Abstract
Background
Treatment of acute lymphoblastic leukemia (ALL) has included the use of prophylactic cranial irradiation in up to 20% of children with high-risk disease despite known cognitive risks of this treatment modality.
Methods
Patients enrolled on the St Jude ALL Total Therapy Study XV, which omitted prophylactic cranial irradiation in all patients, were assessed 120 weeks after completion of consolidation therapy (n = 243) using a comprehensive cognitive battery. χ2 analysis was used to compare the percentage of below-average performers among the entire ALL patient group to the expected rate based on the normative sample. Univariate logistic regression was used to estimate the effect of intensity of chemotherapy (treatment arm), age at diagnosis, and sex on the probability of below-average performance. All statistical tests were two-sided.
Results
Overall, the ALL group had a statistically significantly higher risk for below-average performance on a measure of sustained attention (67.31% more than 1 SD below the normative mean for omission errors, P < .001) but not on measures of intellectual functioning, academic skills, or memory. Patients given higher intensity chemotherapy were at greater risk for below-average performance compared with those given lower intensity therapy on measures of processing speed (27.14% vs 6.25%, P = .009) and academic abilities (Math Reasoning: 18.60% vs 3.90%, P = .008; Word Reading: 20.00% vs 2.60%, P = .007; Spelling: 27.91% vs 3.90%, P = .001) and had higher parent-reported hyperactivity (23.00% vs 9.84%, P = .018) and learning problems (35.00% vs 16.39%, P = .005). Neither age at diagnosis nor sex was associated with risk for below-average cognitive performance.
Conclusions
Omitting cranial irradiation may help preserve global cognitive abilities, but treatment with chemotherapy alone is not without risks. Caregiver education and development of interventions should address both early attention deficits and cognitive late effects.
The prognosis for children diagnosed with acute lymphoblastic leukemia (ALL) has improved dramatically, with 5-year event-free survival rates as high as 79% to 82% among patients treated in the 1990s (1–3). Higher survival rates have resulted in heightened focus on improving quality of life of survivors by reducing treatment-related late effects, including cognitive deficits.
Historically, treatment of childhood ALL with cranial irradiation has been associated with substantial cognitive morbidity (4,5); however, these findings are based on doses generally exceeding those used in modern therapy. When directly compared, treatment with lower dose cranial irradiation (eg, ≤18 Grey [Gy]) used in contemporary clinical trials typically (6–9), but not always (10), results in worse cognitive outcomes than chemotherapy alone on measures of intellectual function as well as more specific cognitive abilities. There is also some evidence (11) to suggest that different chemotherapy regimens carry greater cognitive risk than others based on drugs used (eg, triple intrathecal methotrexate, hydrocortisone and cytarabine [ITMHA] vs intrathecal methotrexate alone; dexamethasone vs prednisone) as well as drug dosage and mode of administration (eg, lower dose oral methotrexate vs high-dose intravenous methotrexate [HDMTX]). Partly because of inconsistent reports of cognitive difference between treatment with lower dose cranial irradiation and intensive chemotherapy, and partly because of concern about increased central nervous system relapse, most pediatric collaborative study groups continue to use prophylactic cranial irradiation in their clinical trials in up to 20% of ALL patients (12).
The St Jude Total Therapy XV study evaluated whether intensification of systemic drugs that affect control of ALL in the central nervous system, together with optimal intrathecal treatment, would allow for complete omission of prophylactic cranial irradiation without compromising overall survival. The clinical trial resulted in 5-year event-free survival of 85.6% and overall survival of 93.5% (13). With additional follow-up, the treatment results remain excellent with a 10-year overall survival rate of 91.0% for all patients, 96.1% for low-risk patients, and 86.0% for standard/high-risk patients. Cognitive outcomes have not been systematically investigated or reported.
Demographic and clinical factors are associated with cognitive late effects in childhood ALL. Intensive treatment has been the most reliable predictor of increased risk (6–9,14–16). Younger age at treatment has been associated with worse cognitive outcomes, most likely resulting from greater vulnerability of the developing brain to neurotoxic agents (6,16–18). However, it is unclear whether this relationship is specific to children receiving cranial irradiation or also holds true for those receiving chemotherapy alone (16). Sex may also be associated with cognitive changes following ALL therapy, with girls more likely to experience deleterious effects (19,20). The mechanism for sex-specific risk is not fully understood but may result from differences in cerebral myelination (21,22). Furthermore, the literature is divided as to whether increased risk in girls is limited to children receiving cranial irradiation (23,24), and sex differences may vary depending on cognitive ability assessed (25). Few studies have been able to examine these demographic and clinical risk factors in a large cohort of prospectively studied patients who received homogeneous treatment and had comprehensive cognitive evaluations.
The first objective of this study was to systematically evaluate cognitive outcomes in a radiation-naive sample treated with contemporary risk-adapted therapy. The second objective was to investigate the predictive value of treatment intensity, age at treatment, and sex with respect to cognitive outcomes. Based on the existing literature, our primary hypothesis was that patients would perform well on global measures of cognitive ability, such as intellectual functioning; however, a subset of them would show evidence of difficulties on measures of attention, particularly on performance-based measures of sustained attention including indices of processing efficiency, which may be more sensitive to central nervous system–directed therapy (14,18,25,26). Secondarily, we predicted that higher treatment intensity and younger age at diagnosis would be risk factors for cognitive problems, whereas female sex would not reliably predict risk when looking across a range of cognitive skills.
Patients and Methods
Patients
All participants were sequentially enrolled on an institutional treatment protocol for ALL, Total Therapy Study XV (ClinicalTrials.gov, NCT00137111), which includes serial cognitive assessment (13). Children were assigned to low-risk or combined standard/high-risk groups based on comprehensive biological and clinical risk classification, which included blast cell immunophenotype and genotype, presenting clinical features, and early treatment response (13). Beginning with remission induction, all patients received ITMHA as central nervous system–directed therapy (13 to 18 treatments in low-risk group and 16 to 25 treatments in standard/high-risk group). During consolidation therapy, HDMTX was given intravenously every other week for four cycles at 2.5g/m2 for low-risk patients and 5.0g/m2 for standard/high-risk patients. During continuation treatment, methotrexate was given intravenously weekly at 40mg/m2 together with daily mercaptopurine for 3 weeks, followed by pulse therapy with vincristine plus dexamethasone at 8mg/m2 per day for 5 days for low-risk patients and at 12mg/m2 per day for 5 days for standard/high-risk patients at week 4. This treatment continued for 120 weeks for girls and 146 weeks for boys, interrupted by two reinduction treatments. At the time of cognitive testing, patients had received no ITMHA for approximately 72 weeks, no HDMTX for approximately 120 weeks, and no corticosteroids for approximately 20 weeks. No patients received prophylactic cranial irradiation.
Children aged 1 to 18 years were enrolled on the St Jude Total Therapy XV protocol between 2000 and 2007. They were excluded from current analysis if they were previously diagnosed with a developmental disorder with known cognitive sequelae (eg, Down syndrome, n = 10), did not speak English as a primary language (n = 16), or were missing psychological data (n = 43; included children with refractory or progressive disease, testing refusals, missed testing appointments, or scheduling problems). The study was approved by the Institutional Review Board at St Jude Children’s Research Hospital. Written informed consent, with assent from the patient as appropriate, was obtained prior to participation.
