Abstract
Background
The Prostate Cancer Prevention Trial (PCPT)—a randomized placebo-controlled study of the efficacy of finasteride in preventing prostate cancer—offered the opportunity to prospectively study effects of finasteride and other covariates on the health-related quality of life of participants in a multiyear trial.
Methods
We assessed three health-related quality-of-life domains (measured with the Health Survey Short Form–36: Physical Functioning, Mental Health, and Vitality scales) via questionnaires completed by PCPT participants at enrollment (3 months before randomization), at 6 months after randomization, and annually for 7 years. Covariate data obtained at enrollment from patient-completed questionnaires were included in our model. Mixed-effects model analyses and a cross-sectional presentation at three time points began at 6 months after randomization. All statistical tests were two-sided.
Results
For the physical function outcome (n = 16 077), neither the finasteride main effect nor the finasteride interaction with time were statistically significant. The effects of finasteride on physical function were minor and accounted for less than a 1-point difference over time in Physical Functioning scores (mixed-effect estimate = 0.07, 95% confidence interval [CI] = −0.28 to 0.42, P = .71). Comorbidities such as congestive heart failure (estimate = −5.64, 95% CI = −7.96 to −3.32, P < .001), leg pain (estimate = −2.57, 95% CI = −3.04 to −2.10, P < .001), and diabetes (estimate = −1.31, 95% CI = −2.04 to −0.57, P < .001) had statistically significant negative effects on physical function, as did current smoking (estimate = −2.34, 95% CI = −2.97 to −1.71, P < .001) and time on study (estimate = −1.20, 95% CI = −1.36 to −1.03, P < .001). Finasteride did not have a statistically significant effect on the other two dependent variables, mental health and vitality, either in the mixed-effects analyses or in the cross-sectional analysis at any of the three time points.
Conclusion
Finasteride did not negatively affect SF–36 Physical Functioning, Mental Health, or Vitality scores.
Research addressing the effect of finasteride, an inhibitor of 5α-reductase (the enzyme that converts testosterone to dihydrotestosterone), on health-related quality of life has been conducted primarily in the context of two medical conditions: benign prostatic hyperplasia and alopecia. Three studies that addressed the effects of finasteride on benign prostatic hyperplasia (1–3) were generally well-powered, with a measurement focus on disease-specific symptoms; improvement associated with finasteride was observed for disease-specific symptoms (except for sexual function) and impact on daily activities or bother items. Less change was observed in these studies for more general measures of health-related quality of life, such as global health-related quality of life, life satisfaction, and worry or concern. Two small studies addressed the impact of finasteride on depression among patients treated for alopecia: one study reported moderate to severe depression or mood disturbance in 19 of 23 patients (4); the other, a single-arm study with 144 patients, reported a statistically significant increase in two measures of depression (ie, more depression) 2 months after treatment with finasteride (5). However, the results of the alopecia studies were preliminary and require confirmation in more patients.
The Prostate Cancer Prevention Trial (PCPT) was a 7-year double-blind, placebo-controlled randomized trial of finasteride as a preventive agent for prostate cancer (6–9). There was a 24.8% reduction in the prevalence of prostate cancer over the 7-year trial duration associated with finasteride treatment (9). In that initial report (9), finasteride was associated with increased sexual dysfunction; however, a subsequent publication demonstrated that the level of sexual dysfunction was minimal and decreased over time (10).
Given the sparse data regarding long-term finasteride use and health-related quality of life, we felt that it was important to monitor this outcome in the PCPT. We have previously reported the baseline health-related quality of life of men who entered the PCPT (11) and on changes in sexual functioning according to PCPT study arm assignment (10). In this report, we examine the effect of finasteride treatment on health-related quality of life, with particular focus on physical function results, over the course of the 7-year study. In this article, we refer to the physical function domain as measured by the Physical Functioning scale.
Subjects and Methods
Participants
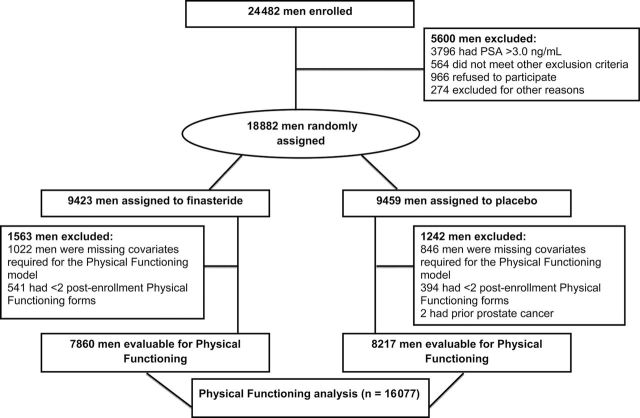
The PCPT enrolled 24 482 men between October 13, 1993, and January 9, 1997. After a 3-month placebo-only run-in period, 18 882 men were randomly assigned to finasteride or placebo. Study criteria required that men were aged 55 years or older at randomization; additional inclusion criteria are described in the primary article (9). The PCPT protocol and consent forms were approved by the institutional review board of each participating research site, and all men who participated in the PCPT provided written informed consent. Figure 1 shows the CONSORT diagram for the sample of participants considered in this analysis of health-related quality of life. The analysis examined the 7-year longitudinal change among PCPT participants in three health-related quality-of-life domains: physical function, mental health, and vitality. However, because results for the mixed-effects models for the three domains were similar, we present only the physical function mixed model results and cross-sectional results. To be included in the physical function analysis, participants were required to have had all covariates measured at time of enrollment (age, race, body mass index, smoking status, Physical Functioning Scale, Mental Health Scale, Vitality Scale, Bodily Pain Scale, education level, marital status, diabetes, hypertension, American Urological Association Symptom Index score, Sexual Activities Scale score, leg pain, and congestive heart failure); in addition, a minimum of two postrandomization Physical Function measures were required. These constraints yielded a sample size of 16 077 participants (Figure 1).
Figure 1.
CONSORT flow diagram for Prostate Cancer Prevention Trial participants who were included in the health-related quality-of-life analyses. The characteristics of these participants are described in Table 1. A total of 16 077 participants had the following characteristics and were included in the Physical Functioning analysis: a Physical Functioning enrollment score and a Physical Functioning score available for at least two subsequent time points. Tables 2 and 3 include all participants who submitted a specific Health Survey Short Form–36 at each time point.
Participants completed questionnaires about their health-related quality of life at enrollment, at 6 months after randomization, and annually for 7 years; health behavior covariates were collected at enrollment only (11). Health-related quality-of-life outcome measures and covariates are briefly described below; more detailed information can be found in other publications (9–11).
Health-Related Quality-of-Life Outcomes and Study Covariates
Health-related quality of life was a secondary endpoint in the PCPT (11) and was measured by the Short Form–36 questionnaire (SF–36) (12–15). The SF–36 allows calculation of eight scales representing different physical and mental health areas (including the Physical Functioning, Mental Health, Vitality, and Bodily Pain scales used in this analysis) and two overall summary measures produced by combining the scores on the eight different scales. The individual scales were preferred over the summary measures for this analysis because each represents a single health area; we wanted to assess potential changes due to treatment over time in each health area without having a negative change in one area obscured by a positive change in another. Individual scales are based on responses to different numbers of questions (range = 1–10 questions). The Physical Functioning and Mental Health scales were considered primary outcomes for this analysis because they could reflect the impact of finasteride on two key aspects of health-related quality of life, and they have excellent measurement properties (12–15); the Vitality scale was examined as a secondary outcome. We also considered the SF–36 Physical Functioning, Mental Health, Vitality, and Bodily Pain measures as covariates. Each SF–36 measure was scored on a scale of 0–100, with higher scores reflecting better health-related quality of life.
Demographic, Clinical Status, Health Behavior, and Genitourinary Status Covariates at Enrollment. Self-reported race was coded as white (non-Hispanic), black (regardless of ethnicity), or other. Education level and marital status were also self-reported. Age at enrollment (in years) was calculated by subtracting the participant’s birth date from the date of enrollment. Data describing body mass index (BMI) and comorbidities (diabetes, hypertension, congestive heart failure, leg pain) were collected by clinical research associates. Bodily pain was measured with the SF–36 scale.
