently diagnosed with prostate cancer will die of this disease has decreased substantially. Although the incidence of prostate cancer has increased greatly in these countries, prostate cancer mortality has not (1,2). Most, if not all, of the increased incidence of prostate cancer can be ascribed to widespread prostate-specific antigen (PSA) screening, which has resulted in a higher proportion of men diagnosed with localized disease and overdiagnosis of nonlethal cancer (3,4). A 2009 study of men older than 65 years in the US Surveillance, Epidemiology, and End Results (SEER) program reported that men with conservatively managed, localized, and well-to-moderately differentiated prostate cancer had a 8%–9% risk of dying from prostate cancer within 10 years of diagnosis (5). According to the SEER database (6), 81% of men diagnosed with prostate cancer in 2001–2007 had localized disease. Thus, most newly diagnosed men will ultimately die from a cause other than their prostate cancer, and the risk of dying from another cause may be modifiable by lifestyle intervention. However, the temporal trends in specific causes of death among prostate cancer patients are not well described. Such information could guide preventive measures that target the overall health of prostate cancer patients.
Many patients with low-risk prostate cancer undergo curative treatment with little or no survival benefit. Treatment for localized prostate cancer also carries considerable reductions in quality of life (7–9). Given the older age at diagnosis of most patients with localized prostate cancer, these men are at substantial risk of dying from a constellation of chronic conditions. As such, lifestyle interventions directed toward reducing the risk of competing causes of death may improve the overall survival of these patients considerably (10).
We used data from population-based databases to perform detailed analyses of the distribution of causes of death among men diagnosed with prostate cancer in Sweden and the United States, two countries with some of the highest incidence rates in the world (83.8 and 95.5 per 100 000 persons, respectively, in 2008) (11). To better assess temporal trends, we analyzed the distribution of specific causes of death by follow-up time, year of diagnosis, and age at diagnosis.
Methods
Study Populations
We analyzed data from population-based cancer registries for men diagnosed with prostate cancer in Sweden between 1961 and 2008 (N = 212 090) and in the United States between 1973 and 2008 (N = 500 788). The Swedish Cancer Registry represents near-complete coverage of all cancers diagnosed in Sweden since 1958 because reporting is required by law for clinicians. Dates and causes of death were ascertained through linkage with the Swedish Cause of Death Register using the unique national registration number that is assigned to each Swedish resident. Analyses were restricted to men who were diagnosed with prostate cancer [International Classification of Diseases (ICD) revision 7 (12) code 177] between January 1, 1961, and December 31, 2008, because cause-of-death data were not available beyond these dates; 99% of prostate cancers are morphologically verified (13).
The US SEER program of the National Cancer Institute has collected data on cancer incidence and mortality from specific registries throughout the United States since 1973. For this analysis, we included all prostate cancer cases [ICD for Oncology, third edition (14) code C619] reported to the nine original SEER registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah) diagnosed between January 1, 1973, and December 31, 2008. The Seattle–Puget Sound and Atlanta registries contributed data from 1974 to 2008 and from 1975 to 2008, respectively (15).
We excluded men for whom the recorded date of prostate cancer diagnosis was at or after their date of death and men with a missing age at diagnosis or who were younger than 30 years or older than 95 years at prostate cancer diagnosis. After applying these criteria, 210 112 Swedish men with prostate cancer (99% of those in the Swedish registry) and 490 341 US men with prostate cancer (98% of those in the SEER database) were included in the analyses.
Outcome Ascertainment
In the Swedish register, cause of death was coded according to ICD revisions 7–10 (12,16–18). Codes for specific causes of death were harmonized by a study clinician (GE). Only the primary causes of death were considered. In SEER, cause of death was classified by ICD revision 8 (16) for men who died before 1979, ICD revision 9 (17) for men who died from 1979 through 1998, and by ICD revision 10 (18) for men who died thereafter. We focused on seven causes of death that together accounted for more than 80% of the reported deaths: prostate cancer, other cancers, ischemic heart disease, cerebrovascular disease, diabetes mellitus (types I and II), chronic obstructive pulmonary disease (COPD), external causes (including trauma and suicide), and also included a category for all other causes of death with a specified ICD revision number and code (including infectious diseases and liver disease). The specific ICD codes included for each endpoint are shown in Supplementary Tables 1 and 2 (available online).
Statistical Analysis
Follow-up time was calculated from date of prostate cancer diagnosis to the recorded date of death, loss to follow-up, or the end of follow-up (December 31, 2008), whichever occurred first (15). Because PSA screening began earlier in the United States than in Sweden, for the purpose of this study we defined the beginning of the “PSA era” as January 1, 1991, in the United States and as January 1, 1994, in Sweden (19).