Cognitive Assessment
Children were tested 120 weeks after completion of consolidation therapy (week 120) using measures standardized on large representative normative samples with demonstrated reliability and validity. All measures were individually administered by trained, master’s level psychological examiners under the supervision of a licensed psychologist within the Psychology Clinic. Participants were administered an age-appropriate Wechsler Intelligence Scale [Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) for patients aged <6 years (27), the Wechsler Intelligence Scale for Children-Third Edition (WISC-III) for patients aged 6–16 years (28), and the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) for patients aged >16 years (29)]. All Wechsler Intelligence Scales yield an age-standardized Full Scale Intelligence Quotient. The majority of participants (59%) were administered the WISC-III, which allows for derivation of Freedom from Distractibility and Processing Speed indices to examine attention and processing speed, respectively. Academic skills were assessed for participants at least 6 years of age using the Wechsler Individual Achievement Test [WIAT, Word Reading, Spelling, and Math Reasoning (30)]. All Wechsler scores have a normative mean of 100 and SD of 15.
Participants at least 6 years of age also were administered the Conners’ Continuous Performance Test (CPT), a computerized measure of sustained attention (31). The CPT provides scores for Omission errors (failing to respond to targets), Reaction Time (processing speed), Reaction Time Variability, d′ (vigilance), and β (risk taking). All scores are age-standardized; for Omission errors, a percentile score is derived, and for the other indices, a T score is derived, with a normative mean of 50 and SD of 10. The Conners’ Parent Rating Scale (CPRS) (32) was also administered as a real-world indicator of attention abilities. CPRS Learning, Impulsive-Hyperactive, and Hyperactive scales were of interest for this study. These scales are age- and sex-standardized.
To assess learning and memory, the age-appropriate California Verbal Learning Test [CVLT-Child, CVLT-C for participants 6–16 years of age (33) and CVLT-Adult, CVLT-A for participants ≥17 years of age (34)] was administered. This verbal memory measure requires the child to recall a list of 15 (CVLT-C) or 16 (CVLT-A) words after each of five exposure trials, after exposure to an interference list, and after short and long delays. Age-standardized scores are provided. The Total recall score is the number of words recalled across five trials and is converted to a T score with a normative mean of 50 and SD of 10. In addition to this global score, variables representing rate of new learning with additional list exposure (Learning Slope), words recalled following a short delay (Short Delay Free Recall), and words recalled following a long delay (Long Delay Free Recall) were also examined. These scores were converted to Z scores, with a normative mean of 0 and SD of 1.
In addition to testing completed at week 120, an Estimated Intelligence Quotient was derived for each participant at study baseline using an abbreviated administration of the Wechsler Intelligence Scale. This score is computed from the Information, Similarities, and Block Design subtests from the WPPSI-R, WISC-III, or WAIS-III using a formula provided by Sattler (35). For children younger than three-and-a-half years of age at study baseline, for whom there is not an age-appropriate Wechsler Intelligence Scale, the Bayley Scales of Infant Development, Second Edition [BSID-II (36)] was administered. The BSID-II yields a global Mental Development Index. Both the Estimated Intelligence Quotient and Mental Development Index have an age-standardized mean of 100 and SD of 15. These scores were provided to characterize the sample at baseline.
Statistical Analyses
Analyses of demographic and clinical variables were performed to characterize the group and compare participants with and without cognitive data to establish group representativeness. For each cognitive measure, the percentage of the sample performing below the average range was calculated. Below-average performance was operationalized as a score more than 1 SD discrepant from the normative sample. χ2 analysis was used to compare these percentages with the expected 16%, based on the normative sample, to identify measures for which there is an excess of below-average performers.
To evaluate effects of treatment risk arm (low, standard/high), age at diagnosis (<5, ≥5 years), and sex, the mean of each cognitive measure for each subgroup was compared with the normative mean using a one-sample t-test. Further, cognitive scores among treatment risk arm, sex, and age at diagnosis subgroups were compared directly using Wilcoxon rank sum tests. Univariate logistic regression analyses were used to estimate the effect of risk arm, age at diagnosis, and sex on the probability of below-average performance. Finally, multivariable logistic regression models were used to investigate the independent effect of relevant clinical variables on probability of below-average performance, including cumulative dexamethasone dose (mg/m2), cumulative HDMTX dose (g/m2), cumulative ITMHA dose (mL; 1mL consisted of 1mg MTX, 2mg hydrocortisone, and 3mg cytarabine), age at diagnosis (<5, ≥5 years), and sex. The Hosmer–Lemeshow Goodness-of-Fit Test was used to test for goodness of fit for logistic regression models. Given the large range of cumulative chemotherapy exposures, odds ratios (OR) were based on doses grouped into therapeutically meaningful units (100mg for dexamethasone, 50mL for ITMHA, and 5g for HDMTX); these units roughly correspond to 2 weeks of dexamethasone treatment, 4–5 ITMHA doses, and 1–2 doses of HDMTX, respectively. All independent variables were entered and retained in each multivariate model.
All tests of statistical significance were two-sided. All P-values were adjusted within each test battery using the Holm–Bonferroni step-down method to address risk for Type I error. Analyses were conducted using SAS version 9.2 (http://www.sas.com; SAS Institute, Cary, NC).
Results
Group Characteristics
The study cohort was on average 6 years of age at the time of diagnosis, largely white, and balanced by sex and treatment risk arm (Table 1). Of 408 patients enrolled on the treatment protocol at St Jude Children’s Research Hospital, 339 participated in at least one cognitive assessment and 243 participated in the week 120 assessment. There were no statistically significant differences among the 339 patients participating in cognitive assessments, the 243 patients with a week 120 cognitive assessment, or the 69 patients without any cognitive assessment on relevant demographic (eg, age, sex, ethnicity) or clinical (eg, treatment risk arm) factors, suggesting that cognitive outcomes were representative of the entire group. Sample size varied for analyses based on age range for cognitive measures, subgroups of interest, and missing data (see Supplementary Table 1, available online).
Table 1.
Patient demographic and clinical characteristics*
| Characteristics | N (%) | Mean (SD) | Range |
|---|---|---|---|
| Sex | |||
| Boys | 131 (53.91) | ||
| Girls | 112 (46.09) | ||
| Ethnicity | |||
| White | 194 (79.84) | ||
| African American | 39 (16.05) | ||
| Other | 10 ( 4.12) | ||
| Risk arm | |||
| Low | 126 (51.85) | ||
| Standard/high | 117 (48.15) | ||
| Age at diagnosis, y | 243 | 6.56 (4.39) | 1.02–18.73 |
| Baseline IQ (EIQ) † | 117 | 101.42 (15.46) | 64.00–142.00 |
| Baseline Bayley MDI‡ | 33 | 92.12 (14.85) | 50.00–116.00 |
| HDMTX dose, g/m2 | |||
| Low-risk arm | 126 | 11.68 (2.14) | 3.56–18.04 |
| Standard/high-risk arm | 117 | 18.61 (3.64) | 7.40–29.30 |
| ITMHA dose, mL | |||
| Low-risk arm | 126 | 150.08 (69.75) | 94.00–856.00 |
| Standard/high-risk arm | 117 | 204.59 (50.12) | 80.00–375.00 |
| Dex dose, mg/m2 | |||
| Low-risk arm | 126 | 1008.18 (177.96) | 175.50–1302.59 |
| Standard/high-risk arm | 117 | 1212.61 (374.11) | 60.34–1690.13 |
* HDMTX = high-dose methotrexate; ITMHA = intrathecal methotrexate, hydrocortisone and ara-C; Dex = dexamethasone.
† EIQ = estimated IQ based on the Block Design, Similarities and Information subtests from the age-appropriate Wechsler scale.
‡ MDI = Mental Development Index from the Bayley Scales of Infant Development, Second Edition, administered to children up to three-and-a-half years of age in absence of an age-appropriate Wechsler scale.