Metabolic equivalent tasks in hours (MET-hours) of physical activity were calculated by assigning specific calorie-burning rates to walking speeds that were self-reported in the health behaviors questionnaire (16–18). A health behaviors questionnaire also collected information on self-reported consumption of beer, wine, and liquor; items were summed to determine drinks per day. Total weekly fat intake in grams was calculated with the Block Diet Screener with the substitution of the nine response categories from the Food Frequency Questionnaire (19). Three categories of smoking status were identified at enrollment and used instead of pack-years (E.R. Gritz, personal communication): current smoker, smoker who quit, and nonsmoker (reference category).
The four-item Sexual Activity Scale (0–100 response scale) was developed for the PCPT and is described in two previous publications (10,11). The American Urological Association (AUA) Symptom Index (20,21) score can range from 0 to 35.
Statistical Methods
Questionnaire submission rates were calculated for each treatment arm by dividing the number of questionnaires submitted by the number of participants who were expected to complete the questionnaire. Scores for each SF–36 scale were calculated according to the instructions provided in the SF–36 manual (14). A hierarchical model was ultimately selected for this analysis. Each participant had a curve fit to his scores over time, and then the parameters of the curve were modeled using covariates of interest. The fit of the curve and modeling of covariates were performed simultaneously as a mixed-effects model, with the curve parameters as random effects and the covariates as fixed effects, using SAS software (version 9.2; SAS Institute Inc, Cary, NC). This method assumes the random effects follow a normal distribution and that missing data are missing at random. The discussion of how these assumptions were tested follows.
Non-normally Distributed Data and Curve Selection. Mean values for the Physical Functioning, Mental Health, and Vitality measures were high in the PCPT population (see Table 2), and the individual scales were slightly skewed. Plots of individual scale trajectories over time generally appeared linear. Therefore, two different curves were considered: a hockey stick model (21), using all scores for a participant over time (pretreatment [enrollment] and posttreatment) with a parameter for a posttreatment change in slope, and a model using posttreatment scores to fit a line with the pretreatment (enrollment) score used as a covariate. The normality of the curve parameters was assessed for each model type. Results of these preliminary models suggested that the line using the enrollment score as a covariate was more appropriate for the physical function outcome; there was no difference between the models for the mental health outcome. For all three measures, the curve was fit as a line using the enrollment score as a covariate. The analysis set for each measure was composed of men with an enrollment score and at least two postenrollment scores (necessary to estimate the intercept and slope of the line for each participant).
Table 2.
Health-related quality-of-life questionnaire submission rates: Physical Function, Mental Health, and Vitality Scales by treatment group and assessment time
| Assessment time | Questionnaire submitted* | Physical Function Score usable† | Mental Health Score usable‡ | Vitality Score usable§ | ||||
|---|---|---|---|---|---|---|---|---|
| Finasteride No. (%) | Placebo No. (%) | Finasteride No. (%) | Placebo No. (%) | Finasteride No. (%) | Placebo No. (%) | Finasteride No. (%) | Placebo No. (%) | |
| Enrollment | 9422 (100) | 9454 (100) | 9420 (100) | 9449 (99.9) | 9399 (99.7) | 9423 (99.6) | 9406 (99.8) | 9435 (99.8) |
| Time after randomization | ||||||||
| 6 mo | 8529 (94.5) | 8711 (94.7) | 8484 (94.0) | 8669 (94.3) | 8476 (93.9) | 8661 (94.2) | 8476 (93.9) | 8665 (94.2) |
| 1 y | 8193 (97.1) | 8591 (97.7) | 8185 (97.0) | 8578 (97.6) | 8174 (96.8) | 8564 (97.4) | 8175 (96.8) | 8565 (97.4) |
| 2 y | 7289 (96.9) | 7902 (96.9) | 7273 (96.7) | 7885 (96.7) | 7256 (96.5) | 7870 (96.5) | 7259 (96.5) | 7868 (96.5) |
| 3 y | 6720 (97.0) | 7330 (96.6) | 6695 (96.6) | 7310 (96.4) | 6679 (96.4) | 7298 (96.2) | 6686 (96.5) | 7297 (96.2) |
| 4 y | 6272 (97.1) | 6917 (97.1) | 6236 (96.5) | 6883 (96.6) | 6220 (96.3) | 6855 (96.2) | 6223 (96.3) | 6854 (96.2) |
| 5 y | 5948 (97.1) | 6515 (96.6) | 5917 (96.6) | 6472 (96.0) | 5884 (96.1) | 6440 (95.5) | 5886 (96.1) | 6442 (95.5) |
| 6 y | 5605 (96.4) | 6176 (96.3) | 5557 (95.6) | 6119 (95.4) | 5532 (95.2) | 6106 (95.2) | 5532 (95.2) | 6107 (95.2) |
| 7 y‖ | 5034 (96.9) | 5462 (96.7) | 4961 (95.5) | 5393 (95.5) | 4941 (95.1) | 5374 (95.1) | 4939 (95.1) | 5374 (95.1) |
* The primary reason for missing forms was a participant going off the assigned prevention agent, including men who were diagnosed with prostate cancer during the trial and men who died. Questionnaire submission rates were calculated for each treatment arm as the number of questionnaires submitted divided by the number of participants expected to complete the questionnaire (ie, the number on study long enough to reach specific assessment point).
† If more than 2 items omitted, the Physical Functioning score is not usable.
‡ If more than 1 item omitted, the Mental Health score is not usable.
§ If more than 1 item omitted, the Vitality score is not usable.
‖ Due to early trial closure, approximately 300 participants completed a final questionnaire at 6 y 6 mo or at 6 y 9 mo instead of at 7 y.
Effect of Missing Data on Model. To determine whether the presence of missing data was biasing estimates of the effect of finasteride vs placebo on health-related quality-of-life outcomes, we compared model coefficients for two sets of participants: the analysis set (those who had all covariates and at least enrollment and two follow-up Physical Functioning scores [n = 16 077], and those who had enrollment and all follow-up assessments [n = 9194]). Model coefficients for these two sets of participants were similar (data not shown). In addition, we applied multiple imputation (using the data present to repeatedly estimate missing values of the covariates and response values). Models with data missing at random are expected to produce stable parameter estimates in multiple imputation as well as estimates that are relatively similar to those produced by running the model on the analysis set. Multiple imputation produced very stable estimates that were similar to (ie, well within the 95% confidence intervals of) the parameters estimated when running the model on the analysis set (data not shown). The comparison of the models on the analysis set and the set of men with all assessments available, and the stable results of multiple imputation, suggest that the missing-at-random assumption is reasonable.
Covariate Selection Our selection of covariates was based on our review of the literature and suggestions from the members of the PCPT Quality-of-Life Advisory Committee. As a result of these deliberations, we identified primary and secondary sets of covariates. Time on study appears in all models (continuous variable, ranging from 0 at 6 months after randomization to 6.5 at 7 years after randomization); this covariate also serves as a surrogate for the effect of aging. The primary (or core) set of covariates, which was always included in the model, included finasteride (0 = no finasteride, 1 = finasteride); age at enrollment (continuous); race (three categories: non-Hispanic white, black, and other, coded with two indicator variables using non-Hispanic white as the referent group); BMI (continuous); current smoking status (three categories: current, former, and nonsmoker, coded with two indicator variables using nonsmokers as the referent group); Physical Functioning score at enrollment (continuous); interaction between Physical Functioning score at enrollment and finasteride; Vitality score at enrollment (continuous); Bodily Pain score at enrollment (continuous); and an interaction term with time for each of these covariates. Secondary covariates included years of post–high school education (continuous, rescaled so that some college or vocational school = 1, college graduate or higher = 2), marital status (0 = single at enrollment, 1 = married or marriage-like relationship at enrollment), MET-hours of exercise walking per week (continuous), weekly total fat intake (in grams, continuous), diabetes (0 = no, 1 = yes), hypertension (0 = no, 1 = yes), AUA Symptom Index score (continuous), Sexual Activity Scale score (continuous), leg pain (0 = no, 1 = yes), and congestive heart failure (0 = no, 1 = yes). All possible subsets of secondary covariate main effects were generated with the restriction that if a covariate was included in a model, its interaction with time was always required to be included (and vice versa). Different variables had different levels of missingness; to compare the fit of these models fairly, a dataset with no missing values for all covariates was generated. The models were all fit on this “no missing values” dataset, and the best model was selected using the Bayesian Information Criterion (22). To further examine the possible effects of finasteride on the three health-related quality-of-life–dependent variables, we examined higher-order interaction models, which were generated by simultaneously adding to each “best” model the two-way interactions of the covariate and finasteride and the three-way interactions of the covariate, finasteride, and time. These higher-order interactions did not statistically significantly improve the model fit: Bayesian Information Criterion values were higher (worse) and differences in log-likelihood were not statistically significant; all P-values were greater than .10.