We assessed the distribution of causes of death among men with prostate cancer by calculating the percentage of total deaths and the cumulative incidence of death from each of the seven selected causes. To analyze time trends, we examined the cumulative incidence of specific causes of death by age at prostate cancer diagnosis (5-year age groups: <65, 65–69, 70–74, 75–79, or ≥80 years), calendar year of prostate cancer diagnosis (1961–1972 [Sweden only], 1973–1980, 1981–1990, 1991–2000, or 2001–2008), and follow-up time (<5, 5–10, or >10 years).
The cumulative incidence of death was assessed by using the CumIncid SAS macro (version 9.2; SAS Institute, Cary, NC). To ensure equal follow-up in both populations, models of 5-year cumulative incidence were restricted to prostate cancers diagnosed before 2004, and analyses of 10-year cumulative incidence were restricted to prostate cancers diagnosed before 1999. Cumulative incidence estimates were also calculated separately for men aged 60–64 years and 75–79 years as representative of younger and older men diagnosed with prostate cancer, because the overall mean age at diagnosis in the combined populations was 70.4 years. To evaluate more recent trends, in a secondary analysis we explored the cumulative incidence of death among men aged 60–64 years and aged 75–79 years who were diagnosed with prostate cancer after January 1, 2000. In all analyses, we used the methods of Kalbfleisch and Prentice (20) to account for competing risks of death.
To graphically assess changes in the distribution of causes of death over time, we fit binomial regression models with a log link function to estimate the relative distribution of the cumulative incidence of death from four broader groups of causes: prostate cancer, ischemic heart disease or cerebrovascular disease, non–prostate cancers, and other causes (including diabetes mellitus, external causes, COPD, and all other causes not previously included) (21). These models assessed the probability of having died from each of these four causes within 5 or 10 years after a prostate cancer diagnosis. Separate models were fitted for each cause of death for the United States and Sweden for men aged 60–64 and 75–79 years. The models included year of diagnosis as a restricted cubic spline with seven knots for a flexible representation of calendar effects. The predicted probabilities of death due to each of the four causes for each calendar year were extracted from the models and plotted to graphically represent the risks of the different causes of death. The binomial regression models did not account for competing causes of death. All statistical analyses were conducted using SAS statistical software (version 9.2). This study was approved by the regional ethics committee in Stockholm, Sweden.
Results
Overall Findings
The distribution of causes of death for both Swedish and US men by age at and year of prostate cancer diagnosis is reported in Table 1. Of the 210 112 Swedish men included in the analysis, 105 423 (50%) were diagnosed during the PSA era (ie, after January 1, 1994), and median follow-up time for all men was 3.8 years (range = 0–48 years). A total of 143 189 Swedish men (68%) died during follow-up. Prostate cancer was the most common cause of death; more than one-third of men diagnosed with prostate cancer (35%, n = 73 829) died from this disease. Prostate cancer accounted for 52% of all reported deaths, whereas ischemic heart disease accounted for 17% of all reported deaths and non–prostate cancers for 6% (Table 2).
Table 1.
Distribution of causes of death among men diagnosed with prostate cancer in the Swedish Cancer Registry, 1961–2008, and the Surveillance, Epidemiology, and End Results (SEER) program, 1973–2008*
| Population characteristics | Swedish Cancer Registry | SEER program | ||||||
| Died from prostate cancer |
Died from other cause |
Censored† | Total | Died from prostate cancer |
Died from other cause |
Censored† | Total | |
| All patients, No. | 73 829 | 69 360 | 66 923 | 210 112 | 78 064 | 180 957 | 231 320 | 490 341 |
| Age at diagnosis in y, No. (%) | ||||||||
| <65 | 13 975 (29.1) | 7370 (15.3) | 26 693 (55.6) | 48 038 | 16 268 (11.2) | 23 930 (16.5) | 104 486 (72.2) | 144 684 |