Cognitive Performance
The results of χ2 analyses revealed that the entire ALL group did not differ statistically significantly from the normative sample with respect to percentage of below-average performers on measures of intellectual functioning (Wechsler scales, Full Scale Intelligence Quotient, Standard Score [SS] = 95.95, % below average = 22.94, 95% confidence interval [CI] = 17.68 to 28.91, P = .12), academic skills (WIAT, Math Reasoning, SS = 100.26, % below average = 11.66, 95% CI = 7.17 to 17.60, P = .80; Word Reading, SS = 100.77, % below average = 11.73, 95% CI = 7.21 to 17.71, P = .80; Spelling, SS = 98.92, % below average = 16.56, 95% CI = 11.21 to 23.18, P = 1.00), or verbal memory (CVLT-Total [T] = 50.15, % below average = 15.19, 95% CI = 9.98 to 21.75, P = 1.00; Learning Slope, Z = −.24, % below average = 17.95, 95% CI = 12.27 to 24.89, P = 1.00; Short Delay Free Recall, Z = 0.05, % below average = 10.26, 95% CI = 5.98 to 16.12, P = .72; Long Delay Free Recall, Z = −.04, % below average = 16.03, 95% CI = 10.65 to 22.74, P = 1.00). However, the entire ALL group differed statistically significantly from normative expectations on the measure of sustained attention (CPT Omissions, % = 84.00, % below average = 67.31, 95% CI = 59.35 to 74.59, P < .001; Hit Reaction Time, T = 48.97, % below average = 26.92, 95% CI = 20.14 to 34.60, P < .018; Variability, T = 58.70, % below average = 46.15, 95% CI = 38.15 to 54.31, P < .001; d′, T = 59.98, % below average = 44.87, 95% CI = 36.91 to 53.03, P < .001; β, T = 71.73, % below average = 63.46, 95% CI = 55.39 to 71.02, P < .001), with greater than 40% of the sample performing below the average range on 4 out of 5 indices.
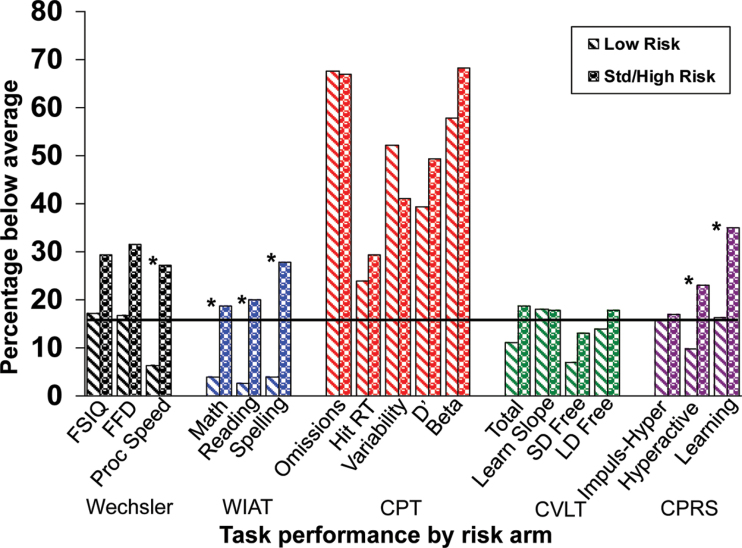
Treatment Intensity. One-sample t-tests indicated that patients on the low-risk arm performed statistically significantly better than the normative sample on Processing Speed (WISC-III) as well as Word Reading, Spelling, and Math Reasoning (WIAT, Table 2). However, patients on the low-risk arm had statistically significantly worse performance than the normative group with respect to sustained attention, including Omission errors, Reaction Time Variability, d′, and β on the CPT. In contrast, patients on the standard/high-risk arm had statistically significantly worse scores than the normative sample on Full Scale Intelligence Quotient, WISC-III Freedom from Distractibility and Processing Speed, and WIAT Spelling, in addition to worse performance than the normative group with respect to Omission errors, Reaction Time Variability, d′, and β on the CPT. Parents also reported statistically significantly higher levels of Learning Problems on the CPRS for children on the standard/high-risk arm (Table 2). Wilcoxon rank sum tests indicated that patients on the low-risk arm performed statistically significantly better than patients on the standard/high-risk arm on all Wechsler indices and all WIAT areas (Table 3). Parents also rated low-risk patients as having statistically significantly fewer problems with Hyperactivity and Learning Problems than standard/high-risk patients. Consistent with these findings, univariate logistic regression revealed a greater probability of below-average performance for patients treated on the standard/high-risk arm relative to the low-risk arm for Processing Speed (WISC-III: 27.14% vs 6.25%, P = .009), all WIAT scores (Math Reasoning: 18.60% vs 3.90%, P = .008; Word Reading: 20.00% vs 2.60%, P = .007; Spelling: 27.91% vs 3.90%, P = .001), as well as Hyperactivity (23.00% vs 9.84%, P = .018) and Learning Problems (35.00% vs 16.39%, P = .005) on the CPRS (Figure 1).
Table 2.
Subgroup performance relative to normative sample*
| Test | Treatment risk arm | Age at diagnosis | Sex | |||||||||||||||||||||
| Low | Standard/high | <5 years | ≥5 years | Boys | Girls | |||||||||||||||||||
| Mean (95% CI) | P† | Mean (95% CI) | P† | Mean (95% CI) | P† | Mean (95% CI) | P† | Mean (95% CI) | P† | Mean (95% CI) | P† | |||||||||||||
| Wechsler, SS‡ | ||||||||||||||||||||||||
| FSIQ | 98.1 (95.5 to 100.7) | .32 | 93.5 (90.3 to 96.7) | <.001 | 94.1 (91.3 to 97.0) | <.001 | 97.8 (94.8 to 100.7) | .27 | 95.3 (92.5 to 98.0) | .003 | 96.7 (93.6 to 99.8) | .12 | ||||||||||||
| FFD | 98.8 (95.5 to 102.1) | .47 | 93.3 (89.7 to 96.9) | <.001 | 93.7 (89.0 to 98.4) | .02 | 96.9 (94.1 to 99.8) | .12 | 95.3 (92.1 to 98.5) | .01 | 96.9 (93.0 to 100.8) | .23 | ||||||||||||
| Proc Speed | 104.8 (101.1 to 108.4) | .04 | 95.3 (91.1 to 99.6) | .03 | 101.5 (96.0 to 107.0) | .59 | 99.1 (95.7 to 102.6) | .62 | 97.1 (93.5 to 100.8) | .12 | 103.8 (99.0 to 108.6) | .23 | ||||||||||||
| WIAT, SS | ||||||||||||||||||||||||
| Math | 103.4 (100.6 to 106.2) | .017 | 97.4 (94.0 to 100.8) | .14 | 100.8 (97.0 to 104.6) | 1.00 | 100.0 (97.2 to 102.9) | 1.00 | 101.1 (98.1 to 104.1) | .96 | 99.1 (95.7 to 102.6) | .83 | ||||||||||||
| Reading | 105.5 (102.1 to 108.8) | .005 | 96.5 (93.2 to 99.8) | .08 | 102.7 (97.8 to 107.7) | .81 | 99.9 (97.1 to 102.7) | 1.00 | 99.9 (96.6 to 103.2) | .96 | 102.0 (98.4 to 105.6) | .83 | ||||||||||||
| Spelling | 104.3 (101.5 to 107.2) | .007 | 94.1 (91.0 to 97.2) | <.001 | 101.4 (97.2 to 105.6) | 1.00 | 97.8 (95.2 to 100.5) | .33 | 96.9 (94.0 to 99.8) | .11 | 101.7 (98.3 to 105.2) | .83 | ||||||||||||
| CPT, T§ | ||||||||||||||||||||||||
| Omissions, % | 82.7 (77.9 to 87.5) | <.001 | 85.1 (81.1 to 89.2) | <.001 | 81.2 (74.1 to 88.3) | <.001 | 85.0 (81.6 to 88.3) | <.001 | 84.0 (80.1 to 87.9) | <.001 | 84.0 (78.9 to 89.1) | <.001 | ||||||||||||
| Hit RT | 50.8 (47.7 to 53.8) | .63 | 47.5 (44.5 to 50.5) | .10 | 45.4 (41.1 to 49.7) | .038 | 50.2 (47.7 to 52.7) | .88 | 49.7 (46.9 to 52.5) | .85 | 47.9 (44.5 to 51.3) | .22 | ||||||||||||