Model Fit Assessments Three mixed-effects models (23) with correlated random time slopes and intercepts were used to estimate the fixed effects of finasteride treatment, time, demographic, and comorbidity covariates for physical function, mental health, and vitality; depending on the outcome variable of interest, the participant’s score at enrollment was included in that model. An interaction of finasteride with enrollment physical function was included in the physical function model; similar interaction terms were not required for the mental health and vitality outcome models, as they did not improve the model fit. The mixed-effects model also provided variance estimates describing participant differences in score curve trajectories (ie, variance estimates for individual slopes and intercepts).
Clinically Significant Predictors. We adjusted the following continuous covariates by subtracting the population median from the unscaled value at enrollment: age, BMI, Mental Health score, Vitality score, Physical Functioning score, Bodily Pain score, Sexual Activity Scale, AUA Symptom Index, MET-hours walking, and weekly total fat intake. The intercept term in each of the three models could be interpreted as the health-related quality-of-life score for the “median man” at 6 months after randomization (ie, a man in the placebo arm of the PCPT who was a nonsmoker, nondiabetic, nonhypertensive, did not have congestive heart failure, was never married, had a high school education or less, had no leg pain, and median scores on all linear covariates at enrollment). To further assist in the interpretation of the model results, we re-expressed the mixed-effects model estimates in three ways. First, the interactions with time are described in cross-sectional fashion at three time points (time 0 = 6 months after randomization, and at 3.5 and 6.5 years after time 0 [4 and 7 years after randomization, respectively]). Second, we compared the effect magnitude relative to the natural variation among similar men (ie, the systematic variability for individuals who share the same covariate profile). To calculate an effect ratio, the mean Physical Functioning score for participants having the condition (eg, diabetes) was subtracted from the mean score for those not having the covariate or condition; the ratio of this difference to the SD in Physical Functioning true scores for men sharing a similar covariate profile produces the effect ratio. This SD is estimated from the square root of the between-person variance of the intercept (ie, the Physical Functioning score when time = 0) from the mixed-effects analysis. Third, we based the expected impact of health-related quality-of-life covariates on a 10-point change in health-related quality of life (24).
According to the SF–36 manual, a sample size of 1364 persons with a repeated measures design has 80% power to detect a 2-point difference between two groups (eg, finasteride and placebo) in a Physical Functioning score change over time (eg, one group remaining stable over time, with the other declining as little as 2 points over time); sample sizes of 824 and 1108 are required to detect a 2-point difference between two groups for a change in score over time for Mental Health and Vitality scores, respectively [see Table 7.4 in Ware et al. (14)].
All analyses were done using SAS software; models were fitted with PROC MIXED in SAS (22). All tests of statistical significance were two-sided; P-values less than .05 were considered statistically significant.
Results
Participant Characteristics and Questionnaire Submission Rates
Demographic and clinical characteristics of study participants were similar between the finasteride and placebo arms (Table 1). At all nine time points, the submission rates for questionnaires with useable scores for the three primary health-related quality-of-life outcomes (Physical Functioning, Mental Health, and Vitality) exceeded 93% (Table 2). Questionnaire submission rates were less than 95% only at the 6-month postrandomization assessment (range = 93.9%–94.7%) (Table 2).
Table 1.
Characteristics of participants included in the physical function model at randomization*
| Characteristics | Finasteride | Placebo |
| (N = 7860) | (N = 8217) | |
| Mean age, y (SD) | 63.12 (5.66) | 63.15(5.66) |
| Age distribution, No. (%) | ||
| <55 y | 1 (0) | 1 (0) |
| 55–59 y | 2467 (31) | 2620 (32) |
| 60–64 y | 2491 (32) | 2472 (30) |
| 65 y | 2901 (37) | 3124 (38) |
| Race, No. (%) | ||
| White | 7269 (92) | 7619 (93) |
| Black† | 293 (4) | 285 (3) |
| Other | 298 (4) | 313 (4) |
| Annual household income distribution, No. (%) | ||
| $0–$10 000 | 16 (0) | 9 (0) |
| $10 001–$30 000 | 3217 (43) | 3221 (41) |
| $30 001–$50 000 | 3627 (48) | 3946 (50) |
| $50 001–$70 000 | 578 (8) | 629 (8) |
| >$70 000 | 84 (1) | 64 (1) |
| Unknown | 338 | 348 |
| Marital status, No. (%) | ||
| Never married | 180 (2) | 204 (2) |
| Previously married‡ | 696 (9) | 790 (10) |
| Presently married or in marriage-like relationship | 6984 (89) | 7223 (88) |
| Education level, No. (%) | ||
| High school graduate, GED, or less | 1485 (19) | 1478 (18) |
| Vocational or training school or some college | 2262 (29) | 2383 (29) |
| College graduate | 1293 (16) | 1365 (17) |
| Post-college education | 2820 (36) | 2991 (36) |
| Smoking status at PCPT enrollment, No. (%) | ||
| Lifetime nonsmoker | 2575 (33) | 2766 (34) |
| Former smoker | 4686 (60) | 4821 (59) |
| Current smoker | 599 (8) | 630 (8) |
| Mean No. cigarettes smoked/day (SD) | 15.24 (8.02) | 14.95 (7.96) |
| Mean No. years of smoking (SD) | 43.90 (5.90) | 44.00 (5.94) |
| Mean AUA Symptom Index score (SD) | 6.66 (4.79) | 6.61 (4.72) |
| Mean Sexual Activity Scale score (SD) | 43.95 (19.71) | 43.78 (19.73) |
| Mean total weekly fat intake, g (SD) | 202.68 (130.43) | 201.65 (131.15) |
| Mean serum HDLC, mg/dL (SD) | 42.88 (11.25) | 42.67 (11.14) |
| Mean BMI, kg/m2 (SD) | 27.68 (4.10) | 27.72 (4.15) |
| Mean MET-hours of walking (SD) | 13.74 (18.44) | 13.19 (17.54) |
| Mean No. alcoholic beverages per day (SD) | 0.79 (1.20) | 0.79 (1.17) |
| Health-related quality-of-life measures, mean (SD) | ||
| Bodily Pain score | 82.43 (18.74) | 82.24 (18.68) |
| Mental Health score | 81.46 (12.91) | 81.34 (12.95) |
| Vitality score | 71.46 (14.87) | 71.17 (15.05) |
| Physical Function score | 88.66 (15.69) | 88.31 (16.41) |
| Comorbidities, No. (%) | ||
| Diabetes | 409 (5) | 473 (6) |
| Hypertension | 2093 (27) | 2227 (27) |
| Leg pain | 1307 (17) | 1407 (17) |
| Congestive heart failure | 49 (1) | 32 (0) |
* GED = general equivalency diploma; PCPT = Prostate Cancer Prevention Trial; AUA = American Urological Association; HDLC = high-density lipoprotein cholesterol; BMI = body mass index; MET = metabolic equivalent.
† Includes black Hispanic.
‡ Divorced, separated, or widower.