| 65–69 | 12 956 (34.1) | 10 428 (27.4) | 14 661 (38.5) | 38 045 | 13 784 (14.7) | 30 496 (32.5) | 49 626 (52.9) | 93 906 |
| 70–74 | 16 991 (37.3) | 16 316 (35.9) | 12 197 (26.8) | 45 504 | 16 123 (16.4) | 41 824 (42.6) | 40 220 (41.0) | 98 167 |
| 75–79 | 15 683 (37.8) | 17 700 (42.7) | 8096 (19.5) | 41 479 | 14 594 (18.5) | 40 310 (51.0) | 24 195 (30.6) | 79 099 |
| ≥80 | 14 224 (38.4) | 17 546 (47.4) | 5276 (14.2) | 37 046 | 17 295 (23.2) | 44 397 (59.6) | 12 793 (17.2) | 74 485 |
| Year of diagnosis, No. (%) | ||||||||
| 1961–1972 | 14 833 (55.0) | 12 001 (44.5) | 124 (0.5) | 26 958 | – | – | – | – |
| 1973–1980 | 12 788 (52.4) | 11 454 (46.9) | 163 (0.7) | 24 405 | 17 166 (37.2) | 28 366 (61.5) | 562 (1.2) | 46 094 |
| 1981–1990 | 18 796 (48.4) | 18 841 (48.5) | 1206 (3.1) | 38 843 | 29 314 (30.1) | 59 626 (61.3) | 8382 (8.6) | 97 322 |
| 1991–2000 | 20 424 (37.5) | 20 251 (37.2) | 13 767 (25.3) | 54 442 | 25 201 (13.6) | 77 093 (41.6) | 83 027 (44.8) | 185 321 |
| 2001–2008 | 6988 (10.7) | 6813 (10.4) | 51 663 (78.9) | 65 464 | 6383 (4.0) | 15 872 (9.8) | 139 349 (86.2) | 161 604 |
| Mean age at diagnosis, y (SD) |
73.0 (8.4) | 75.2 (7.5) | 68.1 (8.4) | 72.2 (8.7) | 72.1 (9.3) | 73.7 (8.3) | 65.6 (8.8) | 69.6 (9.5) |
| Mean age at death, y (SD) | 77.1 (8.4) | 80.8 (7.4) | – | 78.9 (8.1)‡ | 77.0 (9.2) | 80.3 (8.3) | – | 79.3 (8.7)‡ |
*– = not applicable.
†Alive as of December 2008 or lost to follow-up.
‡Includes men who died or were lost to follow-up.
Table 2.
Distribution of causes of death by time between diagnosis and death, separately for the United States and Sweden*
| Mortality status | Swedish Cancer Registry (n = 210 112) | US SEER Registry (n = 490 341) | ||||
| Men with <5 y of follow-up | Men with 5–10 y of follow-up | Men with >10 y of follow-up | Men with <5 y of follow-up | Men with 5–10 y of follow-up | Men with >10 y of follow-up | |
| No. at risk† | 210 112 | 81 914 | 27 776 | 490 341 | 265 368 | 121 274 |
| Alive throughout follow-up, No. (%)‡ |
37 620 (17.9) | 20 119 (24.6) | 9184 (33.1) | 94 890 (19.4) | 71 042 (26.8) | 65 388 (53.9) |
| All deaths, No. (%) | 90 578 (43.1) | 34 019 (41.5) | 18 592 (66.9) | 130 083 (26.5) | 73 052 (27.5) | 55 886 (46.1) |
| Cause-specific death, No. (%)§ | ||||||
| Prostate cancer | 51 342 (56.7) | 15 809 (46.5) | 6678 (35.9) | 47 541 (36.5) | 19 486 (26.7) | 11 037 (19.7) |
| IHD | 14 368 (15.9) | 5853 (17.2) | 3535 (19.0) | 29 507 (22.7) | 18 057 (24.7) | 14 615 (26.2) |
| Cerebrovascular disease | 4115 (4.5) | 2150 (6.3) | 1346 (7.2) | 5854 (4.5) | 3961 (5.4) | 3223 (5.8) |
| Other cancers | 5319 (5.9) | 2408 (7.1) | 1350 (7.3) | 19 486 (15.0) | 11 300 (15.5) | 7993 (14.3) |
| COPD | 1210 (1.3) | 593 (1.7) | 385 (2.1) | 4408 (3.4) | 2914 (4.0) | 2340 (4.2) |
| Diabetes | 527 (0.6) | 298 (0.9) | 206 (1.1) | 1564 (1.2) | 1201 (1.6) | 1007 (1.8) |
| External causes‖ | 1438 (1.6) | 656 (1.9) | 419 (2.3) | 2472 (1.9) | 1639 (2.2) | 1254 (2.2) |
| Other specified causes¶ | 12 259 (13.5) | 6252 (18.4) | 4673 (25.1) | 19 251 (14.8) | 14 494 (19.8) | 14 417 (25.8) |
*SEER = Surveillance, Epidemiology, and End Results; IHD = ischemic heart disease; COPD = chronic obstructive pulmonary disease.
†Number of men alive at the beginning of follow-up window.
‡Men alive at the beginning of the follow-up window who did not die during the specified period of follow-up.
§Percentage of all deaths.
‖Death caused by external causes, including suicide and trauma.
¶Deaths from causes not previously categorized in this analysis, including infectious diseases and liver disease.
Of the 490 341 US men included in the analysis, 327 957 (67%) were diagnosed with prostate cancer during the PSA era (ie, after January 1, 1991). During a median 5.6 years of follow-up (range = 0–36 years), 259 021 men (53%) had died. Prostate cancer was the single most common cause of death (n = 78 064) and accounted for 30% of all reported deaths; however, this percentage corresponds to only 16% of all men diagnosed with prostate cancer who died from this disease. Ischemic heart disease was the second most common cause of death and accounted for 24% of all reported deaths, whereas 15% of all deaths were due to non– prostate cancers (Table 2).