| Variability | 58.4 (55.2 to 61.6) | <.001 | 59.0 (56.0 to 61.9) | <.001 | 60.3 (57.1 to 63.5) | <.001 | 58.1 (55.5 to 60.8) | <.001 | 57.5 (54.8 to 60.2) | <.001 | 60.4 (56.9 to 64.0) | <.001 | ||||||||||||
| d′ | 59.4 (57.0 to 61.7) | <.001 | 60.5 (58.2 to 62.8) | <.001 | 59.4 (56.4 to 62.3) | <.001 | 60.2 (58.2 to 62.2) | <.001 | 58.9 (57.2 to 60.7) | <.001 | 61.5 (58.3 to 64.6) | <.001 | ||||||||||||
| β | 68.0 (63.7 to 72.3) | <.001 | 74.9 (70.6 to 79.1) | <.001 | 63.6 (59.2 to 67.9) | <.001 | 74.5 (70.9 to 78.2) | <.001 | 71.6 (68.0 to 75.3) | <.001 | 71.8 (66.6 to 77.1) | <.001 | ||||||||||||
| CVLT, Z§ | ||||||||||||||||||||||||
| Total (T) | 50.9 (48.4 to 53.3) | .94 | 49.5 (46.7 to 52.4) | 1.00 | 46.0 (42.6 to 49.4) | .09 | 51.7 (49.5 to 54.0) | .37 | 49.2 (46.7 to 51.6) | .83 | 51.6 (48.6 to 54.6) | .59 | ||||||||||||
| Learning Slope | −0.2 (−0.5 to 0.1) | .43 | −0.3 (−0.5 to 0.0) | .08 | −0.1 (−0.6 to 0.3) | 1.00 | −0.3 (−0.5 to 0.1) | .013 | −0.3 (−0.5 to 0.0) | .11 | −0.2 (−0.5 to 0.1) | .37 | ||||||||||||
| SD Free Recall | 0.2 (−0.0 to 0.4) | .43 | −0.1 (−0.3 to 0.2) | 1.00 | −0.1 (−0.4 to 0.2) | 1.00 | 0.1 (−0.1 to 0.3) | .61 | −0.1 (−0.4 to 0.1) | .83 | 0.3 (0.0 to 0.6) | .14 | ||||||||||||
| LD Free Recall | 0.1 (−0.2 to 0.4) | .94 | −0.1 (−0.4 to 0.2) | 1.00 | −0.3(−0.8 to 0.1) | .37 | 0.1 (−0.2 to 0.3) | .61 | −0.1 (−0.4 to 0.1) | .83 | 0.1 (−0.2 to 0.4) | .59 | ||||||||||||
| CPRS, T§ | ||||||||||||||||||||||||
| Impulse-Hyper | 48.6 (46.8 to 50.3) | .22 | 51.8 (49.5 to 54.1) | .13 | 51.2 (49.1 to 53.2) | .46 | 48.8 (46.8 to 50.8) | .47 | 49.6 (47.5 to 51.6) | .97 | 50.5 (48.5 to 52.5) | 1.00 | ||||||||||||
| Hyperactive | 48.1 (46.4 to 49.9) | .10 | 52.8 (50.2 to 55.4) | .08 | 51.3 (49.2 to 53.4) | .46 | 49.0 (46.8 to 51.3) | .47 | 50.8 (48.6 to 52.9) | .97 | 49.6 (47.4 to 51.8) | 1.00 | ||||||||||||
| Learning | 50.3 (48.2 to 52.3) | .79 | 56.8 (53.6 to 60.1) | <.001 | 54.3 (51.5 to 57.1) | .008 | 52.1 (49.5 to 54.6) | .32 | 54.1 (51.3 to 56.9) | .01 | 52.3 (49.8 to 54.7) | .21 | ||||||||||||
* FSIQ = Full Scale Intelligence Quotient; FFD = Freedom from Distractibility Index; WIAT = Wechsler Individual Achievement Test; CPT = Conners’ Continuous Performance Test; RT = Reaction Time; CVLT = California Verbal Learning Test; SD = Short Delay; LD = Long Delay; CPRS = Conners’ Parent Rating Scale. Proc Speed = processing speed; Impulse-Hyper = Impulsivity–Hyperactivity Index; d′ = vigilance; β = risk taking; SS = standard score.
† P-values are based on a one-sample t-test compared with the published normative mean. They have been adjusted using the Holm–Bonferroni step-down method to account for multiple comparisons and reduce the risk of a Type I error. All tests of statistical significance were two-sided.
‡ Scores are reported as SS, which have a normative mean of 100 and SD of 15; T-scores, which have a normative mean of 50 and SD of 10; and Z-scores, which have a normative mean of 0 and SD of 1.
§ Indices on the CPT and CPRS are reverse cued such that a higher score is indicative of worse performance or greater problems.
Table 3.
Subgroup comparison by risk arm, age at diagnosis, and sex*
| Test | By treatment risk arm† | By age at diagnosis† | By sex | |||
| Difference of means (95% CI) | P‡ | Difference of means (95% CI) | P‡ | Difference of means (95% CI) | P‡ | |
| Wechsler, SS§ | ||||||
| FSIQ | 4.6 (0.6 to 8.7) | .03 | −3.6 (−7.7 to 0.4) | .37 | −1.5 (−5.6 to 2.7) | .34 |
| FFD | 5.5 (0.7 to 10.4) | .03 | −3.3 (−8.6 to 2.1) | .67 | −1.6 (−6.6 to 3.4) | .34 |
| Proc Speed | 9.4 (3.8 to 15.0) | .003 | 2.4 (−4.1 to 8.8) | .67 | −6.7 (−12.5 to −0.8) | .03 |
| WIAT, SS | ||||||
| Math | 6.0 (1.6 to 10.4) | .007 | 0.7 (−4.1 to 5.6) | 1.00 | 1.9 (−2.6 to 6.5) | .68 |
| Reading | 9.0 (4.3 to 13.6) | .001 | 2.9 (−2.4 to 8.1) | 1.00 | −2.1 (−7.0 to 2.8) | .68 |
| Spelling | 10.2 (6.0 to 14.4) | <.001 | 3.5 (−1.3 to 8.4) | 1.00 | −4.8 (−9.3 to −0.3) | .06 |
| CPT, T‖ | ||||||
| Omissions (%) | −2.4 (−8.6 to 3.7) | .30 | −3.8 (−10.8 to 3.3) | .25 | 0.0 (−6.2 to 6.3) | .74 |
| Hit RT | 3.3 (−1.0 to 7.6) | .80 | −4.8 (−9.6 to 0.1) | .11 | 1.8 (−2.5 to 6.2) | 1.00 |
| Variability | −0.5 (−4.9 to 3.8) | .80 | 2.2 (−2.8 to 7.1) | .35 | −2.9 (−7.3 to 1.4) | .63 |
| d′ | −1.1 (−4.4 to 2.2) | .80 | −0.8 (−4.6 to 3.0) | .52 | −2.6 (−5.9 to 0.8) | 1.00 |
| β | −6.9 (−12.9 to −0.9) | .15 | −11.0 (−17.7 to −4.2) | .025 | −0.2 (−6.4 to 6.0) | 1.00 |
| CVLT, Z | ||||||
| Total (T) | 1.4 (−2.4 to 5.2) | 1.00 | −5.7 (−9.8 to −1.6) | .006 | −2.4 (−6.3 to 1.4) | .30 |
| Learning Slope | 0.1 (−0.3 to 0.4) | 1.00 | 0.1 (−0.2 to 0.5) | .49 | −0.0 (−0.4 to 0.3) | .91 |
| SD Free Recall | 0.3 (−0.1 to 0.6) | 1.00 | −0.2 (−0.6 to 0.2) | .15 | −0.4 (−0.8 to −0.0) | .08 |
| LD Free Recall | 0.2 (−0.2 to 0.6) | 1.00 | −0.4 (−0.9 to 0.0) | .15 | −0.3 (−0.7 to 0.1) | .30 |
| CPRS, T | ||||||
| Impulse-Hyper | −3.2 (−6.1 to −0.4) | .05 | 2.4 (−0.5 to 5.2) | .25 | −0.9 (−3.8 to 1.9) | 1.00 |
| Hyperactive | −4.7 (−7.7 to −1.6) | .02 | 2.3 (−0.8 to 5.3) | .24 | 1.2 (−1.9 to 4.3) | 1.00 |
| Learning | −6.6 (−10.2 to −2.9) | .02 | 2.3 (−1.5 to 6.0) | .37 | 1.8 (−2.0 to 5.6) | 1.00 |
* FSIQ = Full Scale Intelligence Quotient; FFD = Freedom from Distractibility Index; WIAT = Wechsler Individual Achievement Test; CPT = Conners’ Continuous Performance Test; RT = Reaction Time; CVLT = California Verbal Learning Test; SD = Short Delay; LD = Long Delay; CPRS = Conners’ Parent Rating Scale; Proc Speed = processing speed; Impulse-Hyper = Impulsivity–Hyperactivity Index; d′ = vigilance; β = risk taking; SS = standard score.