Health-Related Quality-of-Life Outcomes
Descriptive Results Table 3 shows sample sizes, mean scores, and SDs by study arm at all time points for each of the three primary health-related quality-of-life outcomes; higher scores reflect better health-related quality of life. In both treatment arms, the Mental Health score increased and the Physical Functioning and Vitality scores decreased over time. However, there were no statistically significant differences in the mean scores for any SF–36 scale between the finasteride and placebo groups at any time point. We, therefore, highlight model results rather than these descriptive results because the models incorporate the effects of covariates.
Table 3.
Quality-of-life scale scores by treatment arm and assessment time for participants with usable scores*
| Assessment time | Physical Functioning score | Mental Health score | Vitality score | |||
|---|---|---|---|---|---|---|
| Finasteride | Placebo | Finasteride | Placebo | Finasteride | Placebo | |
| Enrollment | 88.4 (16.0) | 88.1 (16.6) | 81.2 (13.0) | 81.1 (13.1) | 71.1(15.0) | 70.9(15.2) |
| Time after randomization | ||||||
| 6 mo | 87.6 (16.8) | 87.4 (17.1) | 83.0 (12.8) | 83.4 (12.6) | 71.3 (16.1) | 71.3 (16.2) |
| 1 y | 86.6 (17.5) | 86.7 (17.3) | 83.4 (12.7) | 83.4 (12.8) | 70.5 (16.7) | 70.5 (16.7) |
| 2 y | 85.9 (18.0) | 86.0 (17.7) | 83.7 (12.6) | 83.9 (12.7) | 70.4 (16.9) | 70.4 (16.7) |
| 3 y | 84.9 (18.5) | 85.0 (18.5) | 83.9 (12.8) | 83.9 (12.7) | 69.8 (17.1) | 69.6 (17.4) |
| 4 y | 84.0 (19.1) | 84.2 (19.0) | 84.1 (12.6) | 84.1 (12.6) | 69.6 (17.6) | 69.2 (17.6) |
| 5 y | 82.8 (20.2) | 83.5 (19.1) | 84.0 (13.0) | 84.1 (12.8) | 68.9 (17.8) | 68.9 (17.8) |
| 6 y | 81.9 (20.9) | 82.3 (20.6) | 84.0 (13.0) | 84.3 (12.8) | 68.7 (17.9) | 68.6 (18.3) |
| 7 y | 81.3 (21.0) | 81.8 (20.8) | 84.1 (12.8) | 84.4 (13.0) | 68.5 (18.1) | 68.8 (17.9) |
* Data represent the mean score (SD). Physical Functioning, Mental Health, and Vitality scores can range from 0 to 100, and higher scores reflect better physical function, mental health, or vitality.
Mixed-Effects Model Results: Physical Function The full results of the mixed-effects model analysis for physical function are presented in Table 4. The Physical Functioning score at enrollment was a strong predictor of later physical function (estimate = 0.50, 95% confidence interval [CI] = 0.48 to 0.51, P < .001; time interaction estimate = −0.009, 95% CI = −0.012 to 0.005, P < .001). Neither finasteride as a main effect (estimate = 0.07, 95% CI = −0.28 to 0.42, P = .71) nor the finasteride interaction with time (estimate = −0.08, 95% CI = −0.19 to 0.01, P = .08) had a statistically significant effect on Physical Functioning scores. The interaction of finasteride with the enrollment Physical Functioning score was statistically significant (estimate = 0.03, 95% CI = 0.01 to 0.05, P = .004). This finding indicates that although the intercept of each participant’s curve over time was higher, on average, in the finasteride group than the placebo group, the difference depended on the enrollment Physical Functioning score. The finasteride main effect, finasteride interaction with enrollment Physical Functioning, and finasteride effect over time (interaction) were combined to calculate an overall finasteride effect on Physical Functioning, which was small (see “Discussion” below).
Table 4.
Mixed model results of treatment, covariate, and individual effects on report of physical function during 7 years of follow-up (N = 16 077)*
| Effect | Description | Estimate (95% CI) | P† |
|---|---|---|---|
| Intercept | On-average entry Physical Functioning score | 90.69 (90.08 to 91.30) | <.001 |
| Time | Time (in y) = 0 at first 6-mo visit | −1.20 (−1.36 to −1.03) | <.001 |
| Age‡ | Age (in y) at enrollment (median = 63 y) | −0.25 (−0.28 to −0.22) | <.001 |
| Time × age | Interaction with time | −0.08 (−0.09 to −0.07) | <.001 |
| Finasteride | Treatment arm: finasteride = 1, placebo = 0 | 0.07 (−0.28 to 0.42) | .71 |
| Time × finasteride | Interaction with time | −0.08 (−0.19 to 0.01) | .08 |
| Black race | Race – black: black = 1, non-black = 0 | −0.93 (−1.82 to −0.05) | .04 |
| Time × black race | Interaction with time | −0.08 (−0.33 to 0.16) | .50 |
| Other race | Race – other: non-white and non-black = 1, white or black = 0 | −1.09 (−2.36 to 0.19) | .09 |
| Time × other race | Interaction with time | 0.10 (−0.28 to 0.47) | .62 |
| BMI‡ | BMI at enrollment, (weight in kg)/(height in m)2; 18.5–24.9kg/m2 is considered normal (median = 27.13kg/m2) | −0.29 (−0.33 to −0.25) | <.001 |
| Time × BMI | Interaction with time | −0.05 (−0.06 to −0.04) | <.001 |
| Current smoking | Indicator, current smoker at enrollment: yes = 1, no = 0 | −2.34 (−2.97 to −1.71) | <.001 |
| Time × current smoking | Interaction with time | −0.49 (−0.67 to −0.31) | <.001 |
| Physical Function score‡ | SF–36 Physical Functioning score at enrollment: 0–100, higher = better functioning (median = 95) | 0.50 (0.48 to 0.51) | <.001 |
| Finasteride × Physical Function score | Finasteride interaction with SF–36 Physical Function score at enrollment | 0.03 (0.01 to 0.05) | .004 |
| Time × Physical Function score | Interaction with time | −0.009 (−0.012 to −0.005) | <.001 |
| Vitality score‡ | SF–36 Vitality score at enrollment: 0–100, higher = less fatigue (median = 75) | 0.09 (0.07 to 0.10) | <.001 |
| Time × Vitality score | Interaction with time | 0.01 (0.01 to 0.16) | <.001 |
| Bodily Pain score‡ | SF–36 Bodily Pain score at enrollment: 0–100, higher = less pain (median = 84) | 0.10 (0.09 to 0.11) | <.001 |
| Time × Bodily Pain score | Interaction with time | −0.0004 (−0.0032 to 0.0024) | .78 |
| Variables added to a model containing all of the above variables | |||
| Education level | Years of education at enrollment, beyond high school, rescaled: 0 = high school or less, 1 = some college or vocational school, 2 = college graduate or higher | 0.52 (0.37 to 0.66) | <.001 |
| Time × education level | Interaction with time | 0.06 (0.02 to 0.10) | .005 |
| Marital status | Married at enrollment: 0 = no, 1 = yes | 0.92 (0.41 to 1.43) | <.001 |
| Time × marital status | Interaction with time | 0.19 (0.05 to 0.33) | .008 |
| MET-hours/wk of walking‡ | MET-hours/wk of exercise through walking at enrollment (median = 7) | 0.03 (0.02 to 0.04) | <.001 |
| Time × MET-hours/wk of walking | Interaction with time | −0.0019 (−0.0006 to 0.0045) | .14 |
| Diabetes | Diabetes at enrollment: yes = 1, no = 0 | −1.31 (−2.04 to −0.57) | <.001 |
| Time × diabetes | Interaction with time | −1.04 (−1.24 to −0.83) | <.001 |
| Hypertension | Hypertension at enrollment: yes =1, no = 0 | −0.73 (−1.11 to −0.35) | .000 |
| Time × hypertension | Interaction with time | −0.23 (−0.34 to −0.13) | <.001 |
| Mental Health score‡ | SF–36 Mental Health score at enrollment: 0–100, higher score = better mental health (median = 84) | −0.06 (−0.08 to −0.04) | <.001 |
| Time × Mental Health score | Interaction with time | −0.004 (−0.009 to −0.000) | .05 |
| AUA Symptom Index‡ | AUA score at enrollment: 0–35, higher score = more urinary symptoms (median = 6) | −0.10 (−0.13 to −0.06) | <.001 |
| Time × AUA Symptom Index score | Interaction with time | −0.01 (−0.02 to 0.00) | .16 |
| SAS score‡ | SAS score at enrollment: 0–100, higher score = more sexual activity (median = 39) | −0.03 (−0.04 to −0.02) | .10 |
| Time × SAS score | Interaction with time | −0.003 (−0.005 to −0.0000) | .02 |
| Leg pain | Leg pain at enrollment: yes = 1, no = 0 | −2.57 (−3.04 to −2.10) | <.001 |
| Time × leg pain | Interaction with time | −0.15 (−0.28 to −0.02) | .03 |
| Congestive heart failure | Congestive heart failure at enrollment: yes = 1, no = 0 | −5.64 (−7.96 to −3.32) | <.001 |
| Time × congestive heart failure | Interaction with time | 0.25 (−0.44 to 0.94) | .48 |
* CI = confidence interval; BMI = body mass index; SF–36 = Health Survey Short Form–36; MET = metabolic equivalent; AUA = American Urological Association; SAS = Sexual Activity Scale.