Among Swedish men with at least 5 years of potential follow-up after prostate cancer diagnosis (n = 81 914), 39% of men diagnosed in the pre-PSA era died from prostate cancer (n = 16 946) compared with 14% of men diagnosed in the PSA era (n = 5541). Among US men with at least 5 years of potential follow-up after prostate cancer diagnosis (n = 265 368), 23% of men diagnosed in the pre-PSA era died of prostate cancer compared with 6% of men diagnosed in the PSA era (data not shown).
Temporal Trends
In Sweden, the 5-year cumulative incidence of death from prostate cancer was 25% among men younger than 65 years at prostate cancer diagnosis and 36% among men aged 80 years or older at prostate cancer diagnosis. In this oldest age group, the cumulative incidence of death from ischemic heart disease was 14%. During the first 10 years after prostate cancer diagnosis, a greater percentage of men aged 80 years or older at diagnosis died from causes other than prostate cancer compared with men younger than 65 years at diagnosis (55% vs 28% of deaths, respectively).
In the United States, the 5-year cumulative incidence of death from prostate cancer was considerably lower than in Sweden: 9% among men younger than 65 years at prostate cancer diagnosis and 19% among men aged 80 years or older at diagnosis. Among the oldest men, the cumulative incidence of death from ischemic heart disease—17%—was similar to the cumulative incidence of death from prostate cancer.
Among Swedish men with at least 5 years of potential follow-up time, the 5-year cumulative incidence of death from prostate cancer declined from 41% among those diagnosed in the 1960s to 25% among those diagnosed in the 1990s (data not shown). The 5-year cumulative incidence of death from ischemic heart disease remained constant at approximately 10% for men diagnosed with prostate cancer until the 1980s, after which it declined to 7% among men diagnosed in the 1990s. The 5-year cumulative incidence of death from cerebrovascular disease also stayed constant at just over 3% among men diagnosed in the 1960s and 2% among men diagnosed in the 1990s. The 5-year cumulative incidences of death from chronic obstructive pulmonary disease, external causes, and diabetes (as a primary cause) each remained fairly constant among men diagnosed over the decades from 1961 to 1999 at approximately 1%. Similarly, the 5-year cumulative incidence of death from non–prostate cancers stayed fairly constant at 2% among men diagnosed with prostate cancer in the 1960s and near 3% among those diagnosed in the 1990s. However, the actual proportion of deaths attributable to non–prostate cancers increased from 4% (n = 831) among men diagnosed in the 1960s to 9% among men diagnosed between 2000 and 2008 (n = 1597).
Among all Swedish men diagnosed with prostate cancer, the 10-year cumulative incidence of death from prostate cancer was 43%, and the 5-year cumulative incidence was 29%. By comparison, the 10-year cumulative incidence of death from ischemic heart disease was 13.2%, and the 5-year cumulative incidence was 8.2% (data not shown). When we stratified the analyses by time at risk between prostate cancer diagnosis and death, the percentage of men who died from prostate cancer decreased with longer follow-up (Table 2), whereas the percentage of men who died from either ischemic heart disease or all other causes increased.
In the United States, the 5-year cumulative incidence of death from prostate cancer declined from approximately 24% among men diagnosed with prostate cancer in the 1970s to 8% among those diagnosed in the 1990s, whereas death from ischemic heart disease declined only slightly from 12% for men diagnosed with prostate cancer in the 1970s to 10% for those diagnosed in the 1980s and to 6% among men diagnosed in the 1990s. The 5-year cumulative incidence of death from cerebrovascular disease declined gradually over time from nearly 3% among men diagnosed in the 1970s to 1% among those diagnosed in the 1990s. As we observed with the Swedish population, the 5-year cumulative incidence of death from external causes (0.5%), COPD (1%), and diabetes (0.4%) remained fairly constant among men diagnosed with prostate cancer from the 1970s through the 1990s, as did the cumulative incidence of death from non–prostate cancers (5% among men diagnosed in the 1970s and 4% among men diagnosed in the 1990s). However, non–prostate cancers accounted for an increasing proportion of deaths over time, increasing from 11% among men diagnosed in the 1970s (n = 4086) to 17% among men diagnosed in the 1990s (n = 18 282) and 20% among men diagnosed between 2000 and 2008 (n = 5858).