† Treatment risk arm: low vs standard high; age: <5 y vs ≥5 y.
‡ P-values are based on Wilcoxon rank sum tests comparing subgroups. They have been adjusted using the Holm–Bonferroni step-down method to account for multiple comparisons and reduce the risk of a Type I error. All tests of statistical significance were two-sided.
§ Scores are reported as mean differences based on SS, which have a normative mean of 100 and SD of 15; T-scores, which have a normative mean of 50 and SD of 10; and Z-scores, which have a normative mean of 0 and SD of 1.
‖ Indices on the CPT and CPRS are reverse cued such that a higher score is indicative of worse performance or greater problems.
Figure 1.
Performance related to treatment risk arm. Black line indicates the expected rate of below-average scores (16%). The asterisk signifies a statistically significant difference in rate of below-average performance between low- and standard/high-risk arms based on univariate logistic regression. All statistical tests were two-sided. WIAT = Wechsler Individual Achievement Test; CPT = Conners’ Continuous Performance Test; CVLT = California Verbal Learning Test; CPRS = Conners’ Parent Rating Scale; FSIQ = Full Scale Intelligence Quotient; FFD= Freedom From Distractibility Index; RT = reaction time; SD = short delay; LD = long delay; Proc Speed = processing speed; Impuls-Hyper = Impulsivity–Hyperactivity Index.
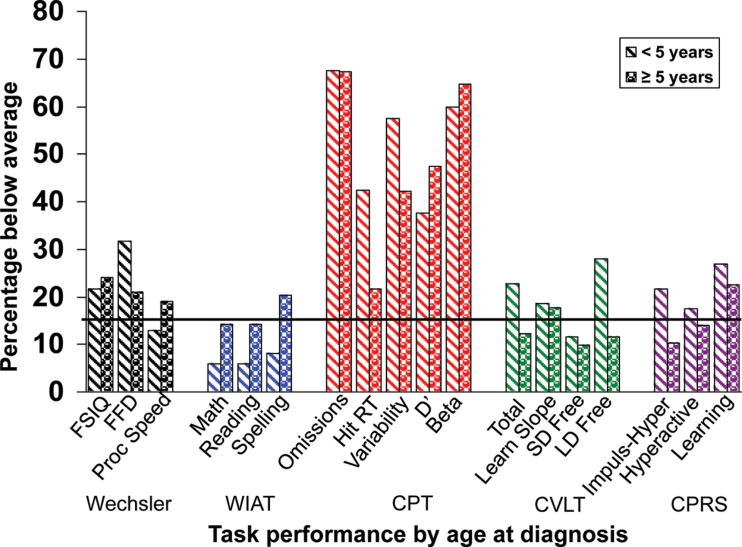
Age at Diagnosis. One-sample t-tests revealed that children diagnosed at a younger age (<5 years) had statistically significantly worse scores than the normative sample on Full Scale Intelligence Quotient (Wechsler FSIQ), WISC-III Freedom from Distractibility (Wechsler FFD), and all indices of sustained attention (CPT) (Table 2). Parents also rated these participants as having statistically significantly greater Learning Problems on the CPRS (Table 2). Children diagnosed at an older age (≥5 years) had statistically significantly worse scores than the normative sample on Omission errors, Reaction Time Variability, d′, and β on the CPT and Learning Slope on the CVLT (Table 2). Wilcoxon rank sum tests revealed that patients diagnosed at a younger age performed statistically significantly worse than children diagnosed at an older age on the Total score for the CVLT (Table 3). Furthermore, the younger age at diagnosis group had a statistically significantly less impulsive response style (β) on the CPT (Table 3). Univariate logistic regression did not reveal a greater probability for below-average performance on any cognitive measure for patients diagnosed at a younger age (Figure 2).
Figure 2.
Performance related to age at diagnosis. Black line indicates the expected rate of below-average scores (16%). All statistical tests were two-sided. WIAT = Wechsler Individual Achievement Test; CPT = Conners’ Continuous Performance Test; CVLT = California Verbal Learning Test; CPRS = Conners’ Parent Rating Scale; FSIQ = Full Scale Intelligence Quotient; FFD = Freedom From Distractibility Index; RT = reaction time; SD = short delay; LD = long delay; Proc Speed = processing speed; Impuls-Hyper = Impulsivity–Hyperactivity Index.
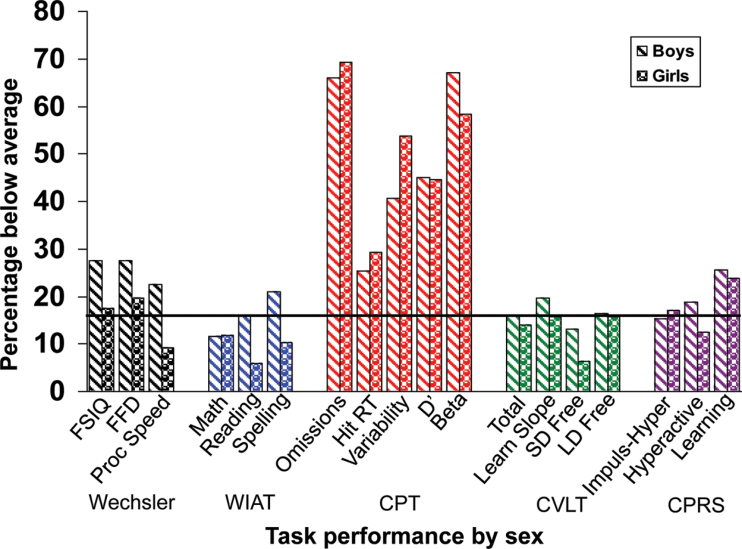
Sex. One-sample t-tests revealed that male participants had statistically significantly worse scores than the normative sample on FSIQ and WISC-III Freedom from Distractibility, and Omission errors, Reaction Time Variability, d′, and β (CPT, Table 2). Parents also rated boys as having statistically significantly greater Learning Problems on the CPRS. Girls performed statistically significantly worse than the normative sample on Omission errors, Reaction Time Variability, d′, and β on the CPT (Table 2). Wilcoxon rank sum tests revealed that girls performed statistically significantly better than boys on Processing Speed (WISC-III, Table 3). Univariate logistic regression did not reveal any differences in risk for below-average performance based on sex for any cognitive measure (Figure 3).