† Two-sided t-test.
‡ Variable adjusted by subtracting median score (median is shown in brackets in description column)
Although most of the regression coefficients were statistically significantly different from zero, the magnitudes of most effect estimates were small. The following covariates were associated with lower Physical Functioning scores and had larger effect estimates relative to other estimates such as the finasteride main effect: diabetes (estimate = −1.31, 95% CI = −2.04 to −0.57, P < .001; time interaction estimate = −1.04, 95% CI = −1.24 to −0.83, P < .001); hypertension (estimate = −0.73, 95% CI = −1.11 to −0.35, P < .001; time interaction estimate = −0.23, 95% CI = −0.34 to −0.13, P < .001); congestive heart failure (estimate = −5.64, 95% CI = −7.96 to −3.32, P < .001; time interaction estimate = 0.25, 95% CI = −0.44 to 0.94, P = .48); leg pain (estimate = −2.57, 95% CI = −3.04 to −2.10, P < .001; time interaction estimate = −0.15, 95% CI = −0.28 to −0.02, P =.03); and current smoking status at enrollment (estimate = −2.34, 95% CI = −2.97 to −1.71, P < .001; time interaction estimate = −0.49, 95% CI = −0.67 to −0.31, P < .001). In addition, participant time on study was also negatively associated with Physical Functioning scores (estimate = −1.20, 95% CI = −1.36 to −1.03, P < .001).
Although the mixed-effects model results for mental health and vitality were generally similar to those for physical function, some differences were observed (data not shown). Finasteride was not associated with change in the Mental Health score over time (estimate = −0.28, 95% CI = −0.48 to 0.07, P = .14; time interaction estimate = 0.02, 95% CI = −0.04 to 0.08, P = .43). The strongest positive predictors of Mental Health over time were enrollment marital status (estimate = 1.28, 95% CI = 0.86 to 1.70, P < .001), the Mental Health score at enrollment (estimate = 0.43, 95% CI = 0.41 to 0.44, P < .001; time interaction estimate = −0.01, 95% CI = −0.02 to −0.01, P < .001), Bodily Pain scores at enrollment where higher scores reflect less pain (estimate = 0.06, 95% CI = 0.05 to 0.07, P < .001), enrollment Vitality score (estimate = 0.04, 95% CI = 0.03 to 0.05, P < .001), and enrollment Physical Functioning score (estimate = 0.03, 95% CI = 0.02 to 0.04, P < .001). The strongest negative predictors of Mental Health scores over time were current smoking at enrollment (estimate = −1.42, 95% CI = −1.98 to −0.86, P < .001), diabetes (estimate = −0.78, 95% CI = −1.39 to −0.17, P = .012; time interaction estimate = −0.29, 95% CI = −0.42 to −0.16, P < .001), the AUA Symptom Index (estimate = −0.14, 95% CI = −0.17 to −0.11, P < .001), and the Sexual Activity Score (estimate = −0.037, 95% CI = −0.045 to −0.030, P < .001).
Finasteride was not associated with change in the Vitality score over time (estimate = −0.05, 95% CI = −0.38 to 0.28, P = .807; time interaction estimate = 0.01, 95% CI = −0.07 to 0.09, P = .083). Positive predictors of change in the Vitality score were black race (estimate = 1.86, 95% CI = 0.96 to 2.76, P < .001), marital status (estimate = 1.11, 95% CI = 0.60 to 1.63, P < .001; interaction with time estimate = 0.23, 95% CI = 0.11 to 0.35, P < .001), the Vitality score at enrollment (estimate = 0.57, 95% CI = 0.56 to 0.59, P < .001; interaction with time estimate = −0.007, 95% CI = −0.010 to 0.003, P < .001), enrollment BMI (estimate = −0.09, 95% CI = −0.13 to −0.05, P < .001; interaction with time estimate = −0.02, 95% CI = −0.03 to −0.01, P < .001), enrollment Physical Function score (estimate = 0.07, 95% CI = 0.06 to 0.08, P < .001), enrollment Bodily Pain score (estimate = 0.06, 95% CI = 0.05 to 0.07, P < .001), MET-hours of walking at enrollment (estimate = 0.04, 95% CI = 0.03 to 0.05, P < .001). Negative predictors of change in the Vitality score were current smoking at enrollment (estimate = −1.81, 95% CI = −2.45 to −1.18, P < .001; time interaction estimate = −0.27, 95% CI = −0.42 to −0.12, P < .001); enrollment leg pain (estimate = −1.61, 95% CI = −2.08 to −1.13, P < .001); diabetes at enrollment (estimate = −1.58, 95% CI = −2.32 to −0.83, P < .001; time interaction estimate = −0.63, 95% CI = −0.81 to −0.46, P < .001); hypertension (estimate = −0.82, 95% CI = −1.21 to −0.44, P < .001); AUA Symptom Index score (estimate = −0.24, 95% CI = −0.27 to −0.20, P < .001); enrollment Mental Health score (estimate = −0.07, 95% CI = −0.09 to −0.06, P < .001); and enrollment Sexual Activity Scale score (estimate = −0.04, 95% CI = −0.05 to −0.03, P < .001).
Cross-sectional Perspective: Physical Function. Because the unfamiliar metrics and model complexity make it difficult to understand the practical meaning of estimates from mixed-effect models, in Table 5 we provide additional interpretations of these data. To understand how interactions with time modified the effects of covariates, we chose three time points: T = 0 (ie, 6 months after randomization), T = 3.5 years after baseline (ie, 4 years after randomization), and T = 6.5 years after baseline (ie, 7 years after randomization). At each time point, we combined the respective covariate and interaction effects to yield a cross-sectional perspective. We calculated our estimates to reflect clinically meaningful differences. For example, a clinically meaningful difference is presence or absence of a comorbidity (ie, diabetes, hypertension, or congestive heart failure). For some linear covariates (eg, BMI or age), a 1-unit difference is clinically meaningful. However, a 1-point difference on an SF–36 scale is clinically negligible. A 10-point difference has been previously documented as clinically meaningful for 0–100 scales (24). Therefore, estimates in Table 5 for the SF–36 scales are multiplied by 10 to display the effect of a 10-point difference.
Table 5.