The 10-year cumulative incidence of death from prostate cancer among all US men diagnosed with prostate cancer was 20%, and the 5-year cumulative incidence was 11%. By contrast, the 10-year cumulative incidence of death from ischemic heart disease was 14%, and the 5-year cumulative incidence was 7%. As we observed with the Swedish population, the percentage of US men who died from prostate cancer decreased with increasing time since diagnosis, whereas the percentage of men who died from ischemic heart disease, cerebrovascular disease, COPD, and diabetes increased (Table 2).
The cumulative incidence of causes of death among men diagnosed in the most recent decade (2000–2008) followed the trends of earlier decades, with lower cumulative incidence of death from prostate cancer compared to earlier decades in both populations. However, the cumulative incidence of death from prostate cancer remained higher in Sweden than in the United States during this time (Supplementary Figure 1, available online). The cumulative incidence of death from prostate cancer among Swedish men aged 60–64 years at prostate cancer diagnosis was more than twice that of US men aged 60–64 years at diagnosis (13.0% vs 4.8%). Among men aged 75–79 years at prostate cancer diagnosis, the cumulative incidence of death from prostate cancer for Swedish men was three times that of US men (30% vs 9.8%). Among men diagnosed with prostate cancer between 2000 and 2008, the cumulative incidence of death from ischemic heart disease in Sweden and the United States was similar among younger (3.2% in the United States, 1.9% in Sweden) and older (10.6% in the United States, 8.9% in Sweden) men (Supplementary Figure 1). The cumulative incidence of death from all causes other than prostate cancer and ischemic heart disease among men diagnosed between 2000 and 2008 was similar to that of men diagnosed during earlier decades, with higher rates from each cause among older men compared with younger men (Supplementary Figure 1, available online).
The 5- and 10-year cause-specific risks of death were plotted by year of diagnosis for Swedish (Figure 1) and US (Figure 2) men aged 60–64 and 75–79 years at diagnosis. In both populations, the risk of death from prostate cancer declined with more recent calendar year of diagnosis, notably among men aged 60–64 years at diagnosis. Among the older men at diagnosis, the decrease in the risk of death from prostate cancer over calendar time was greater in the United States than in Sweden. In the United States, the overall risk of death in both age groups and for both the 5- and 10-year risk calculations was mostly due to causes other than prostate cancer, especially among men diagnosed in recent years. The division between deaths from prostate cancer and deaths from all other causes was less clear in the Swedish data, where prostate cancer remained a considerable cause of death even among men diagnosed in the most recent years. The proportions of deaths from ischemic heart disease and cerebrovascular disease decreased over time in both age groups, while the risks of death from non–prostate cancers and all other causes remained stable or increased slightly.
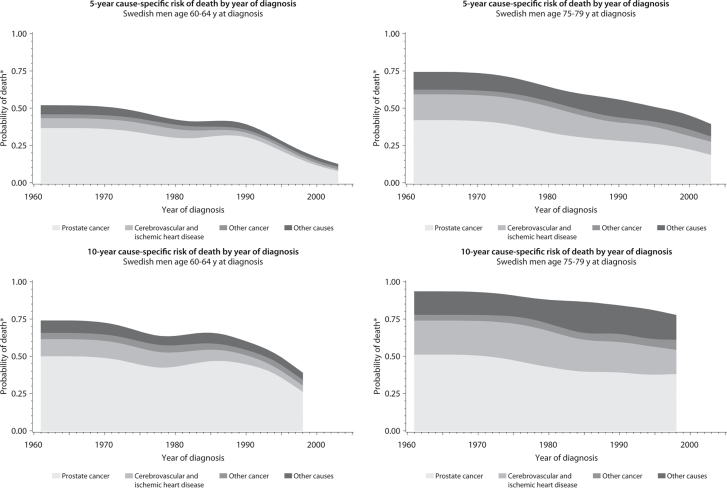
Figure 1.
The distribution of cause-specific cumulative incidence of death among Swedish men with prostate cancer by age and year of diagnosis, 1961–2008. *Risk of cause-specific death, does not account for competing risks. Cancer other than prostate cancer; Other causes, including diabetes mellitus, external causes (including trauma and suicide), chronic obstructive pulmonary disease, and all other specified causes.
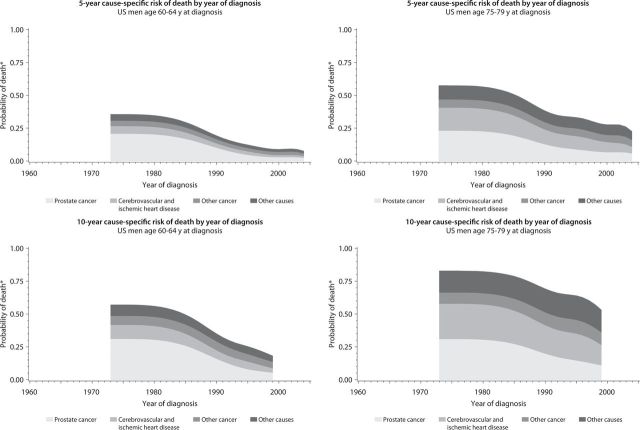
Figure 2.