Figure 3.
Performance related to sex. Black line indicates the expected rate of below-average scores (16%). All statistical tests were two-sided. WIAT = Wechsler Individual Achievement Test; CPT = Conners’ Continuous Performance Test; CVLT = California Verbal Learning Test; CPRS = Conners’ Parent Rating Scale; FSIQ = Full Scale Intelligence Quotient; FFD = Freedom From Distractibility Index; RT = reaction time; SD = short delay; LD = long delay; Proc Speed = processing speed; Impuls-Hyper = Impulsivity–Hyperactivity Index.
Multivariable Models. Multivariable logistic regression models, accounting for sex and cumulative dose of ITMHA, MTX, and dexamethasone, revealed that participants treated at a younger age (<5 years) were at a statistically significantly increased risk (three- to fourfold) for below-average performance on Reaction Time (CPT, OR = 3.91, 95% CI = 1.65 to 9.30, P = .010) and Long Delay Free Recall (CVLT, OR = 4.64, 95% CI = 1.69 to 12.71, P = .01; Table 4). Multivariable models did not identify any statistically significant, independent risk for sex, cumulative dexamethasone, cumulative ITMHA, or cumulative HDMTX dose on any of the cognitive outcome variables.
Table 4.
Multivariable logistic regression of patient and clinical characteristics and cognitive performance*
| Test | Age at diagnosis† | Sex | Cumulative dexamethasone† | Cumulative ITMHA† | Cumulative HDMTX† | |||||
| OR (95% CI) | P‡ | OR (95% CI) | P‡ | OR (95% CI) | P‡ | OR (95% CI) | P‡ | OR (95% CI) | P‡ | |
| Wechsler, SS | ||||||||||
| FSIQ | 1.09 (0.57 to 2.10) | 1.00 | 0.60 (0.31 to 1.16) | .38 | 0.98 (0.88 to 1.09) | .68 | 1.21 (0.95 to 1.53) | .37 | 1.16 (0.79 to 1.71) | .46 |
| FFD | 2.63 (1.04 to 6.65) | .12 | 0.68 (0.28 to 1.65) | .40 | 0.94 (0.83 to 1.06) | .57 | 1.18 (0.73 to 1.91) | .51 | 1.62 (0.91 to 2.89) | .22 |
| Proc Speed | 1.18 (0.36 to 3.85) | 1.00 | 0.45 (0.14 to 1.37) | .38 | 0.90 (0.78 to 1.03) | .37 | 1.48 (0.89 to 2.48) | .37 | 1.84 (0.94 to 3.60) | .22 |
| WIAT, SS | ||||||||||
| Math | 0.56 (0.14 to 2.16) | .80 | 1.60 (0.55 to 4.65) | .41 | 0.96 (.83 to 1.10) | .58 | 1.58 (0.95 to 2.62) | .23 | 1.44 (0.78 to 2.67) | .74 |
| Reading | 0.57 (0.15 to 2.19) | .80 | 0.40 (0.12 to 1.34) | .41 | 0.90 (0.78 to 1.04) | .49 | 1.40 (0.84 to 2.34) | .39 | 1.31 (0.69 to 2.46) | .74 |
| Spelling | 0.44 (0.14 to 1.41) | .51 | 0.52 (0.20 to 1.38) | .41 | 0.93 (0.82 to 1.06) | .58 | 1.19 (0.76 to 1.86 | .46 | 1.37 (0.79 to 2.38) | .74 |
| CPT, T | ||||||||||
| Omissions, % | 1.03 (0.46 to 2.30) | 1.00 | 1.28 (0.62 to 2.65) | 1.00 | 1.05 (0.95 to 1.17) | 1.00 | 1.02 (0.69 to 1.51) | 1.00 | 1.05 (0.67 to 1.65) | 1.00 |
| Hit RT | 3.91 (1.65 to 9.30) | .01 | 1.38 (0.62 to 3.07) | 1.00 | 0.94 (0.84 to 1.05) | 1.00 | 1.29 (0.84 to 1.96) | 1.00 | 1.54 (0.94 to 2.52) | .42 |
| Variability | 1.78 (0.83 to 3.86) | .56 | 1.65 (0.83 to 3.27) | .77 | 0.99 (0.90 to 1.10) | 1.00 | 0.85 (0.58 to 1.24) | 1.00 | 1.29 (0.84 to 2.00) | .75 |
| d′ | 0.73 (0.34 to 1.59) | 1.00 | 1.09 (0.55 to 2.16) | 1.00 | 0.97 (0.88 to 1.07) | 1.00 | 1.18 (0.82 to 1.71) | 1.00 | 1.03 (0.67 to 1.58) | 1.00 |
| β | 0.00 | 1.00 | 0.00 | 1.00 | 0.84 (0.42 to 1.67) | 1.00 | 2.90 (0.25 to 33.88) | 1.00 | 0.05 (0.00 to 4.14) | .72 |
| CVLT, Z | ||||||||||
| Total (T) | 3.02 (1.11 to 8.19) | .09 | 1.12 (0.42 to 3.02) | 1.00 | 1.06 (0.91 to 1.22) | .88 | 1.12 (0.66 to 1.90) | 1.00 | 1.78 (1.01 to 3.14) | .19 |
| Learning Slope | 1.03 (0.40 to 2.66) | .96 | 0.75 (0.31 to 1.84) | 1.00 | 1.07 (0.93 to 1.23) | .88 | 1.00 (0.62 to 1.62) | 1.00 | 0.80 (0.46 to 1.40) | .44 |
| SD Free Recall | 1.88 (0.56 to 6.35) | .62 | 0.48 (0.14 to 1.70) | 1.00 | 0.92 (0.80 to 1.07) | .88 | 1.25 (0.70 to 2.25) | 1.00 | 1.54 (0.80 to 2.93) | .44 |
| LD Free Recall | 4.64 (1.69 to 12.71) | .01 | 0.98 (0.37 to 2.61) | 1.00 | 0.92 (0.81 to 1.05) | .80 | 1.38 (0.81 to 2.33) | .94 | 1.51 (0.87 to 2.64) | .44 |
| CPRS, T | ||||||||||
| Impulse-Hyper | 2.51 (1.13 to 5.60) | .07 | 0.91 (0.43 to 1.92) | 1.00 | 0.91 (0.79 to 1.04) | .52 | 0.94 (0.68 to 1.30) | 1.00 | 1.10 (0.71 to 1.71) | .66 |
| Hyperactive | 1.69 (0.78 to 3.67) | .37 | 0.63 (0.28 to 1.37) | .73 | 1.00 (0.87 to 1.14) | 1.00 | 1.28 (0.98 to 1.69) | .22 | 1.26 (0.81 to 1.95) | .62 |
| Learning | 1.45 (0.76 to 2.75) | .37 | 0.93 (0.49 to 1.76) | 1.00 | 0.98 (0.88 to 1.10) | 1.00 | 1.04 (0.83 to 1.31) | 1.00 | 1.40 (0.97 to 2.02) | .23 |
* FSIQ = Full Scale Intelligence Quotient; FFD = Freedom from Distractibility Index; WIAT = Wechsler Individual Achievement Test; CPT = Conners’ Continuous Performance Test; RT = Reaction Time; CVLT = California Verbal Learning Test; SD = Short Delay; LD = Long Delay; CPRS = Conners’ Parent Rating Scale. Proc Speed = processing speed; Impulse-Hyper = Impulsivity–Hyperactivity Index; d′ = vigilance; β = risk taking; SS = standard score; OR = odds ratio; CI = confidence interval. Odds ratios represent the effects of being <5 years old at the time of diagnosis (referent = ≥5y), female (referent = male), cumulative dexamethasone (per 100mg/m2), cumulative ITMHA (per 50mL) and cumulative HDMTX (per 5g/m2). Dexamethasone, ITMHA, and HDMTX were categorized into therapeutically meaningful units that roughly correspond to 2wk of dexamethasone treatment, 4–5 ITMHA doses, and 1–2 doses of HDMTX, respectively.