Cross-sectional perspective: SF–36 Physical Function outcome (N = 16 077)*
| Effect | Description | Time = 0 | Time = 3.5 y | Time = 6.5 y | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | Effect ratio† | P‡ | Estimate (95% CI) | Effect ratio† | P‡ | Estimate (95% CI) | Effect ratio† | P‡ | ||
| Intercept | On-average entry Physical Functioning score | 90.69 (90.08 to 91.30) | – | <.001 | 90.69 (90.08 to 91.30) | – | <.001 | 90.69 (90.08 to 91.30) | – | <.001 |
| Time | Time in y; time = 0 at first 6-mo visit | 0 | – | – | −4.18 (−4.77 to −3.59) | – | <.001 | −7.77 (−8.87 to −6.67) | – | <.001 |
| Age | Age (in y) at randomization | −0.25 (−0.29 to −0.21) | – | <.001 | −0.54 (−0.58 to −0.50) | – | <.001 | −0.78 (−0.84 to −0.72) | – | <.001 |
| Finasteride§ | Treatment arm; finasteride = 1, placebo = 0 | 0.07 (−0.28 to 0.42) | 0.01 | .71 | −0.21 (−0.60 to 0.18) | −0.02 | .30 | −0.45 (−1.04 to 0.14) | −0.05 | .13 |
| Black race | Yes = 1, no = 0 | −0.93 (−1.81 to −0.05) | −0.10 | .04 | −1.23 (−2.27 to −0.19) | −0.14 | .02 | −1.49 (−3.08 to 0.10) | −0.17 | .07 |
| Other race | Not black, not non-Hispanic white: yes = 1, no = 0 | −1.09 (−2.36 to 0.18) | −0.12 | .09 | −0.75 (−2.28 to 0.78) | −0.08 | .34 | −0.46 (−2.85 to 1.93) | −0.05 | .71 |
| Body mass index | Body mass index at enrollment, (weight in kg)/(height in m)2, 18.5–24.9kg/m2 is considered normal | −0.29 (−0.33 to −0.25) | – | <.001 | −0.46 (−0.50 to −0.42) | – | <.001 | −0.61 (−0.69 to −0.53) | – | <.001 |
| Current smoker | Indicator, current smoker at enrollment: yes = 1,no = 0 | −2.34 (−2.97 to −1.71) | −0.26 | <.001 | −4.06 (−4.80 to −3.32) | −0.45 | <.001 | −5.53 (−6.67 to −4.39) | −0.62 | <.001 |
| Physical Function | SF–36 Physical Functioning score at enrollment (scale: 0–100): higher score = better functioning | 5.00 (4.84 to 5.16) | – | <.001 | 4.70 (4.52 to 4.88) | – | <.001 | 4.44 (4.20 to 4.68) | – | <.001 |
| Vitality|| | SF–36 Energy/Vitality score at enrollment (0–100): higher score = more energy | 0.89 (0.73 to 1.05) | – | <.001 | 1.29 (1.11 to 1.47) | – | <.001 | 1.63 (1.36 to 1.90) | – | <.001 |
| Bodily Pain|| | SF–36 Bodily Pain Scale at enrollment (0–100): higher score = less pain | 0.97 (0.87 to 1.07) | – | <.001 | 0.96 (0.84 to 1.08) | – | <.001 | 0.94 (0.76 to 1.12) | – | <.001 |
| Education level¶ | At enrollment: 3 levels | 0.52 (0.36 to 0.68) | – | <.001 | 0.72 (0.54 to 0.90) | – | <.001 | 0.89 (0.64 to 1.14) | – | <.001 |
| Marital status¶ | At enrollment: 2 levels | 0.92 (0.41 to 1.43) | 0.10 | <.001 | 1.59 (1.00 to 2.18) | 0.18 | <.001 | 2.16 (1.26 to 3.06) | 0.24 | <.001 |
| MET-hours of walking | At enrollment | 0.03 (0.03 to 0.03) | – | <.001 | 0.04 (0.02 to 0.06) | – | <.001 | 0.05 (0.03 to 0.07) | – | <.001 |
| Diabetes | Indicator, diabetes at enrollment: yes = 1, no = 0 | −1.31 (−2.05 to−0.57) | −0.15 | <.001 | −4.94 (−5.80 to −4.08) | −0.55 | <.001 | −8.06 (−9.37 to −6.75) | −0.90 | <.001 |
| Hypertension | Indicator, hypertension at enrollment: yes = 1, no = 0 | −0.73 (−1.12 to−0.34) | −0.08 | <.001 | −1.55 (−2.00 to −1.10) | −0.17 | <.001 | −2.26 (−2.93 to −1.59) | −0.25 | .01 |
| Mental Health|| | SF–36 Mental Health score at enrollment (0–100): higher score = better mental health | −0.60 (−0.76 to−0.44) | – | <.001 | −0.87 (−0.96 to −0.56) | – | <.001 | −1.01 (−1.18 to−0.60) | – | <.001 |
| AUA Symptom Index | AUA SymptomIndex at enrollment (0–35): higher score = worse urinary symptoms | −0.69 (−0.94 to −0.44) | – | <.001 | 0.87 (−1.16 to −0.58) | – | <.001 | −1.01 (−1.46 to−0.56) | – | <.001 |
| Sexual Activity Scale|| | Sexual Activity Scale score at enroll- ment (0–100): higher score = more sexual dysfunction | −0.30 (−0.40 to −0.20) | – | <.001 | −0.40 (−0.50 to −0.30) | – | <.001 | −0.49 (−0.65 to−0.33) | – | <.001 |
| Leg pain | Leg pain at enrollment: yes = 1, no = 0 | −2.57 (−3.04 to −2.10) | −0.29 | <.001 | −3.09 (−3.64 to −2.54) | −0.35 | <.001 | −3.54 (−4.36 to−2.72) | −0.45 | <.001 |
| Congestive heart failure | Congestive heart failure at enrollment: yes = 1, no = 0 | −5.64 (−7.97 to −3.31) | −0.63 | <.001 | −4.76 (−7.56 to −1.96) | −0.53 | <.001 | −4.01 (−8.40 to0.38) | −0.45 | .07 |
* CI = confidence interval; – = not applicable; SF–36 = Health Survey Short Form–36; MET = metabolic equivalent; AUA = American Urological Association.
† Ratio of effect estimate to σβ0, the SD of the random intercept term. Effect ratios are not calculated for continuous variables.
‡ Two-sided t-test.
§ The finasteride effects shown in the table are those expected for the “typical” man having the median Physical Functioning score at enrollment. The impact of finasteride depends on the enrollment Physical Functioning score and varies from a 1-point benefit (for those with the maximum Physical Functioning score at enrollment) to a 1-point deficit (for those with the minimum Physical Functioning score at enrollment). This variation is statistically significant but negligible in real terms.
‖ Estimate represents the expected effect on the Physical Functioning score of a 10-point difference in this covariate at enrollment.
¶ See Table 4 for variable categories.
Finasteride was not statistically significantly associated with the Physical Functioning score at any of the three time points (Table 5). Having diabetes at enrollment was associated with an 8.06-point decrease in the Physical Functioning score at 6.5 years after randomization (95% CI = −9.37 to −6.75, P < .001). Being on study for 7 years was associated with 7.77-point decrease in the Physical Functioning score by the end of the study (95% CI = −8.87 to −6.67, P < .001). Being a current smoker at enrollment was associated with a 5.53-point decrease in the Physical Functioning score at 6.5 years after randomization (95% CI = −6.67 to −0.53, P < .001). Congestive heart failure, leg pain, and hypertension at enrollment were associated with 4.01-point (95% CI = −8.40 to 0.38, P = .073), 3.54-point (95% CI = −4.36 to −2.72, P < .001), and 2.26-point (95% CI = −2.93 to −1.59, P < .001) decreases, respectively, in Physical Functioning scores at 6.5 years after randomization.