The distribution of cause-specific cumulative incidence of death among US men with prostate cancer by year of diagnosis, 1973–2008. *Risk of cause-specific death, does not account for competing risks. Cancer other than prostate cancer; Other causes, including diabetes mellitus, external causes (including trauma and suicide), chronic obstructive pulmonary disease, and all other specified causes.
Discussion
Our analysis revealed important trends in causes of death among men with prostate cancer. In Sweden and the United States, men diagnosed with prostate cancer were more likely to die from another cause, although the division between the likelihood of dying from prostate cancer and the likelihood of dying from all other causes was less distinct in Sweden. We also found that the distribution of causes of death varied by age at and calendar year of diagnosis and the length of follow-up. In both countries, the cumulative incidence of death from prostate cancer was highest among men who were diagnosed during earlier calendar periods, among older men, and during the first 5 years after prostate cancer diagnosis.
Any analysis of trends in disease-specific mortality among prostate cancer patients in the era of PSA screening is influenced by lead time and length biases. Lead time is defined as the amount of time a prostate cancer diagnosis was advanced due to PSA screening. Bias may arise if PSA screening results in an earlier diagnosis but does not impact the disease outcome, thus making PSA screen–detected cases of prostate cancer appear to have artificially prolonged survival. In this study, we sought to incorporate the lead time following prostate cancer diagnosis by examining the distribution and trends in all causes of death among prostate cancer patients, the majority of whom died from causes other than prostate cancer. By examining the cumulative incidence of death from various causes at fixed follow-up times (5 and 10 years), and by stratifying the analyses by the pre- vs post-PSA era, we aimed to account for the impact of lead time from PSA screening, which has been estimated at between 5 and 12 years, depending on the study population and definition used (22–24). However, it is unlikely that we have completely eliminated the considerable influence of lead time from our results. Length bias may also have influenced our analysis of prostate cancer–specific death because prostate cancers detected through screening tend to be slower-growing and less aggressive compared with tumors detected by symptoms, and thus fewer men with screen-detected prostate cancer die from this disease.
We found that the risk of dying from newly diagnosed prostate cancer has declined in both the Swedish and US populations over the time period included in this analysis (Sweden: 1961–2008; United States: 1973–2008). The magnitude of the decrease in disease-specific mortality, particularly during recent years, may be due in part to lead time and overdiagnosis of nonlethal disease as a result of PSA screening. However, the actual proportion of men who died of prostate cancer remains considerably higher in Sweden than in the United States, possibly reflecting the earlier and more aggressive use of PSA screening and curative treatment in the United States (25–28). The discrepancy in screening and treatment practices between countries is also reflected in the relatively older age at diagnosis and shorter median follow-up times for the Swedish men. Ischemic heart disease was the second most common cause of death in both cohorts; however, a greater proportion of US men than Swedish men died from ischemic heart disease (24% vs 17% of reported deaths).
This study has several limitations. First, we had no information on other risk factors for early death, such as smoking history, obesity, diet, or comorbidities. These factors may also differ by age group or between countries and may influence the risk of death from various causes in these groups. Second, we limited the analyses to the primary cause of death and ignored contributing causes, which may or may not have included prostate cancer. Misclassification of cause of death may have resulted in an underestimation of deaths from causes other than prostate cancer, as patients may have been incorrectly recorded as having died from their prostate cancer (attribution bias) (29). The magnitude of attribution bias may differ between the study populations and may have contributed to the observed differences. Third, we restricted our analysis to diabetes as a primary cause of death, and thus diabetic patients who died from other causes directly resulting from their diabetes may have been misclassified, thus underestimating the number of deaths from diabetes. However, we observed similar trends in the cumulative incidence of several causes of death between the study populations, thus supporting this comparison of Swedish and US registry data. Analysis by stage or grade of prostate cancer at diagnosis was outside the scope of this project.
The strengths of this study include the use of reliable population-level data collected over decades spanning the introduction of PSA screening in two countries with high incidence rates of prostate cancer. In addition, the large sample sizes and lengthy follow-up allowed us to describe subtle variations in the distribution of causes of death. The Swedish Cause of Death Register is a highly valid source of mortality data for prostate cancer patients when compared with medical records, especially for younger men and those with localized disease (30). Finally, our analysis of time trends in specific causes of death among men with prostate cancer, which to our knowledge has not previously been investigated in detail, has potentially important clinical implications in the management of men with prostate cancer.