† All clinical factors, age at diagnosis (<5 vs ≥5 y), sex, cumulative dexamethasone (per 100mg/m2), ITMHA (per 50mL), and HDMTX (per 5g/m2), were included in final multiple logistic regressions.
‡ P-values have been adjusted using the Holm–Bonferroni step-down method to account for multiple comparisons and reduce the risk of a Type I error. All tests of statistical significance were two-sided.
Given that below-average performance on the CPT was the most common finding, post hoc analyses were conducted to evaluate the relationship between this performance measure of attention and parent report of attention problems. Pearson correlation coefficients revealed a small but statistically significant relationship between Reaction Time Variability on the CPT and Hyperactivity on the CPRS (r = .18, P = .037) and trends for relationships between Omission errors on the CPT and Hyperactivity on the CPRS (r = .15, P = .078) and Learning Problems on the CPRS (r = .14, P = .093).
Discussion
Overall, study findings were consistent with a priori hypotheses. Omission of prophylactic cranial irradiation from ALL treatment resulted in generally well-preserved cognitive abilities for the group as a whole relative to normative expectations. The entire sample performed well on global measures of cognitive ability without evidence of excess impairment on measures of intellectual functioning, academic abilities, and learning and memory. This contrasts with historical experience with patients receiving 18–24 Gy of cranial irradiation (4,5,7,9,14,37). Problems with sustained attention emerged as the most prominent deficit, with below-average performance in approximately 40% of the sample irrespective of sex, age at treatment, or treatment intensity. This limited association among attention measures and treatment and demographic factors suggests other sources for variability, such as genetic polymorphisms related to pharmacokinetics or pharmacodynamics of chemotherapy.
Higher treatment intensity with chemotherapy was associated with worse performance on measures of processing speed and academics, as well as greater parent report of learning problems. Hence, additional follow-up with potential therapeutic intervention is warranted, especially for half of our patients who received intensive chemotherapy. Younger age at treatment also remained predictive of worse performance on selective indices of sustained attention and memory. The effect of sex was negligible and in the opposite direction of the existing literature that suggests female sex as a risk factor. It may be that sex differences are specific to assessed skill and less likely to be found on language-based measures, for which there is evidence of developmental advantages for girls (37–39).
It is important to note that not all statistically significant findings have clinical impact. Using clinical thresholds to classify average and below-average performers based on normative expectations assists in interpreting study findings. The statistically significantly elevated rate of impairment on a computerized measure of sustained attention, coupled with parent report of attention and learning problems, suggests that attention difficulties are of noteworthy magnitude and affect real-world functioning. These findings corroborated those of a previous study (26), which included a subset of patients from this study, demonstrating similar proportions of below-average performers on a different measure of attention and working memory, with performance related to leukoencephalopathy on neuroimaging.
Current findings should be interpreted in the context of study limitations. Data analyses were cross-sectional, examining cognitive outcomes two-and-a-half years following remission induction. Longitudinal studies including longer term outcomes would allow for further examination of persistence and/or exacerbation of deficits over time. Early emerging attention problems may result in later emerging declines in intellectual functioning and academic achievement (40). It will also be important to compare the cognitive findings of this study with those from other contemporary trials using lower doses of cranial irradiation (eg, 12 Gy), once those results become available. The current study utilized gold standard assessment measures with large normative samples; however, inclusion of a medical control group would allow for isolation of effects of central nervous system–directed treatment from other illness-related experiences (eg, prolonged absences from school). Finally, ecological measures, such as school grades or provision of special education services, would allow for fuller understanding of the clinical impact of cognitive late effects.
The findings of this study highlight the benefits of risk-adapted therapy omitting cranial irradiation but also indicate that treatment with chemotherapy alone is not without risks. Attention as a core and primary cognitive late effect of modern therapy is consistent with the existing literature (14,41,42) as well as findings implicating vulnerability of cerebral white matter to central nervous system–directed therapy (43,44). Post-mortem histological findings (45), with convergent support from structural and functional neuroimaging studies (46,47), suggest that frontal-subcortical pathways continue to develop into the third decade of life, as indicated by ongoing myelination and pruning. Given protracted developmental myelination of the prefrontal cortices and dependence of attention on these brain regions, these skills may be particularly vulnerable to treatment-related neurotoxicity (26,48).
White matter abnormalities in the frontal lobes have been reported in leukemia patients during treatment with MTX (44), and leukoencephalopathy during active therapy appears related to attention and working memory problems detected at completion of therapy (26). Transient changes in white matter may be the result of demyelination, which could lead to decreased axonal density and brain atrophy. Reddick et al. (43) found that ALL survivors who receive chemotherapy alone have white matter volumes larger than ALL patients who receive cranial irradiation but smaller than that of healthy sibling control participants. In this same study (43), smaller white matter volumes were associated with larger deficits in attention, IQ, and academic achievement. In a more recent diffusion tensor imaging study (49), the nine survivors of childhood ALL treated only with chemotherapy had persistently lower white matter volume in frontal lobes than 14 healthy control participants. Furthermore, this reduction in white matter volume corresponded to deficits in attention, visual-constructional skills, mental flexibility, and mathematics.
Current findings indicate that monitoring for late effects and caregiver education should focus on attention abilities early in the survivorship period. Furthermore, development of interventions that address cognitive late effects is imperative. There is encouraging empirical support for emerging pharmacologic and nonpharmacologic remediation approaches targeting attention concerns (50–52).
Funding
This work was supported, in part, by the National Cancer Institute (P30 CA21765 and GM92666 to St Jude Children's Research Hospital, and R01 CA90246 to WER) and the American Lebanese Syrian Associated Charities (ALSAC).
Notes
The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication. We are particularly thankful for the children and families who donated their time to participate in this study. We also wish to thank the psychological examiners who conducted the study assessments: Maggi R. Dunavant, Charlotte L. Fineberg-Buchner, and David B. Hopper.