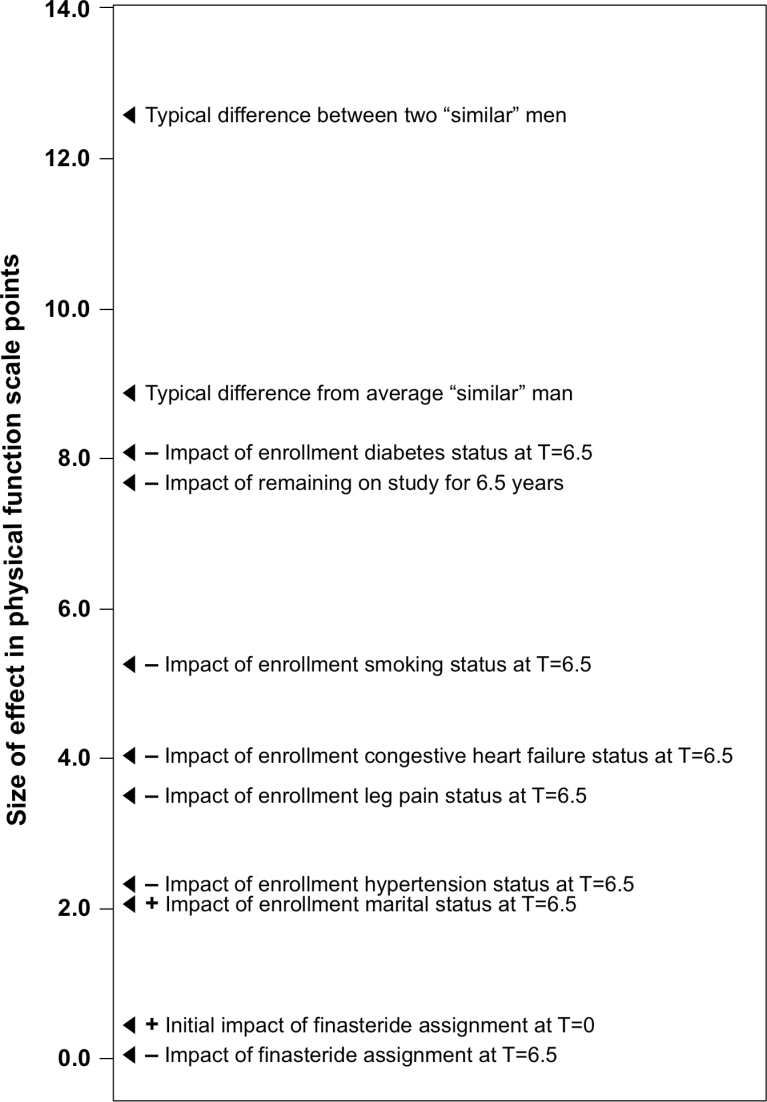
Variability among individual participants in Physical Functioning score was high: the SD of individual intercepts (estimated by maximum likelihood) was approximately 8.92. This is the amount by which a typical man’s expected Physical Functioning score would differ from the average Physical Functioning score for all similar men (those sharing the same treatment assignment, enrollment, Physical Functioning level, and other covariate values). The estimated difference between any two randomly chosen men with the same covariate profile is 12.62 points in Physical Functioning score. Even some of the larger effects (such as the −5.53-point effect at 6.5 years after randomization of being a current smoker at enrollment) are small compared with this systematic inter-individual variability. To expand on the importance of considering inter-individual variation in clinical trial results (25) and to provide context for interpreting the clinical importance of the covariates associated with self-reported physical function, we include Figure 2, which compares the relative expected impacts of covariates on Physical Functioning scores at 6 months after randomization (T = 0) with that at 7 years after randomization (T = 6.5 years).
Figure 2.
Relative levels of treatment, covariate, and individual effects on change in Physical Functioning scores. This figure illustrates the expected impact, in Physical Functioning points (adjusted for Physical Functioning level at T = 0 [6 months after randomization]), of study covariates as well as individual differences (expected difference between two “similar” men and the expected deviation from “similar” men). Individual differences pertain to systematic variation in Physical Functioning among patients sharing the same covariate profile (including receipt of finasteride). Effects on Physical Functioning are also shown at T = 6.5 (7 years after randomization). Covariates associated with a negative impact on Physical Functioning scores are preceded by a minus sign (−) and those associated with a positive impact on Physical Functioning scores are preceded by a plus sign (+).
In addition, in Table 5 we also present the semistandardized effect sizes for dichotomous covariates as a ratio of the estimate to the SD of the random intercept term (this SD scales systematic variability across individuals who share the same predictor profile). This effect ratio is an estimate of how large the average cross-condition effect is compared to the amount a typical patient systematically differs from the within-condition (those who shared a predictor profile) average. The magnitudes of the ratios for the effect of finasteride on Physical Functioning scores over time were very small (effect ratios of 0.01, −0.02, and −0.05 at the three time points), reflecting the clinically unimportant effect of finasteride on Physical Functioning scores. By contrast, 6.5 years after randomization, the effect ratio for having diabetes at study enrollment was −0.90, which is a clinically large effect on Physical Functioning scores (26). Current smoking at enrollment had a moderate effect on Physical Functioning scores, with an effect ratio of −0.62 at 6.5 years after randomization. Both of these variables were more important over time than having other comorbidities at study enrollment with moderate to small effect sizes over time, such as leg pain (effect ratio = −0.45).
Discussion
In this study, we found that taking finasteride over a 7-year period did not affect any of the three primary health-related quality-of-life domains—physical function, mental health, or vitality— either positively or negatively. Other medical and behavioral variables, such as comorbidity status and being a current smoker at study enrollment, had the largest effects on physical function. The findings from the study entry health-related quality-of-life profile for PCPT participants also provided early indication of the negative impact of comorbid medical conditions (particularly diabetes) on Physical Functioning scores at study entry. However, covariate effects for the 7-year trial period were smaller than differences observed between any two men with similar covariate profiles, indicating that natural sources of variation were a major source of differences in reported Physical Functioning scores.
To put our results in perspective, it is important to keep in mind that the PCPT enrolled healthy men (11) interested enough in preventing cancer that they committed to a 7-year trial that addressed the value of finasteride as a preventive agent. Finasteride resulted in a 25% reduction in the 7-year prevalence of histologically proven prostate cancer (9). Although the initial results (9) revealed a higher prevalence of high-grade prostate cancer in men who were randomly assigned to receive finasteride compared with men who received placebo, a later analysis that adjusted for several biases found that finasteride did not increase the risk of high-grade prostate cancer (27). However, it is also important to include health-related quality-of-life outcomes in primary cancer prevention trials to ensure that preventative agents do not negatively impact the quality of life in healthy men, as noted by Rowland et al. (28). To this end, we included the SF–36 measure of health-related quality of life in the PCPT as well as two measures of sexual function (the Sexual Activity Scale and Sexual Problems Scale), because a decrease in sexual function was a known side effect of taking finasteride (7,9). In a previous publication (10), we examined the effects of finasteride on the Sexual Activity Scale and found a very small effect of finasteride on this scale that decreased over time and was much smaller than the typical variability observed between men sharing a similar covariate profile.
Finasteride is approved for the treatment of benign prostatic hyperplasia (29). PCPT participants in the finasteride arm reported a 40%–44% reduction in incident, symptomatic benign prostatic hyperplasia compared with men who received placebo (30,31). These lower urinary tract symptoms have been associated with more falls in men (32). Benign prostatic hyperplasia symptoms are also associated with reduced health-related quality of life (33,34). These findings suggest that the use of finasteride to control benign prostatic hyperplasia symptoms may have health-related quality-of-life benefits that were not measured in the PCPT. Taken together with the other findings discussed above, our results indicate that finasteride is a low-risk preventative agent with minimal impact on health-related quality of life.
A possible limitation of this study is that health-related quality-of-life data were not collected from participants who went off study (either temporarily or permanently). However, we conducted two sets of analyses to evaluate the impact of missing data on health-related quality-of-life estimates and found that both estimates were similar to those generated for participants with all follow-up assessments.
In conclusion, taking finasteride for 7 years did not appear to harm general aspects of health-related quality of life. Mean SF–36 scores for PCPT participants receiving finasteride were very similar to those for participants receiving placebo at 7 years; however, participants who had comorbid medical conditions at study enrollment reported compromised health-related quality of life (11), and this relationship between comorbidity status and health-related quality of life persisted over time. Our results show that natural sources of variability in this heterogeneous population and comorbidity status at study entry, particularly diabetes and current smoking status, had a greater clinically relevant impact on the Physical Functioning score than did finasteride treatment. Our findings reinforce the need to consider individual differences in age, time on study, smoking status, and medical comorbidities when evaluating the effect of different preventive interventions on health-related quality of life.