This analysis describes the emerging landscape of prostate cancer and argues for changes in the management of newly diagnosed patients. This argument would be valid regardless of the reasons for the change in the landscape, including the presence of lead time and length biases. Our data clearly demonstrate that men with newly diagnosed prostate cancer run a greater risk of dying from a cause other than prostate cancer itself. The global health of prostate cancer patients may thus be improved over the long term by encouraging modification of lifestyle factors, such as smoking, physical activity, diet, and obesity, which are strongly related to most common causes of death among patients. For example, lifestyle interventions that target multiple risk factors in populations at high risk for diabetes have shown sustained lifestyle changes and reductions in incident disease with up to 20 years of follow-up (31–34). In addition, risks of all-cause (35) and cancer (36) mortality were elevated across age groups in men and women with the highest body mass index in US cohort studies, suggesting that an intervention targeting obesity in prostate cancer patients might improve survival for all men. An estimated 4.2%–14.2% of all cancer deaths among men have been attributed to overweight and obesity (36). It is also known that smoking cessation increases overall survival; for example, the British Doctors Study found that cigarette smokers died an average of 10 years younger than lifelong nonsmokers (37).
Compared with the benefits of lifestyle intervention, the benefits from curative prostate cancer treatment on disease-specific and overall survival are less clear. The Scandinavian Prostate Cancer Group Study Number 4 randomized trial of radical prostatectomy vs watchful waiting in men with localized prostate cancer found a 6.1% lower cumulative incidence of death from prostate cancer in the surgery group compared with the watchful waiting group after 15 years of follow-up (38). This trial currently represents the best estimate for the effects of radical treatment vs watchful waiting on mortality for men with localized prostate cancer; however, prostate cancers in the trial were largely detected by symptoms and may not be representative of those detected by screening (39). Initial unpublished results (Dr Timothy Wilt, unpublished observations) from the randomized Prostate Cancer Intervention Versus Observation Trial (PIVOT) of US men with largely PSA-detected, clinically localized disease suggest that radical prostatectomy treatment did not reduce disease-specific or overall mortality compared with watchful waiting in the study population, and especially among men with low PSA levels and low-risk disease, although men with high-risk disease may have some benefit (40). The survival benefit from prostatectomy, notwithstanding its detrimental impact on quality of life, is comparably less than the benefits of adhering to a healthy lifestyle pattern to reduce the risks of competing causes of death. Given that our data show competing causes eventually kill more patients than prostate cancer, investing in lifestyle modification may increase both the duration and quality of life beyond that achieved with aggressive prostate cancer treatment alone.
This analysis revealed salient cause-of-death trends, which should affect the clinical management of men who receive a prostate cancer diagnosis. The overall risk of death from causes other than prostate cancer is greater than the overall risk of death from prostate cancer in two countries with high incidence rates. Since the beginning of follow-up in both study populations, the cumulative incidence of prostate cancer–specific death has declined. Although these trends are presumably driven by screening rates, a man diagnosed with prostate cancer today clearly has a higher risk of dying from another cause than from prostate cancer. Our data suggest that a prostate cancer diagnosis may represent a teachable moment when physicians should not only counsel patients on prostate cancer treatment but also on modifying lifestyle factors that will reduce their risk of other chronic conditions. Managing prostate cancer patients with a lifestyle approach could increase the duration and quality of life among men living with prostate cancer, arguably more than aggressive treatment, by addressing increasingly dominant, yet preventable, causes of death.
Supplementary Material
Funding
This work was supported by Karolinska Institutet Distinguished Professor Award (Dnr: 2368/10-221 to HOA); National Institutes of Health National Cancer Institute research training grant (R25 CA098566 to MME); and a postdoctoral grant from Svenska Sällskapet för Medicinsk Forskning (to GE).
Notes
The study sponsors did not have any role in the design of this study, analysis or interpretation of the data, the writing of this article, or the decision to submit the article for publication.