References
- 1. Moricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL_BFM 95 Blood. 2008;111(9):4477–4489 [DOI] [PubMed] [Google Scholar]
- 2. Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia Blood. 2007;109(3):896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital Blood. 2004;104(9):2690–2696 [DOI] [PubMed] [Google Scholar]
- 4. Copeland DR, Fletcher JM, Pfefferbaum-Levine B, et al. Neuropsychological sequelae of childhood cancer in long-term survivors Pediatrics. 1985;75:745–753 [PubMed] [Google Scholar]
- 5. Rowland JH, Glidewell OJ, Sibley RF, et al. Effects of different forms of central nervous system prophylaxis on neuropsychologic function in childhood leukemia J Clin Oncol. 1984;2(12):1327–1335 [DOI] [PubMed] [Google Scholar]
- 6. Jankovic M, Brouwers P, Valsecchi MG, et al. Association of 1800cGy with intellectual function in children with acute lymphoblastic leukaemia Lancet. 1994;344(8917):224–227 [DOI] [PubMed] [Google Scholar]
- 7. Langer T, Martus P, Ottensmeier H, et al. CNS late effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory Ed Pediatr Oncol. 2002;38(5):320–328 [DOI] [PubMed] [Google Scholar]
- 8. Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy Neuropsychol. 2000;14(2):189–200 [DOI] [PubMed] [Google Scholar]
- 9. Spiegler BJ, Kennedy K, Maze R, et al. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate J Clin Oncol. 2006;24(24):3858–3864 [DOI] [PubMed] [Google Scholar]
- 10. Waber DP, Turek J, Catania L, et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 J Clin Oncol. 2007;25(31):4914–4921 [DOI] [PubMed] [Google Scholar]
- 11. Janzen LA, Spiegler BJ. Neurodevelopmental sequelae of pediatric acute lymphoblastic leukemia and its treatment Dev Disabil Res Rev. 2008;14(3):185–195 [DOI] [PubMed] [Google Scholar]
- 12. Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia Lancet Oncol. 2008;9(3):257–268 [DOI] [PubMed] [Google Scholar]
- 13. Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation N Engl J Med. 2009;360(26):2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia Pediatr Blood Cancer. 2007;49(1):65–73 [DOI] [PubMed] [Google Scholar]
- 15. Moore IM, Kramer JH, Wara W, et al. Cognitive function in children with leukemia: effect of radiation dose and time since radiation Cancer. 1991;68(9):1913–1917 [DOI] [PubMed] [Google Scholar]
- 16. Simbert E, Anderson V, Godber T, et al. Risk factors for intellectual and educational sequelae of cranial irradiation in childhood acute lymphoblastic leukaemia Br J Cancer. 1996;73(6):825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, et al. Visuomotor control in survivors of childhood acute lymphoblastic leukemia: Effect of treatment intensity J Int Neuropsychol Soc. 2005;11(5):554–565 [DOI] [PubMed] [Google Scholar]
- 18. Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, et al. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: Effect of treatment intensity Pediatr Blood Cancer. 2005;45(3):281–290 [DOI] [PubMed] [Google Scholar]
- 19. Robison LL, Nesbit ME, Sather HN, et al. Factors associated with IQ scores in long-term survivors of childhood acute lymphoblastic leukemia Am J Pediatr Hematol/Oncol. 1984;6(2):115–1–21 [DOI] [PubMed] [Google Scholar]
- 20. Waber DP, Urion DK, Tarbell NJ, et al. Late effects of central nervous system treatment of acute lymphoblastic leukemia in childhood are sex-dependent Dev Med Child Neurol. 1990;32(3):238–248 [DOI] [PubMed] [Google Scholar]
- 21. De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence Cereb Cortex. 2001;11(6):552–557 [DOI] [PubMed] [Google Scholar]
- 22. Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls Hum Brain Mapp. 2008;29(6):696–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mulhern RK, Fairclough D, Ochs J. A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18-Gy, 24-Gy or no cranial irradiation J Clin Oncol. 1991;9(8):1348–13–56 [DOI] [PubMed] [Google Scholar]
- 24. Schlieper AE, Esseltine DW, Tarshis MA. Cognitive function in longterm survivors of childhood acute lymphoblastic leukemia Pediatr Hematol Oncol. 1989;6(1):1–9 [DOI] [PubMed] [Google Scholar]
- 25. Jain N, Brouwers P, Okcu MF, et al. Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia Cancer. 2009;115(18):4238–4245 [DOI] [PubMed] [Google Scholar]
- 26. Ashford J, Schoffstall C, Reddick WE, et al. Attention and working memory abilities in children treated for acute lymphoblastic leukemia Cancer. 2010;116(9):4638–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence, Revised. San Antonio, TX:: Psychological Corporation;; 1989.. [Google Scholar]
- 28. Wechsler D. Wechsler Intelligence Scale for Children.3rd ed. San Antonio, TX: Psychological Corporation; 1991.. [Google Scholar]
- 29. Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: Psychological Corporation; 1997.. [Google Scholar]
- 30.Wechsler D. Wechsler Individual Achievement Test. New York, NY:: Psychological Corporation;; 1992.. [Google Scholar]
- 31.Conners CK. Conners’ Continuous Performance Test. Toronto, Ontario:: Multi-Health Systems;; 2000.. [Google Scholar]
- 32.Conners CK. Conners’ Rating Scales–Revised Technical Manual. North Tonawanda, New York:: Multi Health Systems;; 2000.. [Google Scholar]
- 33. Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test: Children’s Version New York, NY: Harcourt; 1994.. [Google Scholar]
- 34.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test: Adult Version. New York, NY:: Psychological Corporation;; 1987.. [Google Scholar]
- 35. Sattler JM. Assessment of Children 3rded. San Diego, CA: Jerome M. Sattler, Publisher, Inc; 1992.. [Google Scholar]
- 36. Bayley N. Bayley Scales of Infant Development.2nd ed. San Antonio, TX: The Psychological Corporation; 1993.. [Google Scholar]
- 37. Bauer DJ, Goldfield BA, Reznic JS. Alternative approaches to analyzing individual differences in the rate of early vocabulary development Appl Psycholing. 2002;23(3):313–3–35 [Google Scholar]
- 38. Burman DD, Bitan T, Booth JR. Sex differences in neural processing of language among children Neuropsychologia. 2008;46(5):1349–13–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roulstone S, Loader S, Northsone K. Descriptive data from the Avon longitudinal study of parents and children Early Child Dev Care. 2002;172(3):259–268 [Google Scholar]
- 40. Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors Cancer. 2003;97(10):2512–251–9 [DOI] [PubMed] [Google Scholar]
- 41. Kahalley LS, Conklin HM, Tyc VL, et al. ADHD and secondary ADHD criteria fail to identify many at-risk survivors of pediatric ALL and brain tumor Ped Blood Cancer. 2011;57(1):110–11–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krull Kr, Khan RB, Ness KK, et al. Symptoms of attention-deficit/hyperactivity disorder in long-term survivors of childhood luekemia Ped Blood Cancer. 2011;57(7):1191–119–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia Cancer. 2006;106(4):941–94–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reddick WE, Glass JO, Helton KJ, et al. A quantitative MR imaging assessment of leukoencephalopathy in children treated for acute lymphoblastic leukemia without irradiation Am J Neuroradiol. 2005;26(9):2371–237–7 [PMC free article] [PubMed] [Google Scholar]
- 45. Huttenlocher PR. Synaptic density in human frontal cortex-developmental changes and effects of aging Brain Res. 1979;163(2):195–201 [DOI] [PubMed] [Google Scholar]
- 46. Giedd JN. Structural magnetic resonance imaging of the adolescent brain Ann N Y Acad Sci. 2004;1021:77–85 [DOI] [PubMed] [Google Scholar]
- 47. Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain during postadolescent brain maturations J Neurosci. 2001; 21(22):8819–88–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reddick WE, Conklin HM. Impact of acute lymphoblastic leukemia therapy on attention and working memory in children Expert Rev Hematol. 2010;3(6):655–66–0 [DOI] [PubMed] [Google Scholar]
- 49. Carey ME, Haut MW, Reminger SL, et al. Reduced frontal white matter volume in long-term childhood leukemia survivors: A voxel-based morphometry study Am J Neuroradiol. 2008;29(4):792–79–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Conklin HM, Reddick WE, Ashford J, et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors J Clin Oncol. 2010;28(29):4465–44–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of cognitive remediation program for childhood survivors of pediatric malignancy J Consult Clin Psychol. 2008;76(3):367–3–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hardy KK, Willard VW, Bonner MJ. Computerized cognitive training in survivors of childhood cancer: a pilot study J Pediatr Oncol Nurs. 2011;28(1):27–33 [DOI] [PubMed] [Google Scholar]