Funding
This research was supported by Public Health Service grants no. 5 U10 CA37429 and 2 U10 CA37429-09 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD (to IMT, principal investigator).
Notes
The study agents (finasteride and placebo) were provided by Merck, Inc. Merck, Inc, and the National Cancer Institute (NCI), Division of Cancer Prevention (DCP), also provided small grants to produce videos and to support projects to enhance trial recruitment and adherence. The study sponsor, DCP/NCI, provided input regarding study design but had no role in the following study activities: collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors extend appreciation to PCPT participants who provided health behaviors information at study entry and faithfully completed the health-related quality-of-life and health behaviors forms over the course of the trial. Study clinical research associates and nurses were also instrumental in our ability to achieve such impressive submission rates for the participant-completed forms.
Drs Donaldson, Ganz, Moinpour, Patrick, Shumaker, and Ware were members of the Quality of Life Advisory Committee for the PCPT.
References
- 1. Byrnes CA, Morton AS, Liss CL, Lippert MC, Gillenwater JY. Investigators obotC. Efficacy, tolerability, and effect on health-related quality of life of finasteride versus placebo in men with symptomatic benign prostatic hyperplasia: A community-based study Clin Ther 1995. 17(5):956–969 [DOI] [PubMed] [Google Scholar]
- 2. Girman CJ, Kolman C, Liss CL, et al. Effects of finasteride on health-related quality of life in men with symptomatic benign prostatic hyperplasia Prostate. 1996. 29(2):83–90 [DOI] [PubMed] [Google Scholar]
- 3. Bruskewitz R, Girman CJ, Fowler J, et al. Effect of finasteride on bother and other health-related quality of life aspects associates with benign prostatic hyperplasia Urology. 1999. 54(4):670–678 [DOI] [PubMed] [Google Scholar]
- 4. Altomare G, Capella GL. Depression circumstantially related to the administration of finasteride for androgenetic alopecia J Dermatol. 2002. 29(10):665–669 [DOI] [PubMed] [Google Scholar]
- 5.Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clin Pharmacol. 2006;6::7. doi: 10.1186/1472-6904-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brawley OW, Ford LG, Thompson I, Perlman JA, Kramer BS. 5-alpha-reductase inhibition and prostate cancer prevention Cancer Epidemiol Biomarkers Prev. 1994. 3(2):177–182 [PubMed] [Google Scholar]
- 7. Gormley GJ, Brawley O, Thompson IM. The potential application of finasteride for chemoprevention of prostate cancer Ann N Y Acad Sci. 1995. 768 163–169 [DOI] [PubMed] [Google Scholar]
- 8. Thompson IM, Coltman CA. Screening for prostate cancer: opportunities for prevention Semin Urol Oncol. 1996. 14(2):4–11 [PubMed] [Google Scholar]
- 9. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer N Engl J Med 2003. 340(3):215–224 [DOI] [PubMed] [Google Scholar]
- 10. Moinpour CM, Darke AK, Donaldson GW, et al. Longitudinal analysis of sexual function reported by men in the Prostate Cancer Prevention Trial J Natl Cancer Inst. 2007. 99(13):1025–10–35 [DOI] [PubMed] [Google Scholar]
- 11. Moinpour CM, Lovato LC, Thompson IM,, Jr, et al. Profile of men randomized to the prostate cancer prevention trial: baseline health-related quality of life, urinary and sexual functioning, and health behaviors J Clin Oncol 2000. 18(9):1942–1953 [DOI] [PubMed] [Google Scholar]
- 12. Ware JE,, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection Med Care. 1992. 30(6):473–483 [PubMed] [Google Scholar]
- 13. McHorney C, Ware JE,, Jr, Raczek A. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs Med Care. 1993. 31(3):247–263 [DOI] [PubMed] [Google Scholar]
- 14. Ware JE, Jr, Snow KK, Kosinski MA, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide Boston, MA: Nimrod Press; 1993. [Google Scholar]
- 15. McHorney CA, Ware JE, Jr, Lu RJF, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups Med Care. 1994. 32(1):40–46 [DOI] [PubMed] [Google Scholar]
- 16. Stewart AL, Hays RD, Wells KB, Rogers WH. Development of measures of physical activity/exercise and tests of variations in physical activity levels within chronic disease groups in the medical outcomes study. Report no P-7781. Santa Monica CA: RAND Corporation; 1993. [Google Scholar]
- 17. Stewart AL, Hays RD, Wells KB, Rogers WH, Spritzer KL, Greenfield S. Long-term functioning and well-being outcomes associated with physical activity and exercise in patients with chronic conditions in the medical outcomes study J Clin Epidemiol. 1994. 47(7):719–730 [DOI] [PubMed] [Google Scholar]
- 18. Satia-Abouta J, Patterson RE, Schiller RN, Kristal AR. Energy from fat is associated with obesity in U.S. men: results from the Prostate Cancer Prevention Trial Prev Med. 2002. 34(5):493–501 [DOI] [PubMed] [Google Scholar]
- 19. Block G, Clifford C, Maughton MD, Henderson M, McAdams M. A brief dietary screen for high fat intake J Nutr Educ 1989. 21(5):199–207 [Google Scholar]
- 20. Barry MJ, Fowler F, Jr, O’Leary MP, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia J Urol. 1992. 148(5):1549–1557 [DOI] [PubMed] [Google Scholar]
- 21. Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. Measuring disease-specific health status in men with benign prostatic hyperplasia Med Care. 1995. 33(4):AS145–AS155 [PubMed] [Google Scholar]
- 22. Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 23. Laird NM, Ware JW. Random-effects models for longitudinal data Biometrics 1982. 38(4):963–974 [PubMed] [Google Scholar]
- 24. Sloan JA, Loprinzi CL, Kuross SA, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer J Clin Oncol. 1998. 16(11):3662–3673 [DOI] [PubMed] [Google Scholar]
- 25. Donaldson G. Patient-reported outcomes and the mandate of measurement Qual Life Res 2008. 17(10):1303–13–13 [DOI] [PubMed] [Google Scholar]
- 26. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale: NJ: Lawrence Erlbaum Associates; Publishers; 1988. [Google Scholar]
- 27. Redman MW, Tangen CM, Goodman PJ, Lucia MS, Coltman CA, Jr, Thompson IM. Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach Cancer Prev Res (Phila) 2008. 1(3):174–1–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rowland JH, Varricchio CG, Trimble EL, Gore-Langton RE. Clinical trials referral resource Oncology. 2001. 15(11):1455–1457 [PubMed] [Google Scholar]
- 29. Robert G, Descazeaud A, de la Taille A. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: who are the high-risk patients and what are the best treatment options? Curr Opin Urol. 2011. 21(1):42–48 [DOI] [PubMed] [Google Scholar]
- 30. Parsons J, Schenk JM, Arnold K, et al. Finasteride reduces the risk of incident symptomatic benign prostatic hyperplasia. Paper presented at Late Breaking Science Forum, annual meeting of the American Urological Association; May 14–19, 2011; . Washington, DC [Google Scholar]
- 31. Parsons JK, Schenk JM, Arnold KB, et al. Finasteride reduces the risk of incident clinical benign prostatic hyperplasia European Urology. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parsons JK, Mougey J, Lambert L, et al. Lower urinary tract symptoms increase the risk of falls in older men BJU Int. 2009. 104(1):63–6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Engström G, Henningsohn L, Walker-Engström M-L, Leppert J. Impact on quality of life of different lower urinary tract symptoms in men measured by the Short Form 36 questionnaire Scand J Urol Nephrol. 2006. 40(6):485–494 [DOI] [PubMed] [Google Scholar]
- 34. Coyne KS, Wein AJ, Tubaro A, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS BJU Int. 2009. 103 (s3):4–11 [DOI] [PubMed] [Google Scholar]