References
- 1. Meyer MS, Mucci LA, Andersson SO, et al. Homogeneous prostate cancer mortality in the Nordic countries over four decades. Eur Urol. 2010;58(3):427 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69 90 [DOI] [PubMed] [Google Scholar]
- 3. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981 990 [DOI] [PubMed] [Google Scholar]
- 4. Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst. 2009;101(19):1325 1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11): 1202 1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howlader N, Noone AM, Krapcho M. et al. (Eds). SEER Cancer Statistics Review, 1975–2008 National Cancer Institute; 2011. Bethesda, MD: http://seer.cancer.gov/csr/1975_2009_pops09/ Accessed October 25, 2011. [Google Scholar]
- 7. Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12(9):891 899 [DOI] [PubMed] [Google Scholar]
- 8. Pardo Y, Guedea F, Aguilo F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28(31):4687 4696 [DOI] [PubMed] [Google Scholar]
- 9. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among. prostate-cancer survivors. N Engl J Med. 2008;358(12):1250 1261 [DOI] [PubMed] [Google Scholar]
- 10. Lajous M, Mozaffarian D, Mozaffarian R, Schrag D, Adami HO. Lifestyle prescriptions for cancer survivors and their communities. J Intern Med. 2011;269(1):88 93 [DOI] [PubMed] [Google Scholar]
- 11. GLOBOCAN 2008 v1.2 Cancer Incidence and Mortality Worldwide [database on the Internet]. Lyon, France: International Agency for Research on Cancer 2010.. http://globocan.iarc.fr. Accessed November 15, 2011. [Google Scholar]
- 12. World Health Organization ICD-7. International Statistical Classification of Diseases and Related Health Problems, Seventh Revision Geneva, Switzerland: World Health Organization; 1955. [Google Scholar]
- 13. The National Board of Health and Welfare. Cancer Incidence in Sweden 2005 Stockholm: National Board of Health and Welfare (Socialstyrelsen); 2007. [Google Scholar]
- 14. World Health Organization International Classification of Diseases for Oncology 3rd ed. (ICD-O-3) Geneva, Switzerland: World Health Organization; 1976. [Google Scholar]
- 15. Surveillance, Epidemiology, and End Results (SEER) Program. Research Data (1973-2008), Bethesda, MD: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2011. http://www.seer.cancer.gov. Accessed September 27, 2011. [Google Scholar]
- 16. World Health Organization ICD-8. International Statistical Classification of Diseases and Related Health Problems, Eighth Revision Geneva, Switzerland: World Health Organization; 1968. [Google Scholar]
- 17. World Health Organization ICD-9. International Statistical Classification of Diseases and Related Health Problems, Ninth Revision Geneva, Switzerland: World Health Organization; 1975. [Google Scholar]
- 18. World Health Organization ICD-10. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Vol. 1–3 Geneva, Switzerland: World Health Organization; 1992–1994. [Google Scholar]
- 19. Neppl-Huber C, Zappa M, Coebergh JW, et al. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: additional diagnoses and avoided deaths. Ann Oncol. 2012;23(5):1325 1334 [DOI] [PubMed] [Google Scholar]
- 20.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons; . 1980.
- 21. Robbins AS, Chao SY, Fonseca VP. What’s the relative risk? A method to directly estimate risk ratios in cohort studies of common outcomes. Ann Epidemiol. 2002;12(7):452 454 [DOI] [PubMed] [Google Scholar]
- 22. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374 383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273(4):289 294 [PubMed] [Google Scholar]
- 24. Savage CJ, Lilja H, Cronin AM, Ulmert D, Vickers AJ. Empirical estimates of the lead time distribution for prostate cancer based on two independent representative cohorts of men not subject to prostate-specific antigen screening. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1201 1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003;289(11):1414 1420 [DOI] [PubMed] [Google Scholar]
- 26. Jonsson H, Holmstrom B, Duffy SW, Stattin P. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2011;129(8):1881 1888 [DOI] [PubMed] [Google Scholar]
- 27. Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98(16):1134 1141 [DOI] [PubMed] [Google Scholar]
- 28. Sandblom G, Dufmats M, Nordenskjold K, Varenhorst E. Prostate carcinoma trends in three counties in Sweden 1987-1996: results from a population-based national cancer—register. South-East Region Prostate Cancer Group. Cancer. 2000;88(6):1445 1453 [DOI] [PubMed] [Google Scholar]
- 29. Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer—part II: cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst. 1999;91(12):1025 1032 [DOI] [PubMed] [Google Scholar]
- 30. Fall K, Stromberg F, Rosell J, Andren O, Varenhorst E. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42(4):352 357 [DOI] [PubMed] [Google Scholar]
- 31. Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673 1679 [DOI] [PubMed] [Google Scholar]
- 32. Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783 1789 [DOI] [PubMed] [Google Scholar]
- 33. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383 393 [DOI] [PubMed] [Google Scholar]
- 34. Tuomilehto J, Schwarz P, Lindstrom J. Long-term benefits from lifestyle interventions for type 2 diabetes prevention: time to expand the efforts. Diabetes Care. 2011;34(2suppl):S210 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097 1105 [DOI] [PubMed] [Google Scholar]
- 36. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625 1638 [DOI] [PubMed] [Google Scholar]
- 37. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708 1717 [DOI] [PubMed] [Google Scholar]
- 39. Sonpavde G. Radical prostatectomy versus watchful waiting. N Engl J Med. 2005;353(12):1298 300 [PubMed] [Google Scholar]
- 40. Wilt T. The VA/NCI/AHRQ CSP #407: Prostate Cancer Intervention Versus Observation Trial (PIVOT): main results from a randomized trial comparing radical prostatectomy to watchful waiting in men with clinically localized prostate cancer Paper presented at American Urological Association Annual Meeting; May 2011; Washington, DC [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.